Abstract
While exposure to social stress leads to increased depression-like and anxiety-like behavior, some individuals are more vulnerable than others to these stress-induced changes in behavior. Prior social experience is one factor that can modulate how individuals respond to stressful events. In this study we investigated whether experience-dependent resistance to the behavioral consequences of social defeat was associated with a specific pattern of neural activation. We paired weight-matched male Syrian hamsters in daily aggressive encounters for two weeks, during which they formed a stable dominance relationship. We also included controls that were exposed to an empty cage each day for two weeks. Twenty-four hours after the final pairing or empty cage exposure, half of the subjects were socially defeated in 3, 5-min encounters, while the others were not socially defeated. Twenty-four hours after social defeat, animals were tested for conditioned defeat in a 5-min social interaction test with a non-aggressive intruder. We collected brains following social defeat and processed tissue for c-Fos immunoreactivity. We found that dominants were more likely to counter-attack the resident aggressor during social defeat than were subordinates, and they showed less submissive and defensive behavior at conditioned defeat testing compared to subordinates. Also, social status was associated with distinct patterns of defeat-induced neural activation in select brain regions including the amygdala, prefrontal cortex, hypothalamus, and lateral septum. Our results indicate that social status is an important form of prior experience that predicts both initial coping style and the degree of resistance to social defeat. Further, the differences in defeat-induced neural activation suggest possible brain regions that may control resistance to conditioned defeat in dominant individuals.
Keywords: social defeat, dominance, stress, resilience, coping, conditioned defeat
Conditioned defeat is a naturalistic model of acute social stress in Syrian hamsters. Male hamsters that experience a brief social defeat show a loss of species-typical territorial aggression and an increase in submissive and defensive behavior when later faced with a smaller, nonaggressive intruder. The conditioned defeat model has been used to better understand the behavioral and neurobiological responses to social stress (Huhman, 2006). We recently found that social status can modulate the development of conditioned defeat. Specifically, hamsters that gain dominant social status show less submissive and defensive behavior during conditioned defeat testing compared to subordinates and controls (Morrison et al., 2011). In the current study, our aim was to identify potential brain regions that control resistance to conditioned defeat in dominant hamsters.
In humans, not every individual who experiences stress or trauma suffers deleterious effects (Agaibi and Wilson, 2005). Several factors are known to be important for resilience in humans, including cognitive flexibility (Yehuda et al., 2006), optimism (Charney, 2004), and perceived control over the stressor (Chorpita and Barlow, 1998). However, the neural substrates underlying stress resilience remain unknown. One approach to studying the neurobiology of stress resilience is to investigate the mechanisms by which past experience immunize individuals to the effects of future stressors. For example, prior experience with control over the termination of tail shock provides behavioral immunization to the negative consequences of future uncontrollable tail shocks (Maier and Watkins, 2010). Using this well-characterized model, researchers have shown that prior experience with controllable tail shock reduces the development of learned helplessness as indexed on a variety of measures, including fear conditioning (Amat et al., 2005), shuttle escape learning (Amat et al., 2005), and responses to social defeat (Amat et al., 2010). Further, the ventral medial prefrontal cortex is a key brain region mediating the immunizing effects of prior experience with controllable tail shock (Amat et al., 2006, Amat et al., 2008). Physical exercise also has been shown to disrupt the development of learned helplessness in rats. Individuals that have experienced six weeks of voluntary wheel running are protected from the negative effects of uncontrollable stress on shuttle box escape and freezing behavior (Greenwood et al., 2003). Researchers have demonstrated that voluntary wheel running results in plasticity in several brain regions within the central serotonin system, including the dorsal raphe nucleus, medial prefrontal cortex, locus coeruleus, striatum and amygdala, that converge to promote resistance to learned helplessness (Greenwood and Fleshner, 2011). Finally, housing mice in an enriched environment has been shown to buffer animals against the effects of later social defeat, and lesions of the medial prefrontal cortex block the buffering effect of enriched housing (Lehmann and Herkenham, 2011). In sum, these studies show that several types of prior experience can alter neural signaling in select brain regions to promote resilience to future stress.
Another approach for studying stress resilience and susceptibility is to investigate the mechanisms underlying individual differences in response to stressors. For example, mice can be categorized on their latency to attack intruders, with short-attack latency (SAL) mice being more aggressive and described as having an ‘active’ coping style, and long-attack latency (LAL) mice being less aggressive and described as employing a ‘passive’ coping style. Coping style, as defined in these mice, has been shown to predict susceptibility to the consequences of chronic psychological stress, such that SAL mice are less susceptible (Veenema et al., 2003). Likewise, outbred rats can be categorized as high responders (HR) and low responders (LR) on the basis of their locomotor activity in a mildly stressful novel environment (Piazza et al., 1989). Recently, HR rats have been shown to exhibit greater immobility and a reduced sucrose preference following chronic social defeat compared to LR rats (Calvo et al., 2011, Hollis et al., 2011). Similarly, rats selectively bred on the basis of baseline trait anxiety have been shown to utilize different coping styles when faced with social defeat stress and to exhibit different patterns of c-Fos immunoreactivity following social defeat, including differences in the medial prefrontal cortex (Frank et al., 2006). Altogether, research on individual differences has begun to identify neural mechanisms that control vulnerability to stress.
We used the protein product of the immediate early gene c-fos as a marker of defeat-induced neural activation (Kollack-Walker et al., 1997, Frank et al., 2006, Walker et al., 2009). We predicted that dominant hamsters would show a reduced conditioned defeat response compared to subordinates. Additionally, we hypothesized that reduced conditioned defeat would be accompanied by changes in defeat-induced neural activation in select brain regions. Specifically, we predicted that dominant individuals would show reduced c-Fos immunoreactivity in brain regions that control the development of conditioned defeat and the neuroendocrine stress response such as the amygdala and PVN respectively, and increased c-Fos immunoreactivity in brain regions that contribute to stress resilience such as the medial prefrontal cortex.
2. Experimental Procedures
2.1. Subjects
Subjects were male Syrian hamsters (Mesocricetus auratus) obtained from Charles River Laboratories (Wilmington, MA). Subjects were 3-4 months old (120-180 g) at the start of the study and were individually housed upon arrival. Older hamsters (> 6 months, >190 g) were individually housed and used as resident aggressors for social defeat training. Younger hamsters (approx. 2 months, <120 g) were housed in groups of four and used as non-aggressive intruders for conditioned defeat testing. All animals were housed in polycarbonate cages (12 cm × 27 cm × 16 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. Food and water were available ad libitum. Cages were not changed for one week prior to dominant-subordinate encounters to allow individuals to scent mark their territory. Subjects were handled daily for one week prior to dominant-subordinate encounters to habituate them to the stress of human handling. Animals were housed in a temperature controlled colony room (21 ± 2 °C) and kept on a 14:10 hr light:dark cycle to facilitate aggressive behavior. All behavioral protocols were performed during the first three hours of the dark phase of their cycle. All procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Behavioral Protocols
2.2.1. Dominant-Subordinate Encounters
To allow animals to establish social status, subjects within each cohort were weight-matched (average difference between resident and intruder was ± 2.4 grams) in resident-intruder dyads and paired in daily social encounters for 14 days. Subjects were randomly assigned as a resident or intruder, and all social encounters occurred in the resident’s home cage. The encounter on day 1 was 10 min in duration, while all subsequent encounters were 5 min. We have previously determined that a 10 min encounter on day 1 facilitates the formation of a dominance relationship, and that 5 min encounters on subsequent days maintain the dominance relationship and reduce the chance of wounding (Morrison et al., 2011). Dominant and subordinate animals were identified by the direction of agonistic behavior within each dyad. Daily encounters were digitally recorded for later behavioral analysis. We quantified agonistic behavior during daily encounters using the ethogram described below for conditioned defeat testing. We also included empty cage controls, whose treatment mirrored dominant-subordinate pairs although they were exposed to a clean, empty cage each day.
