Abstract
The controlled delivery of growth factors and cells within biomaterial carriers can enhance and accelerate functional bone formation. The carrier system can be designed with preprogrammed release kinetics to deliver bioactive molecules in a localized, spatiotemporal manner most similar to the natural wound healing process. The carrier can also act as an extracellular matrix-mimicking substrate for promoting osteoprogenitor cellular infiltration and proliferation for integrative tissue repair. This review discusses the role of various regenerative factors involved in bone healing and their appropriate combinations with different delivery systems for augmenting bone regeneration. The general requirements of protein, cell and gene therapy are described, with elaboration on how the selection of materials, configurations and processing affects growth factor and cell delivery and regenerative efficacy in both in vitro and in vivo applications for bone tissue engineering.
Keywords: Bone tissue engineering, controlled delivery, growth factors, cells, scaffolds
1. Introduction
Every year, more than 500,000 bone graft procedures are performed to address bone fractures and other orthopedic-related injuries resulting from a variety of surgical, degenerative and traumatic causes [1, 2]. The current gold standard of treatment is autologous bone, harvested primarily from the patient’s iliac crest or other locations such as the distal femur, proximal tibia, ribs and intramedullary canal [2–4]. Despite its immunocompatibility and excellent osteoconductive properties, autograft bone is of limited supply and presents associated donor-site morbidity [5–7]. Allograft bone from human donors/cadavers or xenograft bone from a non-human are viable alternative treatments. However, risks of disease transmission, infection and host rejection have restricted their use. The application of biological signals and cells to stimulate the host’s natural healing response for functional tissue repair may successfully address the drawbacks of current approaches for bone regeneration.
Direct growth factor and cell delivery has shown great therapeutic potential in preclinical testing and clinical trials, but few have reached commercial success. Bone morphogenetic protein (BMP)-2 and BMP-7, currently regulated by the Food and Drug Administration (FDA), were found to be promising alternatives to autografts in non-union bone defects, open tibial fractures, spinal fusion and accelerated fracture healing [8, 9]. However, currently employed delivery methods experience insufficient local retention and require high amounts of protein to exert a biological effect, especially in larger animal models and humans [10]. Cellular therapy using localized administration of cells to the defect site has been constrained by similar problems. Despite the positive results obtained from clinical applications of cell therapy using autologous or allogeneic bone marrow, plasma and cultured osteoblasts [11, 12], studies show that transplanted cells poorly engraft to local tissue and fail to spread from the injury site, limiting repair and cell viability [13]. Tissue engineering strategies have emerged as promising methods to provide superior regenerative treatment through the combination of biological factors, cells and biomaterial scaffolds.
The controlled delivery of bone regenerative factors can be accomplished via biomaterial carrier systems to facilitate local repair at the defect. The optimal carrier should provide four main functions: 1) site specific delivery of regenerative factors to the defect, 2) local regulation and retention of released factors, 3) enhanced infiltration and proliferation of cells on a three-dimensional substrate and 4) optimized biodegradation for complete tissue regeneration. In addition, the carrier system and its soluble byproducts should also be biocompatible and noncytotoxic as to prevent premature clearance and/or adverse local tissue responses which may lead to delayed wound healing. While there have been many investigations in regards to the material, configuration and processing of carriers, the main challenge lies in balancing the design elements to preserve the essential functions for successful delivery [14]. The carrier must retain proteolytic protection without interfering with the bioactivity and spatiotemporal dosing of encapsulated molecules and biological function of the local microenvironment. This review discusses the role of different bone regenerative factors in bone regeneration and examines the requirements for protein, cell and gene therapy for bone regeneration, with emphasis on the materials and methods for controlled delivery.
2. Bone Regenerative Factors
Bone healing and remodeling is accomplished by a complex, coordinated effort of cells, bioactive factors and extracellular matrix to stimulate the proliferation, differentiation and migration of osteoprogenitor cells [15, 16]. The induction of signaling cascades for tissue repair results from the elevated expression of pro-inflammatory, angiogenic and osteogenic growth factors released by cells in the injury site, many of which have well established roles in embryonic development and skeletal homeostasis [17, 18]. The ability to recapitulate and manipulate those signaling processes on a similar spatiotemporal scale could provide specific control over the regenerative process.
2.1 Growth Factors
Growth factors are soluble signaling molecules that control a wide variety of cellular responses through specific binding of transmembrane receptors on target cells [18]. The ultimate biological response elicited from a growth factor depends on the identity of the growth factor and target cell, cell number, receptor type and other signaling events. Therefore, a critical component in designing a controlled delivery system is selection of the appropriate single or combination of growth factors for maximized tissue repair [19].
2.1.1. Osteogenic Factors
Members of the highly conserved transforming growth factor-β (TGF-β) superfamily play an important role in embryonic development, tissue morphogenesis, cell proliferation and cell differentiation [20]. TGF-βs, activins, growth differentiation factors (GDF), BMPs, and their various isoforms share similar biological activities through their homologous polypeptide structure, which only differs in the C-terminal amino acid sequences [20]. First implicated by Urist in the 1960s for directing osteoblast differentiation, several TGF-β superfamily members have been further linked to the biological processes of bone induction, including mesenchymal cell recruitment, proliferation, and extracellular matrix (ECM) production [21, 22]. Various defect types and animal models have been investigated with TGF-β [23–29] but TGF-β isoforms have only provided limited success for endochondral bone formation in adult non-human primates [20, 30]. BMPs, particularly BMP-2, BMP-4 and BMP-7, are the most extensively studied osteogenic molecules for inducing de novo bone formation in ectopic and orthotopic sites, including critical size defects (CSD) [10, 31–33].
2.1.2. Angiogenic Factors
Vascularization for the transport of oxygen, nutrients, growth and differentiation factors and circulating cells is essential for the formation and homeostasis of bone [34, 35]. The presence of a local microvascular network supports the osteogenic, chondrogenic and mesenchymal stem cells required for bone repair. Angiogenesis is regulated by soluble molecules such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) and insulin-like growth factor (IGF) [34]. Bone research with angiogenic factors has primarily focused on VEGF’s role in neovascularization and osteogenic recruitment [36]. VEGF delivery was found to increase blood vessel density and stimulate slight bone regeneration in rabbit [37, 38] and rat [39–42] critical size bone defects. Recent studies have shown that the combined delivery of VEGF with osteoinductive growth factors synergistically enhances osteogenesis [43–47].
2.1.3. Inflammatory Factors
Fracture healing can be characterized by the three phases of inflammation, renewal and remodeling. Control of inflammation involves the manipulation of proinflammatory cytokines and growth factors temporally and spatially released following bone injury. Studies have shown that inflammatory molecules including tumor necrosis factor-α, interleukins, interferon-γ and prostaglandins stimulate the migration and differentiation of osteoblasts and osteoclasts. Also, their release activates the secondary signal cascade required for enhanced angiogenesis and bone repair. Incorporation of immunomodulatory and anti-inflammatory agents such as peptide factors [48, 49], selective anticytokine therapies, corticosteroids and nonsteroidal anti-inflammatory drugs into bone tissue engineering strategies provides methods to direct the proregenerative and proresorptive effects of inflammatory signals. An in-depth examination of inflammatory factors for bone regeneration can be found in recent reviews [50, 51].
2.1.4. Systemic Factors
Since bone injuries also involve a systemic physiological response, the therapeutic role of systemic factors such as parathyroid hormone (PTH), growth hormone, steroids, calcitonin and Vitamin D in osteogenesis and angiogenesis have also been considered [52]. Although their mechanisms for directing osteogenic activity are not well understood, studies have shown that periodic exposure of PTH can stimulate bone formation in rats and humans [53–55]. Time-and dose-dependent administration of calcitonin and Vitamin D can also induce limited bone formation [56, 57] and osteoblastic differentiation [58], respectively.
2.2. Cells
Successful bone induction using autologous and allogeneic grafts relies on the presence of undifferentiated stem cells with high osteogenic potential to replace injured end stage differentiated cells. Stem cells are characterized by their abilities to self renew and differentiate into a variety of functional specialized cell types. Most cellular therapy strategies for bone regeneration employ adult stem cells, like mesenchymal stem cells (MSCs), due to their potential to differentiate into cells of a particular lineage. MSCs are culture-adherent, multipotent progenitor cells capable of differentiating into bone, cartilage, fat, tendon, muscle and nerve [59, 60]. They have been isolated from various sources including bone marrow, adipose tissue, muscle tissue, amniotic fluid, human placenta, periosteum, cord blood and even peripheral blood [59, 61–63]. The efficacy and survival of MSCs depend on the methods of isolation and ex vivo expansion and manipulation prior to transplantation.
Although MSCs have shown great potential in bone research, the plasticity of embryonic stem (ES) cells is highly desired for replacing the various tissue types affected in bone injuries, enabling widespread, more integrative repair. ES cells are pluripotent, therefore capable of differentiating into cell types from all three germ layers. Despite successful bone regeneration with both human and murine ES cells [64–66], safety and ethical concerns [11, 60] have limited their use. Recent studies have shown that differentiated cells can be genetically reprogrammed to regain their stemness for differentiation into other phenotypes not restricted to their tissue type. Induced pluripotent stem cells, human marrow isolated multilineage inducible cells and vascular smooth muscle cells have shown promise for bone tissue engineering applications [67–69].
3. Protein Therapy
3.1 General Requirements of Growth Factor Carriers
Protein therapy involves the targeted transport and sustained release of therapeutic growth factors using biomaterial carriers, the design requirements of which are similar to many drug delivery vehicles. A carrier system specifically for growth factors should provide protected delivery and regulated time- and dose-dependent release of protein at the target site as well as supportive scaffolding for cell migration and proliferation that will lead to the generation of ECM and vascular networks for enhanced tissue integration and repair.
The main challenge in developing delivery systems for bone tissue engineering stems from the fact that there is no ideal vehicle for all applications. While permanent implants have been utilized for growth factor delivery, degradable systems offer certain benefits for full tissue integration and reduced invasiveness. The shape, size and structure of the bone defect will dictate the required mechanical and degradation characteristics and the mode of delivery (prefabricated or injectable). The optimal carrier should metabolically degrade at a rate of a few weeks to months corresponding to the rate of tissue restoration while maintaining release of the appropriate concentration of protein. High surface area to volume ratio and pore interconnectivity are also required for adequate mass transport between the biological environment and space for cellular ingrowth and neovascularization. Additionally, since the kinetics of growth factor delivery are carrier-dependent, the therapeutic efficacy will rely on the chemistry and interaction of the protein, carrier material and local microenvironment. For example, in the case of delivering BMP-2 for bone regeneration, the ideal carrier would provide sustained release over a period of at least three weeks. Following injury, BMP-2 is released locally into the fracture site from the surrounding bone matrix. BMP-2 expression is further upregulated in differentiating osteoprogenitor cells and MSCs and maintained for about 21 days before returning to normal levels during remodeling [53]. A study by Jeon et al. comparing the long and short term delivery of BMP-2 found that release of bioactive protein over 4 weeks resulted in significantly higher ectopic bone formation and calcium deposition after 8 weeks in vivo than burst release of the same protein dose in 3 days [70]. The results suggest that long term BMP-2 delivery is needed to induce a commitment to the bone phenotype and correlates with previous unsuccessful attempts of bolus growth factor therapy in patients [71].
Discussion of prolonging growth factor retention also highlights the importance of maintaining protein bioactivity over extended periods of time. Although the degree to which protein bioactivity is affected by different methods of growth factor incorporation into the scaffold and carrier sterilization is not comprehensively assessed, studies have shown that encapsulated proteins possess greater stability [72] while modifications to the protein itself may reduce its functionality [8, 73]. Current techniques for evaluating protein bioactivity are limited to in vitro studies with cell lines or ex vivo animal studies with radiolabeled protein, both of which do not provide simple, accurate methods for correlating in vivo growth factor release and biological effect. Kempen et al. investigated a promising non-invasive monitoring method to measure growth factor release and bone formation in vivo using nuclear medicine and radiology techniques [74]. Sequential measurements made with single photon emission computed tomography and micro-computed tomography in association with a scintillation probe setup successfully monitored local retention profiles of radioiodinated BMP-2 (125I-BMP-2) and bone formation in ectopic and orthotopic rat models. Additionally, comparison between non-invasive monitoring and ex vivo analysis showed no significant differences in 125I activity and biodistribution after 8 weeks of follow-up. The non-invasive sequential measurement approach may provide superior ways to optimize present and new carrier systems for growth factor delivery.
3.2 Vehicles for Controlled Protein Delivery
Controlled systems have been accomplished using a variety of natural, synthetic and inorganic materials, each with their own advantages and disadvantages. In vivo applications of different growth factor delivery systems are summarized in Table 1.
Table 1.
Selective in vivo applications of inorganic, natural and synthetic carriers for controlled growth factor delivery
| Material | Growth Factor | Carrier | Animal Model | Key Results | Reference |
|---|---|---|---|---|---|
| Titanium | BMP-2 | Heparin/apatite -coated implant | Rat tibial defect | Increased horizontal and vertical bone formation, BMP-2 presence no effect on bone implant contact | [97] |
| β-TCP/collagen | FGF-2 | Injectable composite | Rabbit segmental defect | Complete healing, marrow formation after 12 weeks | [86] |
| CPC | rhBMP-2 | Porous discs | Rabbit subcutaneous implant | Bone formation and vessel ingrowth after 10 weeks, no implant degradation | [92] |
| CPC + GMP | TGF-β1 | Injectable composite | Rabbit femoral defect | Increased bone density and remodeling after 12 weeks, enhanced degradation | [93] |
| Collagen | BMP-2 | Apatite-coated scaffold | Mouse calvarial CSD | Apatite coating prolonged BMP-2 activity, synergistic enhancement of bone formation and mineralization | [126] |
| nHAp/Collagen/PLA | BMP-2 peptide | Composite scaffold | Rat subcutaneous pocket | Dose-dependent ectopic bone formation over 12 weeks, peptide lower osteoinductivity than BMP-2 protein | [125] |
| Gelatin | rhBMP-2 | Hydrogel | Mouse subcutaneous implant | Osteoprogenitor cell recruitment, de novo bone formation higher with sustained release BMP-2 | [178] |
| Fibrin | BMP-2 | Heparin- conjugated hydrogel | Rat subcutaneous pocket | Increased bone formation, ALP activity, calcium deposition compared to controls; complete hydrogel degradation after 8 weeks | [116] |
| Silk Fibroin | BMP-2 | MP | Rat subcutaneous pocket | Bone formation and calcified ECM with only encapsulated BMP-2, osteocalcin present | [152] |
| PLGA | BMP-7 | MP | Osteopenic ovine vertebral defect | Higher compressive modulus and bone volume for slow release BMP-7; no significant results compared to controls | [148] |
| PLLA + PLGA NP | rhBMP-7 | Composite scaffold | Rat subcutaneous pocket | De novo bone formation with mineralized matrix after 3 weeks with sustained rhBMP-7 release | [132] |
| PPF/gelatin + PLGA MP | BMP- 2/VEGF | Composite Scaffold | Rat ectopic/femoral CSD | Woven/lamellar bone formation with BMP-2 delivery only in ectopic site, no significant synergistic VEGF effect | [44] |
| PPF + GMP | BMP- 2/VEGF | Composite Scaffold | Rat cranial CSD | Dual delivery improved defect bridging and healing time, no effect on vessel formation or total bone volume compared to BMP-2 alone | [45] |
| PLDL | rhBMP- 2/rhTGF- β3 | Porous Scaffold | Rat femoral CSD | Incomplete union, but dense mineralized matrix, sufficient integration/mechanical strength compared to controls | [133] |
ALP, alkaline phosphatase; β-TCP, β-tricalcium phosphate; BMP, bone morphogenetic protein; rhBMP, human recombinant BMP; CaP, calcium phosphate; CSD, critical size defect; FGF, fibroblast growth factor; GMP, gelatin microparticle; MP, microparticle; nHAp, nanohydroxyapatite; NP, nanoparticle; PLGA, poly(DL-lactic-co-glycolic acid); PLLA, poly(L-lactic acid); PLDL, poly(L-lactic acid-co-DL-lactic acid); PPF, poly(propylene fumarate); TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor
3.2.1 Three-Dimensional Scaffolds
Three-dimensional matrices and porous scaffolds are the most common growth factor delivery systems and have been investigated extensively in both experimental and clinical applications. The development of new carriers for bone regeneration focuses on improving local protein retention and sustained release for enhanced osteoinductivity in vivo. Strategies for growth factor incorporation into scaffolds include either non-covalent (surface adsorption, physical entrapment, affinity binding, ionic complexation) or covalent immobilization on or into the delivery system (chemical conjugation), the selection of which depends on the physicochemical properties and interactions between the growth factor, carrier and defect type [75].
3.2.1.1 Inorganic Materials
Inorganic materials such as ceramics have long been used as hard bone replacements for the benefits of high compressive strength, biodegradability and osteoconductivity, but lack intrinsic mechanisms for controlled delivery. Although physical adsorption of proteins to material surfaces is the simplest method of growth delivery, the loading and release of functional adsorbed molecules can be non-efficient [75]. Ziegler et al. found that rhBMP-2 and recombinant basic FGF displayed time-dependent decreases in bioactivity after adsorption on different inorganic carriers in vitro [76]. Alkaline phosphatase (ALP) activity and cell proliferation in human primary osteogenic sarcoma cells was attributed to the release of unbound, bioactive protein within the first hour of incubation. Cells receiving protein released from carriers 24 hours after adsorption showed no expression of ALP or other osteogenic activity. Therefore, methods to strengthen the immobilization of growth factors to material surfaces or encapsulation within carriers may prolong osteoinductive activity.
