Abstract
In the present study, we showed that in vivo administration of an anti-CD25 antibody (PC61) decreased the Th17 response in C57BL/6 (B6) mice immunized with the uveitogenic peptide IRBP1–20, while enhancing the autoreactive Th1 response. The depressed Th17 response was closely associated with decreased numbers of a splenic dendritic cell (DC) subset expressing CD11c+CD3−CD25+ and decreased expansion of γδ T cells. We demonstrated that ablation of the CD25+ DC subset accounted for the decreased activation and the expansion of γδ T cells, leading to decreased activation of IL-17+ IRBP-specific T cells. Our results show that an enhanced Th17 response in an autoimmune disease is associated with the appearance of a DC subset expressing CD25 and that treatment of mice with anti-CD25 antibody causes functional alterations in a number of immune cell types, namely DCs and γδ T cells, in addition to CD25+αβTCR+ regulatory T cells.
Keywords: autoimmunity, CD25+, cell, Dendritic cells, EAU, Interleukin-17, Th17, uveitis
Introduction
Knowledge about factors that affect or regulate the activation of autoreactive T cells is required for of therapeutics for autoimmune diseases. Over the past two decades, circumstantial evidence has been obtained that, in a number of experimental autoimmune diseases, including encephalomyelitis (1–4), arthritis (5,6), and uveitis (7, 8), a major subset of pathogenic autoreactive T cells produce IFN-γ and IL-2 and belong to the Th1 type of CD4 T cells. Recent studies have identified a new and crucial autoreactive T cell subset which produces IL-17, but not IFN-γ or IL-4, designated as Th17 cells (9–13). Studies have shown that the requirements for activation of Th1 and Th17 autoreactive T cells differ (14–18) and that Th1 and Th17 autoreactive T cells may not be regulated by the same cells (19,20). These observations prompted us to determine how Th17 autoreactive T cells differ from Th1 autoreactive T cells in their regulation by functionally counter-reactive cells and molecules, whether different mechanisms leading to the activation of Th1 or Th17 pathogenic T cell subsets could be identified, and whether a single therapeutic strategy could control the pathogenic activity of both types of T cell.
Anti-CD25 monoclonal antibody binds to the α chain of the IL-2 receptor (IL-2R) (21,22). CD25 expression is not restricted to T cells (23) and can easily be detected on human (24–26) and mouse (27–30) dendritic cells (DCs) and myeloid cells. To determine whether Th1 and Th17 pathogenic T cells are regulated by different immunoregulatory mechanisms, we examined whether decreasing the activity of CD25+ regulatory T cells, a treatment found to result in enhanced function of Th1 autoreactive T cells (31–35), would have a similar or a different effect on Th17 autoreactive T cell responses. We found that mice treated with anti-CD25 monoclonal antibody (mAb) before immunization with human interphotoreceptor retinoid-binding protein (IRBP) peptidehad a significantly decreased Th17 response, but an enhanced Th1 response. Mechanistic studies on the effects on functionally different autoreactive T cell responses in anti-CD25mAb-treated mice showed that a decline in the numbers of CD25+ and Foxp3+ T cells was associated with decreased activation of γδTCR+ T cells. Moreover, the altered responses were also evident when in vivo primed T cells from mice with or without prior antibody treatment were stimulated with the immunizing antigen in the presence of splenic APCs from mAb-treated mice, suggesting an involvement of functionally altered APCs in the treated mice. We also showed that approximately 10% of CD11c+CD3− DCs in spleens from immunized mice co-expressed CD25 and that purified CD25+ DCs were much more effective than CD25− DCs in stimulating the activation of IL-17+ autoreactive T cells and γδ T cells. Our data demonstrate that an enhanced Th17 response in an autoimmune disease is associated with the appearance of a DC subset expressing CD25 and that treatment of mice with anti-CD25 mAb causes functional alterations in multiple immune cell types, rather than only CD4+CD25+ T cells.
Methods
Animals and reagents
Female C57BL/6 (B6) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Southern California. Institutional approval was obtained and institutional guidelines regarding animal experimentation followed. Recombinant murine IL-23 and IL-12 were purchased from R & D (Minneapolis, MN). Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies against mouse IFN-γ, IL-17, CD25, CD11c, αβ T cell receptor (TCR), and γδ TCR were purchased from Biolegend (San Diego, CA), while all other antibodies were from BD Bioscience (La Jolla, CA).
Immunization procedure and in vitro stimulation of in vivo primed T cells
B6 mice were immunized subcutaneously over 6 spots at the tail base and on the flank with 200 µl of emulsion containing 150 µg of the uveitogenic peptide IRBP1–20 [amino acids 1–20 of human interphotoreceptor retinoid-binding protein (IRBP; Sigma, St. Louis, MO)] emulsified in complete Freund’s adjuvant (CFA; Difco, Detroit, MI). Concurrently, 200 ng of pertussis toxin (PTX) (Sigma, St. Louis, MO) was injected intraperitoneally. At day 13 post-immunization, T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column, then 1 × 107 cells in 2 ml of RPMI 1640 medium (Cellgro, VA, USA) containing 10% fetal calf serum were added to each well of a 6-well plate (Costar) and stimulated for 48 h with 10 µg/ml of IRBP1–20 in the presence of 1 × 107 irradiated syngeneic spleen cells as antigen-presenting cells (APCs) in the presence of either IL-12 (Th1 polarized) or IL-23 (Th17-polarized) (10 ng/ml), then activated T cell blasts were separated by Ficoll gradient centrifugation and cultured for another 72 h in the same medium used for stimulation without the peptide.
In vivo administration of anti-CD25 antibody
The PC61 (anti-CD25) hybridoma was purchased from the American Type Culture Collection (Manassas, VA) and ascites produced in SCID mice by Taconic (Hudson, New York). Ammonium sulfate-precipitated IgG from PC61 as cites was used for all injections. C57BL/6 mice were injected intraperitoneally with 3 doses of 500 µg of PC61 on days -7, -4, and -1 before immunization on day 0. Flow cytometry was performed on a BD FACScan or FACSCalibur and the results analyzed using CellQuest software (BD Immunocytometry Systems, San Jose, CA).
Cytokine assays
Enriched T cells (3 × 104 cells/well) from the draining lymph nodes and spleens were cultured at 37° C for 48 h in 96-well microtiter plates with irradiated syngeneic spleen APCs (1 × 105) in the presence of IRBP1–20, then a fraction of the culture supernatant was assayed for IL-17 using ELISA kits (R & D).