2.2.2. Social Defeat Training
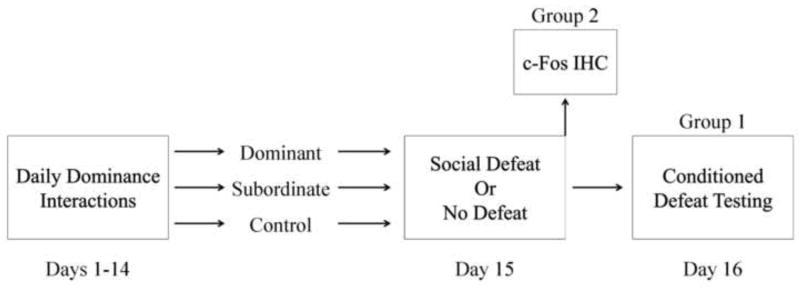
Following two weeks of daily social encounters, half of the subjects were exposed to social defeat training, while the others served as no defeat controls (Fig. 1). Social defeat consisted of subjects being placed in the home cages of three different resident aggressors for three separate 5-min encounters. Resident aggressors are older, heavier male hamsters that have been singly housed for a prolonged period of time and display reliable aggression when faced with intruders. Subjects received a 5-min rest period in their home cage between each aggressive encounter. Dominants and empty cage controls often fought back against the resident aggressor during the first defeat but eventually lost and did not fight back during subsequent defeats. To correct for potential variation in the amount of aggression subjects received, we defined social defeat as starting at the resident aggressor’s first attack that was accompanied by submissive behavior in a subject. No defeat control subjects were placed in the empty home cages of three different resident aggressors for three separate 5-min exposures. We placed no defeat controls in dirty resident aggressor cages to control for olfactory cues that may impact behavior and neural activation.
Figure 1.
A schematic representation of the experimental design. Group 1 animals were euthanized after social defeat training, whereas Group 2 animals experienced conditioned defeat testing.
Social defeats were digitally recorded for later behavioral analysis. We quantified the total aggression produced by the resident aggressors and the total submission displayed by subjects using the ethogram described below for conditioned defeat testing. Due to a technical problem with video transfer, the videos of social defeat sessions for nineteen individuals from Group 2 were lost (dominant, N = 6; subordinate, N = 7; empty cage control, N = 6). However, we maintain written notes about all defeats that were used to assess whether subjects counter attacked the resident aggressor and to ensure a sufficient defeat experience.
2.2.3. Conditioned Defeat Testing
In order to assess the effect of social defeat on subsequent behavior, animals in Group 1 were tested for conditioned defeat 24 hours after social defeat (Fig. 1). Testing consisted of a 5-min social interaction test during which a non-aggressive intruder was placed in the subject’s home cage. Non-aggressive intruders are younger, group-housed animals that display social and nonsocial behavior during conditioned defeat testing, and do not direct agonistic behavior toward the subject. We digitally recorded all conditioned defeat testing sessions and quantified the behavior of subjects using Noldus Observer software (Noldus Information Technology, Wageningen, Netherlands). We quantified the total duration of the following categories of behavior: submissive/defensive (flee, avoid, upright and side defensive postures, tail-up, stretch-attend, head flag); aggressive (chase, attack including bite, upright and side offensive postures); nonagonistic social (sniff, approach); and nonsocial (locomotion, grooming, nesting, feeding). We also recorded the frequency of attacks, flees, and stretch-attend postures. All behavioral scoring was performed by a researcher blind to the experimental conditions of the subject. On a subset of videos, inter-rater reliability on the duration of submissive/defensive behavior was greater than 90%.
2.3. c-Fos Immunohistochemistry
Ninety minutes following the start of social defeat, animals were anesthetized with a cocktail of 93% sodium pentobarbital and 7% isopropyl alcohol (Sleep Away, Webster Veterinary). Then, animals were transcardially perfused with 100ml of 0.1 M phosphate buffered saline (PBS) followed by 100ml of 4% paraformaldehyde solution. Brains were removed and soaked in 4% paraformaldehyde for 24 hours, followed by 0.1 M PBS/30% sucrose solution for 48 hours, and then were stored in cryoprotectant, all at 4°C. A consecutive series of 30 μm coronal sections were cut submerged in 0.1 M PBS on a vibrating microtome, collected into nine vials, and stored as free floating sections in cryoprotectant at 4°C. The collected sections were processed for c-Fos protein immunohistochemistry using the following protocol. Sections were washed five times in PBS + 0.2% Triton before each incubation, which were conducted at room temperature unless otherwise stated. Sections were incubated for 25 min in 0.3% hydrogen peroxide and methanol solution. Sections were then incubated with 0.5% goat serum (GS) in PBS + 0.2% Triton for 25 minutes before being incubated at room temperature for 24 hr in rabbit anti-c-Fos polyclonal primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a final dilution of 1:10,000 in PBS + 0.2% Triton. Sections were then washed five times with PBS + 0.2% Triton, incubated for 60 min in biotinylated secondary anti-rabbit IgG (Vector Laboratories, Burlingame, CA) at a final dilution of 1:200 with 0.5% GS and 0.2% Triton. Sections were then incubated in avidin-biotin-complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) with 0.2% Triton for 60 min, and the peroxidase reaction was visualized using a 15 min incubation in 3,3’-diaminobenzidine (DAB tablet, Sigma-Aldrich, St. Louis, MO) and nickel dissolved in PBS. The sections were washed five times with PBS and 5 times with distilled H2O prior to being mounted onto glass microscope slides. After air-drying, sections were dehydrated using a series of alcohols, cleared with citrisolv and coverslipped using DPX mountant (Sigma-Aldrich, St. Louis, MO). For each brain region, the tissue from all subjects was processed simultaneously.
Images were captured at 10X magnification using an Olympus BX41 microscope. The number of c-Fos immunopositive cells was determined in select brain regions using MCID Core image analysis software (InterFocus Imaging, Cambridge, England). We quantified the number of c-Fos immunopositive cells in the following brain regions: basolateral amygdala (BLA), dorsal medial amygdala (dMeA), ventral medial amygdala (vMeA), lateral amygdala (LA), paraventricular nucleus of the hypothalamus (PVN), lateral regions of the anterior hypothalamus (AH), lateral regions of the ventromedial hypothalamus (VMHL), ventral lateral septum (vLS), dorsal lateral septum (dLS), medial preoptic area (MPOA), bed nucleus of the stria terminalis (BNST), prelimbic cortex (PL), and infralimbic cortex (IL). C-Fos immunoreactivity was not observed in several brain regions of interest including the central amygdala, dorsal hippocampus, dentate gyrus and the nucleus accumbens. For each brain region, we recorded background immunoreactivity in unstained regions of each image. We then defined immunopositive cells as those that showed staining 1.5X darker than the specific background immunoreactivity calculated for each image. Cell counts were limited to the area within defined boxes that were tailored to the size of each brain region (Fig. 2a). A sample image of c-Fos immunoreactivity within a 800 × 660 μm box in the IL is shown in Figure 2b. For each brain region we quantified three sections per individual along a rostral-caudal axis.