Growth factors like BMP and VEGF can be chemically conjugated to the material surface via its primary amine groups using N-hydroxysuccinimide/N-ethylcarbodiimide, N,N-carbonyldiimidazol and reductive amination chemistry [77, 78] or linker molecules like collagen and heparin [79] for prolonged release. Aminosilane chemistry is a common approach for covalent attachment of growth factors to ceramic surfaces like hydroxyapatite (HAp) [80]. Ehlert et al. achieved a five-to-tenfold increase in BMP-2 immobilization using aminosilane linkers on a nanoporous silica coating [81]. Surface modification provides a different method to enhance growth factor binding by changing the surface chemical composition and topology. Biphosphonate-based precipitation and calcium phosphate coprecipitation techniques have shown potential for bone healing by maintaining protein bioactivity and minimizing burst release [82, 83]. Tsurushima et al. developed a FGF-2 release HAp-ceramic buttons capable of promoting bone formation in a round rat craniotomy parietal defect [84]. High and low doses of FGF-2 were precipitated onto the buttons after immersion in supersaturated calcium phosphate solution. Although the doses of FGF-2 were not optimal, FGF-2 release stimulated in vivo BMP-2, FGF, ALP and osteocalcin activity.
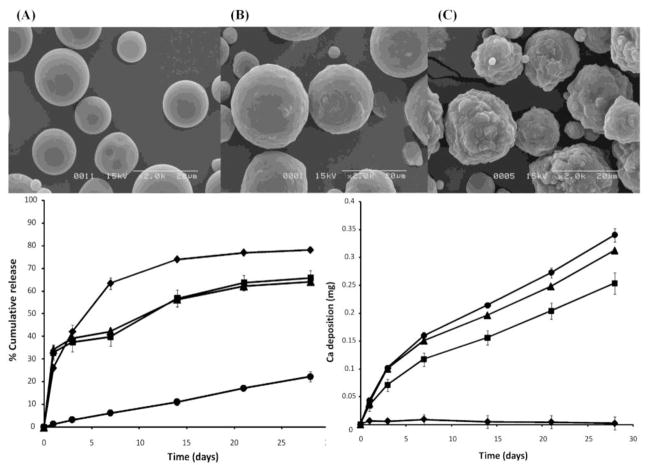
Protein encapsulation can also be performed using composites with ceramics or ceramic derivatives such as calcium phosphate cements (CPC), bioactive glass, HAp and beta tricalcium phosphate (β-TCP) [85, 86]. Ceramic materials provide load bearing [87], retarded delivery [88, 89] and enhanced angiogenic [40, 90] characteristics to natural and synthetic polymeric matrices. CPC composites, in particular, hold great promise in bone tissue engineering applications due to their injectability. CPC can be formulated as an injectable paste that self-sets in vivo which allows for minimally invasive reconstruction of irregular defects without the generation of heat [91]. The delivery of BMP-2 and TGF-β1 in rabbit ectopic, radial and femoral defects from CPC composites have demonstrated an increase in bone formation and remodeling after 8–10 weeks [92–94]. Recent studies with calcium phosphate cement (CPC)/gelatin composite and diopside (CaMgSi2O6) ceramic microspheres show that ceramic microparticle systems can be successfully used as an injectable, biodegradable and osteoinductive delivery vehicles, as demonstrated in Figure 1 [95, 96].
Figure 1.
Scanning electron micrographs of pure gelatin (A), gelatin-10% calcium phosphate (B) and gelatin-40% calcium phosphate microparticle composites (C) and in vitro release (bottom left graph) and mineralization (bottom right graph) characteristics. Composites (■ & ▲) possess bioactive calcifying properties similar to calcium phosphate powder (●) and decreased release rates compared to pure gelatin microparticles (◆). (Reprinted with permission from Leeuwenburgh et al. [95]. Copyright 2010 American Chemical Society.)
Although degradable materials like ceramics are preferred for bone regenerative medicine, tissue engineering principles can be applied to enhance the performance of non-degradable materials. Metals like titanium and stainless steel have been used for many bone applications, but do not possess the clinically desired ability to direct osteoprogenitor function. Common attempts to improve osseointegration and cellular signaling through growth factor delivery involve chemical conjugation with heparin or modification with titanium-binding motifs [97–99]. Surface modification techniques such as plasma spraying, acid-etching, anodic polarization and grit-blasting enhances growth factor binding by changing the surface chemical composition and topology [100–103]. Another surface modification technique is to apply coatings of bioactive polymers and hydrogels that physically entrap molecules against the material surface or within the porous structure to direct osteoinductive cellular function [104–106]. In vivo release profiles of FGF-2 from gelatin microparticles (GMP) embedded in hydroxyapatite-coated titanium nonwoven fabrics (Ti-HAp-GMP) showed less than 15% burst release after one day and more than 60% retention after 7 days in comparison to uncoated Ti and Ti-HAp [107]. Ti-HAp-GMP with bound FGF-2 induced marrow-like bone tissue with mineralization and minor angiogenesis in rabbit skull defects after 6 weeks. Liu et al. achieved ectopic bone formation and ossification in vivo in an ectopic rat model with titanium-alloy discs coated with calcium phosphate and BMP-2 [108]. Osteogenic activity was maintained over 5 weeks with only 60% release of loaded growth factor.
3.2.1.2 Natural Polymers
Polymers of natural origin such as collagen, silk fibroin, hyaluronic acid (HA), starch, fibrin, chitosan and alginate are desirable bone tissue engineering scaffolds due to their biocompatibility, degradability and biomimetic chemical properties. Porous 3D scaffolds, sheets, fibers and other configurations for controlled delivery can be fabricated via freezing, salt-leaching or lyophilization methods. Natural biopolymers provide a signaling ECM-like matrix that facilitates the migration, proliferation and differentiation of osteoprogenitor cells for in vivo manipulation of the regenerative process [109].
As the main protein component of natural ECM, collagen is one of the most investigated natural polymers for tissue engineering scaffolds and its ability for inducing bone formation with delivered growth factors has been well established [110]. Pharmacokinetic and thermodynamic studies show that rhBMP-2 binding to collagen and its isoforms is specific, forming multiple growth factor layers on the collagen surface as a function of proton ion concentration. By modulating the isoelectric point of rhBMP-2 and pH and ionic strength of the carrier solution, increased loading and controlled delivery of BMP-2 for favorable bone induction can be achieved [111, 112]. However, rhBMP-2-soaked collagen sponges, a clinically applied growth factor therapy for bone regeneration, lack sustained release characteristics, exhibiting high burst release and retaining less than 5% protein after a two week time period [113]. Chemical modification of natural polymers with functional groups or linker molecules has displayed improved binding and release. Collagen coupling with perlecan domains, a heparan sulfate proteoglycan, shows equally strong affinity for heparin-binding growth factors such as VEGF, FGF, PDGF and TGF-β as conjugating heparin linker molecules, which have been tested with other biopolymers [114–117]. In a different approach, the growth factor itself can be engineered to increase binding specificity. BMP-2, fused with a collagen binding domain or hexahistidine tag to the N-terminal of the growth factor, shows improved release kinetics and osteogenic activity in vivo when bound to collagen or monoclonal polyhistidine antibody-demineralized bone matrix, respectively [118, 119].
The main challenges affecting the use of natural polymers for scaffolds is their batch-to-batch variability, potential immunogenicity, sterilization-induced inactivation and fabrication costs, especially if they are reconstituted from allogeneic or xenogenic sources. Although purification and recombinant technology has streamlined mass production of biopolymers with defined material properties, the degradation rate and the subsequent growth factor release rate are still difficult to control. Collagen, in particular, is relatively unstable and degrades quickly in the physiological environment due to protease activity. Using more robust natural materials [120, 121], improved crosslinking methods [122, 123] or natural composites with inorganic or synthetic material such as HAp/chitosan/poly(L-lactic acid) (PLLA) or collagen/poly(DL-lactic acid) (PLA) [124–126] can provide greater control over the physical properties. Collagen type I vitrigel, a compact collagen scaffold comprised of vitrified type I collagen fibers, demonstrated enhanced mechanical properties and sustained release of BMP-2 in comparison to collagen type I scaffolds in vitro and in vivo [127]. In a mouse calvarial defect, BMP-2-containing vitrigel showed extensive bone formation and 85% BMP-2 retention after 4 weeks, even though vitrigel and BMP-2-loaded normal collagen scaffold controls experienced similar burst release in the first day. Sustained delivery of BMP-2 was attributed to the dense microarchitecture of the vitrigel scaffold, which deters the rapid degradation of the collagen fibers, thus slowing BMP-2 release. Therefore, modifications in the physical structure or processing of natural polymers can improve their controlled release kinetics.
3.2.1.3 Synthetic Polymers
Synthetic polymers offer great versatility for growth factor delivery due to their simple processing and physical, chemical, and mechanical properties, which can be specifically tailored for different applications. Although there are some issues in terms of inflammatory response, bulk degradation and poor clearance of high molecular weight polymers, rational design and formulation of synthetic materials can provide superior characteristics for controlled delivery of functional protein and bone tissue restoration. Poly(α-hydroxy acids), poly(anhydrides), poly(phosphazenes), poly(ethylene glycol) (PEG), poly(ε-caprolactone) (PCL), poly(propylene fumarate) (PPF), polaxamers, polyurethanes and polyphosphate polymers have all been used as scaffolds for bone tissue engineering [128].
In order to create ECM-mimicking scaffolds capable of tissue ingrowth, synthetic materials require processing to generate uniform and well-distributed porous architectures, which tunes the scaffold degradation rate, mechanical strength and degree of growth factor entrapment. Fabrication techniques such as solvent casting, particulate leaching, freeze drying, thermally induced phase separation, melt molding, phase emulsion, in situ polymerization and gas foaming have all been utilized for scaffold construction [129]. Porous scaffolds created from poly(glycolic acid) (PGA), PLA and its copolymer poly(DL-lactic-co-glycolic acid) (PLGA) have been widely investigated for BMP-2 [130, 131], BMP-7 [132] and TGF-β3 [133] delivery. The primary processing method with those scaffolds is salt-leaching or emulsion freeze drying, which may create irregular, nonuniformly distributed pores that decrease transport and mechanical properties. Sustained released can be obtained through careful design of the pore size, shape and interconnectivity [134, 135]. Supercritical carbon dioxide mixing technology has been used to form PLA scaffolds with controlled porosity and incorporate BMP-2 or VEGF simultaneously in one step [136, 137]. The average pore size was 250 μm and porosity was 70%, as determined by microcomputed tomography. In vivo studies with a VEGF-incorporated PLA scaffold showed increased bone volume and ALP activity 28 days post-implantation in a mouse femur segmental model [137]. Hydrophobic polymers like PPF can be favorable substrates for bone formation as well when processed into porous scaffolds or coupled with micro/nanoparticles for growth factor release. Vehof et al. demonstrated that photocrosslinked and salt-leached PPF scaffolds enhanced bone formation in a rabbit subcritical size cranial defect when coated with TGF-β1 [29]. Kempen et al. reported enhanced bioactivity and ectopic osteoinductivity of BMP-2 when it was released from PLGA microsphere-PPF composites than gelatin hydrogels or microsphere/gelatin hydrogels [72]. The in vivo release profile showed an S-shaped curve over 84 days in a rat subcutaneous model.
Since extended release of growth factors has shown to be more beneficial for osteogenic efficacy, synthetic polymer blends and other scaffold configurations such as films, mats and meshes have also been studied to provide additional control over the release kinetics. Electrospinning is a technique which creates fibrous scaffolds and films through electrostatic repulsion of liquid polymer solutions using a high-voltage source. The nano/micron-sized fibers, reminiscent of ECM collagen fibers, are suitable for cell attachment and growth. Their orientation and geometries for growth factor release can be controlled by collecting the extruded fibers on various static or rotating plates. Sahoo et al. demonstrated that at least one week of sustained basic FGF release with both blended electrospun and coaxial electrospun PLGA scaffolds upregulated ECM protein gene expression in bone marrow stromal cells [138]. Electrospun PLGA-HAp composite scaffolds incorporating BMP-2 showed sustained release and complete bone healing in nude mice tibial defects over 6 weeks [139]. Similarly, PCL-PEG electrospun scaffolds displayed core-shell pore size-dependent release of rhBMP-2 in a rat cranial defect for 8 weeks [140]. The advent of computer-aided design strategies such as solid free form fabrication, application solvent technology and fused deposition modeling have provided additional methods for altering carrier morphology. Rapid prototyping technology enables the fabrication of reproducible scaffolds with precisely controlled geometry for complex shapes as well as manipulation of scaffold surface properties and bioactive factor incorporation to direct desired biological activities [141–143].
3.2.2 Encapsulated Growth Factor Carriers
Since growth factors suffer from rapid degradation, encapsulation within a biomaterial vehicle can provide protection from enzymes and increased protein retention at the target site. Additionally, controlled dosing prevents unwanted cytotoxic and inflammatory effects from superphysiological doses as well as ectopic bone formation, problems encountered by direct administration of protein [144]. The release kinetics of particulate carrier systems are ultimately determined by the rate of carrier degradation, loaded amount of protein, protein diffusion, particulate size and if applicable, the particulate crosslinking extent.
Synthetic polymers such as poly(α-hydroxy acids), poly(orthoesters), poly(anhydrides), poly(amino acids) and copolymers of lactic and glycolic acid have been used for encapsulated delivery for their tunable physicochemical properties. Studies have shown that the release profile of rhBMP-2 from PLGA microparticles is affected by the molecular weight, lactic to glycolic acid ratio, end-group functionalization, and dose of incorporated growth factor. In vivo studies have demonstrated that PLGA microparticles prolonged retention and release of rhBMP-2 and rhBMP-7 to sufficiently bridge rat and ovine critical size cranial defects, respectively [145–148]. The release kinetics were affected greater in vitro by the functionalization of acidic moieties to PLGA end groups, which accelerated the degradation of the carrier, resulting in premature release of encapsulated protein and sub-optimal bone formation. Copolymerization of PLGA with other polymers has been examined to prevent inflammatory response and loss of BMP-2 bioactivity. Poly(ethylene glycol) (PEG)-PLGA diblock copolymers and PLGA-HAp composites were found to be more biocompatible without affecting encapsulation efficiencies and rates of release [149, 150].
Natural carrier materials have also been used for delivering bone growth factors due to their protein affinity and mild processing conditions. Gelatin, silk fibroin and glycidyl methacrylated dextran-PEG microspheres have all been used to efficiently deliver functional BMP-2 in vitro and in vivo [151–153]. One problem concerning naturally derived materials is the poor mechanical stability under physiological conditions, resulting in inadequate protein retention. Employing composite materials or chemical crosslinking methods can also provide the necessary physicochemical properties. In vivo studies with chitosan-alginate or chitosan-collagen microparticles showed enhanced bone formation when delivering Nel-like molecule 1 or TGF-β1 in rat defect models, respectively [154, 155]. Chan et al. fabricated injectable collagen microspheres using a modified emulsion and self-assembled reconstitution process that provided dose-dependent growth factor release based on the degree of photochemical crosslinking [156].
There is continuous effort in developing strategies to precisely control carrier degradation and the growth factor release rate though the incorporation of particulate systems into scaffolds and use of sub-micron particles. VEGF-alginate microparticles incorporated into chitosan and uncapped end group PLA scaffolds exhibited lower burst release and prolonged VEGF release in comparison to microparticle-uncoated chitosan scaffolds and microparticles separately [157]. Niu et al. achieved similar retention rates in vitro using porous nanohydroxyapatite/collagen/PLA scaffolds with chitosan microparticles encapsulating a BMP-2 synthetic peptide [124]. A different method that requires no external scaffold involves the fusion of PLGA microspheres into modular 3D scaffolds using dichloromethane vapor. Release studies of IGF-1 and TGF-β1 in the fused microsphere scaffolds show promise in spatially and temporally releasing multiple growth factors for tissue engineering applications [158]. Submicron and nano-sized particles (NP) for controlled delivery hold several advantages over microparticle carriers in terms of surface functionality due to scaling effects in their physicochemical properties [159, 160]. Increased growth factor encapsulation efficiency is achieved through encapsulation of proteins with the particles and grafting of proteins on the surface [161]. For bone tissue engineering applications, although nanoparticles alone can provide sustained and even sequential release in vitro [162, 163], their incorporation into a scaffold or hydrogel matrix can modulate the kinetic properties for improved repair [164, 165]. Chung et al. developed a heparin-functionalized NP/fibrin gel complex for the release of low doses of rhBMP-2 in a rat critical size calvarial defect [165]. In vivo results showed the efficacy of the BMP-2 heparin-NP/gel complex for higher defect closure, bone density and alkaline phosphatase activity over 4 weeks in comparison to controls, demonstrating that NP-based systems can induce bone regeneration with prolonged release of minimal amounts of growth factor. The main consideration with using nanoparticulate systems is that the increased surface-to-volume ratio leads to higher initial burst release.
