Abstract
The major outward chloride transporter in neurons is the potassium chloride co-transporter 2 (KCC2), critical for maintaining an inhibitory reversal potential for GABAA receptor channels. In a recent study, we showed that Zn2+ regulates GABAA reversal potentials in the hippocampus by enhancing the activity of KCC2 via an increase in its surface expression. Zn2+ initiates this process by activating the Gq-coupled metabotropic Zn2+ receptor mZnR/GPR39. Here, we first demonstrated that mZnR/GPR39 is functional in cortical neurons in culture and then tested the hypothesis that the increase in KCC2 activity is mediated through a SNARE-dependent process. We established the presence of functional mZnR in rat cultured cortical neurons by loading cells with a Ca2+ indicator and exposing cells to Zn2+, which triggered consistent Ca2+ responses that were blocked by the Gq antagonist YM-254890, but not by the metabotropic glutamate receptor antagonist MCPG. Importantly, Zn2+ treatment under these conditions did not increase the intracellular concentrations of Zn2+ itself. We then measured KCC2 activity by monitoring both the rate and relative amount of furosemide-sensitive NH4+ influx via the co-transporter using an intracellular pH sensitive fluorescent indicator. We observed that Zn2+ pretreatment induced a Ca2+-dependent increase in KCC2 activity. The effects of Zn2+ on KCC2 activity were also observed in wild-type mouse cortical neurons in culture, but not in neurons obtained from mZnR/GPR39−/− mice, suggesting that Zn2+ acts via mZnR/GPR39 activation to upregulate KCC2 activity. We next transfected rat cortical neurons with a plasmid encoding botulinum toxin C1 (Botox C1), which cleaves the SNARE proteins syntaxin 1 and SNAP-25. Basal KCC2 activity was similar in both transfected and non-transfected neurons. Non-transfected cells, or cells transfected with marker vector alone, showed a Zn2+-dependent increase in KCC2 activity. In contrast, KCC2 activity in neurons expressing Botox C1 was unchanged by Zn2+. These results suggest that SNARE proteins are necessary for the increased activity of KCC2 following Zn2+ stimulation of mZnR/GPR39.
Keywords: Zinc, metabotropic zinc receptor, GPR39, KCC2, botulinum toxin, SNARE
Zn2+ is found in glutamate-containing synaptic vesicles in neurons that are abundant in the cerebral cortex, limbic structures and auditory brainstem, among other regions (Frederickson et al., 2005, Sensi et al., 2009). Vesicular Zn2+ has been shown to be released in a calcium and activity-dependent manner (Qian and Noebels, 2005, 2006) and can reduce postsynaptic neuronal excitability (Vogt et al., 2000, Smart et al., 2004, Sensi et al., 2009). As such, possible anticonvulsant actions of the metal have been noted (Fukahori and Itoh, 1990, Elsas et al., 2009). Vesicular Zn2+ has also been shown to regulate the Ca2+ sensitivity of release at high firing frequencies (Lavoie et al., 2011). Zinc can modify excitability by allosterically modulating both excitatory and inhibitory neurotransmitter receptors (Hosie et al., 2003, Rachline et al., 2005, Paoletti et al., 2009, Sensi et al., 2009). Recent studies have demonstrated that synaptically released zinc can also directly act on a postsynaptic metabotropic zinc receptor mZnR/GPR39 (Besser et al., 2009, Chorin et al., 2011). Activation of this receptor initiates IP3 signaling via a Gq protein, resulting in intracellular calcium release (Hershfinkel et al., 2001). In hippocampal slices, mZnR activation and subsequent calcium liberation leads to upregulation of potassium/chloride co-transporter 2 (KCC2) activity, inducing a hyperpolarizing shift in the GABAA receptor channel reversal potential (Chorin et al., 2011).
KCC2 is the major outward transporter of chloride in neurons, necessary and sufficient for creating a chloride equilibrium potential negative to the resting membrane voltage (Lu et al., 1999, Lee et al., 2005), thereby rendering GABAA-mediated synaptic potentials inhibitory (Farrant and Kaila, 2007, Viitanen et al., 2010). Increases in KCC2 activity can enhance the inhibitory actions of GABA, which may provide high excitatory or epileptic activity with a self-regulating dampening drive (Huberfeld et al., 2007, Zhu et al., 2008, Khirug et al., 2010). In the present study we tested the hypothesis that a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent process mediates the enhanced activity of KCC2 following mZnR/GPR39 activation.
EXPERIMENTAL PROCEDURES
Cell culture
Primary cortical cultures obtained from embryonic day 16 Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were prepared as described by (Hartnett et al., 1997) in accordance with approved protocols. Embryonic cortices were dissociated with trypsin and, subsequently, the cell suspension was adjusted to 670,000 cells per 35 mm well in six-well tissue culture plates, each containing five 12-mm poly-L-ornithine-treated glass coverslips. Cultures were maintained at 37°C in 5% CO2, in a growth medium composed of a volume-to-volume mixture of 80% Dulbecco’s modified minimal essential medium (DMEM + GlutaMAX-1; Sigma-Aldrich; St Louis, MO, USA), 10% Ham’s F12-nutrients (F-12 + GlutaMAX-1; Sigma-Aldrich) and 10% heat-inactivated and iron supplemented bovine calf serum with 25mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 24U/ml penicillin and 24µg/ml streptomycin. Non-neuronal cell proliferation was inhibited after 2 weeks in culture with 1–2µm cytosine arabinoside, after which the cultures were maintained in growth medium containing 2% serum without F12-nutrients. Rat cultures were utilized at 18–25 days in vitro (DIV).
Mouse cortical neurons were prepared in a similar manner utilizing E15-E17 GPR39−/− or wild type mice from the same genetic background (Moechars et al., 2006). The resulting cell suspension was prepared in growth medium and was adjusted to 1.2×106 cells per 35 mm well (5 poly-L-ornithine-treated glass coverslips each). At 3 DIV the growth medium was changed to one devoid of L-glutamine and with the addition of 25% Neurobasal medium + 2.5% B27 Supplements. Mouse cortical neurons were utilized at 7–12 DIV. The presence of KCC2 at this developmental stage was confirmed by immunoblots (see below) and with functional assays (Titz et al., 2006, Hershfinkel et al., 2009a, Chorin et al., 2011).
Fluorescence imaging
Cells were visualized using an epifluorescence microscope and perfused with a HEPES-buffered physiological salt solution (HBSS: 115 mM NaCl, 2.5 mM KCl, 2.0 mM MgCl2, 10 mM HEPES, 10 mM d-glucose, 1 mM CaCl2; pH 7.2). The response time-course to the agents added via the perfusion were somewhat slower than those reported earlier (Besser et al., 2009; Chorin et al., 2011). This is due to differences in the speed of perfusion and larger recording chamber volume used in the present work. Images were acquired by exciting fluorescent dyes every 10 sec using a computer-controlled monochromator (Polychrome II; TILL photonics, Martinsried, Germany) and CCD camera (IMAGO; TILL photonics). All measurements of relative fluorescence units were background corrected. Each field under observation (20X) contained approximately 15 to 30 neurons, which were selected as regions of interest (ROI) to form individual fluorescence traces. Traces obtained from all neurons in a single coverslip were averaged and considered a single data point, except during transfection studies, where transfected (DsRed+, see below) and non-transfected cells from the same coverslip were evaluated independently. All results are expressed as the mean ± SEM.