Figure 2.
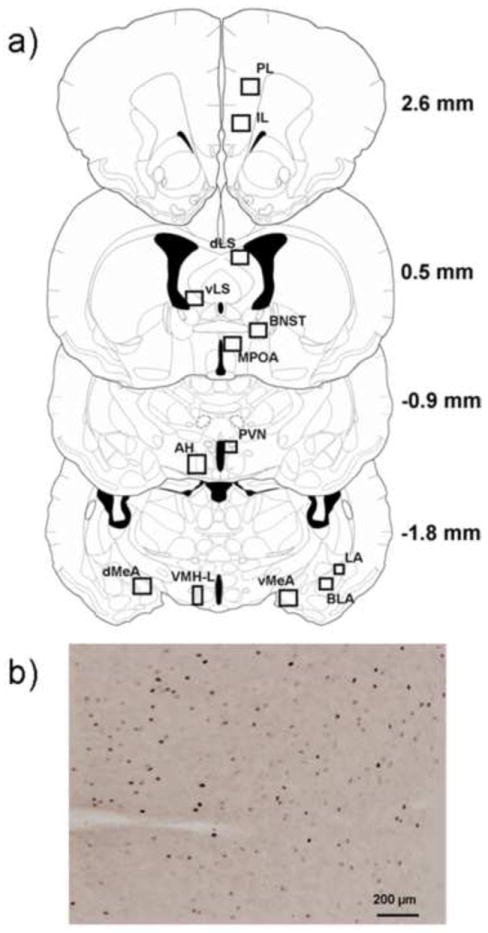
a) The diagrams indicate the location of brain regions selected for c-Fos quantification. The diagrams were modified from the hamster atlas of Morin & Wood and values indicate the distance from bregma (Morin and Wood, 2001). The box sizes used for quantification are as follows (width × height): 260 μm × 280 μm (LA); 440 μm × 400 μm (BLA); 325 μm × 650 μm (VMHL); 439 μm × 330 μm (PVN); 500 μm × 500 μm (dLS, vLS, BNST, MPOA); 870 μm × 660 μm (dMeA, vMeA, PL, IL, AH). b) Representative photomicrograph of the IL from a defeated hamster used for c-Fos quantification. Black dots represent c-Fos immunopositive nuclei.
2.4. Group 1: Conditioned Defeat Behavior
We tested the hypothesis that dominant individuals would show reduced submissive and defensive behavior during conditioned defeat testing compared to subordinates and empty cage controls. Following the daily social encounters or empty cage exposures, dominants, subordinates, and empty cage controls received social defeat (dominant, N = 13; subordinate, N = 13; empty cage control, N = 13) or no defeat control treatment (dominant, N = 13; subordinate, N = 13; empty cage control, N = 12). All subjects underwent conditioned defeat testing. Sample sizes were reduced slightly when eight individuals were removed from statistical analysis because of technical problems with social defeat training or conditioned defeat testing: dominant social defeat (N = 2); subordinate social defeat (N = 1); empty cage control social defeat (N = 5).
2.5. Group 2: Defeat-induced Neural Activation
We tested the hypothesis that dominant individuals would show differences in defeat-induced c-Fos immunoreactivity in select brain regions compared to subordinates and empty cage controls. Following the daily social encounters or empty cage exposures, dominants, subordinates, and empty cage controls received social defeat (dominant, N = 12; subordinate, N = 12; empty cage control, N = 11) or no defeat control treatment (dominant, N = 12; subordinate, N = 12; empty cage control, N = 11). Sample sizes were reduced slightly when two individuals were removed from statistical analysis because of technical problems with the daily dominance interactions: dominant social defeat (N = 1); subordinate no defeat control (N = 1).
2.5. Data Analysis
Data from social defeat training were analyzed with a 1-way analysis of variance (ANOVA) with Social Status as a between-subjects factor. The occurrence of counter attacking during social defeat training was analyzed using a Chi-square test. Conditioned defeat testing data were analyzed with a 2-way ANOVA with Social Status and Defeat Experience as between-subjects factors. For immunohistochemical data, we performed a 3-way repeated measures ANOVA with Social Status and Defeat Experience as between-subjects factors and Brain Region as a within-subjects factor. Following the 3-way ANOVA, we performed a series of planned comparisons using a 2-way ANOVA for each brain region. Significant main effects and interactions were followed by Tukey HSD post hoc tests. Results were considered significant when the α level was P ≤ 0.05.
3. Results
3.1. Dominant-Subordinate Encounters
On average, dominance relationships were decided on day 1.9 ± 0.2. In pairs that did not form a dominance relationship during the first encounter, both animals were often aggressive until one animal submitted during the second or third encounter (personal observation). In all cases, dominance relationships were stable once established (i.e. – dominance status never switched). The average duration of submissive/defensive behavior displayed by subordinates is shown in Fig. 3a. Subordinates displayed elevated and stable submissive/defensive behavior during the pairings compared to dominants. Consistent with the duration of submissive/defensive behavior, subordinates show a high and stable level of fleeing from the dominant (average number of flees per day: 3.0 ± 0.6). After dominance relationships were formed, subordinates never displayed aggressive behavior. Dominant individuals displayed a high and stable level of aggressive behavior during the daily encounters (Fig. 3b). Also, dominants consistently attacked subordinates (average number of attacks per day: 3.1 ± 0.5).
Figure 3.
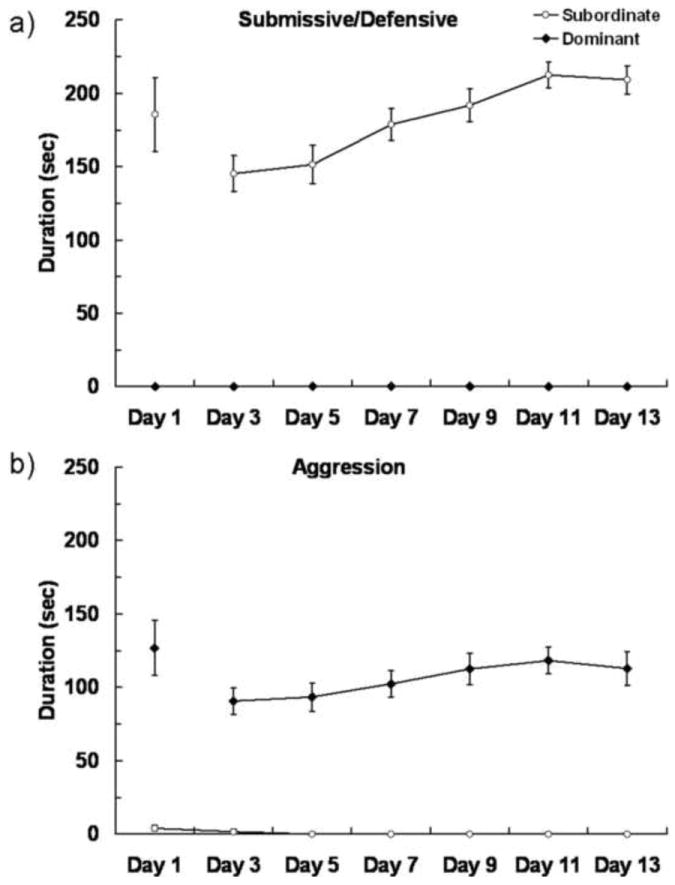
Agonistic behavior during daily encounters. Encounters were 10 min on day 1 and 5 min on days 2–14. a) Duration (mean ± SE) of submissive/defensive behavior displayed by subordinates from both Group 1 (N = 25) and Group 2 (N = 23) during fourteen days of encounters. Dominants never displayed submissive/defensive behavior. b) Duration (mean ± SE) of aggressive behavior displayed by dominants from both Group 1 (N = 24) and Group 2 (N = 23) throughout the encounters. Group 1 and Group 2 did not significantly differ in agonistic behavior and therefore the data were combined for presentation.