The harsh processing conditions and low loading efficiencies of particulate systems have led to the development of lipid-based vehicles for growth factor delivery. Lipid-based carriers are well-established for gene and drug therapy for their abilities of injectable, targeted delivery, biocompatibility and ease of fabrication. The efficacy of loading and dosing depends on the composition and interaction of the drug, liposome and accompanying shells/matrices [166–168]. In a study by Haidar et al., single administration of hybrid core-shell nanoparticles consisting of cationic liposome core and self-assembling layer-by-layer anionic alginate and cationic chitosan for slow release of rhBMP-7 induced bone formation in a rabbit distraction osteogenesis model [169]. Another group obtained sustained release of bioactive BMP-2 over 2 weeks using a lipid-based microtubule system that modulated release through hydrolysis of the microtubule ends [170]. Both studies demonstrate that lipid-based carriers have potential as injectable carriers for localized delivery; however, further investigations are required to assess if growth factor release can be sustained for a physiologically relevant timeframe for full bone regeneration.
3.2.3. Hydrogels
Controlled delivery of growth factors to a bone defect can also be achieved with the release from a hydrophilic hydrogel network, which is dependent on the physicochemical properties of the polymer structure and bioactive factor, type and density of crosslinker and target release kinetics. Drug loading for hydrogels is achieved via physical entrapment, either with drug absorption post-fabrication or in situ encapsulation, with ligands with specific affinity for the active agent or with tethering of metabolically cleavable linker molecules [171]. Both synthetic and natural materials have been used as hydrogel carriers of osteoinductive factors including gelatin [172], alginate [122, 173], fibrin [116], hyaluronic acid [174] and PEG-based polymers [175]. All provide a tissue-compatible substrate for cell attachment and growth while delivering functional proteins in a predictable and controlled manner over time.
For many hydrogel systems, biomolecule release occurs through the diffusion of molecules as a function of porosity, degradation or the swelling of the hydrogel network. Hydrogel permeability and swelling can be precisely controlled by using ionic, physical (UV radiation, thermal) and covalent crosslinking (glutaraldehyde, carbodiimide, acrylate/thiol chemistry) methods. Stable HA and alginate gels have been fabricated in a mild and efficient manner using photocrosslinking techniques. Patterson et al. fabricated slow, intermediate and fast-release glycidyl-methacrylated HA (HA-GMA) hydrogels by modulating the degree of interchain crosslinks with increasing concentrations of HA-GMA or adding 1-vinyl-2-pyrrolidinone as a co-monomer [176]. The release rate of BMP-2 directly correlated with the hydrolysis of the ester linkages of the crosslinks, which was confirmed by gel permeation chromatography analysis. Growth factor release from chemically crosslinked gelatin has shown similar kinetics for BMP-2 in vivo. Studies in a rabbit segmental defect and mouse subcutaneous implantation model suggest that there is an optimum hydrogel water content and crosslinking density for synchronized delivery and bone formation [177, 178].
The main challenge with diffusion- and swelling-dependent release mechanisms is that increased crosslinking extent reduces cytocompatibility and effective control over small biomolecules and proteins. Additionally, the release kinetics are influenced by the mode and rate of hydrogel degradation, hydrogel-protein interaction and inclusion of multiple phases (polymers or particulate carriers) or cleavable sequences within the network. Therefore, chemically-controlled growth factor delivery may be a better mechanism in order to provide reproducible release profiles from hydrogels [171]. A variety of chemically-dependent release systems have been developed with PEG-based hydrogels due to their biocompatibility, hydrophilicity and versatility in tailoring their physicochemical properties. Introducing hydrolytically or enzymatically cleavable functional units along to the PEG backbone can impart specific degradation characteristics for protein delivery. Copolymerization of PEG with PLA, PGA or chemical intermediaries with end-capped acrylate or sebacic acid acrylate groups can produce crosslinkable and degradable hydrogels with labile ester bonds [179–182]. The delivery of BMP-2 with oligo(poly(ethylene glycol) fumarate) (OPF), a copolymer of PEG and fumaric acid, and PEG-PLA has been examined in vitro [183] and in vivo for bone and soft tissue formation [184, 185]. BMP-2 efficacy for bone remodeling and healing can be synergistically enhanced by incorporating proteolytic peptides susceptible to matrix metalloproteinases or plasmin [186, 187] or providing topographical or peptide cues to stimulate specific cellular responses [188–190].
3.2.4 Stimulus Responsive Polymers
The success of sustained growth factor release at therapeutic concentrations for controlling cellular function and tissue regeneration has been demonstrated in aforementioned studies. However, few systems have addressed the cooperative biological signaling events of cells as a function of the changes in their dynamic microenvironment. Incorporating stimulus-responsive elements into growth factor delivery vehicles is one biomimetic strategy to obtain specific growth factor release triggered by selective physical, biochemical and external stimuli [191].
Temperature and pH-sensitive polymers are among the most widely investigated physical stimulus-responsive materials and can be combined to form dual-responsive delivery systems. Poly(N-isopropylacrylamide) (PNiPAAm) [192–194], poly(organophosphazenes) [195], PEG-based di/tri block copolymers [196], PCL [197] and their derivatives as well as biomimetic materials like chitosan, dextran and elastin-like peptides [198, 199] undergo a reversible phase transition at the lower critical solution temperature, which can be exploited for drug delivery in physiological conditions. Release of molecules from pH-dependent carriers like poly(acrylic acid) relies on the reversible volumetric swelling and deswelling of acidic and basic pendant groups. Kim et al. developed a pH-and thermosensitive hydrogel for BMP-2 delivery by adding pH-sensitive sulfamethazine oligomers (SMO) end groups to thermosensitive block copolymer made of poly(ε-caprolactone-co-lactic acid) (PCLA) and PEG units [200]. SMO-PCLA-PEG-PCLA-SMO hydrogels displayed functional BMP-2 activity over a narrow pH and temperature range, leading to elevated ALP activity and mineralization over 7 weeks in vivo. Although the release kinetics and local BMP-2 concentration were not measured, the study demonstrates that carrier systems can be designed for controlled release in specific environmental conditions; conversely, environmentally triggered systems also can be manipulated to improve growth factor retention during long term storage.
Biochemically triggered release of growth factors can be achieved through incorporation of cleavable peptides for enzymatic degradation. Matrix metalloproteinase (MMP) peptide crosslinkers confer proteolytic sensitivity to synthetic and natural hydrogels for synchronized tissue remodeling and hydrogel degradation [201–203]. MMP-sensitive PEG and HA hydrogels with BMP-2 delivery both showed improved bone regeneration in rat calvarial defects [186, 202]. Alternatively, enzyme-cleavable peptides can be used to attach proteins and prodrugs for delayed release and activation in response to cell infiltration [204, 205].
Although drug delivery in response to magnetic, ultrasound, irradiation and electric stimuli has shown promise, the delivery of growth factors and proteins via external triggers for bone tissue engineering remains limited to experimental trials. Magnetic biohybrid scaffolds formulated from collagen-based scaffolds dip-coated in aqueous ferrofluid particles or crosslinked with magnetite nanoparticles have been investigated as novel delivery vehicles. Growth factor loading is accomplished through absorption onto the particle surface and guided into the scaffold with an externally applied magnetic field [206, 207]. The primary strength of the approach is the ability to control reloading and spatiotemporal release of different proteins from an external source after implantation in vivo, allowing for creation of desired growth factor gradients and long term sustained release. Additionally, preliminary in vitro studies suggest that the magnetic scaffolds and their degradation byproducts are biocompatible. However, the influence of the magnetization on osteogenic differentiation as well as magnetization stability for the duration of the tissue repair process has not been thoroughly examined.
3.2.5 Multifunctional Delivery Platforms
Biomimetic strategies capable of orchestrating the appropriate time, concentration and spatial profiles of growth factors and other signaling molecules with target cells and ECM may enhance and accelerate effective tissue repair. Although the multitude of signaling mechanisms of the wound healing response is not fully elucidated, the current understanding suggests that the delivery of single proteins inadequately stimulates endogenous repair mechanisms. Numerous studies in both humans and murine fracture models show that expression of various bioactive factors at the site of injury is tightly regulated during the fracture healing and subsequent remodeling periods [15]. The immediate injury response (hours to 3 days) is marked by the release of pro-inflammatory cytokines and growth factors such interleukin (IL)-1, IL-6, TNF-α, FGF, PDGF and TGF-β1 from the systemic circulation and inflammatory cells, initiating the signaling events for matrix deposition and progenitor cell recruitment. Secretion of other growth factors including BMPs, GDFs, IGFs, TGF-βs and angiogenic molecules from MSC and osteoprogenitor populations occurs in the later phases of fracture healing (5–21 days) and returns to normal levels during remodeling. Although certain growth factors like GDF-5 and GDF-10 or VEGF may not play a direct regulatory role in osteogenesis like BMPs (as earlier described), they aid in cartilage mineralization during endochondral bone formation and induction of new vessels, respectively. Reviews by Dimitriou et al. and Tsiridis et al. provide further detail into the temporal expression of the growth factors involved in fracture healing [53, 208].
Therefore, local administration of multiple growth factors with the proper spatiotemporal kinetics may enhance functional tissue restoration with lower doses of loaded protein. Designing carriers capable of pre-programmed release of multiple factors as well as regulating cell activity remains a challenge. Key growth factors and their appropriate combinations and dosing for promoting specific regenerative responses remain unclear [209]. Controlled multiple growth factor delivery and its effects on tissue regeneration remains a strong area of research in tissue engineering.
3.2.5.1 Simultaneous and Sequential Delivery
One strategy for multiple growth factor release involves simultaneous delivery of two growth factors locally to a defect. Since individual growth factors possess different functions depending on the target tissue, the appropriate combination of growth factors may act cooperatively to regulate complete tissue formation. Richardson et al. reported the first dual delivery system consisting of a PDGF-encapsulated PLGA microsphere and VEGF-incorporated PLGA scaffold composite for angiogenesis [210]. Dual delivery of PDGF and VEGF demonstrated enhanced blood vessel density and maturation in comparison to bolus administration of VEGF or PDGF alone in a subcutaneous pocket model. The synergistic effects of dual growth factor delivery have been further examined in vitro and in vivo with other particulate scaffold composites for numerous tissue engineering applications including bone (BMP-2 with VEGF, TGFβ-3 or IGF-1) [45, 211–213] and cartilage formation (IGF-1 and TGFβ-1) [214]. Embedding multiple growth factors in charged polyelectrolyte films on scaffolds or beads using layer-by-layer technology can also provide controlled release via hydrolytic degradation of the multilayer coat [215, 216]. Using another technique, Choi et al. achieved dual release of BMP-2 and dexamethasone from PLGA-core/alginate-shell microcapsules fabricated with coaxial electro-dropping [217]. The temporal release of each component in the injectable system was regulated based on their location (shell or core) within the capsule.
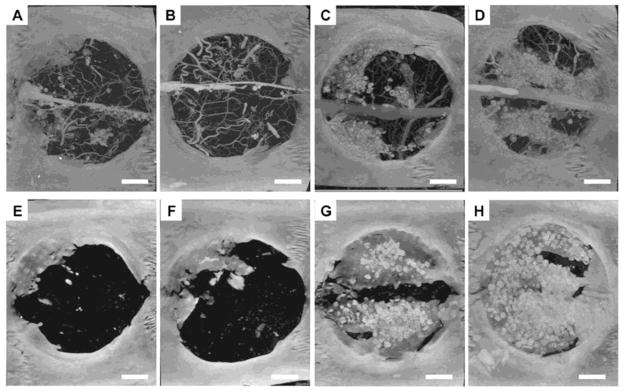
Studies have shown that sequential and spatiotemporal release may lead to improved tissue regeneration by providing physiologically relevant release profiles and spatial gradients that mimic the natural healing response. Various composite systems have been developed to recapitulate the early expression of angiogenic factors and subsequent upregulation of osteogenic factors of bone healing in vivo by releasing VEGF and BMP-2 at different rates; however, enhanced bone formation has only been achieved in ectopic, but not orthotopic models. Initial BMP-2 and VEGF release studies by Patel et al. and Young et al. using GMP-loaded PPF scaffolds found that bone formation in a rat calvarial CSD was BMP-2 dose-dependent [45, 218]. There were significant beneficial effects of VEGF on bone regeneration in the presence of BMP-2 at 4 weeks, but not 12 weeks, as seen in Figure 2. In a separate study by Kempen et al., suboptimal bone formation was found in a rat femoral defect with sequential release of VEGF and BMP-2 from PLGA microsphere-loaded PPF scaffold-gelatin hydrogel composites over 8 weeks [44]. The lack of long term VEGF impact suggests that VEGF action occurs in the early stages of healing and may be inhibited by the large initial burst effect of the system or inherent vascularity of the model. In contrast, current understanding of the angiogenesis as a part of fracture healing indicates that VEGF acts in the late stages of healing prior to remodeling, while PDGF and angiopoietin (1–2) are upregulated immediately after injury [53, 208]. Manipulating VEGF release to achieve the appropriate temporal expression or in conjunction to early release of other angiogenic molecules may improve the synergistic effects of VEGF-BMP-2 for augmenting bone regeneration for extended periods of time.
Figure 2.
Microcomputed tomography images of bone regeneration in rat calvarial critical size defect at 4 (top row) and 12 (bottom row) weeks with no growth factor delivery (Panels A & E), VEGF delivery only (Panels B & F), BMP-2 delivery only (Panels C & G) and VEGF/BMP-2 dual delivery (Panels D & H). Bone formation with dual delivery is higher at 4 weeks and comparable at 12 weeks to BMP-2 delivery alone. Scale bar represents 200 μm. (Reprinted with permission from Patel et al. [45])
Sequential release of BMP-2 and BMP-7 has also been investigated with nanocapsules of PLGA and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and PLGA scaffolds loaded with poly(4-vinyl pyridine)/alginic acid polyelectrolyte microspheres [164, 219]. For both studies, suppressed proliferation of rat MSCs occurred with early release of BMP-2 and later release of BMP-7, indicating increased osteogenic activity, but ALP activity varied. The differing results indicate that the correct growth factor combinations, animal models and carrier properties greatly affect osteogenic differentiation and should be considered when designing delivery systems.
3.2.5.2 Spatially Controlled Delivery
Another factor to account for is the spatial presentation of growth factors to mimic the natural concentration gradients in living systems. Spatially controlled growth factor delivery provides topological cues for cell infiltration and differentiation. A promising approach for osteochondral defects was developed by creating linear gradients of rhBMP-2 and rhIGF-1-incorporated silk fibroin microspheres immobilized in a 3D porous silk scaffold [220]. To prepare single growth factor gradient scaffolds, a gradient maker concurrently eluted silk solution with decreasing volumes of a separate growth factor-loaded microparticle solution into a cylindrical glass mold. Sodium chloride particles were added with the solution mixtures and leached to create porous scaffolds. Reverse gradients were created by adding a different growth factor-loaded microparticles in the silk solution and following the same experimental protocol. Simultaneous release of encapsulated rhBMP-2 and rhIGF-1 promoted osteogenic and chondrogenic differentiation of hMSCs over 5 weeks along the gradients. Multilayered scaffolds are a unique platform that enables multiple growth factor delivery through incorporation of different phases into scaffolds for differential release kinetics. However, multilayered scaffolds can also employ spatially controlled release of bioactive factors to engineer interfacial tissues for orthopedic applications. For example, the cartilage-bone interface between the hyaline cartilage layer and underlying bone consists of separate zones with distinctive ECM composition and mechanical properties [221]. Holland et al. developed a bilayered OPF hydrogel with a bottom bone-forming layer and a top cartilage-forming that incorporated TGF-β1 and IGF-1 to mimic the structure of rabbit osteochondral defects [222]. Although no synergistic effects for cartilage formation was observed from the growth factor combination in vivo, TGF-β1 and IGF-1 were released at different rates only to the cartilage-forming layer. Analogously, bilayered OPF hydrogels developed by Guo et al. demonstrated enhanced chondrogenic differentiation of encapsulated rabbit MSCs in vitro with delivery of TGF-β1 or TGF-β3 in the chondrogenic layer and co-culture with pre-differentiated osteogenic cells in the lower bone-forming layer [223, 224].
3.2.5.3 Platelet-Rich Plasma
An alternative, yet controversial approach for delivering effective combinations of growth factors for tissue is the use of platelet-rich plasma (PRP), an autologous plasma product enriched with growth factor and protein reservoir units like PDGF, VEGF, IGF and TGF-β. PRP preparation involves platelet activation with commercially available bovine thrombin or more immunocompatible substances like calcium chloride, thrombin receptor activator peptide-6 or sodium citrate, as discussed in other reviews [225]. PRP shows promise for bone tissue repair via controlled delivery from hydrogels and scaffolds in conjunction with cells or BMP or as a carrier material for other growth factors and proteins [226–230]. However, the full range of PRP’s benefits is unknown and has shown variable results in clinical settings and experimental studies. Kretlow et al. demonstrated that the inclusion of rat PRP to uncultured bone marrow mononuclear cells within a fibrin glue-scaffold hybrid construct had no effect on cell differentiation or bone formation in a rat calvarial CSD [231]. Although the use of fibrin glue may have influenced PRP function, successful bone regeneration was ultimately dependent on the presence of cells and scaffold material.