Scoring of experimental autoimmune uveitis (EAU)
The mice were examined three times a week for clinical signs of EAU by indirect fundoscopy. The pupils were dilated using 0.5% tropicamide and 1.25% phenylephrine hydrochloride ophthalmic solutions and fundoscopic grading of disease performed using the scoring system described previously (36). For histopathological evaluation, whole eyes were collected at the end of the experiment and immersed for 1 h in 4% glutaraldehyde in phosphate buffer, pH 7.4, then were transferred to 10% formaldehyde in phosphate buffer until processed. The fixed and dehydrated tissues were embedded in methacrylate, then 5 µm sections were cut through the pupillary-optic nerve plane and stained with hematoxylin and eosin. Presence or absence of disease was evaluated blind by examining six sections cut at different levels for each eye. Disease was graded pathologically based on cellular infiltration and structural changes (37).
Intracellular staining and FACS analysis
For intracellular staining, T cells (2 × 105 in 100 µl of PBS) were incubated for 4 h with 50 ng/ml of PMA, 1 µg/ml of ionomycin, and 1 µg/ml of brefeldin A (Sigma-Aldrich), then were washed, fixed, permeabilized overnight with Cytofix/Cytoperm buffer (eBioscience), intracellularly stained with antibodies against IFN-γ and IL-17, and analyzed on a FACScalibur flow cytometer.
Limiting dilution analysis (LDA)
B6 mice were immunized with IRBP1–20 emulsified in CFA, then the spleen and draining lymph nodes were removed 13 days later, a single cell suspension prepared, and T cells enriched by nylon wool adherence. They were then seeded in two sets in 96-well flat-bottomed culture plates containing irradiated spleen cells (1 × 105 per well) under Th1 or Th17 polarizing conditions, with one set of plates containing an optimal dose of immunizing peptide (10 µg/ml). Activation of IRBP1–20-specific T cells was estimated by comparing the proliferation and IFN-γ and IL-17 production of graded numbers (3,000–200,000/well) of T cells in the presence and absence of IRBP1–20 under polarizing conditions, 24 replicate wells being used for each cell density. After 44 h of incubation, the plates were centrifuged and 50 µl of supernatant harvested for lymphokine assays and the rest of the cultures pulsed with 3H-thymidine for 6 h, harvested, and counted in a β-counter. Positive microcultures were defined as those in which lymphokine activity or incorporated thymidine exceeded the mean activity in control cultures (no responder cells) by more than three standard deviations (38–40). The frequency of responder T cells was obtained from the minimum estimates of precursor frequency calculated using a program developed to analyze the LDA data acquired (41,42). This program uses the Poisson distribution to calculate the frequency of responder T cells with 99% confidence limits.
Statistical analysis
Experiments were repeated at least twice, usually three or more times. Experimental groups were typically composed of four mice. The figures show data from a representative experiment. Differences between the values for different groups were examined using the two-tailed t test.
Results
Treatment of B6 mice with anti-mouse CD25 antibody (PC61) decreases the Th17 autoreactive T cell response
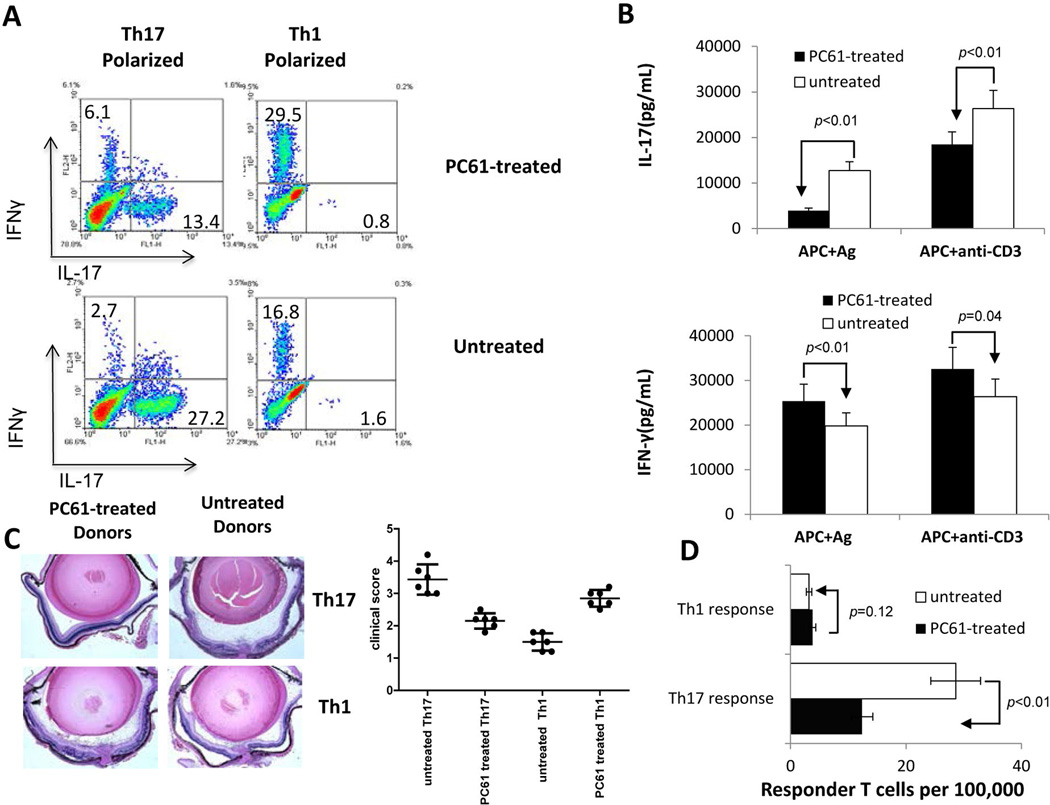
Previous studies have shown that treatment of mice with an anti-mouse CD25 antibody enhances auto reactivity by abolishing the function of regulatory T cells (31–35). To determine whether antibody treatment affected both Th1 and Th17 autoreactive T cells, B6 mice were randomly divided into two groups, one of which was injected with 3 doses of anti-CD25 antibody (PC61) and the other with an isotype-matched rat IgGon days -7, -4, and -1 before immunization with IRBP1–20 emulsified in CFA (see Materials and Methods), or remained untreated. In vivo primed T cells, collected on day 13 post-immunization, were re-stimulated in vitro for two days with immunizing peptide and syngeneic APCs under either Th1 or Th17 polarizing conditions (culture medium containing IL-12 or IL-23, respectively) and the activated T cells intracellularly stained with antibodies specific for IFN-γ, IL-17, or αβ T cell receptor (TCR) and