3.2. Social Defeat Stress
Social Status did not alter the total amount of aggression subjects received (F(2,45) = 1.325, P = 0.277) or the total amount of submissive behavior subjects produced (F(2,45) = 1.478, P = 0.239) during social defeat training (Table 1). However, Social Status influenced whether individuals counter attacked the resident aggressor during the first social defeat encounter. Dominant individuals almost always counter attacked the resident aggressor (21 out of 22 dominants), whereas subordinate individuals never counter attacked (0 out of 24 subordinates; χ2 (1, N = 46) = 42.1, P < 0.001). Also, empty cage controls displayed an intermediate level of counter aggression (9 out of 19 controls). Specifically, more empty cage controls counter attacked than did subordinates (χ2 (1, N = 43) = 14.4, P < 0.001), but fewer empty cage controls counter attacked than did dominants (χ2 (1, N = 41) = 12.0, P < 0.001).
Table 1.
Total durations (mean ± SEM) of behavior during social defeat training.
| Dominant (N = 16) | Subordinate (N = 17) | Empty Cage Control (N = 13) | P | |
|---|---|---|---|---|
| Aggression Received (sec) | 486 ± 27 | 433 ± 25 | 492 ± 17 | ns |
| Submission Produced (sec) | 741 ± 26 | 785 ± 20 | 721 ± 36 | ns |
3.3. Conditioned Defeat Testing
Subjects from Group 1 were tested for conditioned defeat behavior. Subjects with dominant social status prior to social defeat training showed reduced conditioned defeat behavior at testing (Fig. 4). We found a significant Defeat Experience × Social Status interaction on the duration of submissive/defensive behavior displayed at conditioned defeat testing (F(2,63) = 3.187, P = 0.048). Although defeated subjects showed more submissive/defensive behavior than non-defeated subjects, the effects of social defeat depended on social status. Among defeated subjects, dominant individuals showed less submissive/defensive behavior at conditioned defeat testing than did subordinates (P = 0.016). The pattern of behavior was slightly different for non-defeated subjects, such that subordinates displayed more submissive/defensive behavior at conditioned defeat testing compared to both dominants (P = 0.034) and empty cage controls (P = 0.039). We found a significant main effect of Defeat Experience on the frequency of stretch-attend postures, such that defeated individuals displayed more stretch-attend postures than did non-defeated individuals (F(1,63) = 19.027, P < 0.0001; defeated dominant: 1.27 ± 0.27, defeated subordinate: 0.92 ± 0.23, defeated empty cage control: 1.0 ± 0.38, non-defeated dominant: 0.08 ± 0.08, non-defeated subordinate: 0.69 ± 0.24, non-defeated empty cage control: 0.08 ± 0.08). There was no effect of Defeat Experience or Social Status on the frequency of flees (P > 0.05, data not shown).
Figure 4.
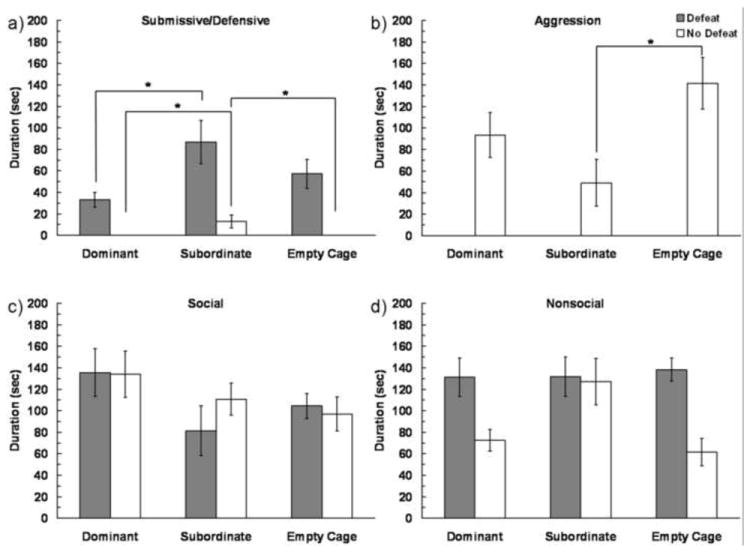
Durations (mean ± SE) of submissive/defensive, aggressive, social and nonsocial behaviors are shown during a 5 min test with a non-aggressive intruder. Subjects represented by gray bars received social defeat training 24 h prior to testing, while subjects represented by white bars were non-defeated controls. An asterisk (*) indicates a significant difference between the bracketed bars (P > 0.05).
We also found a significant Defeat Experience × Social Status interaction on the duration of aggressive behavior displayed at conditioned defeat testing (F(2,63) = 3.369, P = 0.041) (Fig. 4b). Defeated subjects did not show aggression during conditioned defeat testing, and in non-defeated subjects, subordinates displayed significantly less aggression at conditioned defeat testing than did empty cage controls (P = 0.016). Additionally, there was a main effect of Defeat Experience on the frequency of attacks, such that non-defeated subjects attacked the non-aggressive intruder more than did defeated subjects (F(1,63) = 23.88, P < 0.0001; defeated subjects: 0 ± 0, non-defeated subjects: 3.7 ± 1.1). There was a main effect of Defeat Experience on nonsocial behavior displayed at conditioned defeat testing (F(1,63) = 8.872, P = 0.004) (Fig. 4d). There were no significant differences in the duration of social behavior displayed at conditioned defeat testing (Fig. 4c).
3.4. c-Fos Immunohistochemistry
Prior to correcting for violations of sphericity, we found a significant 3-way interaction such that the interaction between Social Status and Defeat Experience depended on Brain Region (F(24,456) = 1.69, P = 0.023). However, because Mauchly’s test indicated that the assumption of sphericity had been violated (χ2 = 518.7, P < 0.001), the degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity. Following the Greenhouse-Geisser correction the 3-way interaction was no longer significant (F(7.494,142.380) = 1.69, P = 0.112). Nevertheless, we proceeded with a series of planned comparisons for each brain region.
Subjects that received social defeat showed an increased number of c-Fos immunopositive cells compared to no defeat controls in several brain regions including the PL, BNST, MPOA, AH, PVN, LA, BLA, and dMeA (P < 0.05 in all brain regions, Table 2). In each of these brain regions we did not find an effect of Social Status or a Defeat Experience × Social Status interaction.