To measure intracellular free Zn2+, neurons were loaded with FluoZin-3 AM (5µM for 30 min; prepared in HBSS), a non-ratiometric (excitation: 485nm, emission: 520nm), highly Zn2+-selective fluorescent indicator. Following acquisition of baseline fluorescence (~5 min), a treatment of 200 µM ZnCl2, a concentration sufficient to activate the mZnR/GPR39 (Hershfinkel et al., 2001, Besser et al., 2009), was perfused into the chamber for 2.5 min and subsequently monitored for 10 min in Zn2+-free HBSS. Cells were also treated with 200 µM ZnCl2 plus 5 µM pyrithione, a zinc ionophore, for 2.5 min. Neuronal free Zn+2 was then chelated by 20 µM N,N,N’,N’-tetrakis-(2-pyridalmethyl)-ethylenediamine (TPEN), a membrane-permeant Zn+2 chelator. ΔF values were measured as the difference between the average baseline fluorescence before the treatment and the average of data points encompassing the peak fluorescence after each treatment.
The ratiometric, calcium sensitive, fluorescent dye Fura-2 AM (5 µM for 30 min; prepared in HBSS) was used to measure intracellular Ca2+ in cortical neurons following activation of the mZnR/GPR39. Fluorescent measurements at 510nm emission were taken as a ratio of the signals obtained upon excitation by 340nm/380nm. Baseline fluorescence was measured for 5 min and cells were subsequently treated with 200µM ZnCl2 and then 300µM glutamate (2.5 min each). The ΔF for each treatment was measured as the difference between the average baseline fluorescence just before the treatment and at peak after the treatment was administered. A separate group of coverslips was pretreated with 10 µM YM-254890, a Gq antagonist (Takasaki et al., 2004), for 15 min, directly before being placed into the perfusion chamber.
KCC2 activity was measured using the ratiometric, pH-sensitive fluorescent dye BCECF AM (2’,7’-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester) during exposure to 5 mM NH4Cl (Titz et al., 2006, Hershfinkel et al., 2009a, Chorin et al., 2011). NH4+, as a surrogate for K+, can be transported into neurons by KCC2 and induce a decrease in intracellular pH. Cells were loaded with 1.5 µM BCECF-AM (prepared in HBSS containing 1% BSA) for 15 minutes and rinsed for 20 min. Fluorescent measurements were taken as a ratio of the signals obtained upon excitation by 440 nm/485 nm using a 510 nm emission filter. Baseline fluorescence was monitored for 5 min and then cells were treated with NH4Cl (5 mM, 5 min). To monitor the effects of Zn2+ on KCC2 activity ZnCl2 (200 µM, 2 min) was added, followed by a 1 min rinse, prior to addition of NH4Cl. Some cells were treated with bumetanide (1 µM), furosemide (100 µM) or BAPTA-AM (13 mM). The activity of KCC2 was measured as both the rate of change in BCECF fluorescence (slope; a.u./min, where a.u. are arbitrary units of fluorescence) and the total change in intracellular BCECF fluorescence (ΔF a.u.; See Fig. 1). Note that results are presented as positive numbers for clarity, although acidification causes decreases in BCECF fluorescence.
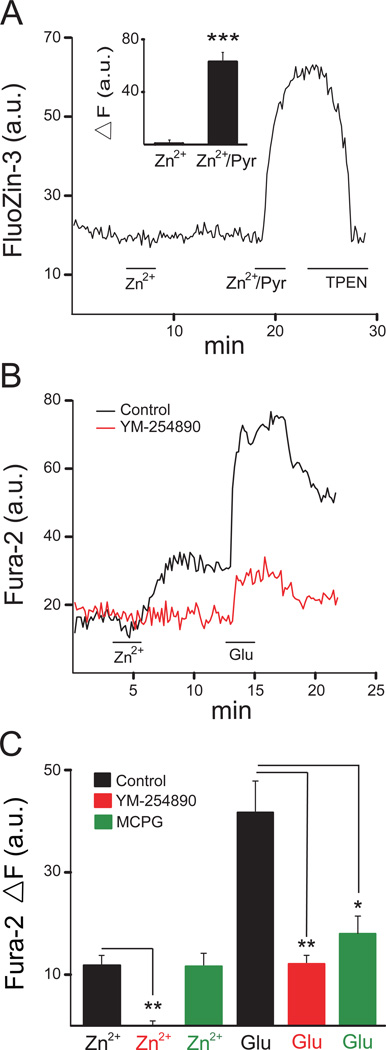
Figure 1.
Zn2+ induces Gq-mediated Ca2+ signals in cortical neurons without altering intracellular Zn2+ levels. (A) Average fluorescence change from rat cortical neurons in a single coverslip loaded with the Zn2+ indicator FluoZin-3 AM. Neurons were subsequently exposed to 200µM ZnCl2 (Zn2+), 200µM ZnCl2 with 5µM pyrithione (Zn2+/Pyr) and 20µM N,N,N’,N’-tetrakis-(2-pyridalmethyl)-ethylenediamine (TPEN). Inset: mean (± SEM: n=8) changes in fluorescence relative to baseline (ΔF) for the treatments described above; *** P<0.001 (paired t test) (B) Average fluorescence change from neurons in a single coverslip loaded with the Ca2+ indicator Fura-2 AM. Cells were treated with 200µM ZnCl2 (Zn2+) and 300µM glutamate (Glu) in the absence or presence of the Gq inhibitor YM-254890 (10µM). (C) Mean ΔF Fura-2 values (± SEM) for control (n=7), YM-254890 treated (n=5), and MCPG (500 µM; n=3) treated-coverslips. The ZnCl2 and glutamate responses were significantly inhibited by YM-254890; only the glutamate response was inhibited by MCPG; *P<0.05; **P<0.01 (Kruskal-Wallis/Mann-Whitney).
Neuronal Transfection
Rat cortical neurons at DIV19–23 were transfected in a 24 well plate with 1.5 µg of plasmid DNA (1 part DsRed-expressing plasmid: 1 part botulinum toxin C1-expressing plasmid or empty parent vector) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). DNA-lipid complexes were allowed to form for 30 min at room temperature before addition to the cortical cultures for approximately 3.5 to 4 hours, during which time cells were maintained at 37°C, 5% CO2. Twenty-four hours after the transfection, cells were utilized for imaging studies as described above.