analyzed by flow cytometry. As shown in Fig. 1A, the number of IL-17+ T cells among the responder T cells obtained from PC61-treated mice was significantly decreased by about 50%, contrasting with the number of IFN-γ+ T cells, which was increased about two-fold, as compared to mice that were untreated with antibody or treated with an irrelevant control antibody. ELISA tests gave results similar to those for intracellular staining, showing that T cells from PC61-treated mice produced increased amounts of IFN-γ, but decreased amounts of IL-17 (Fig. 1B). To determine whether IRBP-specific T cells from PC61-treated mice had increased or decreased pathogenic activity, 2 × 106 Th1 or Th17 polarized activated T cells from each group were transferred to naïve recipients and the development of experimental autoimmune uveitis (EAU) in the recipient mice followed by fundoscopy and pathological examination. Fig. 1C shows a set of representative pathologic pictures along with a summarized plot to show that IL-17+ IRBP-specific T cells from PC61-treated mice showed significantly decreased uveitogenic activity, whereas the uveitogenic activity of IFN-γ+ IRBP-specific T cells from the same mice was slightly enhanced. To determine whether the antibody treatment directly affected the in vivo priming of Th1 and Th17 autoreactive T cells, we performed limiting dilution assays (LDA) to measure the frequencies of in vivo primed Th1 and Th17 IRBP-specific T cells in PC61-treated and non-treated mice. As shown in Fig. 1D, the average frequency of IRBP-specific Th17 responder T cells was decreased by up to 50% in PC61-treated mice (12 cells per 100,000 responder T cells) comparedto untreated mice (25 cells per 100,000 responder T cells), whereas the frequency of IFN-γ+ IRBP-specific T cells was increased from approximately 3 to 5 cells per 100,000 responder T cells.
Fig. 1. Injection of B6 mice with anti-mouse CD25 antibody (PC61) decreases the Th17 autoreactive T cell response.
A). Splenic T cells from IRBP1–20/CFA-immunized mice with or without prior PC61 treatment were enriched by passage through nylon wool and stimulated for 48 h with an optimal dose of IRBP1–20 (10 µg/ml) under Th1 or Th17 polarized conditions and the activated T cells separated by Ficoll gradient centrifugation on day 3, then cultured under the same polarized conditions for a further 5 days and intracellularly stained with PE-conjugated anti-IFN-γ antibodies and FITC-conjugated anti-IL-17 antibodies, followed by FACS analysis.
B) IFN-γ and IL-17 levels in the culture supernatants after the 48 h incubation with peptide measured by ELISA.
C) IRBP-specific T cells (2 × 106/mouse) from untreated and PC61-treated IRBP-immunized mice activated under Th1 or Th17 polarized conditions were adoptively transferred to syngeneic B6 mice. Pathologic examination was conducted 10 days after disease induction. A set of representative pathologic slides is shown along with summarized in vivo results.
D) Responder T cell numbers evaluated by LDA as detailed in the Materials and Methods.
The results shown are representative of those from >5 experiments.
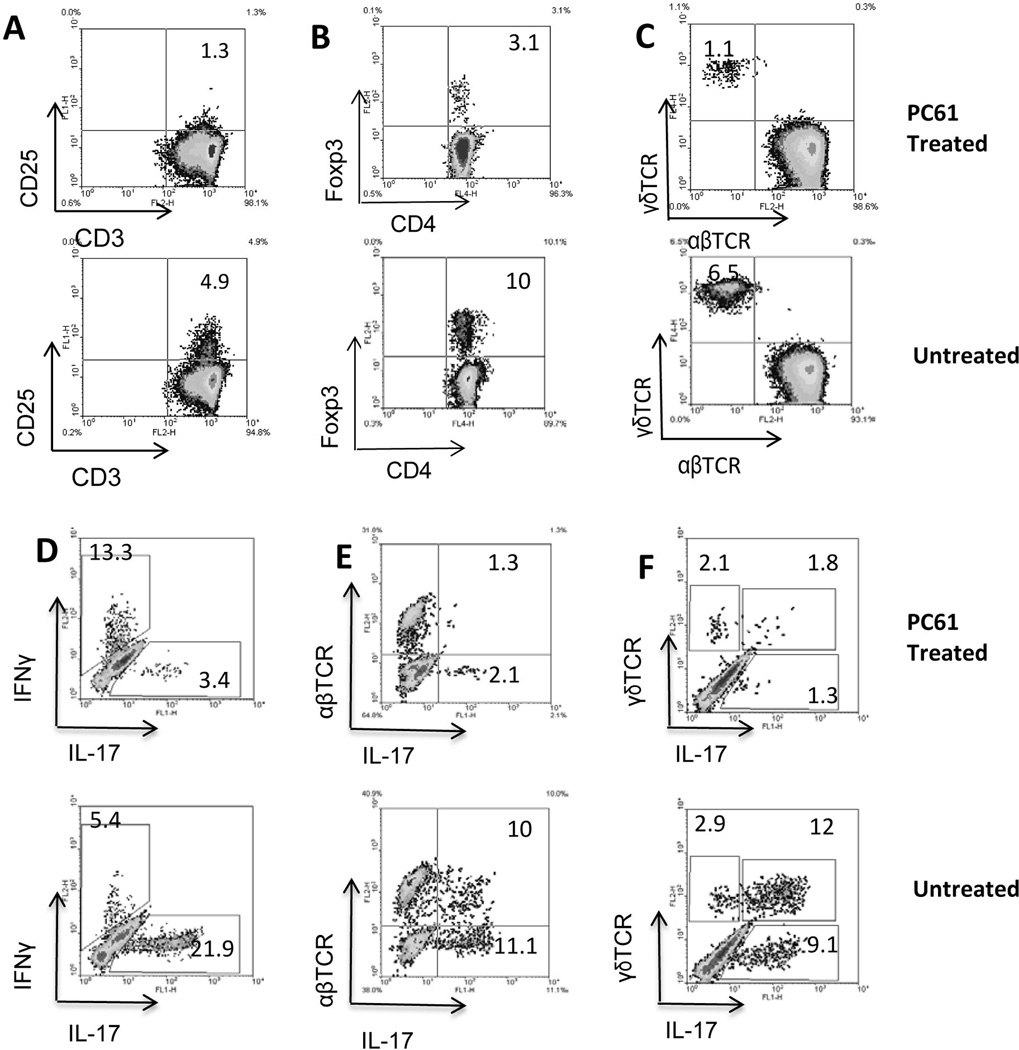
Decreased activation of γδ T cells in PC61-treated mice
To determine the mechanism by which in vivo administration of PC61 decreased the generation of IL-17+ autoreactive T cells, we examined cellular components in the spleen before (Fig. 2A–C) and after (Fig. 2D–F) in vitro activation. As shown in Fig. 2A, before immunization, approximately 5% of splenic T cells from immunized mice expressed CD25 and most of this T cell population disappeared after antibody administration. In parallel, there was a significant decrease in the numbers of Foxp3+ cells (Fig. 2B) and γδ T cells (Fig. 2C). After stimulation with the immunizing antigen and expansion under Th17 polarized conditions for 8 days, the number of IL-17+ T cells decreased > 6-fold in PC61-treated mice compared to untreated mice (Fig. 2D). Since IL-17+ T cells contain both αβ and γδ T cells, we then separately assessed the IL-17+αβTCR+ and IL-17+γδTCR+ T cells and found that the number of IL-17+αβTCR+ T cells dropped more than 5-fold (Fig. 2E) and the number of IL-17+γδTCR+ T cells declined even more dramatically (Fig. 2F).