Table 2.
Number of c-Fos immunopositive cells (mean ± SEM) following social defeat training. In each brain region, social defeat increased the number of c-Fos immunopositive cells compared to no defeat controls, although dominance status did not alter c-Fos immunoreactivity (asterisk indicates main effect of social defeat).
| Social Defeat* | No Defeat | |||||
|---|---|---|---|---|---|---|
| Brain Region | Dominant (N = 8-11) | Subordinate (N = 8-12) | Empty Cage (N = 8-11) | Dominant (N = 10-12) | Subordinate (N = 9-10) | Empty Cage (N = 8-11) |
| PL | 36.8 ± 5.1 | 28.2 ± 5.0 | 35.9 ± 6.0 | 15.0 ± 2.4 | 10.4 ± 2.1 | 11.5 ± 2.9 |
| BNST | 2.0 ± 0.6 | 2.1 ± 0.7 | 1.2 ± 0.4 | 0.8 ± 0.4 | 0.7 ± 0.4 | 0.9 ± 0.6 |
| MPOA | 18.1 ± 3.3 | 13.1 ± 3.3 | 12.4 ± 2.0 | 5.3 ± 1.2 | 4.0 ± 1.4 | 4.0 ± 1.2 |
| Hypothalamus | ||||||
| AH | 41.6 ± 8.4 | 34.9 ± 2.8 | 41.3 ± 6.7 | 7.0 ± 1.3 | 4.3 ± 1.5 | 6.3 ± 1.6 |
| PVN | 19.7 ± 3.5 | 16.9 ± 3.0 | 16.6 ± 3.6 | 3.7 ± 0.7 | 3.1 ± 0.6 | 2.3 ± 1.3 |
| Amygdala | ||||||
| LA | 2.2 ± 0.6 | 1.7 ± 0.3 | 1.7 ± 0.5 | 0.6 ± 0.2 | 0.2 ± 0.2 | 0.4 ± 0.2 |
| BLA | 3.7 ± 0.8 | 2.1 ± 0.4 | 2.8 ± 0.4 | 1.4 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| dMeA | 49.1 ± 5.1 | 33.2 ± 4.3 | 47.0 ± 5.1 | 8.2 ± 1.6 | 9.3 ± 3.8 | 12.1 ± 3.8 |
Note: Sample sizes vary because some individuals were excluded from data analysis due to poor tissue quality. AH – anterior hypothalamus, BLA – basolateral amygdala, BNST – bed nucleus of the stria terminalis, dMeA – dorsal medial amygdala, LA – lateral amygdala, MPOA – medial preoptic area, PL – prelimbic cortex, PVN – paraventricular nucleus of the hypothalamus
In the dLS we found an effect of Social Status on the number of c-Fos immunopositive cells (F(2,56) = 67.24, P = 0.037), but no effect of Defeat Experience or a Defeat Experience × Social Status interaction (Fig. 5). Specifically, subordinate individuals had more c-Fos immunopositive cells in the dLS than did empty cage controls (P = 0.025).
Figure 5.
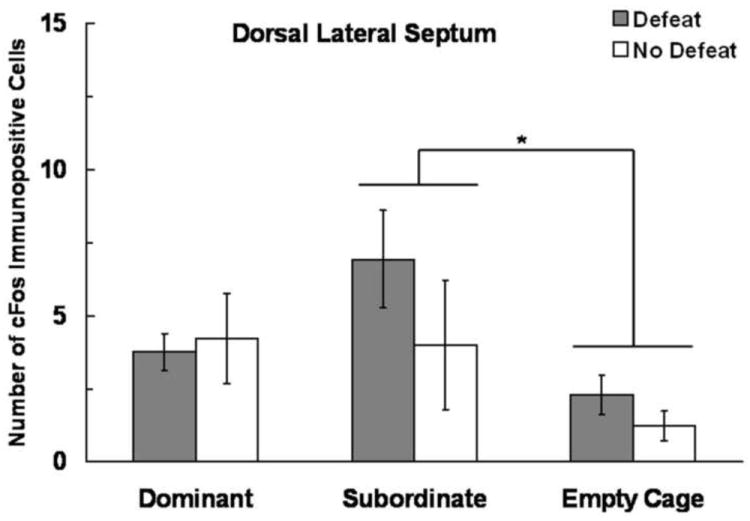
Number (mean ± SE) of c-Fos immunopositive cells in the dLS measured following social defeat training. Subjects represented by gray bars received social defeat training 24 h prior to testing, while subjects represented by white bars were non-defeated controls. We found a main effect of social status, and an asterisk (*) indicates a significant difference between bracketed bars (P > 0.05).
In the IL and vLS we found main effects of both Defeat Experience and Social Status on the number of c-Fos immunopositive cells, but no interaction (Fig. 6). Defeated individuals displayed more c-Fos immunopositive cells compared to non-defeated individuals in both the IL (F(1,60) = 39.64, P < 0.0001) and the vLS (F(1,56) = 32.21, P < 0.0001). Additionally, Social Status altered the number of c-Fos immunopositive cells in both the IL (F(2,60) = 5.107, P = 0.009) and in the vLS (F(2,56) = 3.621, P = 0.033). In the IL, dominants had significantly more c-Fos immunopositive cells than did subordinates (P = 0.018). In the vLS, dominant individuals had a nearly significant increase in the number of c-Fos immunopositive cells compared to empty cage controls (P = 0.053).
Figure 6.
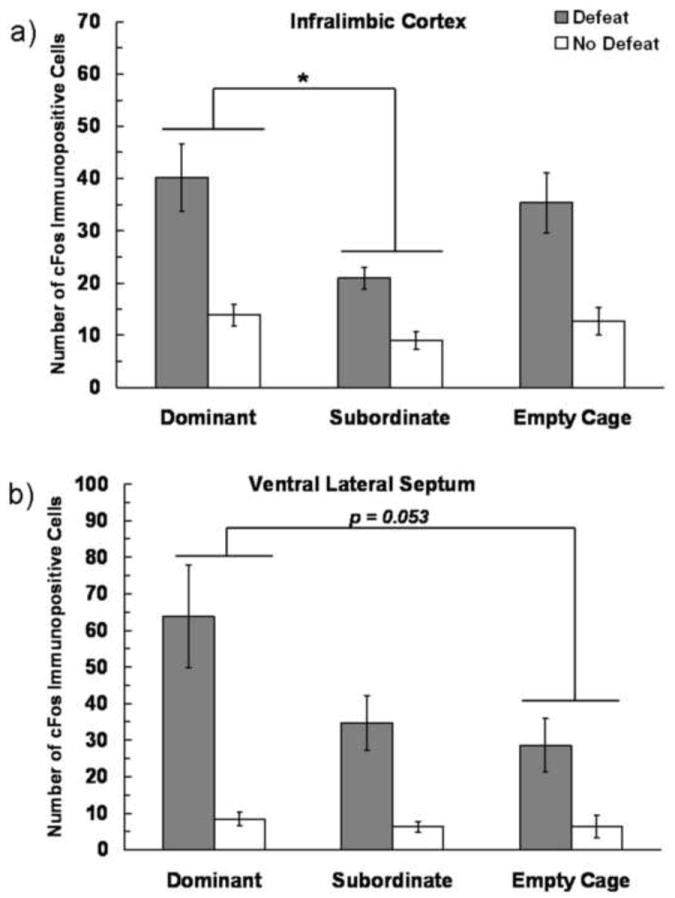
Number (mean ± SE) of c-Fos immunopositive cells measured in the a) IL and b) vLS measured following social defeat training. Subjects represented by gray bars received social defeat training, while subjects represented by white bars were non-defeated controls. We found main effects of both defeat experience and social status, and an asterisk (*) indicates a significant difference between bracketed bars (P > 0.05).