Genotyping and immunobloting
GPR39−/− (Moechars et al., 2006) were kindly provided by Diederik Moechars (Janssen Pharmaceutical Companies of Johnson & Johnson, Beerse, Belgium). DNA was isolated from mouse-tail biopsy samples utilizing the Genetra Puregene Mouse Tail Kit (Qiagen Inc., Valencia, CA, USA). Wild-type (WT) selection primers, 5′ACCCTCATCTTGGTGTACCT3′ and 5′ATGTAG-CGCTCAAAGCTGAG3′ and knockout (KO) selection primers 5′GGAACTCTCACT-CGACCTGGG3′ and 5′GCAGCGCATCGCCTTCTATC3′, were obtained from Integrated DNA Technologies (Coralville, IA, USA). 10X PCR Reaction Buffer was obtained from USB Corporation (Cleveland, OH, USA), and all other PCR components were obtained from Promega Corporation (Madison, WI, USA). Polymerase chain reaction (PCR) was used to screen for genotypes. Two PCR reaction tubes, one for each set of primers, were prepared for each DNA sample and contained 100 ng DNA in a 2 µl volume. The reaction mixture contained: 1x PCR reaction buffer, 2 mM MgCl2, 0.2 mM dNTP mix, 1 µM of each of the two primers and 0.025 U/µl GoTaq DNA polymerase. The total volume of 24 µl per reaction was adjusted with nuclease-free Water. PCR reactions were performed in an automatic thermal cycler. An initial cycle of 5 minutes at 95 °C, was followed by 34 cycles, each cycle consisting of, 30 seconds at 95 °C, 30 seconds at 64 °C and 30 seconds at 72 °C. During the last 4 cycles, the 72 °C incubation was increased to 1, 2, 4 and 8 minutes prior to the cooling of the samples to 4 °C. PCR products were visualized utilizing agarose gel electrophoresis, with the WT and KO reactions yielding a 311-bp and 262-bp band, respectively (Moechars et al., 2006; Fig. 3A, inset).
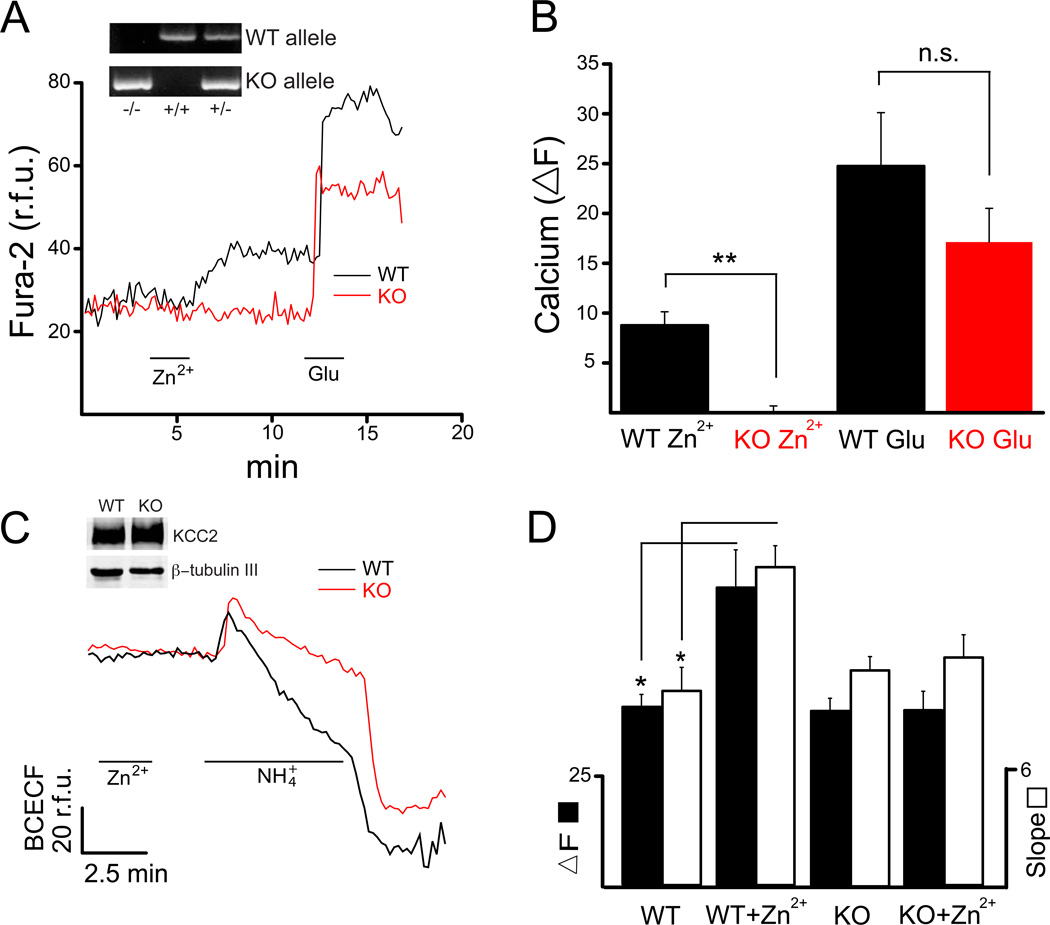
Figure 3.
GPR39−/− mice lack Zn2+-mediated upregulation of KCC2 activity. (A) Average Fura-2 fluorescence change in cells in single coverslips obtained from wild type (WT; black) and GPR39 KO (red) mice, treated with both 200 µM ZnCl2 (Zn2+) and 300 µM glutamate (Glu) Inset: Genotyping results for GPR39−/−, GPR39+/+, and GPR39+/− mice using the procedures described in Experimental Procedures. (B) Mean ΔF values (± SEM) from WT (n=4) and KO (n=6) coverslips. KO neurons had essentially no response to Zn2+, but the response to Glu was not significantly different to WT neuron responses; **P<0.01 (Mann-Whitney). (C) Average BCECF fluorescence change in WT and KO neurons from single coverslips pretreated with Zn2+. Inset: Immunoblots denoting similar levels of KCC2 protein expressed in GPR39+/+ (WT) and GPR39−/− (KO) mouse cortical cultures at the in vitro stage used for the functional assays in this study; βtubulin III used as a loading control. (D) NH4+ transport, mediated by KCC2 activity was measured in terms of both ΔF (black) and slope (white) for WT (n’s=4,4) and KO (n’s=7,10) coverslips in the absence and presence of Zn2+ pretreatment. WT KCC2 activity is significantly increased upon pretreatment with Zn2+; *P<0.05 (Mann-Whitney).
Protein samples from cultured mouse cultured neurons were obtained for Western blot analysis. The protein concentrations of the samples were measured with a bicinchoninic acid assay. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis was carried out using the Mini Protean 3 System (Bio-Rad, Hercules, CA, USA). Samples with equal amounts of protein were run on 6% gels. Separated proteins were transferred onto a 0.2 µm nitrocellulose membrane (Bio-Rad). Membranes were blocked with 1% bovine serum albumin in PBS with 0.05% Tween 20 (PBST) at room temperature for 1h, and probed with anti-KCC2 antibody (Millipore, Billerica, MA, USA). After washing (3X) in PBST, blots were incubated with Li-Cor IRDye infrared dye secondary antibodies (Li-Cor, Lincoln, NE, USA) at room temperature for 1h. Membranes were stripped and reprobed with and antibody to the neuronal-specific marker β-tubulin, class III (Covance, Princeton, NJ, USA). We observed similar levels of KCC2 protein expression in cortical cultures derived from either GPR39−/− or WT littermate mice at DIV similar to those used for the physiological measurements (Fig. 3C, inset).