4. Cell Therapy
Cellular regenerative strategies offer a different means to recapitulate the bone healing process by providing cell populations that directly participate in the assembly of new tissue and secrete trophic factors to regulate local cellular activity for augmented repair. Biomaterial carriers for controlled delivery of cells function similarly to those for growth factor delivery, in which they act as targeted carriers for delivery as well as supportive ECM-like substrates for cell adhesion, migration and growth. Additional criteria for cell delivery technologies include carrier permeability, biocompatibility and biodegradability to maintain the viability of cells during administration and for the duration of the regenerative process [14]. MSCs, the main source of cells for bone tissue engineering applications, are exempt from most of the ethical and supply concerns with other cell types, making them ideal for cell therapy. Additionally, MSCs possess the ability to differentiate down the osteogenic lineage with high proliferative potential in vitro. There are four primary approaches for engineering MSC-based cell delivery systems with enhanced osteoinductive capacity: 1) delivery of cells within an injectable or prefabricated scaffolds 2) pre-culture/co-delivery of cells with osteoinductive growth factors or co-culture with other cell types, 3) delivery of cellular or acellular bioreactor cultured scaffolds and 4) delivery of genetically modified cells for expression of osteogenic genes. Experimental results for the different approaches of cell delivery systems in vivo are shown in Table 2.
Table 2.
In vivo applications of cell therapy for bone tissue engineering
| Mode of Delivery | Cell type | Animal model | Key Results | Reference |
|---|---|---|---|---|
| CaP-coated titanium scaffold | RMBC | Rat subcutaneous pocket | Supported RBMC attachment and proliferation; no ectopic bone formation | [288] |
| Gelatin/alginate scaffold | rBMSC | Mouse subcutaneous implant | Observed cell attachment and neovascularization; suppression of differentiation markers despite predifferentiation | [232] |
| CPC/alginate scaffold | rBMSC | Rat calvarial CSD | Scaffold directed osteogenic differentiation of cells, bony tissue observed after 6 weeks | [236] |
| MPEG/PCL hydrogel | rBMSC | Rat subcutaneous implant | Dexamethasone dose-dependent differentiation and bone formation; little cell migration from center to periphery | [197] |
| PEG-MMP hydrogel | rBMSC/BMP-2 | Rat calvarial CSD | Bone formation greater in scaffold-BMP-2 groups; undifferentiated rBMSCs had no/inhibitory effect on osteoinduction | [186] |
| ECM-titanium mesh (acellular) | rBMSC | Rat subcutaneous implant | Tissue response of pregenerated ECM-Ti similar to plain Ti; no bone formation, but some vascularization observed | [256] |
| ECM-HAp microparticles (acellular) | Rat calvarial osteoblast and dermal fibroblast | Rat calvarial defect | Compared to HAp control, greater integrated bone formation; no complete bridging or difference between types of cell-generated ECM | [289] |
| Cell transplantation | BMP-2/VEGF expressing mouse MSC | Mouse tibial defect | Augmented bone healing, neoangiogenesis and bone strength with dual expressing cells | [265] |
| Collagen disks | BMP-4/VEGF expressing MDSC | Rat intramuscular pocket/calvarial CSD | Cartilage mineralization, capillary ingrowth in ectopic site; increased bone formation, enhanced angiogenesis with less dual expressing cells compared to BMP4 or VEGF alone | [47] |
| PLGA scaffolds | Runx2/Osterix expressing ADSC | Nude mouse subcutaneous implantation | Extensive mineralization and bone formation with individual or dual genes after 6 weeks | [263] |
| Gelfoam | Ex vivo VEGF- transfected fibroblast | Rabbit tibial defect | Accelerated complete bridging, higher vessel density compared to controls | [266] |
ADSC, adipose-derived stem cell; BMP, bone morphogenetic protein; CaP, calcium phosphate; CPC, calcium phosphate cement; CSD, critical size defect; ECM, extracellular matrix; HAp, hydroxyapatite; MDSC, muscle-derived stem cell; MMP, matrix metalloproteinase; MPEG, methoxy poly(ethylene glycol); PCL, poly(ε-caprolactone); PEG, poly(ethylene glycol); PLGA, poly(lactic-co-glycolic acid); rMBC, rat marrow bone cell; rBMSC, rat bone marrow stromal/mesenchymal stem cell; Ti, titanium; VEGF, vascular endothelial growth factor
4.1 Cell-laden Scaffolds
The simplest method for cell delivery utilizes scaffolds to provide the appropriate physical and chemical properties for controlling the cell response without co-administration of exogenous growth factors. A variety of cell-compatible scaffolds have been formulated with natural and synthetic materials [224, 232, 233]. However, injectable ceramic-based composites consisting of CPC and cytocompatible hydrogels may have better potential as effective bone regenerative systems by possessing osteoconductive characteristics for seeded MSCs with the added benefit of injectability for simple cell encapsulation and delivery [234]. Ceramic composite scaffolds consisting of CPC and cytocompatible hydrogels have demonstrated favorable osteoconductive characteristics for seeded MSCs with the added benefit of injectability for simple cell encapsulation and delivery. In one study, rat MSCs cultured on CPC-chitosan composites showed a several-fold increase in ALP activity in comparison to FDA-regulated CPC scaffolds and tissue culture plastic in vitro, indicating lineage-specific differentiation towards the osteogenic phenotype [235]. Another study showed that direct deposition of injectable CPC-alginate solutions formed degradable fibrous scaffolds capable of stimulating porosity-dependent differentiation of MSCs [236]. In vivo implantation of the porous composite scaffolds within a rat critical size calvarial defect showed near complete closing of the defect over 6 weeks. Other injectable materials like thermoresponsive PNiPAAm or microsphere formulations also show some potential for directing MSC differentiation, without possessing any intrinsic osteoinductivity. Klouda et al. demonstrated that PNiPAAm-based constructs were capable of mineralizing in vitro, which may provide osteogenic cues for encapsulated cells [237].
4.2 Co-delivery of Cells with Bioactive Factors
To enhance their osteogenic efficacy, MSCs can be chemically or physically manipulated in vitro to stimulate their differentiation towards a specific lineage prior to scaffold delivery. Most commonly, osteogenic medium with dexamethasone, β-glycerol phosphate and ascorbic acid has been used to enhance osteoblastic differentiation of MSCs ex vivo/in vitro. MSC culture medium can also be supplemented with growth factors like BMPs and TGF-βs for controlling their bone-forming activity. The main problem is that MSC proliferation is greatly hindered by their early induction down the osteogenic lineage, reducing the number of viable cells for delivery. Also, the BMP and TGF-β half life is short, requiring large amounts to maintain the differentiated MSC phenotype. Co-delivery of growth factors and MSCs in various controlled delivery vehicles has been widely investigated, as discussed in earlier sections. Incorporation of growth factors is used to stimulate transplanted cell activity and differentiation as well as recruit undifferentiated osteoprogenitor cells into the carrier. Reciprocally, the cells deposit bone-like matrix to induce bone formation and defect closure. Numerous studies have shown that growth factor and MSC co-delivery possesses regenerative potential in vitro and in vivo [174, 186, 202]; however, it is unclear if the synergistic effects for enhancing bone formation are significant. Recent studies using fluorescent carboxyfluoresceine-diacetate-succinimidyl-ester-labeled MSCs have shown that post-transplant survival after 7 days is very low, suggesting that improved delivery techniques and further understanding is needed for optimal cell efficacy [238]. Co-culture of MSCs with other cell types such as endothelial cells (EC) may address the drawbacks of growth factor-mediated cell differentiation. Cross-talk between different cell types, especially MSCs, osteoblasts and ECs, is vital to the formation of new bone as well as the success of tissue engineered cell constructs due to the need for an adequate blood supply [239]. Additionally, MSC and EC interaction leads to local release of VEGF and osteogenic proteins like BMP-2, respectively, which promote osteoblastic differentiation and angiogenesis. Numerous studies have shown the positive effect of EC co-culture on vascularization of cell-laden scaffolds in vitro and in vivo [240, 241]. Cell proliferation and microvessel-like networks were enhanced in the constructs compared to single cell cultures. Furthermore, MSCs in co-culture experienced higher expression levels of osteocalcin and ALP and higher release of VEGF and BMP-2 in vitro. However, similar to dual VEGF/BMP-2 growth factor delivery, there are mixed results in the beneficial effects of co-culture-induced vascularization on bone formation in vivo. In studies by Sun et al. and Tao et al., MSC/EC co-delivery into rat intramuscular pockets showed significant differences in bone formation and mineralization compared to empty, MSC only and EC only scaffolds [240, 242]. Similar results for improved osteogenesis due to increased vascularity were found in a rabbit segmental defect model [243]. After 12 weeks, porous β-TCP scaffolds consisting of MSCs and MSC-induced ECs achieved complete vascularization. By 16 weeks, full bony union was also complete and 3-point bending tests showed that the mechanical properties of the new bone almost matched that of the original. Koob et al. did not observe the same trends in regenerating mice CSD over 6 weeks with a combination of MSC/HUVEC and processed bovine cancellous bone [244]. Despite significant differences in vascularity, no significant differences were found in bone formation between co-culture and control groups. Further investigation into other cell types such as osteoblasts instead of MSCs [245–248] or different EC sources [249, 250] may provide new understanding of the physical and biochemical aspects of angiogenic and osteogenic cell-cell communication in order to improve co-culture therapy.
4.3 Dynamic Culture of MSCs
Alternatively, cells may be cultured within a dynamic 3D environment in order to mimic the natural transport and biomechanical conditions in vivo. Bioreactors such as spinner flasks, rotating flasks and flow perfusion systems provide engineered fluid stresses for stimulating bone differentiation and mineralization of MSCs [251, 252]. In particular, flow perfusion bioreactor systems better control the shear stresses and uniform transport of nutrients to cell-seeded scaffolds, leading to higher seeding efficiencies, cell attachment and expression of osteogenic markers such as osteopontin, osteocalcin, bone sialoprotein and collagen 1α1 [251, 253]. Even without osteogenic supplements present in the culture medium, flow perfusion culture of rat MSCs on titanium fiber meshes showed increased calcium deposition, osteopontin expression and ALP activity compared to static culture, demonstrating that mechanical stimulation greatly influences the osteoblastic phenotype [254]. A novel bioreactor strategy involves the generation of bioactive ECM coatings on biologically inert scaffolds for the presentation of biological signaling cues for bone regeneration. The mineralized matrix deposited by osteoblasts in vivo plays a large role in mediating bone formation. The native ECM consists of a complex arrangement of collagen, glycoproteins and a variety of osteoinductive factors for promoting cell attachment and matrix mineralization, which cannot be easily replicated in vitro through scaffold, cell or growth factor delivery alone. Initial studies utilizing a combinatorial approach showed that culturing rat MSCs under engineered culture conditions in a flow perfusion bioreactor promoted formation of bone-like ECM coating on titanium meshes. Increased MSC osteogenic differentiation was attributed to the cell-synthesized ECM coating, which rendered the titanium mesh osteoinductive. The pregenerated ECM/titanium scaffolds expressed higher calcium content and ALP activity in comparison to plain titanium mesh in the absence of dexamethasone, which support the bone-forming capabilities of the ECM [255]. However, in vitro generated ECM/titanium constructs showed no observed osteoinductive properties in vivo, due to either the non-degradability of the scaffold, quality of the ECM coating or lack of seeded MSCs to facilitate cell-matrix interaction [256]. Studies on the osteoinductivity of cell-generated ECM coatings on biodegradable materials such as PCL, β-TCP and HAp have shown promise in vitro [257–260]. Acellular pregenerated ECM/HAp microparticles demonstrated cooperative effects for improved, but incomplete bone formation in rat calvarial defect against collagen and HAp controls. This suggests that cell-synthesized coatings may provide synergistic effects to bioactive materials [261].
4.4 Stem Cell-based Gene Therapy
Another method of cell therapy involves the genetic manipulation of cells in vitro to improve their differentiation potential and osteoinductive effect in vivo through the secretion of osteogenic factors. The cells act as an engineered protein factory and overexpress osteogenic genes such as BMP-2, VEGF and LIM mineralization protein-1 and transcription factors, Osterix and Runx2, individually or combinatorially [32, 262, 263]. Transduced MSCs derived from bone marrow, fat and muscle cells have all shown bone forming abilities in numerous studies [47, 264–266], without apparent differences in efficacy between the cell types [267]. In vitro studies have suggested that stem cell-based gene therapy has superior osteogenic potential over exogenous growth factor delivery. Meinel et al. found that adenovirus-BMP-2 transduced hMSCs expressed higher levels of osteogenic proteins than BMP-2 delivery following the same temporal profile [268]. A potential problem of stem cell-based gene therapy is the overexpression of growth factors, leading to unwanted ectopic or heterotrophic bone formation. Incorporating inducible promoters with osteogenic genes provides an ON-OFF pharmacologically-modulated system for regulating therapeutic transgene expression. Tetracycline (Tet) and rapamycin regulatable systems for controlling gene expression will be discussed in the next section.
5. Gene Therapy
Gene therapy offers an alternative means to achieve controlled delivery of protein for bone regeneration through the transfer of nucleic acids to somatic cells for sustained therapeutic expression of osteoinductive factors. The advantage of this approach over protein therapy is that appropriate concentrations of functional growth factors are produced stably at the site of interest to regulate cell activity. There are two main gene therapy strategies for bone repair. As discussed earlier, stem-cell based gene therapy involves the delivery of ex vivo genetically engineered cell populations that act as the carrier for therapeutic genes. A more straightforward approach is to use direct gene therapy, in which therapeutic genes are delivered directly in vivo via viral or nonviral vectors. The efficiency of direct gene therapy for bone repair has been demonstrated with different vectors in various animal models [269]. Table 3 describes select in vivo studies of gene therapy for bone tissue engineering.
Table 3.
In vivo applications of gene delivery vectors for bone tissue engineering
| Mode of Delivery | Gene | Animal Model | Key Results | Reference |
|---|---|---|---|---|
| Adenovirus/silk scaffolds | BMP-7 + rBMSC | Mouse calvarial defect | Bone deposits within pores, enhanced expression of osteogenic markers; no effect with transduced BMSC | [283] |
| Adenovirus/gelatin sponge | BMP-2 | Rat calvarial CSD | Two-fold increase in bone area and volume fraction compared to AdBMP-2 alone | [281] |
| Adenovirus | VEGF-A | Rat femoral defect | Accelerated bone healing and increased, but unorganized vessel formation | [42] |
| Adeno-associated virus | BMP-2 | Rat intramuscular injection | Local gene transduction, mature bone formation with AAV- BMP-2 delivery compared to controls | [270] |
| Moloney leukemia virus | LMP-1 | Rat femoral defect | Increased bony union and mineral deposition without heterotopic bone formation compared to BMP-4 delivery | [262] |
| Murine leukemia virus | Cox-2 | Rat femoral defect | Complete fracture healing in 21 days with persistent Cox-2 expression; no ectopic bone formation observed | [290] |
| Plasmid GAM | VEGF | Rabbit radial CSD | Enhanced bone and vessel formation after 12 weeks; response leveled off with higher VEGF concentrations | [37] |
| Cassette vector/electroporation | BMP- 2/BMP-7 | Rat intramuscular injection | Compared to controls, calcified, bone-like tissue after 10 days with dual delivery | [274] |
| Nucleofected hMSCs | hBMP- 2/hBMP-9 | Mouse intramuscular injection | Mature bone formation with transient expression of transgenes, hBMP-9 higher osteoinductivity than hBMP-2 | [275] |
| Plasmid/sonoporation | rhBMP-9 | Mouse intramuscular injection | Moderate ectopic bone formation over 5 weeks, less comparable to electroporation in same model | [276] |
Ad, adenovirus; AAV, adeno-associated virus; BMP, bone morphogenetic protein; Cox-2, cyclooxygenase-2; CSD, critical size defect; GAM, gene-activated matrix; hBMP, human BMP; hMSC, human mesenchymal stem cell; LMP-1, LIM mineralization protein-1; rhBMP, human recombinant BMP; VEGF, vascular endothelial growth factor
The limiting factor of gene therapy is development of vehicles capable of overcoming the cellular barriers to efficiently transfect cells with the genes of interest for the stable expression of transgene encoded proteins at therapeutic levels in vivo [14]. Controlled delivery of genetic material to cells via viral and nonviral vectors has shown great therapeutic potential for orthopedic applications, but they each have distinct disadvantages. Virus-mediated delivery exploits the intrinsic infection ability of viruses such as adenoviruses [42], adeno-associated viruses (AAV) [270] and retroviruses like lentivirus [262, 271, 272] as the vector for efficient gene delivery. Although the use of viral vectors leads to higher transgene expression and transduction efficiency, they possess inherent immunological risks, limited tropism and size limitations on the inserted transgene [272]. Controlled gene delivery using non-viral vectors involves either the direct injection of naked DNA to cells or delivery within cationic liposomes or natural or synthetic polymer-complexes [273]. Since non-viral systems have nonspecific cell targeting abilities, their transfection efficiency and transgene expression levels are low. On the other hand, they possess little immunogenicity or gene size restrictions and easy handling, making them suitable for mass manufacture. Additionally, in vivo transfection efficiencies can be improved through physical means using electroporation [274], nucleofection [275] and sonoporation [276]. One interesting approach to address cell-selectivity and vector localization is to incorporate genetic material with engineered biomaterial scaffolds for transient expression of encoded proteins from infiltrating cells. Gene activated matrix (GAM) technology has shown promise for bone and osteochondral defects by entrapping VEGF, BMP-2, BMP-4 and BMP-2/TGF-β1 coding plasmids [37, 277–279] and are capable of controlled released via cleavable peptide linkages [280]. Alternatively, lyophilized adenovirus and recombinant AAV can also be incorporated in GAM carriers like silk fibroin and HAp scaffolds, showing improved DNA delivery for enhanced bone formation [281–283].