Fig. 2. Phenotypic change of T cells in PC61-injected mice.
B6 mice were randomly separated into two groups (n=8) and immunized with IRBP1–20/CFA with or without a prior treatment with PC61. After 13 days, splenic T cells were enriched and stimulated with the immunizing peptide. The phenotype of the T cells was analyzed either before (A–C) or after (D–F) in vitro stimulation.
A–C) Effect of PC61 injection on CD25+CD3+ cells (A), Foxp3+ cells (B), and γδ T cells (E).
D–F) After in vitro stimulation with the immunizing antigen and expansion under Th17 polarized conditions, the activated T cells were stained with the indicated antibodies and analyzed by flow cytometer. The experiments were repeated more than 5 times and the results of one representative experiment are shown.
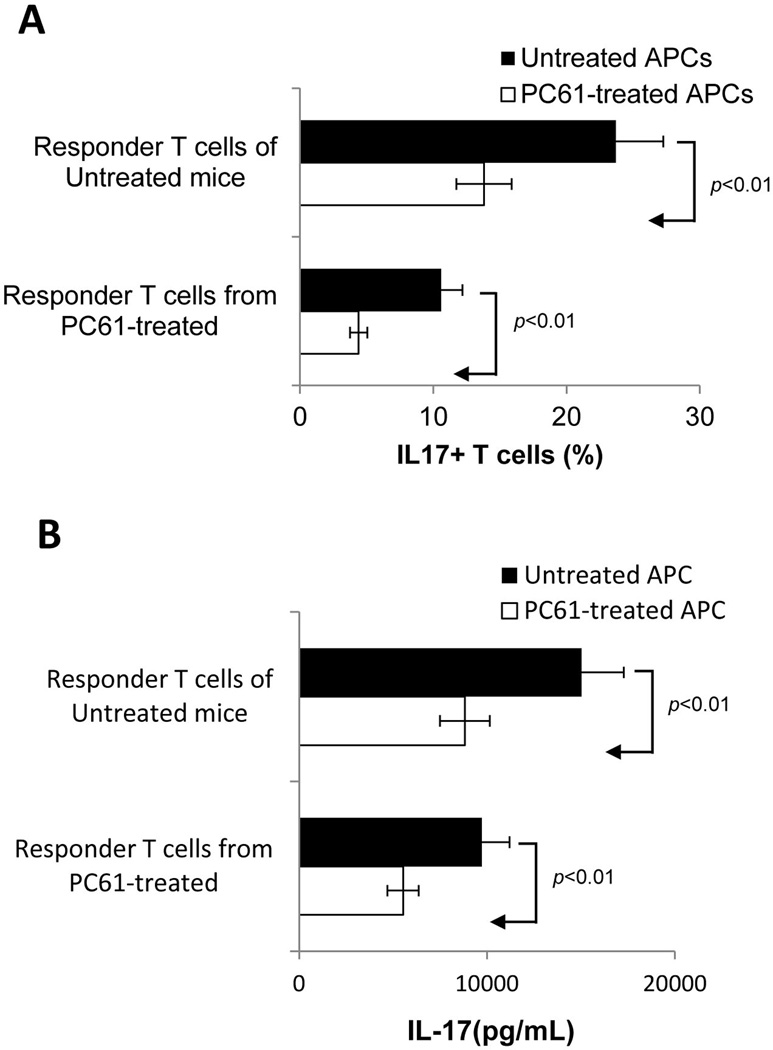
Splenic APCs from PC61-treated mice show a decreased ability to stimulate IL-17+ autoreactive T cells in vitro
To determine whether the antibody treatment has an effect on immune cells other than CD25+ T cells, we performed crisscross experiments in which in vivo primed T cells from PC61-treated or untreated mice were stimulated with immunizing antigen in the presence of splenic APCs from PC61-treated or untreated mice. When in vivo primed T cells were stimulated for 5 days in vitro with the immunizing peptidein the presence of APCs from PC61-treated mice, the activated T cells contained significantly fewer IL-17+ cells, regardless of whether the T cells were from antibody-treated or non-treated mice, and T cells from PC61-treated mice showed a significantly decreased response compared to untreated mice (Fig. 3A). Consistent with the intracellular staining analysis, ELISA tests showed that the use of APCs from PC61-treated mice resulted in less activation of IL-17+ T cells (Fig. 3B), but slight enhancement of the activation of IFN-γ+ IRBP-specific T cells (data not shown). These data show that splenic APCs from PC61-treated mice are functionally altered in their ability to stimulate Th17 and Th1 T cells.
Fig. 3. Crisscross tests showing that dysfunction of splenic APCs accounts for the decreased generation of IL-17+ IRBP-specific T cells in PC61-treated mice.
A). Responder T cells from immunized PC61-treated or non-treated mice were stimulated in vitro for 48 h with IRBP1–20 peptide in the presence of splenic APCs from PC61-treated or non-treated mice and the percentage of IL17+ T cells measured.