RESULTS
Zinc-triggered activation of Gq protein-mediated Ca2+ signaling in cortical neurons
It has been shown that an intracellular Zn2+ rise is capable of inhibiting KCC2 activity (Hershfinkel et al., 2009a). Thus, in order to assess the effect of extracellular Zn2+ on the upregulation of KCC2 via mZnR/GPR39 activation (Chorin et al., 2011), we first investigated whether a ZnCl2 treatment at a concentration sufficient to activate the receptor (Besser et al., 2009) could, in and of itself, cause intracellular Zn2+ levels to increase. Cultures were loaded with the zinc selective fluorescent dye FluoZin-3 AM and exposed to 200 µM ZnCl2 for 2.5 min. We found that treatment with 200 µM ZnCl2 alone caused no substantial change in FluoZin-3 fluorescence for at least a 10-minute period after the treatment was administered (Fig. 1A, inset). Cells were then treated with 200 µM ZnCl2 plus 5 µM pyrithione, a zinc ionophore, directly followed by 20 µM N,N,N’,N’-tetrakis-(2-pyridalmethyl)-ethylenediamine (TPEN), a membrane-permeant Zn+2 chelator (Fig. 1A). The Zn2+/pyrithione treatment led to a considerable increase in fluorescence, which was quenched by TPEN (Fig. 1A, inset). These results indicate that Zn2+ treatment alone, under the conditions utilized here, did not substantially raise intracellular levels of this metal. Again, this was an important control to perform since intracellular Zn2+ blocks KCC2 activity, regardless of whether cells express the mZnR or not (Hershfinkel et al., 2009; Chorin et al., 2011).
We next loaded cells with Fura-2 AM to measure intracellular Ca2+ changes in cortical neurons upon activation of metabotropic receptor activity. Zinc (200 µM) and glutamate (300 µM)-induced responses were monitored in the same preparations. We performed further trials with cultures pretreated (15 min) with 10 µM YM-254890, a Gq antagonist (Takasaki et al., 2004; Fig. 1B). We observed a relatively small, but consistent, Ca2+ response following treatment with 200 µM ZnCl2 in rat cortical neurons in culture. Zn2+ induced a relative change in fluorescence (ΔF) of 11.8 ± 3.30 a.u. (arbitrary units; n=7), which was completely eliminated by YM-254890 (ΔF=−0.067 ± 0.967 a.u.; n=5). By comparison, glutamate induced a mean ΔF of 41.7 ± 11.5 a.u. (n=7) that was reduced to 11.9 ± 2.77 a.u. (n=5) in the presence of YM-254890 (Fig. 1C). The metabotropic glutamate receptor antagonist MCPG (500 µM) did not alter Zn2+-mediated Ca2+ responses (ΔF=11.4 ± 2.3 a.u.; n=3), while markedly inhibiting glutamate-induced responses (ΔF=18.1 ± 3.4 a.u.; n=3; Fig. 1C). These results suggests that the Zn2+-mediated responses were entirely mediated by a Gq-protein, and that a substantial component of the glutamate Ca2+ responses measured in our preparation were likely triggered by activation of group I metabotropic glutamatergic receptors, which are similarly coupled to Gq proteins (van Dam et al., 2004, Nicoletti et al., 2011). Our data strongly indicate that rat cortical neurons in culture express a metabotropic zinc receptor.
Zn2+-induced increase in KCC2 activity in rat cortical neurons
Rat cortical cultures were next loaded with BCECF-AM, a ratiometric pH sensitive fluorescent dye, and exposed to 5 mM NH4Cl to measure functional KCC2 activity in neurons. Experiments were performed under control conditions and following a 2 min exposure to 200 µM ZnCl2 (Fig. 2A). KCC2 transports NH4+ into neurons, thereby causing cellular acidification and decreasing BCECF fluorescence (following a transient alkalynization due passive entry of NH3 into cells (Titz et al., 2006). As such, KCC2 activity can be quantified as both the total change in fluorescence or the rate of change (Fig. 2A), during the NH4Cl treatment. KCC2 activity in control neurons (n=9) resulted in a mean BCECF ΔF of 26.7 ± 3.15 a.u. and a mean slope of 8.10 ± 0.78 a.u./min. In contrast, cultures pretreated (2 min) with 200 µM Zn2+ (n=9) had substantially increased KCC2 activity, with a mean BCECF ΔF of 36.9 ± 1.96 a.u. and slope of 10.7 ± 0.54 a.u./min (Figs. 2B, 2C). These results demonstrate that Zn2+ pre-treatment enhances KCC2 activity in cortical neurons.
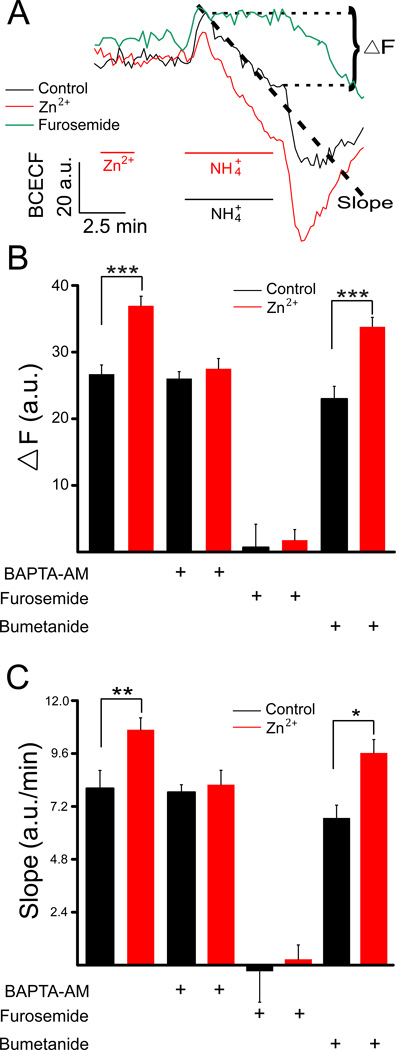
Figure 2.
Zn2+ enhances KCC2 co-transporter activity. (A) Average fluorescence change from rat cortical neurons from single coverslips loaded with the fluorescent pH indicator BCECF. Signals were obtained from control cells (black), as well as from neurons treated with 200 µM ZnCl2 for 2 min as shown (red). An example of a furosemide (100 µM)-treated preparation is also shown, as this drug inhibits the KCC2 activity and NH4+ transport (green). NH4+ transport, mediated by KCC2 (mean ± SEM), was quantified by calculating the slope during intracellular acidification. In addition, the net change in fluorescent signals (ΔF), as denoted in the figure, was measured. The slopes were calculated from the initial 200 s of acidification phase of the traces (Chorin et al., 2011). KCC2 activity, measured both in terms of ΔF (B) and slope (C), under control conditions and following Zn2+, was determined in cells exposed to vehicle (n’s=9,9), 13 mM BAPTA-AM (n’s=4,5), 100 µM furosemide (n’s=4,6), or 1 µM bumetanide (n’s=5–6); *P<0.05, **P<0.01, ***P<0.001 (unpaired t-test).