In the VMHL and vMeA we found main effects of both Defeat Experience and Social Status on the number of c-Fos immunopositive cells, and a significant interaction (Fig. 7). The effect of Defeat Experience on the number of c-Fos immunopositive cells depended on Social Status in the VMHL (F(2,53) = 8.039, P = 0.001) and in the vMeA (F(2,52) = 3.768, P = 0.03). In the VMHL, defeated subordinates showed significantly fewer c-Fos immunopositive cells than did both defeated dominants (P = 0.006) and defeated empty cage controls (P = 0.012). In contrast, non-defeated subordinate individuals had significantly more c-Fos immunopositive cells in the VHML than did non-defeated dominant individuals (P = 0.031). In the vMeA, defeated subordinates had fewer c-Fos immunopositive cells than did both defeated dominants (P = 0.024) and defeated empty cage controls (P = 0.049).
Figure 7.
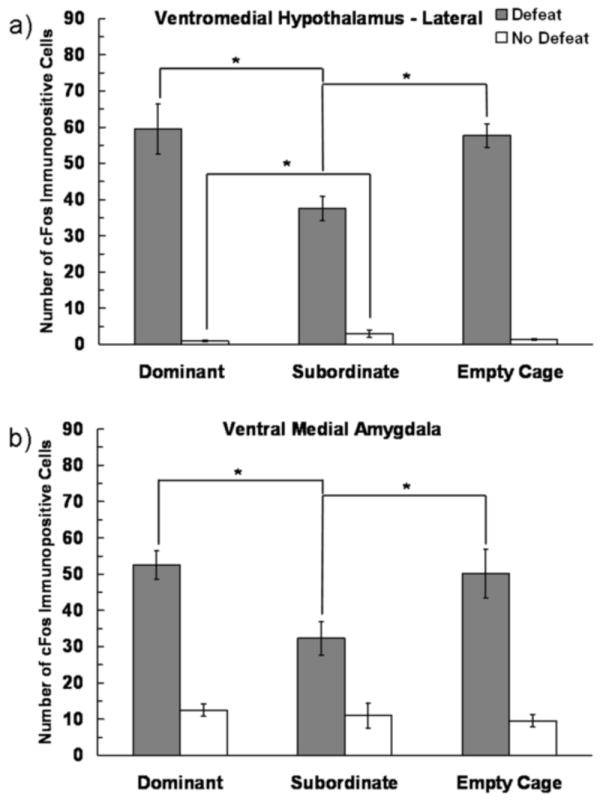
Number (mean ± SE) of c-Fos immunopositive cells in the VHML and vMeA measured following social defeat training. Subjects represented by gray bars received social defeat training, while subjects represented by white bars were non-defeated controls. We found a significant interaction between defeat experience and social status. An asterisk (*) indicates a significant difference between bracketed bars (P > 0.05).
4. Discussion
4.1. Behavioral responses of dominant and subordinate animals
We found that social status alters how non-defeated male Syrian hamsters respond to a novel conspecific. Subordinate individuals were less likely to display normal territorial aggression and instead displayed more submissive/defensive behavior compared to dominants and empty cage controls. These data are consistent with a large literature indicating that subordinates display increased stress-related behavior when they continue to receive aggression from familiar animals in their social group (Blanchard et al., 1995, Kramer et al., 1999). However, species differ in the extent to which social status alters agonistic behavior in future encounters with novel opponents. For example, we found that subordinate hamsters respond to novel opponents with less aggression than do dominants. Mice show a similar pattern of agonistic behavior such that subordinates are less aggressive against novel opponents than are dominants (Kloke et al., 2011). In contrast, opponent recognition is particularly salient in other species such as green anoles because both dominant and subordinate lizards respond to unfamiliar intruders with aggressive signals (Forster et al., 2005, Ling et al., 2010).
Social defeat potentiates the difference in future agonistic behavior between dominants and subordinates such that it leads to reduced conditioned defeat in dominants. Specifically, we found that dominants showed lower levels of submissive and defensive behavior during conditioned defeat testing than did subordinates, while empty cage controls were intermediate. At present it is unclear whether dominants are resistant to the effects of social defeat or whether subordinates are susceptible or both. However, we believe that dominants show at least some resistance to conditioned defeat for two reasons. First, the duration of submissive and defensive behavior observed in subordinates in the current study is similar to the durations observed in vehicle-treated animals from our previous conditioned defeat studies (Cooper et al., 2005, Cooper et al., 2008, Cooper and Huhman, 2010, Morrison and Cooper, 2012). Second, in our previous study examining the effects of social status, subordinates and empty cage controls show similar levels of submissive and defensive behavior at conditioned defeat testing, while dominants were significantly lower than both (Morrison et al., 2011). Altogether, our results indicate that social status modulates behavioral responses to stressful events in Syrian hamsters.
We found that when dominant individuals were attacked during social defeat, they initially responded by counter attacking their opponent. We observed a similar response to social defeat among dominant individuals in a previous study (Morrison et al., 2011). Others have shown that offensive aggression is associated with a proactive coping style as indicated by measures such as increased shock probe burying (Koolhaas et al., 2010), increased active avoidance in a shuttle box (Benus et al., 1989), and increased struggling during a forced swim test (Veenema et al., 2005). Also, when rats display a proactive coping style during social defeat, the effects of social defeat are reduced (Walker et al., 2009). In our study, several empty cage controls counter-attacked the resident aggressor during social defeat, indicating that both dominants and empty cage controls exhibited a proactive coping style. In contrast, all subordinate animals initially responded to resident aggressors with submissive and defensive behavior. Although our subjects differed in their initial reaction to the resident aggressor, they did not differ in the total amount of aggression received. We and others have argued that the amount of aggression received during social defeat does not correlate with submissive and defensive behavior produced at conditioned defeat testing (Jasnow and Huhman, 2001, Solomon et al., 2009, Morrison and Cooper, 2012).
Because several empty cage controls initially displayed a dominant-like proactive coping style during social defeat, it is perhaps not surprising that they also showed partial resistance to conditioned defeat. Empty cage control animals were socially isolated for over three weeks prior to social defeat to replicate the housing conditions of dominants and subordinates. Several previous studies suggest that extended periods of social isolation alter hamster agonistic behavior. Solomon and colleagues found that 4 weeks of social isolation led to a decrease in submissive behavior during conditioned defeat testing (Solomon et al., 2009). Others have also found that prolonged periods of social isolation in both male and female Syrian hamsters increase aggressive behavior when confronted with a novel opponent (Brain, 1972, Wise, 1974). Thus, empty cage controls may gain partial resistance to conditioned defeat due to prolonged social isolation, and the behavioral and immunohistochemical results must be interpreted with this caveat in mind.