A major concern of gene therapy is the ability to control transgene expression, as the overexpression of proteins leads to unwanted adverse side effects and abnormal tissue formation. Inclusion of Tet or its analog doxycycline (Dox) and rapamycin inducible promoter systems in gene vectors provides strict regulation over protein synthesis via presence or absence of the inducer molecule. Tet-ON and Tet-OFF systems have been used to regulate the expression of osteogenic genes like Runx2, BMP-2 and BMP-4 in vitro and in vivo [284–286]. In all cases, bone mineralization and osteoblastic differentiation was only observed in the cases where transgene expression was induced to occur, indicating successful exogenous control over ectopic and orthotopic bone formation. Reversible, dose-dependent BMP-2 synthesis has also been reported using a two-component rapamycin binding transcription factor that dimerizes to activate transgene expression [287]. The rapamycin-controlled system demonstrated sustained, high levels of BMP-2 after induction that reversibly dropped to basal concentrations within 10 days and led to well-integrated bone formation in a rat calvarial CSD. Although inducible promoters have demonstrated their efficacy in vivo, unregulated gene expression is possible through interfering molecules, which should be considered when designing gene therapy vectors.
6. Considerations and Conclusions
In living systems, the successful regeneration of bone requires the coordinated effort of cells and growth factors in a time, concentration and site specific fashion. Continued efforts in understanding the complex biological mechanisms of the wound healing process provide new knowledge for engineering the restoration of functional tissue. A wide variety of biomaterial carriers have shown great potential for the controlled delivery of growth factors and cells to direct the necessary cellular activity and signaling for facilitating bone repair. Scaffolds derived from inorganic, natural or synthetic materials in various configurations can provide differential release kinetics through the manipulation of their physicochemical properties. Currently, the use of biomaterial composites and tissue-compatible smart materials like hydrogels and stimulus responsive polymers enables the most successful delivery by sustaining long term bioactive factor activity with the local environment. However, as certain studies have shown, the combinatorial delivery of bioactive factors with different spatial and temporal profiles such as sequential VEGF/BMP-2 delivery or TGF-β/IGF-1 delivery in bilayered scaffolds can enhance tissue regeneration in comparison to long term release of a single factor. Therefore, as the field moves towards the development of multifunctional platforms to better recapitulate the natural bone regenerative process, integration of bioactive factor spatiotemporal kinetics with the appropriate biomaterial carriers will be key to designing innovative treatments.
Efficacy of future delivery systems can be enhanced through the study and manipulation of the biological factors themselves. Identification of the most effective individual (BMPs, TGF-βs) or combinations of growth factors (BMP/VEGF, BMP-2/BMP-7) and their release profiles in different defects has the potential to improve the osteoinductive efficacy in vivo. Gene therapy is a promising method of growth factor delivery in vivo, but warrants further investigation into safer, more efficient delivery vehicles as well as new osteogenic genes. Localization of the foreign genetic material would also prevent unwanted transgene expression in non-target cells. Pre-differentiated MSC populations for delivery can also be examined. Improved isolation, enrichment and in vitro manipulation techniques for MSCs have been shown to maximize therapeutic efficacy. Understanding the temporal mechanisms of bone formation using MSCs and identification of new sources of MSCs and their differential capacity for regeneration in various applications also can enhance cell therapeutics for tissue repair.
Although further investigation is needed in the optimization of material and growth factor combinations, controlled delivery of bioactive factors via biomaterial carriers shows great potential for maximizing bone repair. With improved experimental animal models that mimic not only the human defect but the human response, controlled delivery systems will no doubt provide efficient methods to clinically regenerate bone in the future.
Acknowledgments
This work was funded by the National Institutes of Health (NIH; R01 DE17441, R01 AR57083 and R01 AR48756). T.N.V. is also supported by the T32 grant from the National Institutes of Health Baylor’s Scientific Training Program for Dental Academic Researchers.
Abbreviations
- ADSC
adipose derived stem cell
- ALP
alkaline phosphatase
- BMP
Bone morphogenetic protein
- Cox-2
cyclooxygenase-2
- CPC
calcium phosphate cement
- CSD
critical size defect
- ECM
extracellular matrix
- ES
embryonic stem (cell)
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- GDF
growth differentiation factor
- GMP
gelatin microparticle
- HA
hyaluronic acid
- HAp
hydroxyapatite
- hMSC
human mesenchymal stem cell
- IGF
insulin-like growth factor
- MDSC
muscle derived stem cell
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- NP
nanoparticle
- OPF
oligo(poly(ethylene glycol) fumarate)
- PCL
poly(ε-caprolactone)
- PCLA
poly(ε-caprolactone-co-lactic acid)
- PDGF
platelet derived growth factor
- PEG
poly(ethylene glycol)
- PGA
poly(glycolic acid)
- PLA
poly(DL-lactic acid)
- PLGA
poly(DL-lactic-co-glycolic acid)
- PLLA
poly(L-lactic acid)
- PNiPAAm
poly(N-isopropylacrylamide)
- PPF
poly(propylene fumarate)
- PRP
platelet rich plasma
- PTH
parathyroid hormone
- rBMSC
rat bone marrow stromal cell
- rhBMP
recombinant human BMP
- SMO
sulfamethazine oligomer
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- β-TCP
β-tricalcium phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenwald AS, Oxon DP, Boden SD, Goldberg VM, Khan Y. Bone graft substitutes: facts, fictions & applications. American Academy of Orthopaedic Surgeons; 2001. [DOI] [PubMed] [Google Scholar]
- 2.Laurencin KY, El-Amin CY. Bone graft substitutes. Exp Rev Med Dev. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop. 2008;466:2973–2980. doi: 10.1007/s11999-008-0538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conway JD. Autograft and nonunions: morbidity with intramedullary bone graft versus iliac crest bone graft. Orthop Clin North Am. 2010;41:75–84. doi: 10.1016/j.ocl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Rhim R, Li L, Martha J, Swaim BH, Banco RJ, Jenis LG, Tromanhauser SG. Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J. 2009;9:886–892. doi: 10.1016/j.spinee.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz CE, Martha JF, Kowalski P, Wang DA, Bode R, Li L, Kim DH. Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual Life Outcomes. 2009;7:49. doi: 10.1186/1477-7525-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury. 2007;38(Suppl 1):S75–80. doi: 10.1016/j.injury.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. J Bone Joint Surg. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Nauth A, Ristevski B, Li R, Schemitsch EH. Growth factors and bone regeneration: how much bone can we expect? Injury. 2011;42:574–579. doi: 10.1016/j.injury.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Seeherman H, Wozney JM. Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytok Growth Fact Rev. 2005;16:329–345. doi: 10.1016/j.cytogfr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Jäger M, Hernigou P, Zilkens C, Herten M, Li X, Fischer J, Krauspe R. Cell therapy in bone healing disorders. Orthop Rev. 2010;2:79–87. doi: 10.4081/or.2010.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S-J, Shin Y-W, Yang K-H, Kim S-B, Yoo M-J, Han S-K, Im S-A, Won Y-D, Sung Y-B, Jeon T-S, Chang C-H, Jang J-D, Lee S-B, Kim H-C, Lee S-Y. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(Ossron) injection to treat fractures. BMC Musculoskel Dis. 2009;10:20. doi: 10.1186/1471-2474-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Malafaya PB, Silva GA, Baran ET, Reis RL. Drug delivery therapies II. Strategies for delivering bone regenerating factors. Mater Sci. 2002;6:297–312. [Google Scholar]
- 15.Barnes GL, Kostenuik PJ, Gerstenfeld LC, Einhorn TA. Growth Factor Regulation of Fracture Repair. J Bone Miner Res. 1999;14 doi: 10.1359/jbmr.1999.14.11.1805. [DOI] [PubMed] [Google Scholar]
- 16.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19:459–466. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Laffargue P, Hildebrand HF, Rtaimate M, Frayssinet P, Amoureux JP, Marchandise X. Evaluation of human recombinant bone morphogenetic protein-2-loaded tricalcium phosphate implants in rabbits’ bone defects. Bone. 1999;25:55S–58S. doi: 10.1016/s8756-3282(99)00134-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J Royal Soc Interf. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S-HS, Heungsoo Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Ramoshebi LN, Matsaba TN, Teare J, Renton L, Patton J, Ripamonti U. Tissue engineering: TGF-beta superfamily members and delivery systems in bone regeneration. Exp Rev Mol Med. 2002;4:1–11. doi: 10.1017/S1462399402004969. [DOI] [PubMed] [Google Scholar]
- 21.Celeste A, Iannazzi J, Taylor R, Hewick R, Rosen V, Wang E, Wozney J. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 23.Aufdemorte T, Fox W, Holt R, McGuff H, Ammann A, Beck L. An intraosseous device for studies of bone-healing: The effect of transforming growth-factor beta. J Bone Joint Surg. 1992;74:1153–1161. [PubMed] [Google Scholar]
- 24.Bosch C, Melsen B, Gibbons R, Vargervik K. Human recombinant transforming growth factor-[beta] in healing of calvarial bone defects. J Craniofac Surg. 1996;7:300–310. doi: 10.1097/00001665-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Heckman J, Ehler W, BBP, ATB, LCH, MT, BBD Bone morphogenetic protein but not transforming growth factor-beta enhances bone formation in canine diaphyseal nonunions implanted with a biodegradable composite polymer. J Bone Joint Surg. 1999;81:1717–1729. doi: 10.2106/00004623-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 26.McKinney L, Hollinger JO. A bone regeneration study: transforming growth factor-[beta]1 and its delivery. J Craniofac Surg. 1996;7:36–45. [PubMed] [Google Scholar]
- 27.Nikolidakis D, Meijer GJ, Oortgiesen DA, Walboomers XF, Jansen JA. The effect of a low dose of transforming growth factor beta1 (TGF-beta1) on the early bone-healing around oral implants inserted in trabecular bone. Biomaterials. 2009;30:94–99. doi: 10.1016/j.biomaterials.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Ueda H, Hong L, Yamamoto M, Shigeno K, Inoue M, Toba T, Yoshitani M, Nakamura T, Tabata Y, Shimizu Y. Use of collagen sponge incorporating transforming growth factor-beta1 to promote bone repair in skull defects in rabbits. Biomaterials. 2002;23:1003–1010. doi: 10.1016/s0142-9612(01)00211-3. [DOI] [PubMed] [Google Scholar]
- 29.Vehof JWM, Fisher JP, Dean D, van der Waerden J-PCM, Spauwen PHM, Mikos AG, Jansen JA. Bone formation in transforming growth factor beta-1-coated porous poly (propylene fumarate) scaffolds. J Biomed Mater Res. 2002;60:241–251. doi: 10.1002/jbm.10073. [DOI] [PubMed] [Google Scholar]
- 30.Ripamonti U. Soluble osteogenic molecular signals and the induction of bone formation. Biomaterials. 2006;27:807–822. doi: 10.1016/j.biomaterials.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Fischer J, Kolk A, Wolfart S, Pautke C, Warnke PH, Plank C, Smeets R. Future of local bone regeneration - Protein versus gene therapy. Int J Oral Maxillof Surg. 2011;39:54–64. doi: 10.1016/j.jcms.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM, Gazit D. Review: gene- and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 2007;13:1135–1150. doi: 10.1089/ten.2007.0096. [DOI] [PubMed] [Google Scholar]
- 33.Kirker-Head C. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev. 2000;43:65–92. doi: 10.1016/s0169-409x(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 34.Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res. 2006;21:183–192. doi: 10.1359/JBMR.050917. [DOI] [PubMed] [Google Scholar]
- 35.Kanczler J, Oreffo R. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cells Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 36.Gerber HP, Ferrara N. Angiogenesis and bone growth. Tr Cardiovasc Med. 2000;10:223–228. doi: 10.1016/s1050-1738(00)00074-8. [DOI] [PubMed] [Google Scholar]
- 37.Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, Simank H-G, Richter W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20:2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 38.Kleinheinz J, Stratmann U, Joos U, Wiesmann H. VEGF-activated angiogenesis during bone regeneration. J Oral Maxillofac Surg. 2005;63:1310–1316. doi: 10.1016/j.joms.2005.05.303. [DOI] [PubMed] [Google Scholar]
- 39.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21 doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 40.Leach JK, Kaigler D, Wang Z, Krebsbach PH, Mooney DJ. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249–3255. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 41.Street J, Bao M, DeGuzman L, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RAD, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. [Google Scholar]
- 42.Tarkka T, Sipola A, Jamsa T, Soini Y, Yla-Herttuala S, Juha T, Hautala T. Adenoviral VEGF-A gene transfer induces angiogenesis and promotes bone formation in healing osseous tissues. J Gene Med. 2003;5:560–566. doi: 10.1002/jgm.392. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y-C, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 44.Kempen DHR, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJA. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 45.Patel ZS, Young S, Tabata Y, Jansen JA, Wong MEK, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng H, Usas A, Olshanski A, Ho AM, Gearhart B, Cooper GM, Huard J. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 47.Peng H, Wright V, Usas A, Gearhart B, Shen H-c, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell – expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheller MR, Crowther RS, Kinney JH, Yang J, Di Jorio S, Breunig T, Carney DH, Ryaby JT. Repair of rabbit segmental defects with the thrombin peptide, TP508. J Orthop Res. 2004;22:1094–1099. doi: 10.1016/j.orthres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Wozney JM, Seeherman HJ. Protein-based tissue engineering in bone and cartilage repair. Curr Opin Biotech. 2004;15:392–398. doi: 10.1016/j.copbio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;00:1–10. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carano RAD, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–989. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- 53.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Kempen DHR, Lu L, Hefferan TE, Creemers LB, Heijink A, Maran A, Dhert WJA, Yaszemski MJ. Enhanced bone morphogenetic protein-2-induced ectopic and orthotopic bone formation by intermittent parathyroid hormone (1–34) administration. Bone. 2010;16:3769–3777. doi: 10.1089/ten.tea.2010.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 56.Almeida JD, Arisawa EA, da Rocha RF, Carvalho YR. Effect of calcitonin on bone regeneration in male rats: a histomorphometric analysis. Int J Oral Maxillof Surg. 2007;36:435–440. doi: 10.1016/j.ijom.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Weiss RE, Singer FR, Gorn AH, Hofer DP, Nimni ME. Calcitonin stimulates bone formation when administered prior to initiation of osteogenesis. J Clin Invest. 1981;68:815–818. doi: 10.1172/JCI110319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosetti M, Boccafoschi F, Leigheb M, Cannas MF. Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomol Eng. 2007;24:613–618. doi: 10.1016/j.bioeng.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Caplan AI. New era of cell-based orthopedic therapies. Tissue Eng Part B Rev. 2009;15:195–200. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee K, Chan CK, Patil N, Goodman SB. Cell therapy for bone regeneration--bench to bedside. J Biomed Mater Res B Appl Biomater. 2009;89:252–263. doi: 10.1002/jbm.b.31199. [DOI] [PubMed] [Google Scholar]
- 61.Lang I, Schweizer A, Hiden U, Ghaffari-Tabrizi N, Hagendorfer G, Bilban M, Pabst MA, Korgun ET, Dohr G, Desoye G. Human fetal placental endothelial cells have a mature arterial and a juvenile venous phenotype with adipogenic and osteogenic differentiation potential. Differentiation. 2008;76:1031–1043. doi: 10.1111/j.1432-0436.2008.00302.x. [DOI] [PubMed] [Google Scholar]