B). ELISA test. IL-17 levels in the culture supernatants after 48 h incubation with peptide and APCs assessed by ELISA.
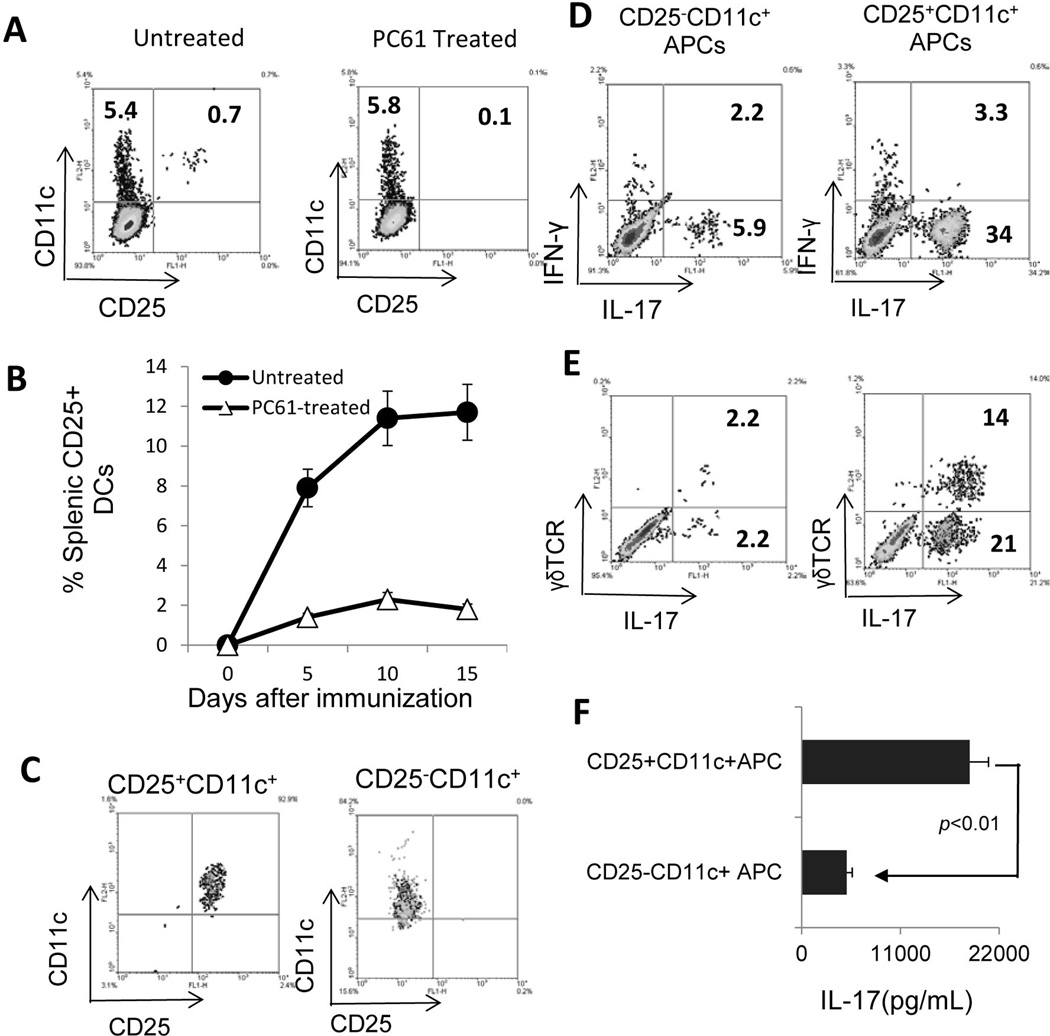
CD25+CD11c+ cells are more effective than CD25−CD11c+ cells in stimulating activation of IL-17+ IRBP-specific T cells and γδ T cells
To determine the mechanism underlying the functional change in splenic APCs from PC61-treated mice, we examined whether the injected antibody depleted a subset of APCs that express CD25. As shown in Fig. 4A, approximately 10% of the CD11c+ cells in the spleen of immunized mice expressed CD25 and this cell population disappeared in mice pretreated with PC61 (Fig 4A&B). Phenotypic studies showed that the CD25+CD11c+ cells were CD3−CD161− (data not shown). To further determine whether CD25+CD11c+ cells were functionally distinct from their CD25− counterparts, we magnetically separated the CD25+ and CD25− fractions from immunized mice (Fig. 4C), stimulated the in vivo primed T cells with the immunizing peptide in the presence of CD25+CD11c+ or CD25−CD11c+ cells as APCs for 5 days, and then stained the activated T cells with antibodies and performed flow cytometry. As shown, the CD25+CD11+ cells showed a significantly decreased ability to stimulate the activation of both IL-17+ IRBP-specific T cells (Fig. 4D) and IL-17+γδ T cells (Fig. 4E) compared to the CD25−CD11+ cells. Furthermore, responder T cells produced larger amounts of IL-17 when they were stimulated by CD25+CD11c+ DCs than they were stimulated by CD25−CD11c+ DCs (Fig. 4F).
Fig. 4. CD25+CD11c+ cells are more effective than CD25−CD11c+ cells instimulating the activation of IL-17+ IRBP-specific T cells and γδTCR+ T cells.
A&B) Splenic cells of immunized mice, with or without PB61 injection, were stained for expression of CD11c and/or CD25.
C) CD25+CD11c+ and CD25−CD11c+ DCs separated using magnetic beads
D&E) Splenic T cells isolated from immunized B6 mice were stimulated with the immunizing peptide IRBP1–20 in the presence of CD25−CD11c+ (upper) or CD25+CD11c+(lower) DCs for 5 days and the activated T cells were stained for expression of IL-17 (4D) and IFN-γ (4E).
F) ELISA assay. Culture supernatant of immunized splenic T cells were tested for IL-17 production after 48h stimulation with the immunizing peptide IRBP1–20 in the presence of either CD25−CD11c+ or CD25+CD11c+DCs.