Additional cultures were exposed to 13 mM BAPTA-AM, a Ca2+ chelator, to inhibit the Zn2+-dependent mZnR/GPR39 response. KCC2 activity in cells exposed to BAPTA-AM alone (n=4) exhibited a mean ΔF of 26.0 ± 1.03 a.u. and a mean slope of 7.62 ± 0.30 a.u./min, similar to control cells (Figs. 2B, 2C). Zn2+ pre-treatment under these conditions, however, did not induce an increase in KCC2 activity (ΔF 27.5 ± 1.54 a.u.; slope of 8.16 ± 0.66 a.u./min; n=5; Figs. 2B, 2C), suggesting that the rise in intracellular Ca2+ induced by Zn2+ is necessary for upregulation of KCC2 activity.
Neurons were then treated with furosemide (100 µM), a KCC2 and NKCC1 (sodium/potassium/chloride co-transporter 1) inhibitor, or bumetanide (1µM), a NKCC1 inhibitor (Fig. 2). KCC2 activity in cells exposed to furosemide, either in the absence or presence of a Zn2+ pretreatment, was nearly completely inhibited (control: ΔF=0.65 ± 3.49 a.u., slope of −0.30 ± 1.44 a.u./min, n=4; Zn2+: ΔF=1.77 ± 1.60 a.u., slope of 0.36 ± 0.66 a.u./min, n=6). In contrast, bumetanide-treated cells behaved essentially the same as non-treated cells (control: ΔF= 22.9 ± 1.91 a.u., slope of 6.66 ± 0.60 a.u./min, n=5; Zn2+: ΔF=33.9 ± 1.42 a.u., slope of 9.6 ± 0.60 a.u./min, n=6). These results confirm that the NH4Cl induced changes in BCECF fluorescence are mediated by KCC2.
Absence of Zn2+-dependent upregulation of KCC2 in GPR39−/−-derived mouse cortical neurons
Experiments were next performed on mouse cortical cultures obtained from GPR39−/− (KO) and wild-type (WT) littermate mice in order to determine whether the Zn2+-triggered Ca2+ responses and effects on KCC2 activity were mediated via activation of the mZnR/GPR39 (Besser et al., 2009, Chorin et al., 2011). In neurons loaded with Fura-2 AM, WT mice cultures (n=4) showed consistent Ca2+ responses to Zn2+ and glutamate (Figs. 3A, 3B), with mean ΔF values of 8.8 ± 1.4 a.u. and 24.8 ± 5.4 a.u., respectively, similar to those observed in rat cultures (Figs. 1B). In contrast, neurons from KO cultures (n=6) showed mean Zn2+ and glutamate ΔF values of −0.5 ± 0.4 a.u. and 17.1 ± 3.4 a.u., respectively. Glutamate responses were not significantly different between WT and KO cultures, although there was a trend to decreased responses in KO cells (Fig. 3B). In contrast, Zn2+-induced Ca2+ responses were completely absent in KO cultures, indicating that GPR39 and the mZnR are likely the same molecule (Chorin et al., 2011).
We next measured KCC2 activity with the BCECF assay (Fig. 3C) and found that WT neurons had an average ΔF and slope value of 40.6 ± 3.31 a.u. and 10.2 ± 1.32 a.u./min under control conditions (n=4) and significantly increased values of 73.8 ± 12.3 a.u. and 19.02 ± 3.60 a.u./min when pretreated with Zn2+ (n=4; Fig. 3D). KO cells showed an average ΔF and slope values of 40.3 ± 2.91 a.u. and 10.8 ± 0.66 a.u./min under control conditions (n=7), which were essentially identical to WT cells, indicating that the basal KCC2 activity is similar in these neurons. In distinct contrast to the WT cells, the mean ΔF and slope values remained unchanged (40.2 ± 4.36 a.u. and 11.4 ± 1.14 a.u./min; n=10) following Zn2+ treatment (Fig 3D). These results strongly indicate that mZnR/GPR39 activation is responsible for the increase in KCC2 activity following Zn2+ exposure.
Zn2+-mediated up-regulation of KCC2 is SNARE dependent
In a recent study we observed that activation of the mZnR/GPR39 by Zn2+ in mouse hippocampal slices led to an increase in the surface expression of KCC2, as determined following in situ biotinylation labeling of the transporter (Chorin et al., 2011). This phenomenon could be the result of a stabilization of existing KCC2 molecules at the plasma membrane (e.g. Lee et al., 2007), an increase in exocytotic insertion of additional transporters, or a combination of both processes. To distinguish between these possibilities, we transfected rat cortical neurons with a plasmid encoding botulinum toxin C1 (Botox C1). This toxin cleaves the SNARE proteins syntaxin 1 and SNAP-25, which are required for exocytotic insertion of some membrane proteins, including the insertion of potassium channels following an apoptotic stimulus in cortical neurons in vitro (Pal et al., 2006).
Neurons were transfected with a plasmid encoding Botox C1 or an empty vector, in combination with a plasmid encoding the marker protein DsRed, used to identify positively transfected neurons. We have previously demonstrated that there is a 90% probability that plasmids transfected together will express together in the same neuron (Santos and Aizenman, 2002). A typical field contained 1–4 positively transfected neurons, out of a total of ~20 (Fig. 4A). We first ensured that Zn2+-triggered Ca2+ responses remained unchanged in Botox C1-expressing cells, as this toxin may have affected the normal trafficking of mZnR/GPR39. Ca2+ responses as a result of either Zn2+ or glutamate treatment, however, remained unchanged in these cells (Fig. 4B). We then monitored KCC2 activity in neurons loaded with BCECF; Figures 4C and 4D show representative individual experiments, and Figures 4E and 4F summarize our results. Neurons transfected with the empty vector under control conditions (n=6 coverslips) had a mean ΔF=37.6 ± 3.81 a.u. and slope of 9.31 ± 0.78 a.u./min; non-transfected neurons on these same coverslips showed a similar mean ΔF=38.3 ± 4.92 a.u. and slope of 9.28 ± 0.48 a.u./min. As expected, following Zn2+ treatment, neurons transfected with the empty vector (n=9) had an increased mean ΔF=54.5 ± 3.66 a.u. and slope of 14.3 ± 0.96 a.u./min, and non-transfected neurons of the same coverslips behaved similarly (ΔF=60.9 ± 5.24 a.u.; slope of 14.5 ± 0.60 a.u./min). These data demonstrate that the transfection process does not affect either basal or Zn2+-stimulated KCC2 activity in neurons.