4.2. Defeat-induced neural activation
Our data suggest that there is a distinct set of brain regions that responds to social defeat with increased activation. Neural activation following aggressive encounters has been examined previously using the detection of immediate early gene products. For example, in hamsters losing an aggressive encounter increases c-fos mRNA in a wide range of brain regions compared to winning (Kollack-Walker et al., 1997). Similarly, a single social defeat in Lister rats increases c-Fos protein expression in select basal forebrain and brainstem nuclei compared to non-defeated controls (Martinez et al., 1998). In the current study, we extended previous work to examine the effects of social status on defeat-induced neural activation. In some brain regions, such as subregions of the amygdala and hypothalamus, social defeat resulted in a similar increase in neural activation among all groups. Thus, the reduced conditioned defeat observed in dominants is not associated with differences in defeat-induced neural activation in these brain regions. In contrast, there were several brain regions in which social status altered defeat-induced neural activation. With the exception of the dLS, dominant individuals showed a greater increase in c-Fos immunoreactivity following social defeat than did subordinates. These findings are consistent with some recent studies on neural activation and coping styles in rats. For example, rats bred for low trait anxiety display an active coping style during social defeat and increased c-Fos expression in several regions of the frontal cortex and decreased c-Fos expression in several subregions of the amygdala compared to rats bred for high trait anxiety (Frank et al., 2006). On the other hand, rats that responded to social defeat with an active coping style have also been shown to exhibit less defeat-induced c-Fos expression in the medial prefrontal cortex, central amygdala, and medial amygdala compared to rats with a passive coping style (Walker et al., 2009). Some of the inconsistencies in the literature may be related to how active and passive coping styles are defined. In some cases an active coping style is indicted by high levels of defensive behavior and counter aggression during social defeat whereas in other areas it is characterized by high levels of rearing and grooming and low levels of freezing during social defeat.
The differences we observed in defeat-induced c-Fos expression provide insight into variation in the development of conditioned defeat. For example, we found that dominants have significantly more c-Fos immunoreactivity in the IL compared to subordinates. The IL is a subregion of the ventromedial prefrontal cortex (vmPFC), which controls executive functions such as working memory, decision making, and inhibitory response control (Dalley et al., 2004). Previous work in a learned helplessness model shows that experience with behavioral control activates the vmPFC, and that this experience of control and subsequent activation of the vmPFC confers resilience to later uncontrollable stress. Pharmacological studies show that inactivation of the vmPFC with muscimol impairs the resilience conferred by prior experience with control (Amat et al., 2006) and activation of the vmPFC with picrotoxin during exposure to uncontrollable stress results in that uncontrollable stress producing resilience (Amat et al., 2008). The IL has also been shown to be important in mediating the protective effect of enriched housing on the negative consequences of social defeat. Housing mice in an enriched environment results in increased FosB/ΔFosB immunostaining in the IL following social defeat, and lesioning the IL prior to environmental enrichment eliminates the resilience to social defeat (Lehmann and Herkenham, 2011). Together, this study suggests that activation of the IL during both the environmental enrichment and subsequent social defeat is necessary for resilience to social defeat. It is possible that a similar mechanism may be at work in conditioned defeat, such that activation of the IL during social defeat is necessary for conditioned defeat resistance.
The vMeA is another brain region that may contribute to resistance to conditioned defeat. A large body of literature indicates that the MeA detects biologically relevant odors and modulates several types of behavior, including sexual behavior (Lehman et al., 1980), fearful behavior (Li et al., 2004), and defensive behavior (Blanchard et al., 2005). Social defeat in rats has been shown to increase c-Fos immunoreactivity in MeA cells that contain corticotropin-releasing factor type-2 receptor mRNA (Fekete et al., 2009). Within the conditioned defeat paradigm, researchers have shown that injection of muscimol into the MeA reduces the acquisition of conditioned defeat, although injection of a protein synthesis inhibitor into the MeA does not (Markham and Huhman, 2008). These data suggest that neural transmission in the MeA is necessary for the development of conditioned defeat behavior. However, in the current study we found that subordinates, who have robust conditioned defeat behavior, had the lowest level of defeat-induced c-Fos expression in the vMeA. Because we do not know the phenotype of c-Fos positive cells it is hard to integrate these seemingly disparate findings, although one possibility is that dominants have increased c-Fos expression in GABAergic interneurons.
We also found that social status alters defeat-induced c-Fos immunoreactivity in the dLS and vLS. A recent study suggests that neural transmission in the LS is associated with the expression of increased submissive and defensive behavior and decreased aggressive behavior at conditioned defeat testing, although protein synthesis-dependent plasticity in the LS plays little role in the development of conditioned defeat (McDonald et al. 2012). These results suggest that the pattern of c-Fos expression observed in our study is likely related to how individuals respond to social defeat training and not the development of conditioned defeat. We found that subordinates displayed increased c-Fos expression in the dLS compared to controls, which might represent an anxiogenic neural response in subordinates. This interpretation would be consistent with previous lesion or pharmacological inactivation studies which indicate an anxiogenic role for the LS in elevated plus maze and defensive burying tests (Pesold and Treit, 1992, Trent and Menard, 2010). We also found that dominants displayed increased c-Fos expression in the vLS compared to controls, which might be related to the initial counter aggression they exhibited during social defeat training. Several lines of research indicate that neural transmission in the LS is associated with decreased aggression in Syrian hamsters (Potegal et al., 1981a, b, McDonald et al., 2012). Thus, the elevated c-Fos expression in the vLS of dominants might reflect initial counter aggression or the rapid suppression of this proactive coping style.
The VMHL is a key brain region controlling hamster aggressive behavior. Arginine vasopressin injected into the VMHL acts at V1a receptors to facilitate offensive aggression (Delville et al., 1996), and animals that attack intruders have increased c-Fos expression in the VMHL (Delville et al., 2000). We have previously shown that dominant hamsters have increased V1a receptor binding in the VHML (Cooper et al., 2005). It is possible that the elevated c-Fos expression in the VMHL of dominants and empty cage controls is related to an up-regulation of V1a receptors. Also, defeat-induced c-Fos expression in the VMHL may be related to the counter attacking initially shown by dominants and empty cage controls during social defeat. In sum, our data suggest that defeat-induced neural activation in brain regions critical for aggressive behavior is associated with conditioned defeat resistance.
4.4. Conclusion
We have demonstrated that social status is an important factor determining how individuals initially respond to social defeat and their degree of resistance to the development of conditioned defeat. Dominant individuals show a proactive coping style during social defeat and reduced conditioned defeat behavior at testing. These behavioral changes are associated with a distinct pattern of defeat-induced neural activation in brain regions such as the IL. This paradigm provides a valuable model for studying the neural basis of resistance to social stress.
Highlights.
Dominant individuals show reduced conditioned defeat compared to subordinates.
Social status leads to modest differences in baseline neural activation.
Social status produces distinct patterns of defeat-induced neural activation.
Defeat-induced neural activation in the prefrontal cortex is linked to resilience.
Acknowledgments
We thank our team of undergraduates, most notably Travis Goode and Cody Swallows. This work was supported by NIH grant R21 MH085230 and a University of Tennessee Professional Development Award.