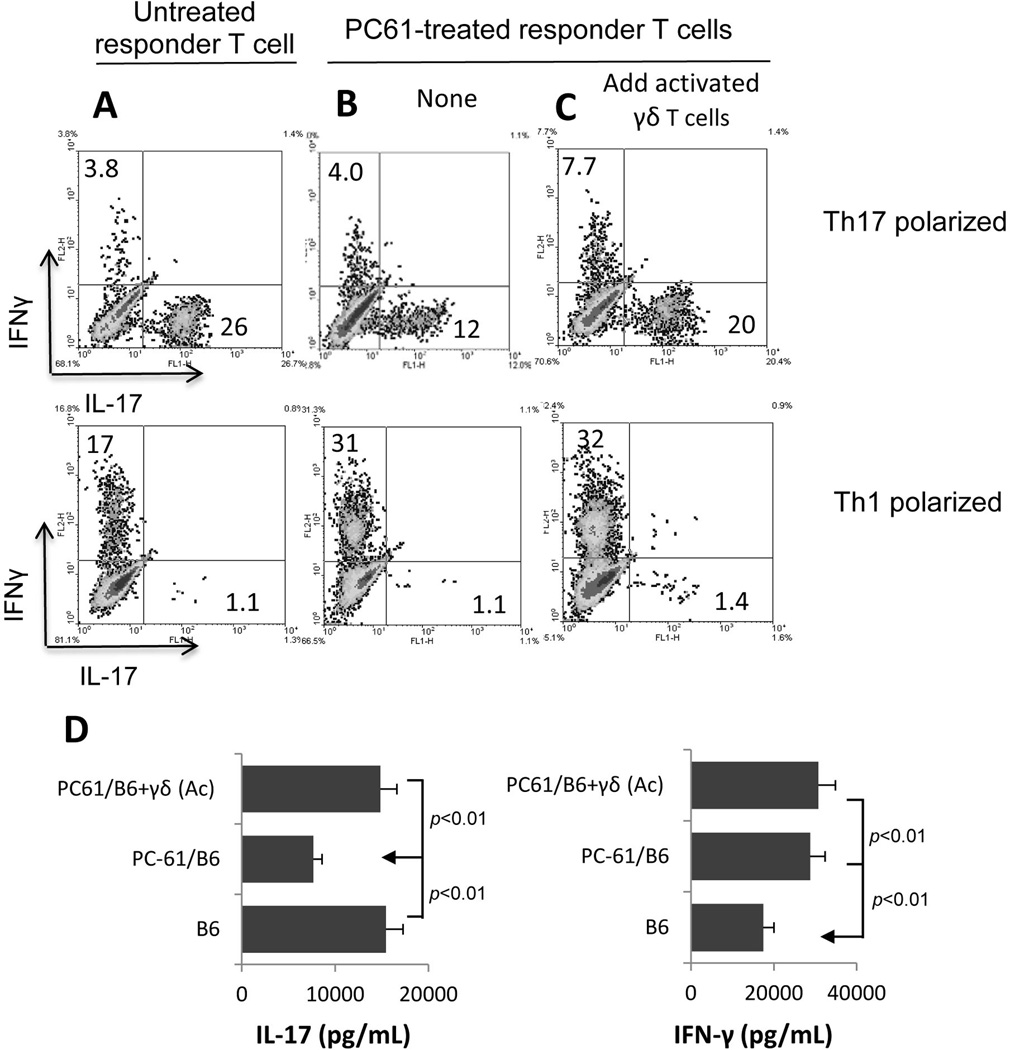
The Th17 response of PC61-treated mice is restored by addition of a small number of activated γδ T cells to the responder T cells
To test the possibility that the decrease in the number of γδ T cells contributed to the hypo-responsiveness of Th17 autoreactive T cells, we added a small number (2%) of γδ T cells pre-activated by exposure to anti-CD3 antibody for 2 days to the in vivo primed T cells from PC61-treated mice before stimulating with the immunizing antigen and APCs under Th1 or Th17 polarized conditions. As shown in Fig. 5, Th17 polarized T cells from PC61-treated mice generated a significantly lower number of IL17+ T cells (Fig. 5B) than those from untreated mice (Fig. 5A). However, addition of 2% of activated γδ T cells restored the intensity of the Th17 response, suggesting that γδ T cells are at least partially responsible for the activation of Th17 autoreactive T cells in this system.
Fig. 5. Restoration of the Th17 response by addition of activated γδ T cells to functionally defective T cells.
A–C). In vivo primed T cells from IRBP1–20/CFA-immunized mice with (B&C) or without (A) prior treatment with PC61 were stimulated for 5 days with immunizing peptide under Th1 or Th17 polarized conditions (A and B) or after addition of 2% of activated γδ T cells (2 × 104/well), then activated T cells were intracellularly stained for IFN-γ and IL-17 and analyzed by FACS for numbers of IL-17+or IF-Nγ+T cells.
D) IFN-γ and IL-17 levels in the culture supernatants at 48 h were assessed by ELISA.
Discussion
Approximately 10% of peripheral CD4+ cells and less than 1% of CD8+ cells in normal unimmunized adult mice express the IL-2R α-chain (CD25) (32,43). Studies have shown that cells within the CD4+ T subset that constitutively express CD25 have immunosuppressive activity (31,44,45). This conclusion was supported by the demonstration that treatment of mice with antibodies specific for mouse CD25 leads to an increased frequency and severity of autoimmune diseases (31–35) and that functional abnormalities in the CD25+ T cell population are closely associated with an increased development of autoimmune diseases (43,46). Regardless of whether the administered anti-CD25 antibody eliminated (33,47–49) or inactivated (50,51) CD25+CD4+ T cells, the fact remains that the antibody caused altered function of T cells expressing CD4 and CD25. However, these previous studies did not examine whether the antibody caused dysregulated function of different autoreactive T cell subsets, such as Th1 and Th17 autoreactive T cells.
Regulatory T cells participate in the maintenance of peripheral tolerance and prevention of autoimmunity (31–35). One of the experimental tools used to assess the effect of regulatory T cells in vivo is to inject mice with an anti-CD25 antibody. In this study, we found that mice treated with an anti-CD25 (PC61) antibody had decreased Th17 responses, which were associated with a decreased activation/expansion of γδ T cells. Furthermore, a decreased number of CD25+ cells among splenic DCs (CD11c+CD3−CD25+ cells) probably accounted for the reduced Th17 response and the diminished activation of γδ TCR+ T cells. Thus, treatment of mice with anti-CD25 antibodies directly or indirectly caused functional alterations in a number of immune cells, namely DCs and γδ T cells, in addition to CD25+αβTCR+ regulatory T cells.