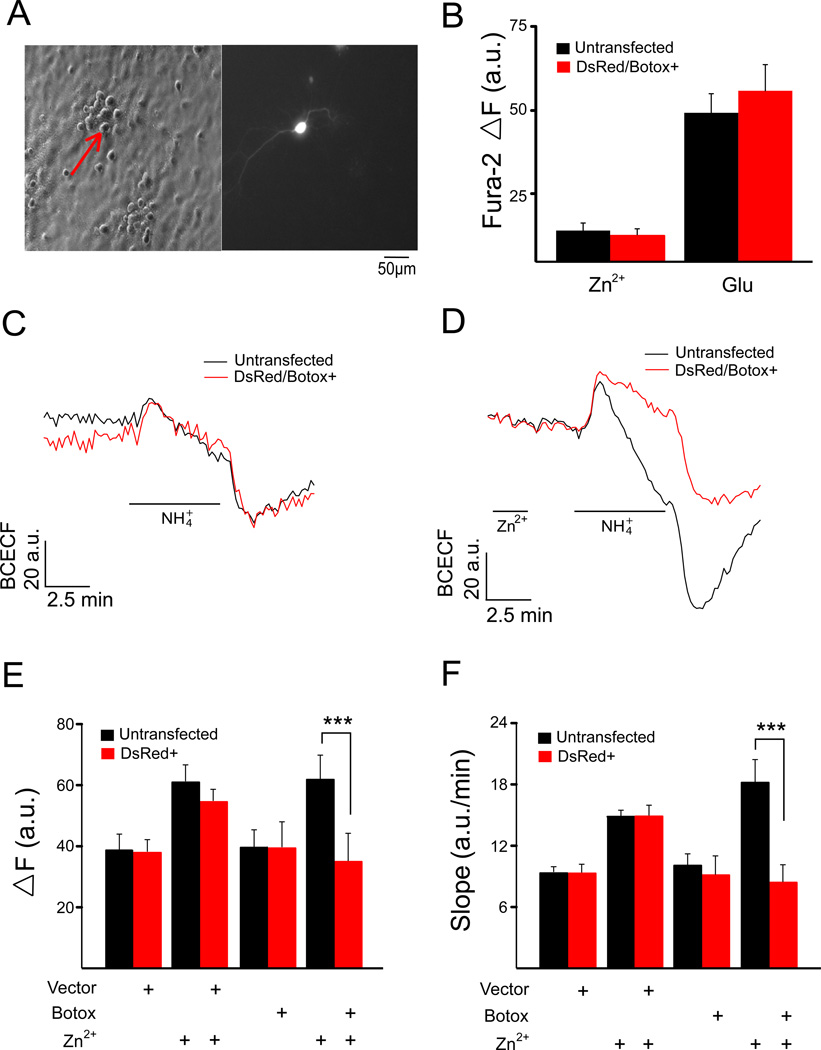
Figure 4.
KCC2 activity in botulinum toxin C1 (Botox C1)-expressing neurons. (A) Co-transfection with a DsRed expressing plasmid was used to locate Botox C1-transfected rat cortical neurons on coverslips. (B) Mean ΔF Fura-2 values (± SEM) for Ca2+ responses in untransfected and DsRed/Botox C1-expressing neurons from the same coverslips (n=3) treated with 200 µM ZnCl2 (Zn2+) and 300 µM glutamate (Glu). Fluorescence change of transfected (DsRed+ or DSRed/Botox C1+) and untransfected cells from the same coverslip in the absence (C) and presence (D) of a 2 min Zn2+ pretreatment. KCC2 activity, measured in terms of both ΔF (E) and slope (F) (mean ± SEM) for empty-vector and Botox C1 transfected rat cortical neuron, along with matched untransfected cells for each condition, in the absence and presence of Zn2+ pretreatment (n=6–9 coverslips per group). Zn2+-treated Botox C1-expressing cells showed significantly lower KCC2 activity when compared to the untransfected cells from the same coverslip; ***P<0.001 (paired t test).
Under control conditions, Botox C1 expressing cells had mean ΔF and slope values (39.4 ± 8.19 a.u. and 9.06 ± 1.80 a.u./min; n=9) that were very similar to the non-transfected cells on the same coverslips (39.6 ± 5.83 a.u. and 10.0 ± 1.14 a.u./min). This suggests that Botox C1-sensitive SNARE proteins are not involved in the normal recycling process of KCC2 molecules, which have been reported to undergo rapid turnover with constitutive endocytosis (Rivera et al., 2004).
Under Zn2+-stimulated conditions however, we observed that Botox C1-expressing cells lacked the increase in KCC2 activity (34.8 ± 8.97 a.u. and 8.40 ± 1.62 a.u./min; n=9). The non-transfected cells on the same coverslips showed the expected increase in KCC2 activity following Zn2+ treatment (61.7 ± 8.03 a.u. and 18.2 ± 2.28 a.u./min). These results strongly suggest that SNARE proteins cleaved by Botox C1 are necessary for the increased activity of the co-transporter following activation of mZnR/GPR39.
DISCUSSION
In this study, we have investigated the upregulation of KCC2 activity following mZnR/GPR39 activation in cortical neurons in culture. Extracellular applications of Zn2+ produced an increase in intracellular Ca2+ in rat and WT mouse cortical neurons, but failed to do so in the presence of YM245890, a Gq antagonist (Takasaki et al., 2004), or in neurons obtained from GPR39−/− mice. These results indicate that the mZnR/GPR39 metabotropic response is present in cultured cortical neurons. The rise in intracellular Ca2+ release following Zn2+ stimulation was necessary for the furosemide-sensitive, and bumetanide insensitive, increase in KCC2 activity. Finally, the up-regulation of KCC2 activity induced by Zn2+ required the Botox C1-sensitive SNARE proteins. These results indicate that an exocytotic process is involved in the mZnR/GPR39-dependent upregulation of KCC2 activity. Surprisingly, basal KCC2 activity in Botox C1-expressing neurons remained unchanged, suggesting that syntaxin and/or SNAP-25 are not recruited for the recycling process of the co-transporter under normal conditions (Rivera et al., 2004, Lee et al., 2007).
Regulation of KCC2 activity has been tightly linked to several physiological and pathophysiological processes. For instance, decreases in KCC2 activity have been associated with injurious stimuli, including sustained interictal-like activity (Rivera et al., 2002, Rivera et al., 2004), pilocarpine-induced status epilepticus (Lee et al., 2010), cerebral ischemia (Jaenisch et al., 2010), and the onset of neuropathic pain (Zhang et al., 2008). Interestingly, intracellular Zn2+, released from metal binding sites within cells during oxygen glucose deprivation, also reduces KCC2 activity (Hershfinkel et al., 2009b). A decrease in KCC2 function can be sufficiently severe to render GABA-mediated synaptic potentials excitatory (Viitanen et al., 2010). On the other hand, a single seizure event in the hippocampus was recently reported to induce an increase in KCC2 activity, rendering GABA-mediated inhibition more effective (Khirug et al., 2010).
