Abstract
An important goal of neurotoxicological research is to provide relevant and accurate risk assessment of environmental and pharmacological agents for populations and individuals. Owing to the challenges of human subject research and the real possibility of species specific toxicological responses, neuronal lineages derived from human embryonic stem cells (hESCs) and human neuronal precursors have been offered as a potential solution for validation of neurotoxicological data from model organism systems in humans. More recently, with the advent of induced pluripotent stem cell (iPSC) technology, there is now the possibility of personalized toxicological risk assessment, the ability to predict individual susceptibility to specific environmental agents, by this approach. This critical advance is widely expected to facilitate analysis of cellular physiological pathways in the context of human neurons and the underlying genetic factors that lead to disease. Thus this technology opens the opportunity, for the first time, to characterize the physiological, toxicological, pharmacological and molecular properties of living human neurons with identical genetic determinants as human patients. Furthermore, armed with a complete clinical history of the patients, human iPSC (hiPSC) studies can theoretically compare patients and at risk groups with distinct sensitivities to particular environmental agents, divergent clinical outcomes, differing co-morbidities, and so forth. Thus iPSCs and neuronal lineages derived from them may reflect the unique genetic blueprint of the individuals from which they are generated. Indeed, iPSC technology has the potential to revolutionize scientific approaches to human health. However, before this overarching goal can be reached a number of technical and theoretical challenges must be overcome. This review seeks to provide a realistic assessment of hiPSC technology and its application to risk assessment and mechanistic studies in the area of neurotoxicology. We seek to identify, prioritize, and detail the primary hurdles that need to be overcome if personalized toxicological risk assessment using patient-derived iPSCs is to succeed.
Keywords: Neurotoxicology, Developmental Toxicology, Human stem cells, induced pluripotent stem cells, environment
1. Introduction
The field of toxicology has seen rapid innovation in the past two decades by the advent of stem cell technology. Perhaps the first major successful use of stem cells for the study of toxicity was the Embryonic Stem Cell Test (EST) developed by Speilmann and colleagues (Heuer et al., 1993; Spielmann et al., 1997). This approach differentiates mouse embryonic stem cells (ESCs) into cardiomyocytes in the presence of potential developmentally toxic agents (Heuer et al., 1993; Seiler and Spielmann, 2011). Although this method utilizes mouse stem cells, and focuses on differentiation into beating cardiomyocytes, the method has been broadly hailed for its ingenuity (Laustriat et al., 2010; Scholz et al., 1999; Wobus and Loser, 2011). However, the method has notable shortcomings in its application to neurotoxicology. For example, although the EST correctly classified the majority of known embryotoxic chemicals tested, it is known that the EST in some cases failed to correctly classify methylmercury as a developmental toxicant (Genschow et al., 2004). There are several potential reasons for these shortcomings of the EST – including species-specific toxicities and tissue-type specific toxicities. Recently, Bremer et al. sought to address both of these issues by adapting the principles of the EST to toxicity testing in human ESCs (hESCs) undergoing neuronal differentiation (Stummann et al., 2009). Their study showed greater sensitivity of early-developing neural precursors over maturing neuronal cells to methylmercury toxicity (i.e. greater changes in expression of key early neurodevelopmental markers versus more mature neuronal markers) (Stummann et al., 2009). Other groups have also provided proof-of-principle experiments demonstrating the potential of hESCs to evaluate developmental toxicity (Pal et al., 2011). However, ethical and regulatory concerns about the use of cells derived from human embryos have limited adoption of hESC based toxicity testing (Leist et al., 2008; Vojnits and Bremer, 2010).