Anti-CD25 monoclonal antibody binds to the α chain of the IL-2R (21,22). Expression of CD25 is not restricted to T cells (23) and can easily be detected on human (24–26) and mouse DCs (27–30) and myeloid cells. We were able to show that approximately 10% of CD11c+ cells in the spleens of immunized mice expressed CD25 and that purified CD3−CD11c+CD25+ cells had a greater ability than CD11c+CD25− to stimulate the activation of IL-17+ uveitogenic T cells and γδ T cells. It is likely that an increase in this DC subset enhances the Th17 response via pathways involving γδ T cell activation, whereas removal of this DC subset diminishes Th17 responses.
We have previously reported that γδ T cells play a major role in the activation of Th17 autoreactive T cells (52–54). γδ-deficient mice (TCR-δ−/−) have decreased activation of Th17 autoreactive T cells, and the transfer of a small number of γδ T cells to TCR-δ−/−mice restores the Th17 response (52–54). We showed that activation of γδ T cells promoted the generation of the Th17 response in vitro and in vivo and thus promoted the development of EAU. Moreover, the enhancing and suppressing effect of γδ T cells on EAU is convertible and dependent on their state of activation (52). In the present study, we found that the activation or expansion of γδ T cells was significantly inhibited in PC61-treated mice and that the decreased number of the CD25+ DC subset accounted for the hypo-function of γδ T cells. These observations support our previous finding that γδ T cells play a major role in regulating Th17 autoreactive T cell responses in EAU (52–54).
The possibility that the decreased number of γδ T cells in PC61-treated mice was due to the elimination of activated γδ T cells expressing CD25 was not supported by our studies, as we failed to demonstrate any CD25+γδ T cells among freshly isolated T cells from either naïve or immunized mice (data not shown). The mechanism by which activated γδ T cells cause an increased autoreactive T cell response remains to be determined. It is likely that the recorded changes are accompanied by a serial of reciprocal interactions between γδ and αβ T cells and between γδ and DCs. Clarification of these issues may help to understand the mechanism by which Th17 responses are regulated and the mechanism by which γδ T cells regulate the Th17 response. The observation that specifically activated DCs or DC subsets are important for γδT cell activation and function should help in efforts to manipulate the Th17 response by acting on γδT cell activation.
There is an unresolved question over whether previously identified regulatory T cells that suppress Th1 cells can also suppress Th17 cells. Indeed, there are suggestions that, in contrast to IFN-γ production, IL-17 production and/or Th17 cell development may not be effectively downregulated by previously identified regulatory T cells (19,20). Thus, efforts aimed at identifying factors that regulate the generation and expansion of Th17 autoreactive T cells is important.
Acknowledgments
This work was supported in part by NIH grants EY018827, EY017373, and EY003040.
Abbreviations
- EAU
experimental autoimmune uveitis
- DC
dendritic cell
- IRBP
interphotoreceptor retinoid-binding protein
- LDA
limiting dilution analysis
References
- 1.Ben-Nun A, Wekerle H, Cohen IR. Vaccination against autoimmune encephalomyelitis using attenuated cells of a T lymphocyte line reactive against myelin basic protein. Nature. 1981;292:60–61. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- 2.Acha-Orbea H, Mitchell DJ, Timmermann L, Wraith DC, Tausch GS, Waldor MK, Zamvil SS, McDevitt HO, Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988;54:263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- 3.Acha-Orbea H, Steinman L, McDevitt HO. T cell receptors in murine autoimmune diseases. Ann. Rev. Immunol. 1989;7:371–405. doi: 10.1146/annurev.iy.07.040189.002103. [DOI] [PubMed] [Google Scholar]
- 4.Sun D, Coleclough C, Hu X. Heterogeneity of rat encephalitogenic T cells elicited by variants of the MBP(68–88) peptide. Eur. J. Immunol. 1995;25:1687–1692. doi: 10.1002/eji.1830250631. [DOI] [PubMed] [Google Scholar]
- 5.Myers LK, Stuart JM, Kang AH. A CD4 cell is capable of transferring suppression of collagen- induced arthritis. J. Immunol. 1989;143:3976–3980. [PubMed] [Google Scholar]
- 6.Gustafsson K, Karlsson M, Andersson L, Holmdahl R. Structures on the I-A molecule predisposing for susceptibility to type II collagen-induced autoimmune arthritis. Eur. J. Immunol. 1990;20:2127–2131. doi: 10.1002/eji.1830200935. [DOI] [PubMed] [Google Scholar]
- 7.Caspi RR, Roberge FG, McAllister CG, El-Saied M, Kuwabara T, Gery I, Hanna E, Nussenblatt RB. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J. Immunol. 1986;136:928–933. [PubMed] [Google Scholar]
- 8.Shao H, Peng Y, Liao T, Wang M, Song M, Kaplan HJ, Sun D. A shared epitope of the interphotoreceptor retinoid-binding protein (IRBP) recognized by the CD4+ and CD8+ autoreactive T cells. J. Immunol. 2005;175:1851–1857. doi: 10.4049/jimmunol.175.3.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trend Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 Plays an Important Role in the Development of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal S, Ghilardi N, Xie MH, De Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 16.Bouguermouh S, Fortin G, Baba N, Rubio M, Sarfati M. CD28 Co-Stimulation Down Regulates Th17 Development. PLoS ONE. 2009;4:e5087. doi: 10.1371/journal.pone.0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40-CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2009;106:876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purvis HA, Stoop JN, Mann J, Woods S, Kozijn AE, Hambleton S, Robinson JH, Isaacs JD, Anderson AE, Hilkens CMU. Low-strength T-cell activation promotes Th17 responses. Blood. 2010;116:4829–4837. doi: 10.1182/blood-2010-03-272153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor RA, Malpass KH, Anderton SM. The Inflamed Central Nervous System Drives the Activation and Rapid Proliferation of Foxp3+ Regulatory T Cells. J. Immunol. 2007;179:958–966. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 21.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 22.Kendall A. The Interleukin 2 Receptor. In: Frank JD, editor. Advances in Immunology. Academic Press; 1988. p. 165. [Google Scholar]