In general, studies reporting changes in KCC2 activity have had two main features in common: a) the dependence of the change of transport rate on the activation of a kinase or phosphatase pathway (e.g. Strange et al., 2000, Rinehart et al., 2009), and b) a change in the surface expression of the transporter (Rivera et al., 2004). In some instances, phosphorylation of KCC2 alters its surface expression by internalization, which may be followed by degradation of the protein. For example, a pilocarpine model of status epilepticus results in tyrosine phosphorylation of residues Y903 and Y1087 of KCC2, a process that leads to decreased surface stability and subsequent lysozomal degradation of the co-transporter (Lee et al., 2010). In contrast, the aforementioned single seizure event in the hippocampus, induced by the excitotoxin kainate, leads to an increase in the surface expression of KCC2 by yet to be defined kinase-dependent process (Khirug et al., 2010). On a similar vein, PKC-dependent phosphorylation of KCC2 at residue S940 was shown to increase the surface stability of the transporter by decreasing its rate of internalization in neurons (Lee et al., 2007). This same S940 residue is also the target of protein phosphatase 1, a Ca2+-dependent enzyme that is activated following excitotoxic NMDA receptor activation, and dephosphorylation of KCC2 via this process leads to increased internalization (Lee et al., 2011).
Therefore, signaling cascades that lead to enhanced surface expression of KCC2, for the most part, have demonstrated a stabilization of the transporter in the membrane with decreased rates of internalization and/or degradation. What is unclear is whether and what fraction of the internalized KCC2 molecules are normally recycled and reinserted in the cell membrane. Our results suggest that under normal conditions the SNARE proteins syntaxin and SNAP-25 are not involved in the recycling of internalized KCC2 molecules. However, the fact that the enhancement of KCC2 activity following activation of mZnR/GPR39 is SNARE dependent suggests that a cytoplasmic pool of transporter molecules (Balakrishnan et al., 2003) can be made available for a differentially regulated exocytotic process following Zn2+- stimulated Ca2+ signaling.
Increases in intracellular calcium, such as those triggered by mZnR/GPR39 activation, are well-known signals for regulated SNARE-dependent exocytosis. For example, it has been demonstrated that vasopressin triggers an intracellular calcium mobilization that is associated with exocytotic insertion of aquaporin-2 (Balasubramanian et al., 2008). SNARE-dependent vesicle fusion has also been shown to regulate the surface expression of glutamate receptors (Selak et al., 2009; Lau et al., 2010) and H+-ATPase (Banerjee et al., 2001), among other proteins. Phosphorylation-mediated increases in KCC2 surface expression have heretofore been shown to be the result of a decrease in internalization rates. As such, our findings that Zn2+-dependent upregulation of KCC2 activity is blocked by the expression of Botox C1 point to a previously unreported, SNARE-dependent increased insertion of the transporter.
KCC2 activity is required for establishing the chloride gradient responsible for GABAA receptor-mediated synaptic inhibition (Lu et al., 1999, Lee et al., 2005). Consequently, increases in the surface expression of the co-transporter can lead to pronounced hyperpolarizing shifts in the GABAA reversal potential (Khirug et al., 2010). Hence, the upregulation of KCC2 activity by extracellular Zn2+ may be responsible for the previously suggested anticonvulsant properties of this micronutrient (Goldberg and Sheehy, 1982, Blasco-Ibanez et al., 2004, Takeda et al., 2005, Ganesh and Janakiraman, 2008). Indeed, ZnT3 KO mice, which lack synaptically released zinc, exhibit increased susceptibility to kainate-induced seizures (Cole et al., 2000). Thus, by regulating excitability, mZnR/GPR39 activity can limit neuronal firing and may provide a novel therapeutic target in epilepsy and related neurological disorders.
Establish presence of metabotropic zinc receptor (mZnR) in cortical neurons
Identify the mZnR as the G protein-linked receptor GPR39
Activation of mZnR/GPR39 leads to enhanced activity of KCC2
Enhanced KCC2 activity is mediated by a SNARE-dependent process
Acknowledgements
We thank Diederik Moechars for the GPR30−/− mice and Astella Pharma, Inc. for the gift of YM-254890. We also thank Thanos Tzounopoulos, University of Pittsburgh School of Medicine, for helpful discussions. This work was supported by US National Institutes of Health grants NS043277 (EA) and DC004199 (KK), Israel Science Foundation grant 585,05 (MH), and US-Israel Bi-national Science Foundation grant BSF2007121 (EA and MH).
Abbreviations
- a.u.
arbitrary units
- BCECF AM
2’,7’-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester
- DIV
days in vitro
- DMEM
Dulbecco’s modified minimal essential medium
- GPR39
G protein-linked receptor 39
- HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)
- KCC2
potassium chloride co-transporter 2
- mZnR
metabotropic zinc receptor
- MCPG
(RS)-α-Methyl-4-carboxyphenylglycine
- SNAP-25
synaptosomal-associated protein 25
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- TPEN
N,N,N’,N’-tetrakis-(2-pyridalmethyl)-ethylenediamine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balasubramanian L, Sham JS, Yip KP. Calcium signaling in vasopressin-induced aquaporin-2 trafficking. Pflugers Arch. 2008;456:747–754. doi: 10.1007/s00424-007-0371-7. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Li G, Alexander EA, Schwartz JH. Role of SNAP-23 in trafficking of H+-ATPase in cultured inner medullary collecting duct cells. Am J Physiol Cell Physiol. 2001;280:C775–C781. doi: 10.1152/ajpcell.2001.280.4.C775. [DOI] [PubMed] [Google Scholar]
- Besser L, Chorin E, Sekler I, Silverman WF, Atkin S, Russell JT, Hershfinkel M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J Neurosci. 2009;29:2890–2901. doi: 10.1523/JNEUROSCI.5093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco-Ibanez JM, Poza-Aznar J, Crespo C, Marques-Mari AI, Gracia-Llanes FJ, Martinez-Guijarro FJ. Chelation of synaptic zinc induces overexcitation in the hilar mossy cells of the rat hippocampus. Neurosci Lett. 2004;355:101–104. doi: 10.1016/j.neulet.2003.10.053. [DOI] [PubMed] [Google Scholar]
- Chorin E, Vinograd O, Fleidervish I, Gilad D, Herrmann S, Sekler I, Aizenman E, Hershfinkel M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J Neurosci. 2011;31:12916–12926. doi: 10.1523/JNEUROSCI.2205-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Robbins CA, Wenzel HJ, Schwartzkroin PA, Palmiter RD. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000;39:153–169. doi: 10.1016/s0920-1211(99)00121-7. [DOI] [PubMed] [Google Scholar]