- 62.Sun H, Feng K, Hu J, Soker S, Atala A, Ma PX. Osteogenic differentiation of human amniotic fluid-derived stem cells induced by bone morphogenetic protein-7 and enhanced by nanofibrous scaffolds. Biomaterials. 2010;31:1133–1139. doi: 10.1016/j.biomaterials.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Singh M, Bonewald LF, Detamore MS. Signalling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3:398–404. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 64.Harkness L, Mahmood A, Ditzel N, Abdallah BM, Nygaard JV, Kassem M. Selective isolation and differentiation of a stromal population of human embryonic stem cells with osteogenic potential. Bone. 2011;48:231–241. doi: 10.1016/j.bone.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 65.Kärner E, Unger C, Sloan AJ, Ährlund-Richter L, Sugars RV, Wendel M. Bone matrix formation in osteogenic cultures derived from human embryonic stem cells in vitro. Stem Cell Dev. 2007;16:39–52. doi: 10.1089/scd.2006.0010. [DOI] [PubMed] [Google Scholar]
- 66.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 67.D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 68.Li F, Bronson S, Niyibizi C. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J Cell Biochem. 2010;109:643–652. doi: 10.1002/jcb.22440. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu T, Tanaka T, Iso T, Doi H, Sato H, Kawai-Kowase K, Arai M, Kurabayashi M. Notch signaling induces osteogenic differentiation and mineralization of vascular smooth muscle cells: role of Msx2 gene induction via Notch-RBP-Jk signaling. Arterioscler Thromb Vasc Biol. 2009;29:1104–1111. doi: 10.1161/ATVBAHA.109.187856. [DOI] [PubMed] [Google Scholar]
- 70.Jeon O, Song SJ, Yang HS, Bhang S-H, Kang S-W, Sung MA, Lee JH, Kim B-S. Long-term delivery enhances in vivo osteogenic efficacy of bone morphogenetic protein-2 compared to short-term delivery. Biochem Biophys Res Comm. 2008;369:774–780. doi: 10.1016/j.bbrc.2008.02.099. [DOI] [PubMed] [Google Scholar]
- 71.Tabata Y. Regenerative inductive therapy based on DDS technology of protein and gene. J Drug Target. 2006;14:483–495. doi: 10.1080/10611860600844879. [DOI] [PubMed] [Google Scholar]
- 72.Kempen DHR, Lu L, Hefferan TE, Creemers LB, Maran A, Classic KL, Dhert WJA, Yaszemski MJ. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245–3252. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanzaki S, Takahashi T, Kanno T, Ariyoshi W, Shinmyouzu K, Tujisawa T, Nishihara T. Heparin inhibits BMP-2 osteogenic bioactivity by binding to both BMP-2 and BMP receptor. J Cell Physiol. 2008;216:844–850. doi: 10.1002/jcp.21468. [DOI] [PubMed] [Google Scholar]
- 74.Kempen DH, Yaszemski MJ, Heijink A, Hefferan TE, Creemers LB, Britson J, Maran A, Classic KL, Dhert WJ, Lu L. Non-invasive monitoring of BMP-2 retention and bone formation in composites for bone tissue engineering using SPECT/CT and scintillation probes. J Control Release. 2009;134:169–176. doi: 10.1016/j.jconrel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schliephake H. Application of bone growth factors--the potential of different carrier systems. Oral Maxillof Surg. 2010;14:17–22. doi: 10.1007/s10006-009-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ziegler J, Anger D, Krummenauer F, Breitig D, Fickert S, Guenther K-P. Biological activity of recombinant human growth factors released from biocompatible bone implants. J Biomed Mater Res A. 2008;86:89–97. doi: 10.1002/jbm.a.31625. [DOI] [PubMed] [Google Scholar]
- 77.Kang SM, Kong B, Oh E, Choi JS, Choi IS. Osteoconductive conjugation of bone morphogenetic protein-2 onto titanium/titanium oxide surfaces coated with non-biofouling poly(poly(ethylene glycol) methacrylate) Coll Surf B Biointerfaces. 2010;75:385–389. doi: 10.1016/j.colsurfb.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 78.Shi Z, Neoh KG, Kang E-T, Poh C, Wang W. Titanium with surface-grafted dextran and immobilized bone morphogenetic protein-2 for inhibition of bacterial adhesion and enhancement of osteoblast functions. Tissue Eng Part A. 2009;15:417–426. doi: 10.1089/ten.tea.2007.0415. [DOI] [PubMed] [Google Scholar]
- 79.Stadlinger B, Pilling E, Huhle M, Mai R, Bierbaum S, Scharnweber D, Kuhlisch E, Loukota R, Eckelt U. Evaluation of osseointegration of dental implants coated with collagen, chondroitin sulphate and BMP-4: an animal study. Int J Oral Maxillof Surg. 2008;37:54–59. doi: 10.1016/j.ijom.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 80.Liu H, Webster TJ. Ceramic/polymer nanocomposites with tunable drug delivery capability at specific disease sites. J Biomed Mater Res A. 2010;93:1180–1192. doi: 10.1002/jbm.a.32614. [DOI] [PubMed] [Google Scholar]
- 81.Ehlert N, Hoffmann A, Luessenhop T, Gross G, Mueller PP, Stieve M, Lenarz T, Behrens P. Amino-modified silica surfaces efficiently immobilize bone morphogenetic protein 2 (BMP2) for medical purposes. Acta Biomater. 2011;7:1772–1779. doi: 10.1016/j.actbio.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 82.Schuessele A, Mayr H, Tessmar J, Goepferich A. Enhanced bone morphogenetic protein-2 performance on hydroxyapatite ceramic surfaces. J Biomed Mater Res A. 2009;90:959–971. doi: 10.1002/jbm.a.31745. [DOI] [PubMed] [Google Scholar]
- 83.Wernike E, Hofstetter W, Liu Y, Wu G, Sebald H-J, Wismeijer D, Hunziker EB, Siebenrock K-A, Klenke FM. Long-term cell-mediated protein release from calcium phosphate ceramics. J Biomed Mater Res A. 2010;92:463–474. doi: 10.1002/jbm.a.32411. [DOI] [PubMed] [Google Scholar]
- 84.Tsurushima H, Marushima A, Suzuki K, Oyane A, Sogo Y, Nakamura K, Matsumura A, Ito A. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010;6:2751–2759. doi: 10.1016/j.actbio.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 85.Habraken W, Wolke J, Jansen J. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:234–248. doi: 10.1016/j.addr.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 86.Komaki H, Tanaka T, Chazono M, Kikuchi T. Repair of segmental bone defects in rabbit tibiae using a complex of beta-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials. 2006;27:5118–5126. doi: 10.1016/j.biomaterials.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 87.Chu T-MG, Warden SJ, Turner CH, Stewart RL. Segmental bone regeneration using a load-bearing biodegradable carrier of bone morphogenetic protein-2. Biomaterials. 2007;28:459–467. doi: 10.1016/j.biomaterials.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fei Z, Hu Y, Wu D, Wu H, Lu R, Bai J, Song H. Preparation and property of a novel bone graft composite consisting of rhBMP-2 loaded PLGA microspheres and calcium phosphate cement. J Mater Sci Mater Med. 2008;19:1109–1116. doi: 10.1007/s10856-007-3050-5. [DOI] [PubMed] [Google Scholar]
- 89.Xu HHK, Weir MD, Simon CG. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent Mater. 2008;24:1212–1222. doi: 10.1016/j.dental.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gerhardt L-C, Widdows KL, Erol MM, Burch CW, Sanz-Herrera JA, Ochoa I, Stämpfli R, Roqan IS, Gabe S, Ansari T, Boccaccini AR. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials. 2011;32:4096–4108. doi: 10.1016/j.biomaterials.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 91.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release. 2006;113:102–110. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 92.Kroese-Deutman HC, Ruhé PQ, Spauwen PHM, Jansen JA. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants inserted at an ectopic site in rabbits. Biomaterials. 2005;26:1131–1138. doi: 10.1016/j.biomaterials.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 93.Link DP, van den Dolder J, van den Beucken JJ, Wolke JG, Mikos AG, Jansen JA. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-beta 1 loaded gelatin microparticles. Biomaterials. 2008;29:675–682. doi: 10.1016/j.biomaterials.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 94.Seeherman HJ, Azari K, Bidic S, Rogers L, Li XJ, Hollinger JO, Wozney JM. rhBMP-2 delivered in a calcium phosphate cement accelerates bridging of critical-sized defects in rabbit radii. J Bone Joint Surg. 2006;88:1553–1565. doi: 10.2106/JBJS.E.01006. [DOI] [PubMed] [Google Scholar]
- 95.Leeuwenburgh SCG, Jo J, Wang H, Yamamoto M, Jansen JA, Tabata Y. Mineralization, biodegradation, and drug release behavior of gelatin/apatite composite microspheres for bone regeneration. Biomacromolecules. 2010;11:2653–2659. doi: 10.1021/bm1006344. [DOI] [PubMed] [Google Scholar]
- 96.Wu C, Zreiqat H. Porous bioactive diopside (CaMgSi(2)O(6)) ceramic microspheres for drug delivery. Acta Biomater. 2010;6:820–829. doi: 10.1016/j.actbio.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 97.Ishibe T, Goto T, Kodama T, Miyazaki T, Kobayashi S, Takahashi T. Bone formation on apatite-coated titanium with incorporated BMP-2/heparin in vivo. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:867–875. doi: 10.1016/j.tripleo.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 98.Kashiwagi K, Tsuji T, Shiba K. Directional BMP-2 for functionalization of titanium surfaces. Biomaterials. 2009;30:1166–1175. doi: 10.1016/j.biomaterials.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 99.Wolf-brandstetter C, Lode A, Hanke T, Scharnweber D, Worch H. Influence of modified extracellular matrices on Ti6AL4V implants on binding and release of VEGF. J Biomed Mater Res A. 2006;79:882–894. doi: 10.1002/jbm.a.30826. [DOI] [PubMed] [Google Scholar]
- 100.Beutner R, Michael J, Förster A, Schwenzer B, Scharnweber D. Immobilization of oligonucleotides on titanium based materials by partial incorporation in anodic oxide layers. Biomaterials. 2009;30:2774–2781. doi: 10.1016/j.biomaterials.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 101.Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23:844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 102.Puleo D, Kissling R, Sheu M. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 2002;23:2079–2087. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 103.Balasundaram G, Shimpi TM, Sanow WR, Storey DM, Kitchell BS, Webster TJ. Molecular plasma deposited peptides on anodized nanotubular titanium: an osteoblast density study. J Biomed Mater Res A. 2011;98:192–200. doi: 10.1002/jbm.a.33105. [DOI] [PubMed] [Google Scholar]
- 104.Choi J, Konno T, Takai M, Ishihara K. Controlled drug release from multilayered phospholipid polymer hydrogel on titanium alloy surface. Biomaterials. 2009;30:5201–5208. doi: 10.1016/j.biomaterials.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Morra M, Cassinelli C, Cascardo G, Cahalan P, Cahalan L, Fini M, Giardino R. Surface engineering of titanium by collagen immobilization. Surface characterization and in vitro and in vivo studies. Biomaterials. 2003;24:4639–4654. doi: 10.1016/s0142-9612(03)00360-0. [DOI] [PubMed] [Google Scholar]
- 106.Paital SR, Dahotre NB. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater Sci Eng R. 2009;66:1–70. [Google Scholar]
- 107.Ichinohe N, Kuboki Y, Tabata Y. Bone regeneration using titanium nonwoven fabrics combined with fgf-2 release from gelatin hydrogel microspheres in rabbit skull defects. Tissue Eng Part A. 2008;14:1663–1671. doi: 10.1089/ten.tea.2006.0350. [DOI] [PubMed] [Google Scholar]
- 108.Liu Y, de Groot K, Hunziker EB. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone. 2005;36:745–757. doi: 10.1016/j.bone.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced drug delivery reviews. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 110.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Bone. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 111.Luca L, Rougemont A-L, Walpoth BH, Gurny R, Jordan O. The effects of carrier nature and pH on rhBMP-2-induced ectopic bone formation. J Control Release. 2010;147:38–44. doi: 10.1016/j.jconrel.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 112.Morin R, Kaplan D, Perez-Ramirez B. Bone morphogenetic protein-2 binds as multilayers to a collagen delivery matrix: an equilibrium thermodynamic analysis. Biomacromolecules. 2006;7:131–138. doi: 10.1021/bm050461i. [DOI] [PubMed] [Google Scholar]
- 113.Uludag H, D’Augusta D, Palmer R, Timony G, Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in rat ectopic model. J Biomed Mater Res. 1999;46:193–202. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 114.Edlund U, Dånmark S, Albertsson A-C. A strategy for the covalent functionalization of resorbable polymers with heparin and osteoinductive growth factor. Biomacromolecules. 2008;9:901–905. doi: 10.1021/bm701267u. [DOI] [PubMed] [Google Scholar]
- 115.Ho Y-C, Mi F-L, Sung H-W, Kuo P-L. Heparin-functionalized chitosan-alginate scaffolds for controlled release of growth factor. Int J Pharm. 2009;376:69–75. doi: 10.1016/j.ijpharm.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 116.Yang HS, La W-G, Bhang SH, Jeon J-Y, Lee JH, Kim B-S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng Part A. 2010;16:1225–1233. doi: 10.1089/ten.TEA.2009.0390. [DOI] [PubMed] [Google Scholar]
- 117.Yang WD, Gomes RR, Alicknavitch M, Farach-Carson MC, Carson DD. Perlecan domain I promotes fibroblast growth factor 2 delivery in collagen I fibril scaffolds. Tissue Eng. 2005;11:76–89. doi: 10.1089/ten.2005.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen B, Lin H, Wang J, Zhao Y, Wang B, Zhao W, Sun W, Dai J. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials. 2007;28:1027–1035. doi: 10.1016/j.biomaterials.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 119.Zhao Y, Zhang J, Wang X, Chen B, Xiao Z, Shi C, Wei Z, Hou X, Wang Q, Dai J. The osteogenic effect of bone morphogenetic protein-2 on the collagen scaffold conjugated with antibodies. J Control Release. 2010;141:30–37. doi: 10.1016/j.jconrel.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 120.Kim HD, Valentini RF. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002;59:573–584. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 121.Wongpanit P, Ueda H, Tabata Y, Rujiravanit R. In vitro and in vivo release of basic fibroblast growth factor using a silk fibroin scaffold as delivery carrier. J Biomater Sci Polym Ed. 2010;21:1403–1419. doi: 10.1163/092050609X12517858243706. [DOI] [PubMed] [Google Scholar]
- 122.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258–266. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He Q, Zhao Y, Chen B, Xiao Z, Zhang J, Chen L, Chen W, Deng F, Dai J. Improved cellularization and angiogenesis using collagen scaffolds chemically conjugated with vascular endothelial growth factor. Acta Biomater. 2011;7:1084–1093. doi: 10.1016/j.actbio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 124.Niu X, Feng Q, Wang M, Guo X, Zheng Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111–117. doi: 10.1016/j.jconrel.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 125.Wu B, Zheng Q, Guo X, Wu Y, Wang Y, Cui F. Preparation and ectopic osteogenesis in vivo of scaffold based on mineralized recombinant human-like collagen loaded with synthetic BMP-2-derived peptide. Biomed Mater. 2008;3:044111. doi: 10.1088/1748-6041/3/4/044111. [DOI] [PubMed] [Google Scholar]
- 126.Yang H, La W, Bhang S, Lee T, Lee M, Kim B-S. Apatite-coated collagen scaffold for bone morphogenetic protein-2 delivery. Tissue Eng Part A. 2011;17:1–12. doi: 10.1089/ten.TEA.2010.0702. [DOI] [PubMed] [Google Scholar]
- 127.Zhao J, Shinkai M, Takezawa T, Ohba S, Chung U-I, Nagamune T. Bone regeneration using collagen type I vitrigel with bone morphogenetic protein-2. J Biosci Bioeng. 2009;107:318–323. doi: 10.1016/j.jbiosc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Puppi D, Chiellini F, Piras AM, Chiellini E. Polymeric materials for bone and cartilage repair. Prog Polym Sci. 2010;35:403–440. [Google Scholar]
- 129.Stevens B, Yang Y, Mohandas A, Stucker B, Nguyen KT. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater. 2008;85:573–582. doi: 10.1002/jbm.b.30962. [DOI] [PubMed] [Google Scholar]
- 130.Lin Z-Y, Duan Z-X, Guo X-D, Li J-F, Lu H-W, Zheng Q-X, Quan D-P, Yang S-H. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J Control Release. 2010;144:190–195. doi: 10.1016/j.jconrel.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 131.Schliephake H, Weich HA, Dullin C, Gruber R, Frahse S. Mandibular bone repair by implantation of rhBMP-2 in a slow release carrier of polylactic acid--An experimental study in rats. Biomaterials. 2008;29:103–110. doi: 10.1016/j.biomaterials.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 132.Wei G, Jin Q, Giannobile WV, Ma PX. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087–2096. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oest ME, Dupont KM, Kong H-J, Mooney DJ, Guldberg RE. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007;25:941–950. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 134.Kim J, Yaszemski MJ, Lu L. Three-dimensional porous biodegradable polymeric scaffolds fabricated with biodegradable hydrogel porogens. Tissue Eng Part C Methods. 2009;15:583–594. doi: 10.1089/ten.tec.2008.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Whang K, Goldstick TK, Healy KE. A biodegradable polymer scaffold for delivery of osteotropic factors. Biomaterials. 2000;21:2545–2551. doi: 10.1016/s0142-9612(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 136.Yang XB, Whitaker MJ, Sebald W, Clarke N, Howdle SM, Shakesheff KM, Oreffo ROC. Human osteoprogenitor bone formation using encapsulated bone morphogenetic protein 2 in porous polymer scaffolds. Tissue Eng. 2004;10:1037–1045. doi: 10.1089/ten.2004.10.1037. [DOI] [PubMed] [Google Scholar]