- 23.Toossi Z, Sedor JR, Lapurga JP, Ondash RJ, Ellner JJ. Expression of functional interleukin 2 receptors by peripheral blood monocytes from patients with active pulmonary tuberculosis. J. Clin. Invest. 1990;85:1777–1784. doi: 10.1172/JCI114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holter W, Goldman CK, Casabo L, Nelson DL, Greene WC, Waldmann TA. Expression of functional IL 2 receptors by lipopolysaccharide and interferon-gamma stimulated human monocytes. J. Immunol. 1987;138:2917–2922. [PubMed] [Google Scholar]
- 25.Pasare C, Medzhitov R. Toll Pathway-Dependent Blockade of CD4+CD25+ T Cell-Mediated Suppression by Dendritic Cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 26.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human Plasmacytoid Dendritic Cells Activated by CpG Oligodeoxynucleotides Induce the Generation of CD4+CD25+ Regulatory T Cells. J. Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 27.Crowley M, Inaba K, Witmer-Pack M, Steinman RM. The cell surface of mouse dendritic cells: FACS analyses of dendritic cells from different tissues including thymus. Cellular Immunology. 1989;118:108–125. doi: 10.1016/0008-8749(89)90361-4. [DOI] [PubMed] [Google Scholar]
- 28.Ardavin C, Shortman K. Cell surface marker analysis of mouse thymic dendritic cells. Eur. J. Immunol. 1992;22:859–862. doi: 10.1002/eji.1830220334. [DOI] [PubMed] [Google Scholar]
- 29.Kronin V, Vremec D, Shortman K. Does the IL-2 receptor alpha chain induced on dendritic cells have a biological function? Int. Immunol. 1998;10:237–240. doi: 10.1093/intimm/10.2.237. [DOI] [PubMed] [Google Scholar]
- 30.Fukao T, Koyasu S. Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur. J. Immunol. 2000;30:1453–1457. doi: 10.1002/(SICI)1521-4141(200005)30:5<1453::AID-IMMU1453>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor a-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 33.Taguchi O, Takahashi T. Administration of anti-interleukin-2 receptor a antibody in vivo induces localized autoimmune disease. Eur. J. Immunol. 1996;26:1608–1612. doi: 10.1002/eji.1830260730. [DOI] [PubMed] [Google Scholar]
- 34.McHugh RS, Shevach EM. Cutting Edge: Depletion of CD4+CD25+ Regulatory T Cells Is Necessary, But Not Sufficient, for Induction of Organ-Specific Autoimmune Disease. J. Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 35.Kohm AP, Williams JS, Miller SD. Cutting Edge: Ligation of the Glucocorticoid-Induced TNF Receptor Enhances Autoreactive CD4+ T Cell Activation and Experimental Autoimmune Encephalomyelitis. J. Immunol. 2004;172:4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- 36.Thurau SR, Chan CC, Nussenblatt RB, Caspi RR. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin. Exp. Immunol. 1997;109:370–376. doi: 10.1046/j.1365-2249.1997.4571356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp. Eye Res. 2006;82:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Cerottini JC, MacDonald HR. Limiting dilution analysis of alloantigen-reactive T lymphocytes. V. Lyt phenotype of cytolytic T lymphocyte precursors reactive against normal and mutant H-2 antigens. J. Immunol. 1981;126:490–496. [PubMed] [Google Scholar]
- 39.Kelso A, MacDonald HR, Smith KA, Cerottini JC, Brunner KT. Interleukin 2 enhancement of lymphokine secretion by T lymphocytes: analysis of established clones and primary limiting dilution microcultures. J. Immunol. 1984;132:2932–2938. [PubMed] [Google Scholar]
- 40.Romero P, Rod Dunbar P, Valmori D, Pittet MI, Ogg GS, Rimoldi D, Chen JL, Liénard D, Cerottini JC, Cerundolo V. Ex Vivo Staining of Metastatic Lymph Nodes by Class I Major Histocompatibility Complex Tetramers Reveals High Numbers of Antigen-experienced Tumor-specific Cytolytic T Lymphocytes. J. Exp. Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun D, Wilson DB, Cao L, Whitaker JN. The role of regulatory T cells in Lewis rats resistant to EAE. J. Neuroimmunol. 1997;78:69–78. doi: 10.1016/s0165-5728(97)00176-8. [DOI] [PubMed] [Google Scholar]
- 42.Sun D, Whitaker JN, Wilson DB. Regulatory T cells in experimental allergic encephalomyelitis. I. Frequency and specificity analysis in normal and immune rats of a T cell subset that inhibits disease. Int. Immunol. 1999;11:307–315. doi: 10.1093/intimm/11.3.307. [DOI] [PubMed] [Google Scholar]
- 43.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Exp. Med. 1998;188:287–296. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 44.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 45.Shevach EM. Regulatory T Cells in Autoimmmunity. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata, Shimizu M, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 47.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, Kuchroo VK. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA. 2004;101:15434–15439. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wing K, Lindgren S, Kollberg G, Lundgren A, Harris RA, Rudin A, Lundin S, Suri-Payer E. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells. Eur. J Immunol. 2003;33:579–587. doi: 10.1002/eji.200323701. [DOI] [PubMed] [Google Scholar]
- 49.Takeuchi M, Keino H, Kezuka T, Usui M, Taguchi O. Immune Responses to Retinal Self-Antigens in CD25+CD4+ Regulatory T-Cell-Depleted Mice. Invest. Ophthalmol. Vis. Sci. 2004;45:1879–1886. doi: 10.1167/iovs.02-1030. [DOI] [PubMed] [Google Scholar]
- 50.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, Miller SD. Cutting Edge: Anti-CD25 Monoclonal Antibody Injection Results in the Functional Inactivation, Not Depletion, of CD4+CD25+ T Regulatory Cells. J. Immunol. 2006;176:3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Xiao L, Shi BY, Qian YY, Bai HW, Chang JY, Cai M. Short-term anti-CD25 monoclonal antibody treatment and neogenetic CD4+CD25high regulatory T cells in kidney transplantation. Transplant Immunology. 2008;19:69–73. doi: 10.1016/j.trim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Nian H, Shao H, O'Brien BA, Born WK, Kaplan HJ, Sun D. Activated γδ cells promote the activation of uveitogenic T cells and exacerbate EAU development. Invest. Ophthalmol. Vis. Sci. 2011;52:5920–5927. doi: 10.1167/iovs.10-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nian H, Shao H, Zhang G, Born WK, O'Brien R, Kaplan HJ, Sun D. Regulatory effect of T cells on IL-17+ uveitogenic T cells. Invest. Ophthalmol. Vis. Sci. 2010;51:4661–4667. doi: 10.1167/iovs.09-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cui Y, Shao H, Lan C, Nian H, O'Brien RL, Born WK, Kaplan HJ, Sun D. Major Role of γδ T Cells in the Generation of IL-17+ Uveitogenic T Cells. J. Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]