Pioneering studies have revealed both the feasibility as well as clear advantages for use of stem cell based approaches for neurotoxicological risk assessment. Although the fundamentals of stem cell culture are outside the scope of this review, a number of book chapters and review articles are available on this topic (Neely et al., 2011; Park et al., 2008; Takahashi et al., 2007). Studies using murine stem cells have identified mRNA based expression markers for assessment of neurodevelopmental toxicity (Kuegler et al., 2010; Theunissen et al., 2011). Comparative studies using hESC derived neurons versus rodent primary neuronal cultures have revealed important differences in sensitivity, reproducibility, and dynamic ranges by toxicity measures examining neurite outgrowth and cytotoxicity; suggesting further work is needed in developing and interpreting hESC-derived neurotoxicity tests (Harrill et al., 2011). Indeed, toxicogenomic approaches revealed key differences on the influence of a developmental neurotoxicant on expression profiles between in vivo models, stem-cell based in vitro models and primary tissue/cell culture based models – yet also identified examples of coherent responses from the in vitro ESC-based models and in vivo measures (Robinson et al., 2011). Furthermore, predictive neurotoxicity testing by hESC-based neuronal differentiation approaches has proven successful in discriminating chemicals and pharmaceuticals with known developmental neurotoxicity (Buzanska et al., 2009). A related approach to hESC-based neurotoxicology has been to start developmentally down-stream of the pluripotent state and utilize multipotent human neuroprogenitors as a starting point for developmental neurotoxicity testing (Breier et al., 2008; Harrill et al., 2010; Harrill et al., 2011; Moors et al., 2009; Schreiber et al., 2010; Tofighi et al., 2011a; Tofighi et al., 2011b). Neuralization of pluripotent stem cells or neuroprogenitors can be accomplished either by adherent culture-based neuronal differentiation or a neurosphere suspension culture, which may be followed by subsequent plating, differentiation and migration. A discussion of the advantages and disadvantages of these two approaches has been recently reviewed by Breier and colleagues (Breier et al., 2010).
In this review, we seek to describe the methods of generating hiPSCs, explore the utility of this technology in the field of neurotoxicology, and discuss technical challenges for these applications. In addition, we will outline the process of generating and maintaining hiPSCs for toxicity testing, characterize multiple exposure paradigms, and attempt to predict the future of the field.
2. The promise of iPSC technology for neurotoxicology
A number of recent reviews have described potential applications of hESC and hiPSC technology to toxicology, pharmacology and the study of human diseases that have environmental contributions to their etiology (Anson et al., 2011; Heng et al., 2009; Marchetto et al., 2011; Saha and Jaenisch, 2009; Vojnits and Bremer, 2010; Winkler et al., 2009; Wobus and Loser, 2011). Here we focus on the promise and roadblocks specifically for neurotoxicological applications. An important advantage of a patient-specific iPSC approach to neurotoxicology is that environmental risk for an individual may be evaluated without a priori knowledge of the genetic risk factors. A complex relationship of environmental and genetic risk factors underlies many neurodevelopmental and neurodegenerative diseases – yet identification of causative factors has been severely hampered by the lack of suitable experimental models to account for the combinatorial influence of diverse toxicants and the inherent variation in human susceptibility and exposure. This complexity and variation of genetic and environmental influences between individuals also complicate epidemiological studies to identify contributors. For example, a link between pesticide exposure and Parkinson’s disease (PD) became suspected in 1983 with the discovery that exposure to 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP), a compound with structural similarity to the pesticide paraquat, causes a selective degeneration of dopaminergic neurons in the substantia nigra (Elbaz and Moisan, 2008; Langston et al., 1983). Despite epidemiological evidence potentially linking pesticide use with risk for PD, discerning the role of specific pesticides in human disease has been difficult (Dick et al., 2007a; Dick et al., 2007b; Frigerio et al., 2009; Frigerio et al., 2006; Kamel et al., 2007). Likewise, studies have found links between PD and exposure to Mn, Pb, and other metals (Coon et al., 2006; Finkelstein and Jerrett, 2007). Interestingly, a recent study of a Chinese cohort found an association between blood Mn levels and PD, yet no differences in exposure were seen between control and disease groups (Fukushima et al., 2009). This raises the possibility that genetic risk factors may predispose some people to accumulate levels of this environmental toxicant thereby selectively increasing their risk for disease. The advent of hiPSC technology may provide researchers a method to test this and similar hypotheses, by allowing the evaluation of selective sensitivity to neurotoxicants across individual patients.
The utility of human pluripotent stem cell technology towards human toxicological risk assessment rests on the idea that cells differentiated from patient-derived stem cells can serve as a model system for understanding the role of human genetic factors in modulating the vulnerability of differentiated cells to specific toxicants. A number of technical as well as theoretical hurdles need to be overcome before the utility of this approach can be realized. For example, efficiency and consistency of stem cell derivation, contribution of epigenetic changes, protocols for differentiation, exposure paradigms, and assessment of toxicity need to be optimized. Perhaps, though, the most fundamental issue that must be addressed upfront is whether the methods for generating patient-derived pluripotent stem cells are capable of yielding a consistent model of sensitivity to environmental toxicants. Direct tests are needed for the hypothesis that iPSCs derived from distinct individuals can be differentiated into neurons that exhibit toxicological sensitivity profiles specific to the subject from which they are derived. In other words, it needs to be shown that neuronal cells from multiple iPSC lines made from the same patient show more similarity in their sensitivity to specific neurotoxicants than neuronal cells from hiPSC lines of different patients. This simple question is fundamental for the development of an iPSC-based personalized toxicological risk assessment.