- 137.Kanczler JM, Ginty PJ, Barry JJA, Clarke NMP, Howdle SM, Shakesheff KM, Oreffo ROC. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials. 2008;29:1892–1900. doi: 10.1016/j.biomaterials.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 138.Sahoo S, Ang LT, Goh JC-H, Toh S-L. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A. 2010;93:1539–1550. doi: 10.1002/jbm.a.32645. [DOI] [PubMed] [Google Scholar]
- 139.Fu Y-C, Nie H, Ho M-L, Wang C-K, Wang C-H. Optimized bone regeneration based on sustained release from three-dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol Bioeng. 2008;99:996–1006. doi: 10.1002/bit.21648. [DOI] [PubMed] [Google Scholar]
- 140.Srouji S, Ben-David D, Lotan R, Livne E, Avrahami R, Zussman E. Slow release human recombinant bone morphogenetic protein-2 embedded within electrospun scaffolds for regeneration of bone defect: in vitro and in vivo evaluation. Bone. 2011;17:269–277. doi: 10.1089/ten.TEA.2010.0250. [DOI] [PubMed] [Google Scholar]
- 141.Rai B, Teoh SH, Hutmacher DW, Cao T, Ho KH. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials. 2005;26:3739–3748. doi: 10.1016/j.biomaterials.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 142.de Mulder ELW, Buma P, Hannink G. Anisotropic porous biodegradable scaffolds for musculoskeletal tissue engineering. Materials. 2009;2:1674–1696. [Google Scholar]
- 143.Sawyer AA, Song SJ, Susanto E, Chuan P, Lam CXF, Woodruff MA, Hutmacher DW, Cool SM. The stimulation of healing within a rat calvarial defect by mPCL-TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials. 2009;30:2479–2488. doi: 10.1016/j.biomaterials.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 144.Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Anim Res. 2011;17:1389–1399. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ruhé PQ, Boerman OC, Russel FGM, Spauwen PHM, Mikos AG, Jansen JA. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release. 2005;106:162–171. doi: 10.1016/j.jconrel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 146.Bodde EWH, Boerman OC, Russel FGM, Mikos AG, Spauwen PHM, Jansen JA. The kinetic and biological activity of different loaded rhBMP-2 calcium phosphate cement implants in rats. J Biomed Mater Res A. 2008;87:780–791. doi: 10.1002/jbm.a.31830. [DOI] [PubMed] [Google Scholar]
- 147.Félix Lanao RP, Leeuwenburgh SCG, Wolke JGC, Jansen JA. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater. 2011;7:3459–3468. doi: 10.1016/j.actbio.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 148.Phillips FM, Turner S, Seim HB, MacLeay J, Toth CA, Pierce AR, Wheeler DL. In vivo BMP-7 (OP-1) enhancement of osteoporotic vertebral bodies in an ovine model. Spine J. 2006;6:500–506. doi: 10.1016/j.spinee.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 149.Lochmann A, Nitzsche H, von Einem S, Schwarz E, Mäder K. The influence of covalently linked and free polyethylene glycol on the structural and release properties of rhBMP-2 loaded microspheres. J Control Release. 2010;147:92–100. doi: 10.1016/j.jconrel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 150.Shen H, Hu X, Yang F, Bei J, Wang S. An injectable scaffold: rhBMP-2-loaded poly(lactide-co-glycolide)/hydroxyapatite composite microspheres. Acta Biomater. 2010;6:455–465. doi: 10.1016/j.actbio.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 151.Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4:1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bessa PC, Balmayor ER, Azevedo HS, Nürnberger S, Casal M, van Griensven M, Reis RL, Redl H. Silk fibroin microparticles as carriers for delivery of human recombinant BMPs. Physical characterization and drug release. Tissue Eng Part C Meth. 2010;16:937–945. doi: 10.1002/term.245. [DOI] [PubMed] [Google Scholar]
- 153.Chen F, Wu Z, Sun H, Wu H, Xin S, Wang Q-t, Dong G, Ma Z, Huang S, Zhang Y, Jin Y. Release of bioactive BMP from dextran-derived microspheres: a novel delivery concept. Int J Pharm. 2006;307:23–32. doi: 10.1016/j.ijpharm.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 154.Lee J-Y, Kim K-H, Shin S-Y, Rhyu I-C, Lee Y-M, Park Y-J, Chung C-P, Lee S-J. Enhanced bone formation by transforming growth factor-beta1-releasing collagen/chitosan microgranules. J Biomed Mater Res A. 2006;76:530–539. doi: 10.1002/jbm.a.30434. [DOI] [PubMed] [Google Scholar]
- 155.Lee M, Li W, Siu RK, Whang J, Zhang X, Soo C, Ting K, Wu BM. Biomimetic apatite-coated alginate/chitosan microparticles as osteogenic protein carriers. Biomaterials. 2009;30:6094–6101. doi: 10.1016/j.biomaterials.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chan OCM, So K-F, Chan BP. Fabrication of nano-fibrous collagen microspheres for protein delivery and effects of photochemical crosslinking on release kinetics. J Control Release. 2008;129:135–143. doi: 10.1016/j.jconrel.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 157.De la Riva B, Nowak C, Sánchez E, Hernández A, Schulz-Siegmund M, Pec MK, Delgado A, Evora C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur J Pharm Biopharm. 2009;73:50–58. doi: 10.1016/j.ejpb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 158.Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Sequential release of bioactive IGF-I and TGF-beta 1 from PLGA microsphere-based scaffolds. Biomaterials. 2008;29:1518–1525. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 159.Kim K, Fisher JP. Nanoparticle technology in bone tissue engineering. J Drug Target. 2007;15:241–252. doi: 10.1080/10611860701289818. [DOI] [PubMed] [Google Scholar]
- 160.Zhang S, Uluda H. Nanoparticulate systems for growth factor delivery. Pharm Res. 2009;26:1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 161.Mercado AE, Jabbari E. Effect of encapsulation or grafting on release kinetics of recombinant human bone morphogenetic protein-2 from self-assembled poly(lactide-co-glycolide ethylene oxide fumarate) nanoparticles. Microsc Res Techniq. 2010;73:824–833. doi: 10.1002/jemt.20846. [DOI] [PubMed] [Google Scholar]
- 162.Chen L, Liu L, Li C, Tan Y, Zhang G. A new growth factor controlled drug release system to promote healing of bone fractures: Nanospheres of recombinant human bone morphogenetic-2 and polylactic acid. J Nanosci Nanotechnol. 2011;11:3107–3114. doi: 10.1166/jnn.2011.3820. [DOI] [PubMed] [Google Scholar]
- 163.Yilgor P, Hasirci N, Hasirci V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J Biomed Mater Res A. 2009;93:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 164.Nandagiri VK, Gentile P, Chiono V, Tonda-Turo C, Matsiko A, Ramtoola Z, Montevecchi FM, Ciardelli G. Incorporation of PLGA nanoparticles into porous chitosan-gelatin scaffolds: Influence on the physical properties and cell behavior. J Mech Behav Biomed. 2011;4:1318–1327. doi: 10.1016/j.jmbbm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 165.Chung Y-I, Ahn K-M, Jeon S-H, Lee S-Y. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle – hydrogel complex. J Control Release. 2007;121:91–99. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 166.Mourtas S, Fotopoulou S, Duraj S, Sfika V, Tsakiroglou C, Antimisiaris SG. Liposomal drugs dispersed in hydrogels. Effect of liposome, drug and gel properties on drug release kinetics. Coll Surf B Biointerfaces. 2007;55:212–221. doi: 10.1016/j.colsurfb.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 167.Dai C, Wang B, Zhao H, Li B. Factors affecting protein release from microcapsule prepared by liposome in alginate. Coll Surf B Biointerfaces. 2005;42:253–258. doi: 10.1016/j.colsurfb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 168.Wang H, Zhao P, Su W, Wang S, Liao Z, Niu R, Chang J. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials. 2010;31:8741–8748. doi: 10.1016/j.biomaterials.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 169.Haidar ZS, Tabrizian M, Hamdy RC. A hybrid rhOP-1 delivery system enhances new bone regeneration and consolidation in a rabbit model of distraction osteogenesis. Growth Factors. 2010;28:44–55. doi: 10.3109/08977190903367788. [DOI] [PubMed] [Google Scholar]
- 170.Johnson MR, Lee H-J, Bellamkonda RV, Guldberg RE. Sustained release of BMP-2 in a lipid-based microtube vehicle. Acta Biomater. 2009;5:23–28. doi: 10.1016/j.actbio.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin C-C, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 172.Young S, Wong M, Tabata Y, Mikos AG. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release. 2005;109:256–274. doi: 10.1016/j.jconrel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 173.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, Guldberg RE. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Park Y, Sun K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28:1830–1837. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 175.Lin C-C, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Patterson J, Siew R, Herring SW, Lin ASP, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31:6772–6781. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yamamoto M, Takahashi Y, Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006;12:1305–1311. doi: 10.1089/ten.2006.12.1305. [DOI] [PubMed] [Google Scholar]
- 178.Kimura Y, Miyazaki N, Hayashi N, Otsuru S, Tamai K, Kaneda Y, Tabata Y. Controlled release of bone morphogenetic protein-2 enhances recruitment of osteogenic progenitor cells for de novo generation of bone tissue. Tissue Eng Part A. 2010;16:1263–1270. doi: 10.1089/ten.TEA.2009.0322. [DOI] [PubMed] [Google Scholar]
- 179.Kim J, Hefferan TE, Yaszemski MJ, Lu L. Potential of hydrogels based on poly(ethylene glycol) and sebacic acid as orthopedic tissue engineering scaffolds. Tissue Eng Part A. 2009;15:2299–2307. doi: 10.1089/ten.tea.2008.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang Y, Wu X, Han Y, Mo F, Duan Y, Li S. Novel thymopentin release systems prepared from bioresorbable PLA-PEG-PLA hydrogels. Int J Pharm. 2010;386:15–22. doi: 10.1016/j.ijpharm.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 181.Bencherif SA, Sheehan JA, Hollinger JO, Walker LM, Matyjaszewski K, Washburn NR. Influence of cross-linker chemistry on release kinetics of PEG-co-PGA hydrogels. J Biomed Mater Res A. 2009;90:142–153. doi: 10.1002/jbm.a.32069. [DOI] [PubMed] [Google Scholar]
- 182.Cho E, Kutty JK, Datar K, Lee JS, Vyavahare NR, Webb K. A novel synthetic route for the preparation of hydrolytically degradable synthetic hydrogels. J Biomed Mater Res A. 2009;90:1073–1082. doi: 10.1002/jbm.a.32172. [DOI] [PubMed] [Google Scholar]
- 183.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J Control Release. 2002;83:53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 184.Shin H, Quinten Ruhé P, Mikos AG, Jansen JA. In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials. 2003;24:3201–3211. doi: 10.1016/s0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 185.Kaito T, Myoui A, Takaoka K, Saito N, Nishikawa M, Tamai N, Ohgushi H, Yoshikawa H. Potentiation of the activity of bone morphogenetic protein-2 in bone regeneration by a PLA-PEG/hydroxyapatite composite. Biomaterials. 2005;26:73–79. doi: 10.1016/j.biomaterials.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 186.Terella A, Mariner P, Brown N, Anseth K, Streubel S-O. Repair of a calvarial defect with biofactor and stem cell-embedded polyethylene glycol scaffold. Arch Fac Plast Surg. 2010;12:166–171. doi: 10.1001/archfacial.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jo YS, Rizzi SC, Ehrbar M, Weber FE, Hubbell JA, Lutolf MP. Biomimetic PEG hydrogels crosslinked with minimal plasmin-sensitive tri-amino acid peptides. J Biomed Mater Res A. 2009;93:870–877. doi: 10.1002/jbm.a.32580. [DOI] [PubMed] [Google Scholar]
- 188.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials. 2004;25:895–906. doi: 10.1016/s0142-9612(03)00602-1. [DOI] [PubMed] [Google Scholar]
- 189.Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, Mikos AG. Modulation of differentiation and mineralization of marrow stromal cells cultured on biomimetic hydrogels modified with Arg-Gly-Asp containing peptides. J Biomed Mater Res A. 2004;69:535–543. doi: 10.1002/jbm.a.30027. [DOI] [PubMed] [Google Scholar]
- 190.Gkioni K, Leeuwenburgh SCG, Douglas TEL, Mikos AG, Jansen JA. Mineralization of hydrogels for bone regeneration. Tissue Eng Part A. 2010;16:577–585. doi: 10.1089/ten.TEB.2010.0462. [DOI] [PubMed] [Google Scholar]
- 191.Mano JF. Stimuli-responsive polymeric systems for biomedical applications. Adv Eng Mater. 2008;10:515–527. [Google Scholar]
- 192.Garbern JC, Hoffman AS, Stayton PS. Injectable pH- and Temperature-Responsive Poly (N-isopropylacrylamide-co-propylacrylic acid) Copolymers for Delivery of Angiogenic Growth Factors. Biomacromolecules. 2010;11:1833–1839. doi: 10.1021/bm100318z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shi J, Liu L, Liu X, Sun X, Cao S. Inorganic-organic hybrid alginate beads with LCST near human body temperature for sustained dual-sensitive drug delivery. Polym Adv Technol. 2008;19:1467–1473. [Google Scholar]
- 194.Na K, Kim SW, Sun BK, Woo DG, Yang HN, Chung HM, Park KH. Osteogenic differentiation of rabbit mesenchymal stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite and bone morphogenic protein-2 (BMP-2) Biomaterials. 2007;28:2631–2637. doi: 10.1016/j.biomaterials.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 195.Chun C, Lim HJ, Hong K-Y, Park K-H, Song S-C. The use of injectable, thermosensitive poly(organophosphazene)-RGD conjugates for the enhancement of mesenchymal stem cell osteogenic differentiation. Biomaterials. 2009;30:6295–6308. doi: 10.1016/j.biomaterials.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 196.He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release. 2008;127:189–207. doi: 10.1016/j.jconrel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 197.Kim MS, Kim SK, Kim SH, Hyun H, Khang G, Lee HB. In vivo osteogenic differentiation of rat bone marrow stromal cells in thermosensitive MPEG-PCL diblock copolymer gels. Tissue Eng. 2006;12:2863–2873. doi: 10.1089/ten.2006.12.2863. [DOI] [PubMed] [Google Scholar]
- 198.Patois E, Osorio-da Cruz S, Tille J-C, Walpoth B, Gurny R, Jordan O. Novel thermosensitive chitosan hydrogels: in vivo evaluation. J Biomed Mater Res A. 2009;91:324–330. doi: 10.1002/jbm.a.32211. [DOI] [PubMed] [Google Scholar]
- 199.Bessa PC, Machado R, Nürnberger S, Dopler D, Banerjee A, Cunha AM, Rodríguez-Cabello JC, Redl H, van Griensven M, Reis RL, Casal M. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J Control Release. 2010;142:312–318. doi: 10.1016/j.jconrel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 200.Kim HK, Shim WS, Kim SE, Lee K-H, Kang E, Kim J-H, Kim K, Kwon IC, Lee DS. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng Part A. 2009;15:923–933. doi: 10.1089/ten.tea.2007.0407. [DOI] [PubMed] [Google Scholar]
- 201.Lutolf MP, Schmoekel HG, Metters AT, Webers FE, Fieldst GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kim J, Kim IS, Cho TH, Kim HC, Yoon SJ, Choi J, Park Y, Sun K, Hwang SJ. In vivo evaluation of MMP sensitive high-molecular weight HA-based hydrogels for bone tissue engineering. J Biomed Mater Res A. 2010;95:673–681. doi: 10.1002/jbm.a.32884. [DOI] [PubMed] [Google Scholar]
- 203.Chung EH, Gilbert M, Virdi AS, Sena K, Sumner DR, Healy KE. Biomimetic artificial ECMs stimulate bone regeneration. J Biomed Mater Res A. 2006;79:815–826. doi: 10.1002/jbm.a.30809. [DOI] [PubMed] [Google Scholar]
- 204.Arrighi I, Mark S, Alvisi M, von Rechenberg B, Hubbell JA, Schense JC. Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials. 2009;30:1763–1771. doi: 10.1016/j.biomaterials.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 205.Ulijn RV, Bibi N, Jayawarna V, Thornton PD, Todd SJ, Mart RJ, Smith AM, Gough JE. Bioresponsive hydrogels. Mater Today. 2007;10:40–48. [Google Scholar]
- 206.Bock N, Riminucci A, Dionigi C, Russo A, Tampieri A, Landi E, Goranov VA, Marcacci M, Dediu V. A novel route in bone tissue engineering: magnetic biomimetic scaffolds. Acta Biomater. 2010;6:786–796. doi: 10.1016/j.actbio.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 207.Tampieri A, Landi E, Valentini F, Sandri M, D’Alessandro T, Dediu V, Marcacci M. A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology. 2011;22:1–8. doi: 10.1088/0957-4484/22/1/015104. [DOI] [PubMed] [Google Scholar]
- 208.Tsiridis E, Upadhyay N, Giannoudis P. Molecular aspects of fracture healing: which are the important molecules? Injury. 2007;38(Suppl 1):S11–25. doi: 10.1016/j.injury.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 209.Chen F-M, Zhang M, Wu Z-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 210.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 211.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 212.Chen F-M, Chen R, Wang X-J, Sun H-H, Wu Z-F. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials. 2009;30:5215–5224. doi: 10.1016/j.biomaterials.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 213.Kanczler JM, Ginty PJ, White L, Clarke NMP, Howdle SM, Shakesheff KM, Oreffo ROC. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242–1250. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 214.Park H, Temenoff JS, Tabata Y, Caplan AI, Raphael RM, Jansen JA, Mikos AG. Effect of dual growth factor delivery on chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in injectable hydrogel composites. J Biomed Mater Res A. 2009;88:889–897. doi: 10.1002/jbm.a.31948. [DOI] [PubMed] [Google Scholar]