Abbreviations
- AH
anterior hypothalamus
- ANOVA
analysis of variance
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- dLS
dorsal lateral septum
- dMeA
dorsal medial amygdala
- GS
goat serum
- IL
infralimbic cortex
- LA
lateral amygdala
- MPOA
medial preoptic area
- PBS
phosphate buffered saline
- PL
prelimbic cortex
- PVN
paraventricular nucleus of the hypothalamus
- vLS
ventral lateral septum
- vMeA
ventral medial amygdala
- VMHL
lateral ventromedial hypothalamus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agaibi CE, Wilson JP. Trauma, PTSD, and resilience: a review of the literature. Trauma Violence Abuse. 2005;6:195–216. doi: 10.1177/1524838005277438. [DOI] [PubMed] [Google Scholar]
- Amat J, Aleksejev RM, Paul E, Watkins LR, Maier SF. Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience. 2010;165:1031. doi: 10.1016/j.neuroscience.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Watkins LR, Maier SF. Activation of the ventral medial prefrontal cortex during an uncontrollable stressor reproduces both the immediate and long-term protective effects of behavioral control. Neuroscience. 2008;154:1178–1186. doi: 10.1016/j.neuroscience.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benus RF, Bohus B, Koolhaas JM, van Oortmerssen GA. Behavioural strategies of aggressive and non-aggressive male mice in active shock avoidance. Behav Processes. 1989;20:1–12. doi: 10.1016/0376-6357(89)90008-9. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CA, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsibity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen BS, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Brain PF. Effects of isolatin/grouping on endocrine function and fighting behavior in male and female golden hamsters (Mesocricetus auratus waterhouse) Behav Biol. 1972;7:349–357. doi: 10.1016/s0091-6773(72)80106-8. [DOI] [PubMed] [Google Scholar]
- Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 2011;183:81–89. doi: 10.1016/j.neuroscience.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: the role of control in the early environment. Psychol Bull. 1998;124:3–12. doi: 10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Blocking corticotropin-release factor-2 receptors, but not corticotropin-releasing factor-1 receptors of glucocorticoid feedback, disrupts the development of conditioned defeat. Physiol Behav. 2010;101:527–532. doi: 10.1016/j.physbeh.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Karom M, Huhman KL, Albers HE. Repeated agonistic encounters in hamsters modulate AVP V1a receptor binding. Horm Behav. 2005;48:545–551. doi: 10.1016/j.yhbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Delville Y, Mansour K, Ferris CF. Serotonin blocks vasopressin-facilitated offensive aggression: interactions within the ventrolateral hypothalamus of golden hamsters. Physiol Behav. 1996;59:813–816. doi: 10.1016/0031-9384(95)02166-3. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Watt MJ, Korzan WJ, Renner KJ, Summers CH. Opponent recognition between male green anoles (Anolis carolinensis) Anim Behav. 2005;69:733–740. [Google Scholar]
- Frank E, Salchner P, Aldag JM, Salome N, Singewald N, Landgraf R, Wigger A. Genetic predisposition to anxiety-related beahvior determines coping style, neuroendocrine responses, and neuronal activation during social defeat. Behav Neurosci. 2006;120:60–71. doi: 10.1037/0735-7044.120.1.60. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, stress resistance, and central serotonergic systems. Exerc Sport Sci Rev. 2011;39:140–149. doi: 10.1097/JES.0b013e31821f7e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis F, Duclot F, Gunjan A, Kabbaj M. Individual differences in the effect of social defeat on anhedonia and histone acetylation in the rat hippocampus. Horm Behav. 2011;59:331–337. doi: 10.1016/j.yhbeh.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: Can they inform us about human psychopathology? Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Kloke V, Jansen F, Heiming RS, Palme R, Lesch KP, Sachser N. The winner and loser effect, serotonin transporter genotype, and the display of offensive aggression. Physiol Behav. 2011;103:565–574. doi: 10.1016/j.physbeh.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front Neuroendocrinol. 2010;31:307–321. doi: 10.1016/j.yfrne.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kramer M, Hiemke C, Fuchs E. Chronic psychosocial stress and antidepressant treatment in tree shrews: time-dependent behavioral and endocrine effects. Neurosci Biobehav Rev. 1999;23:937–947. doi: 10.1016/s0149-7634(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Herkenham M. Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. J Neurosci. 2011;31:6159–6173. doi: 10.1523/JNEUROSCI.0577-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Ling TJ, Summers CH, Renner KJ, Watt MJ. Opponent recognition and social status differentiate rapid neuroendocrine responses to social challenge. Physiol Behav. 2010;99:571–578. doi: 10.1016/j.physbeh.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- McDonald MM, Markham CM, Norvelle A, Albers HE, Huhman KL. GABA(A) receptor activation in the lateral septum reduces the expression of conditioned defeat and increases aggression in Syrian hamsters. Brain Res. 2012;1439:27–33. doi: 10.1016/j.brainres.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. San Diego: Academic Press; 2001. [Google Scholar]
- Morrison KE, Cooper MA. A role for 5-HT1A receptors in the basolateral amygdala in the development of conditioned defeat in Syrian hamsters. Pharmacol Biochem Behav. 2012;100:592–600. doi: 10.1016/j.pbb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Swallows CL, Cooper MA. Effects of dominance status on conditioned defeat and expression of 5-HT1A and 5-HT2A receptors. Physiol Behav. 2011;104:283–290. doi: 10.1016/j.physbeh.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Treit D. Excitotoxic lesions of the septum produce anxiolytic effects in the elevated plus-maze and the shock-probe burying tests. Physiol Behav. 1992;52:37–47. doi: 10.1016/0031-9384(92)90431-z. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Potegal M, Blau A, Glusman M. Effects of anteroventral septal lesions on intraspecific aggression in male hamsters. Physiol Behav. 1981a;26:407–412. doi: 10.1016/0031-9384(81)90167-0. [DOI] [PubMed] [Google Scholar]
- Potegal M, Blau A, Glusman M. Inhibition of intraspecific aggression in male hamsters by septal stimulation. Physiological Psychology. 1981b;9:213–218. [Google Scholar]
- Solomon MB, Karom M, Norvelle A, Markham CA, Erwin WD, Huhman KL. Gonadal hormones modulate the display of conditioned defeat in male Syrian hamsters. Horm Behav. 2009;56:423–428. doi: 10.1016/j.yhbeh.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent NL, Menard JL. The ventral hippocampus and the lateral septum work in tandem to regulate rats’ open-arm exploration in the elevated plus-maze. Physiol Behav. 2010;101:141–152. doi: 10.1016/j.physbeh.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Cremers TI, Jongsma ME, Steenbergen PJ, de Boer SF, Koolhaas JM. Differences in the effects of 5-HT1A receptor agonists on forced swimming behavior and brain 5-HT metabolism between low and high aggressive mice. Psychopharmacology (Berl) 2005;178:151–160. doi: 10.1007/s00213-004-2005-5. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM. Genetic selection for coping style predicts stressor susceptibility. J Neuroendocrinol. 2003;15:256–267. doi: 10.1046/j.1365-2826.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- Walker FR, Masters LM, Dielenberg RA, Day TA. Coping with defeat: acute glucocorticoid and forebrain responses to social defeat vary with defeat episode behaviour. Neuroscience. 2009;162:244–253. doi: 10.1016/j.neuroscience.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Wise DA. Aggression in the female golden hamster: effects of reproductive state and social isolation. Horm Behav. 1974;5:235–250. doi: 10.1016/0018-506x(74)90032-4. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann N Y Acad Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]