Additional technical considerations for early experimental applications of iPSC technology to neurotoxicological research are necessary. These include the importance of studying cell autonomous mechanisms of neurotoxicity. Owing to challenges in differentiation of specific neuronal populations and the consequential challenge in developing models of human neuronal circuits, it is likely unfeasible to explore neuronal network based toxicity until a better understanding of cell-autonomous toxicity is examined. For example, direct toxicity due to impaired mitochondrial function or effects of toxicants on specific cell signaling cascades need to be examined before realization of the down-stream consequences to neuronal network activity or intercellular signaling can be understood. Prototypical bench assays of toxicological measures need to be developed before automation or high-throughput screening of toxicants can be performed. Detailed examination of differentiation methods and the developmental trajectories and marker expression, as well as method-based influences on neurological phenotypes, need to be established before the impact of neurotoxicants on differentiation and neuronal activity can be interpreted in the context of predicting human neurodevelopmental toxicity. Indeed, validation of toxicological outcomes in iPSC-based models might best begin with human subjects (individuals with known genetic risk factors) and toxicants for which clearly understood human gene-environment interactions exist to facilitate confidence in interpreting data for situations where risk factors are unknown.
The study of the interactions between neurotoxicants and neurological disorders is an especially exciting future application of hiPSC technology, given the complexities of the diverse and ill-defined environmental and genetic risk factors. Furthermore, the ability to differentiate neurons and glia from patient hiPSCs offers the potential to examine changes in both the development and maintenance of neural function resulting from complex genetic inheritance patterns and toxicant perturbation (Liu and Zhang, 2011). The ability to expand, maintain, culture, and differentiate hiPSCs may enable utilization of this resource by a broad range of laboratories throughout the US and around the world, expanding both the scope and depth of research into human disease.
3. Methods of hiPSC generation
In 2007 Yamanaka showed for the first time the possibility of transforming adult human fibroblasts to pluripotent stem cells using four defined transcription factors (Takahashi et al., 2007). This ground breaking discovery led the way to ample research in an attempt to both understand the molecular basis of stem cell induction and possible ways to improve it. Induced pluripotent stem cells (iPSCs) exhibit the typical characteristics of the inner mass-derived human embryonic stem cells, including self-renewal and the potential to differentiate to cell types of any of the three germ layers. In the original work by Yamanaka, after screening for 24 potential genetic targets, a set of four retrovirus-carried genes, namely OCT4, SOX2, c-MYC, and KLF4, were chosen to transduce human adult dermal fibroblasts. Further study of these factors revealed that except for OCT4, the requirement for these factors is not stringent. c-MYC, for example, was shown to be dispensable mainly for safety purposes, with a modest decrease in efficiency (Nakagawa et al., 2008). Similarly, c-MYC and KLF-4 could be replaced with NANOG and LIN28 with no significant effect on the outcome of stem cell induction (Yu et al., 2007).
Because of serious drawbacks associated with the use of retroviruses for transduction, including persistent expression and insertional mutagenesis, alternative methods have been developed to deliver genes into the target cells. One of the strategies employs a doxycyclin inducible expression of the transgenes (Brambrink et al., 2008). This diminishes the likelihood of constitutive transgene activation and could serve as an indicator of pluripotency since fully reprogrammed cells are not dependent on the exogenous factors for their self-renewal. Another way to control viral gene expression utilizes genes flanked by loxP sites, which could later be excised by transiently expressing Cre recombinase. Alternatively, PiggyBac transposons, mobile genetic elements that could be inserted into the genome, could be used instead of the transgenes. This is an attractive method as they can be removed by transient expression of transposases (Yusa et al., 2009). Non-integrating vectors have also been used successfully to induce pluripotency. Initial trials involved transduction of mouse cells with adenovirus and non-viral methods including plasmid transfections (Okita et al., 2008; Stadtfeld et al., 2008). Adenovirus was later proved to be capable of inducing pluripotency in human dermal fibroblasts as well (Zhou and Freed, 2009). Interestingly, Yamanaka’s group has recently reported a highly efficient reprogramming of human dermal fibroblasts and dental pulp cells using episomal vectors carrying the four classical transcription factors in addition to L-MYC and P53 shRNA (Okita et al., 2011). Finally, extensive research is being carried out to screen for compounds that might permit the process of iPSC induction without the introduction of genetic materials (Lin et al., 2009).