- 215.Facca S, Cortez C, Mendoza-Palomares C, Messadeq N, Dierich A, Johnston APR, Mainard D, Voegel J-C, Caruso F, Benkirane-Jessel N. Active multilayered capsules for in vivo bone formation. Proc Natl Acad Sci U S A. 2010;107:3406–3411. doi: 10.1073/pnas.0908531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shah NJ, Macdonald ML, Beben YM, Padera RF, Samuel RE, Hammond PT. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials. 2011;32:6183–6193. doi: 10.1016/j.biomaterials.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Choi DH, Park CH, Kim IH, Chun HJ, Park K, Han DK. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J Control Release. 2010;147:193–201. doi: 10.1016/j.jconrel.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 218.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M, Mikos AG. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Basmanav FB, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29:4195–4204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 220.Wang X, Wenk E, Zhang X, Meinel L, Vunjak-Novakovic G, Kaplan DL. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang PJ, Temenoff JS. Engineering orthopedic tissue interfaces. Tissue Eng Part B Rev. 2009;15:127–141. doi: 10.1089/ten.teb.2008.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Holland TA, Bodde EWH, Cuijpers VMJI, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthr Cart. 2007;15:187–197. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 223.Guo X, Liao J, Park H, Saraf A, Raphael RM, Tabata Y, Kasper FK, Mikos AG. Effects of TGF-beta3 and preculture period of osteogenic cells on the chondrogenic differentiation of rabbit marrow mesenchymal stem cells encapsulated in a bilayered hydrogel composite. Acta Biomater. 2010;6:2920–2931. doi: 10.1016/j.actbio.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Guo X, Park H, Liu G, Liu W, Cao Y, Tabata Y, Kasper FK, Mikos AG. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials. 2009;30:2741–2752. doi: 10.1016/j.biomaterials.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Anitua E, Sanchez M, Orive G, Andia I. Delivering growth factors for therapeutics. Tr Pharmacol Sci. 2007;29:37–41. doi: 10.1016/j.tips.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 226.Lu HH, Vo JM, Chin HS, Lin J, Cozin M, Tsay R, Eisig S, Landesberg R. Controlled delivery of platelet-rich plasma-derived growth factors for bone formation. J Biomed Mater Res A. 2008;86:1128–1136. doi: 10.1002/jbm.a.31740. [DOI] [PubMed] [Google Scholar]
- 227.Hokugo A, Sawada Y, Hokugo R, Iwamura H, Kobuchi M, Kambara T, Morita S, Tabata Y. Controlled release of platelet growth factors enhances bone regeneration at rabbit calvaria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:44–48. doi: 10.1016/j.tripleo.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 228.Gallego L, Junquera L, García E, García V, Alvarez-Viejo M, Costilla S, Fresno MF, Meana A. Repair of rat mandibular bone defects by alveolar osteoblasts in a novel plasma-derived albumin scaffold. Tissue Eng Part A. 2010;16:1179–1187. doi: 10.1089/ten.TEA.2009.0517. [DOI] [PubMed] [Google Scholar]
- 229.Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbühl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29:3983–3992. doi: 10.1016/j.biomaterials.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 230.Bi L, Cheng W, Fan H, Pei G. Reconstruction of goat tibial defects using an injectable tricalcium phosphate/chitosan in combination with autologous platelet-rich plasma. Biomaterials. 2010;31:3201–3211. doi: 10.1016/j.biomaterials.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 231.Kretlow JD, Spicer PP, Jansen JA, Vacanti CA, Kasper FK, Mikos AG. Uncultured marrow mononuclear cells delivered within fibrin glue hydrogels to porous scaffolds enhance bone regeneration within critical-sized rat cranial defects. Tissue Eng Part A. 2010;16:3555–3568. doi: 10.1089/ten.tea.2010.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang C, Frei H, Rossi FM, Burt HM. The differential in vitro and in vivo responses of bone marrow stromal cells on novel porous gelatin – alginate scaffolds. J Tissue Eng Regen Med. 2009;3:601–614. doi: 10.1002/term.201. [DOI] [PubMed] [Google Scholar]
- 233.Chan BP, Hui TY, Wong MY, Yip KHK, Chan GCF. Mesenchymal stem cell-encapsulated collagen microspheres for bone tissue engineering. Tissue Eng Part C Meth. 2010;16:225–235. doi: 10.1089/ten.tec.2008.0709. [DOI] [PubMed] [Google Scholar]
- 234.Park S-H, Tofighi A, Wang X, Strunk M, Ricketts T, Chang J, Kaplan DL. Calcium phosphate combination biomaterials as human mesenchymal stem cell delivery vehicles for bone repair. J Biomed Mater Res B Appl Biomater. 2011;97:235–244. doi: 10.1002/jbm.b.31805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Moreau JL, Xu HHK. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold. Biomaterials. 2009;30:2675–2682. doi: 10.1016/j.biomaterials.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee G-S, Park J-H, Shin US, Kim H-W. Direct deposited porous scaffolds of calcium phosphate cement with alginate for drug delivery and bone tissue engineering. Acta Biomater. 2011;7:3178–3186. doi: 10.1016/j.actbio.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 237.Klouda L, Perkins KR, Watson BM, Hacker MC, Bryant SJ, Raphael RM, Kasper FK, Mikos AG. Thermoresponsive, in situ cross-linkable hydrogels based on N-isopropylacrylamide: fabrication, characterization and mesenchymal stem cell encapsulation. Acta Biomater. 2011;7:1460–1467. doi: 10.1016/j.actbio.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zimmermann G, Gierloff M, Hedderich J, Acil Y, Wiltfang J, Terheyden H. Survival of transplanted rat bone marrow-derived osteogenic stem cells in vivo. Tissue Eng Part A. 2011;17:1147–1156. doi: 10.1089/ten.TEA.2009.0577. [DOI] [PubMed] [Google Scholar]
- 239.Grellier M, Bordenave L, Amedee J. Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol. 2009;27:562–571. doi: 10.1016/j.tibtech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 240.Sun H, Qu Z, Guo Y, Zang G, Yang B. In vitro and in vivo effects of rat kidney vascular endothelial cells on osteogenesis of rat bone marrow mesenchymal stem cells growing on polylactide-glycoli acid (PLGA) scaffolds. Biomed Eng Online. 2007;6:41. doi: 10.1186/1475-925X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xue Y, Xing Z, Hellem S, Arvidson K, Mustafa K. Endothelial cells influence the osteogenic potential of bone marrow stromal cells. Biomed Eng Online. 2009;8:34. doi: 10.1186/1475-925X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tao J, Sun Y, Wang QG, Liu CW. Induced endothelial cells enhance osteogenesis and vascularization of mesenchymal stem cells. Cells Tissues Organs. 2009;190:185–193. doi: 10.1159/000218139. [DOI] [PubMed] [Google Scholar]
- 243.Zhou J, Lin H, Fang T, Li X, Dai W, Uemura T, Dong J. The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Biomaterials. 2010;31:1171–1179. doi: 10.1016/j.biomaterials.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 244.Koob S, Torio-Padron N, Stark GB, Hannig C, Stankovic Z, Finkenzeller G. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A. 2011;17:311–321. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- 245.Choong CS, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12:2521–2531. doi: 10.1089/ten.2006.12.2521. [DOI] [PubMed] [Google Scholar]
- 246.Grellier M, Granja PL, Fricain JC, Bidarra SJ, Renard M, Bareille R, Bourget C, Amedee J, Barbosa MA. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials. 2009;30:3271–3278. doi: 10.1016/j.biomaterials.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 247.Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407–4415. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 248.Yu H, Vandevord PJ, Gong W, Wu B, Song Z, Matthew HW, Wooley PH, Yang SY. Promotion of osteogenesis in tissue-engineered bone by pre-seeding endothelial progenitor cells-derived endothelial cells. J Orthop Res. 2008;26:1147–1152. doi: 10.1002/jor.20609. [DOI] [PubMed] [Google Scholar]
- 249.Fuchs S, Hofmann A, Kirkpatrick CJ. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577–2588. doi: 10.1089/ten.2007.0022. [DOI] [PubMed] [Google Scholar]
- 250.Hofmann A, Ritz U, Verrier S, Eglin D, Alini M, Fuchs S, Kirkpatrick CJ, Rommens PM. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials. 2008;29:4217–4226. doi: 10.1016/j.biomaterials.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 251.Yeatts AB, Fisher JP. Bone tissue engineering bioreactors: dynamic culture and the influence of shear stress. Bone. 2011;48:171–181. doi: 10.1016/j.bone.2010.09.138. [DOI] [PubMed] [Google Scholar]
- 252.Rauh J, Milan F, Gu K-P, Stiehler M. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011;17:263–280. doi: 10.1089/ten.TEB.2010.0612. [DOI] [PubMed] [Google Scholar]
- 253.Bancroft GN, Sikavitsas VI, Mikos AG. Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003;9:549–554. doi: 10.1089/107632703322066723. [DOI] [PubMed] [Google Scholar]
- 254.Holtorf HL, Jansen JA, Mikos AG. Flow perfusion culture induces the osteoblastic differentiation of marrow stromal cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72:326–334. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 255.Datta N, Pham QP, Sharma U, Sikavitsas VI, Jansen JA, Mikos AG. In vitro generated extracellular matrix and fluid shear stress synergistically enhance 3D osteoblastic differentiation. Proc Natl Acad Sci U S A. 2006;103:2488–2493. doi: 10.1073/pnas.0505661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pham QP, Kasper FK, Mistry AS, Sharma U, Yasko AW, Jansen JA, Mikos AG. Analysis of the osteoinductive capacity and angiogenicity of an in vitro generated extracellular matrix. J Biomed Mater Res A. 2009;88:295–303. doi: 10.1002/jbm.a.31875. [DOI] [PubMed] [Google Scholar]
- 257.Liao J, Guo X, Nelson D, Kasper FK, Mikos AG. Modulation of osteogenic properties of biodegradable polymer/extracellular matrix scaffolds generated with a flow perfusion bioreactor. Acta Biomater. 2010;6:2386–2393. doi: 10.1016/j.actbio.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kang Y, Kim S, Khademhosseini A, Yang Y. Creation of bony microenvironment with CaP and cell-derived ECM to enhance human bone-marrow MSC behavior and delivery of BMP-2. Biomaterials. 2011;32:6119–6130. doi: 10.1016/j.biomaterials.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A. 2010;16:431–440. doi: 10.1089/ten.tea.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Deutsch ER, Guldberg RE. Stem cell-synthesized extracellular matrix for bone repair. Journal of Materials Chemistry. 2010;20:8942–8951. [Google Scholar]
- 261.Tour G, Wendel M, Tcacencu I. Cell-derived matrix enhances osteogenic properties of hydroxyapatite. Tissue Eng Part A. 2011;17:127–137. doi: 10.1089/ten.TEA.2010.0175. [DOI] [PubMed] [Google Scholar]
- 262.Strohbach CA, Rundle CH, Wergedal JE, Chen S-T, Linkhart TA, Lau K-HW, Strong DD. LMP-1 retroviral gene therapy influences osteoblast differentiation and fracture repair: a preliminary study. Calcif Tissue Int. 2008;83:202–211. doi: 10.1007/s00223-008-9163-0. [DOI] [PubMed] [Google Scholar]
- 263.Lee J-S, Lee J-M, Im G-I. Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials. 2011;32:760–768. doi: 10.1016/j.biomaterials.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 264.Jabbarzadeh E, Starnes T, Khan YM, Jiang T, Wirtel AJ, Deng M, Lv Q, Nair LS, Doty SB, Laurencin CT. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: a combined gene therapy-cell transplantation approach. Proc Natl Acad Sci U S A. 2008;105:11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. J Orthop Res. 2009;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 267.Miyazaki M, Zuk PA, Zou J, Yoon SH, Wei F, Morishita Y, Sintuu C, Wang JC. Comparison of human mesenchymal stem cells derived from adipose tissue and bone marrow for ex vivo gene therapy in rat spinal fusion model. Spine. 2008;33:863–869. doi: 10.1097/BRS.0b013e31816b45c3. [DOI] [PubMed] [Google Scholar]
- 268.Meinel L, Hofmann S, Betz O, Fajardo R, Merkle HP, Langer R, Evans CH, Vunjak-Novakovic G, Kaplan DL. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials. 2006;27:4993–5002. doi: 10.1016/j.biomaterials.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 269.Pelled G, Ben-Arav A, Hock C, Reynolds DG, Yazici C, Zilberman Y, Gazit Z, Awad H, Gazit D, Schwarz EM. Direct gene therapy for bone regeneration: gene delivery, animal models, and outcome measures. Tissue Eng Part B Rev. 2010;16:13–20. doi: 10.1089/ten.teb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Chen Y, Luk KD, Cheung KM, Xu R, Lin MC, Lu WW, Leong JC, Kung HF. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Ther. 2003;10:1345–1353. doi: 10.1038/sj.gt.3301999. [DOI] [PubMed] [Google Scholar]
- 271.Miyazaki M, Sugiyama O, Zou J, Yoon SH, Wei F, Morishita Y, Sintuu C, Virk MS, Lieberman JR, Wang JC. Comparison of lentiviral and adenoviral gene therapy for spinal fusion in rats. Spine. 2008;33:1410–1417. doi: 10.1097/BRS.0b013e3181761003. [DOI] [PubMed] [Google Scholar]
- 272.Phillips JE, Gersbach CA, García AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–229. doi: 10.1016/j.biomaterials.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 273.Partridge KA, Oreffo ROC. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10:295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 274.Kawai M, Maruyama H, Bessho K, Yamamoto H, Miyazaki J-I, Yamamoto T. Simple strategy for bone regeneration with a BMP-2/7 gene expression cassette vector. Biochem Bioph Res Comm. 2009;390:1012–1017. doi: 10.1016/j.bbrc.2009.10.099. [DOI] [PubMed] [Google Scholar]
- 275.Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M, Li JINZ, Helm GA, Gazit DAN, Gazit Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006;12:877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 276.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15:257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 277.Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein Sa, McCauley LK, Davidson BL, Roessler BJ. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chen J, Chen H, Li P, Diao H, Zhu S, Dong L, Wang R, Guo T, Zhao J, Zhang J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32:4793–4805. doi: 10.1016/j.biomaterials.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 279.Kolk A, Haczek C, Koch C, Vogt S, Kullmer M, Pautke C, Deppe H, Plank C. A strategy to establish a gene-activated matrix on titanium using gene vectors protected in a polylactide coating. Biomaterials. 2011;32:6850–6859. doi: 10.1016/j.biomaterials.2011.05.071. [DOI] [PubMed] [Google Scholar]
- 280.Kim HS, Yoo HS. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: in vitro and in vivo evaluation. J Control Release. 2010;145:264–271. doi: 10.1016/j.jconrel.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 281.Hu W-W, Wang Z, Hollister SJ, Krebsbach PH. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14:891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- 282.Jang J-H, Schaffer DV, Shea LD. Engineering Biomaterial Systems to Enhance Viral Vector Gene Delivery. Mol Ther. 2011;19:1407–1415. doi: 10.1038/mt.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang Y, Fan W, Nothdurft L, Wu C, Zhou Y, Crawford R, Xiao Y. In vitro and in vivo evaluation of adenovirus combined silk fibroin scaffolds for bone morphogenetic protein-7 gene delivery. Tissue Eng Part C Meth. 2011;17:789–797. doi: 10.1089/ten.tec.2010.0453. [DOI] [PubMed] [Google Scholar]
- 284.Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, Galun E, Gazit Z, Jorgensen C, Gazit D. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9:587–595. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 285.Gersbach CA, Le Doux JM, Guldberg RE, García AJ. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006;13:873–882. doi: 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- 286.Peng H, Usas A, Gearhart B, Young B, Olshanski A, Huard J. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004;9:885–894. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 287.Koh J-T, Ge C, Zhao M, Wang Z, Krebsbach PH, Zhao Z, Franceschi RT. Use of a stringent dimerizer-regulated gene expression system for controlled BMP2 delivery. Mol Ther. 2006;14:684–691. doi: 10.1016/j.ymthe.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 288.Vehof JWM, van den Dolder J, de Ruijter JE, Spauwen PHM, Jansen J. Bone formation in CaP-coated and noncoated titanium fiber mesh. J Biomed Mater Res A. 2003;64:417–426. doi: 10.1002/jbm.a.10288. [DOI] [PubMed] [Google Scholar]
- 289.Tour G, Wendel M, Tcacencu I. Cell-derived matrix enhances osteogenic properties of hydroxyapatite. Tissue Eng Part A. 17:127–137. doi: 10.1089/ten.TEA.2010.0175. [DOI] [PubMed] [Google Scholar]
- 290.Rundle CH, Strong DD, Chen S-t, Linkhart TA, Sheng MH, Wergedal JE, Lau KW. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat. J Gene Med. 2008;10:229–241. doi: 10.1002/jgm.1148. [DOI] [PubMed] [Google Scholar]