- Elsas SM, Hazany S, Gregory WL, Mody I. Hippocampal zinc infusion delays the development of afterdischarges and seizures in a kindling model of epilepsy. Epilepsia. 2009;50:870–879. doi: 10.1111/j.1528-1167.2008.01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- Fukahori M, Itoh M. Effects of dietary zinc status on seizure susceptibility and hippocampal zinc content in the El (epilepsy) mouse. Brain Res. 1990;529:16–22. doi: 10.1016/0006-8993(90)90806-m. [DOI] [PubMed] [Google Scholar]
- Ganesh R, Janakiraman L. Serum zinc levels in children with simple febrile seizure. Clin Pediatr (Phila) 2008;47:164–166. doi: 10.1177/0009922807306165. [DOI] [PubMed] [Google Scholar]
- Goldberg HJ, Sheehy EM. Fifth day fits: an acute zinc deficiency syndrome? Arch Dis Child. 1982;57:633–635. doi: 10.1136/adc.57.8.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnett KA, Stout AK, Rajdev S, Rosenberg PA, Reynolds IJ, Aizenman E. NMDA receptor-mediated neurotoxicity: a paradoxical requirement for extracellular Mg2+ in Na+/Ca2+-free solutions in rat cortical neurons in vitro. J Neurochem. 1997;68:1836–1845. doi: 10.1046/j.1471-4159.1997.68051836.x. [DOI] [PubMed] [Google Scholar]
- Hershfinkel M, Kandler K, Knoch ME, Dagan-Rabin M, Aras MA, Abramovitch-Dahan C, Sekler I, Aizenman E. Intracellular zinc inhibits KCC2 transporter activity. Nat Neurosci. 2009a;12:725–727. doi: 10.1038/nn.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfinkel M, Kandler K, Knoch ME, Dagan-Rabin M, Aras MA, Abramovitch-Dahan C, Sekler I, Aizenman E. Intracellular zinc inhibits KCC2 transporter activity. Nat Neurosci. 2009b;12:725–727. doi: 10.1038/nn.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfinkel M, Moran A, Grossman N, Sekler I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc Natl Acad Sci U S A. 2001;98:11749–11754. doi: 10.1073/pnas.201193398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie AM, Dunne EL, Harvey RJ, Smart TG. Zinc-mediated inhibition of GABA(A) receptors: discrete binding sites underlie subtype specificity. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- Huberfeld G, Wittner L, Clemenceau S, Baulac M, Kaila K, Miles R, Rivera C. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch N, Witte OW, Frahm C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. Stroke. 2010;41:e151–e159. doi: 10.1161/STROKEAHA.109.570424. [DOI] [PubMed] [Google Scholar]
- Khirug S, Ahmad F, Puskarjov M, Afzalov R, Kaila K, Blaesse P. A single seizure episode leads to rapid functional activation of KCC2 in the neonatal rat hippocampus. J Neurosci. 2010;30:12028–12035. doi: 10.1523/JNEUROSCI.3154-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010;30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie N, Jeyaraju DV, Peralta MR, 3rd, Seress L, Pellegrini L, Toth K. Vesicular zinc regulates the Ca2+ sensitivity of a subpopulation of presynaptic vesicles at hippocampal mossy fiber terminals. J Neurosci. 2011;31:18251–18265. doi: 10.1523/JNEUROSCI.4164-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Chen CX, Liu YJ, Aizenman E, Kandler K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur J Neurosci. 2005;21:2593–2599. doi: 10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat Neurosci. 2011;14:736–743. doi: 10.1038/nn.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Jurd R, Moss SJ. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol Cell Neurosci. 2010;45:173–179. doi: 10.1016/j.mcn.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HH, Walker JA, Williams JR, Goodier RJ, Payne JA, Moss SJ. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J Biol Chem. 2007;282:29777–29784. doi: 10.1074/jbc.M705053200. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Moechars D, Depoortere I, Moreaux B, de Smet B, Goris I, Hoskens L, Daneels G, Kass S, Ver Donck L, Peeters T, Coulie B. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology. 2006;131:1131–1141. doi: 10.1053/j.gastro.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal SK, Takimoto K, Aizenman E, Levitan ES. Apoptotic surface delivery of K+ channels. Cell Death Differ. 2006;13:661–667. doi: 10.1038/sj.cdd.4401792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2009;158:126–136. doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Exocytosis of vesicular zinc reveals persistent depression of neurotransmitter release during metabotropic glutamate receptor long-term depression at the hippocampal CA3-CA1 synapse. J Neurosci. 2006;26:6089–6095. doi: 10.1523/JNEUROSCI.0475-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J, Maksimova YD, Tanis JE, Stone KL, Hodson CA, Zhang J, Risinger M, Pan W, Wu D, Colangelo CM, Forbush B, Joiner CH, Gulcicek EE, Gallagher PG, Lifton RP. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138:525–536. doi: 10.1016/j.cell.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S, Aizenman E. Functional expression of muscle-type nicotinic acetylcholine receptors in rat forebrain neurons in vitro. Methods Find Exp Clin Pharmacol. 2002;24:63–66. doi: 10.1358/mf.2002.24.2.677127. [DOI] [PubMed] [Google Scholar]
- Selak S, Paternain AV, Aller MI, Pico E, Rivera R, Lerma J. A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron. 2009;63:357–371. doi: 10.1016/j.neuron.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- Strange K, Singer TD, Morrison R, Delpire E. Dependence of KCC2 K-Cl cotransporter activity on a conserved carboxy terminus tyrosine residue. Am J Physiol Cell Physiol. 2000;279:C860–C867. doi: 10.1152/ajpcell.2000.279.3.C860. [DOI] [PubMed] [Google Scholar]
- Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, Kobori M. A novel Galphaq/11-selective inhibitor. J Biol Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- Takeda A, Yamada K, Minami A, Nagano T, Oku N. Enhanced excitability of hippocampal mossy fibers and CA3 neurons under dietary zinc deficiency. Epilepsy Res. 2005;63:77–84. doi: 10.1016/j.eplepsyres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Titz S, Hormuzdi S, Lewen A, Monyer H, Misgeld U. Intracellular acidification in neurons induced by ammonium depends on KCC2 function. Eur J Neurosci. 2006;23:454–464. doi: 10.1111/j.1460-9568.2005.04583.x. [DOI] [PubMed] [Google Scholar]
- van Dam EJ, Kamal A, Artola A, de Graan PN, Gispen WH, Ramakers GM. Group I metabotropic glutamate receptors regulate the frequency-response function of hippocampal CA1 synapses for the induction of LTP and LTD. Eur J Neurosci. 2004;19:112–118. doi: 10.1111/j.1460-9568.2004.03103.x. [DOI] [PubMed] [Google Scholar]
- Viitanen T, Ruusuvuori E, Kaila K, Voipio J. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 2010;588:1527–1540. doi: 10.1113/jphysiol.2009.181826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Liu LY, Xu TL. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience. 2008;152:502–510. doi: 10.1016/j.neuroscience.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Zhu L, Polley N, Mathews GC, Delpire E. NKCC1 and KCC2 prevent hyperexcitability in the mouse hippocampus. Epilepsy Res. 2008;79:201–212. doi: 10.1016/j.eplepsyres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]