4. Special considerations and technical challenges for iPSC-based neurotoxicological applications
A number of obstacles stand between these promises of iPSC technology and their practical application. This review seeks to find a realistic optimism for what can reasonably be accomplished in the coming years as well as detail some of the key roadblocks that must be addressed before the hope of such applications can be realized.
4.1 Generation, maintenance and selection of appropriate iPSCs for toxicity testing
The process of iPSC induction encompasses multiple steps with many possible hurdles. The efficiency of reprogramming varies greatly depending on many overlapping technical and biological factors. Although different labs report the efficiency of reprogramming in different ways, the overall rates are not satisfactory. Indeed, ever since the first human iPSC was made, extensive work has been focused on improving both the quality and efficiency of the reprogramming process (Figure 1(4.1)). Adding small molecules to manipulate certain signaling pathways has attracted special attention recently because of the reproducibility and consistency of the results. Most commonly, the targets of these attempts are either the epigenetic state of the reprogrammed cells or certain cellular pathways responsible for cell growth and fate determination. Certain epigenetic characteristics are particularly pervasive in hESCs, and screening for molecules that alter the epigenetic state of the reprogrammed cells has identified some compounds that add to the reprogramming efficiency (Rada-Iglesias and Wysocka, 2011). For example, treating partially reprogrammed cells with the DNA methyltransferase inhibitor 5-aza-cytidine (AZA) has been found to both improve the reprogramming efficiency and to induce a rapid and stable transition to fully reprogrammed iPSCs (Huangfu et al., 2008; Mikkelsen et al., 2008). Finally, it has been shown that iPSC generation is markedly enhanced by p53 gene suppression, and implementation of this knowledge dramatically improves reprogramming by episomal vectors (Hong et al., 2009; Okita et al., 2011). It remains to be determined what impact the choice of reprogramming method may have on neurotoxicological outcome measures of neuronal lineages derived from hiPSCs.
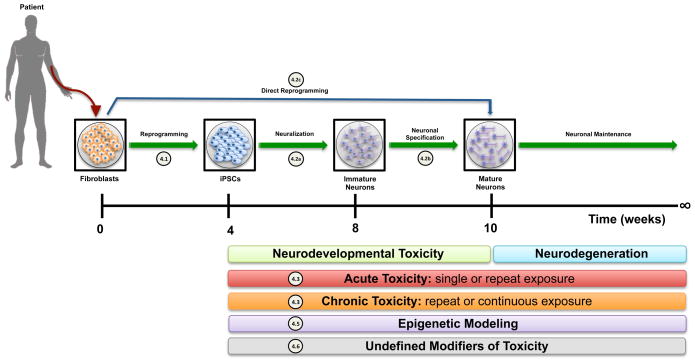
Fig 1. Generation of human induced pluripotent stem cells for neurotoxicological studies.
Schematic representation of the generation and differentiation of hiPSCs into mature neurons. Approximate timeline of this process are indicated underneath each stage of development. The colored bars indicate the types of toxicological assays and the periods in which they can be performed. The circled numerical cross-references adjacent to individual steps or experimental paradigms highlight the sub-chapter in the text discussing specific technical challenges.
Selection of iPSC lines appropriate for neurotoxicological studies relies on their behavior in culture, validation of their pluripotency characteristics (i.e. assessment of their pluripotency), their ability to differentiate with good efficiency to the required cell type, and the fidelity of their genome and epigenome. Here we will consider the first two points, and we will leave the later points to subsequent sections.
Characterization of pluripotency in iPSC lines has rested heavily on the degree by which they show similarities to ESCs in their colony and cellular morphology, molecular and expression characteristics, and their qualitative and quantitative capacity to differentiate into the three embryonic germ layers. Research has delineated defining characteristics of hESCs that may be used as a reference in the process of pluripotency validation (Initiative et al., 2007). Some of these classical characteristics, which are widely used to assess the pluripotency of iPSCs, include the expression of certain biomolecular markers such as the tissue-nonspecific alkaline phosphatase, and the expression of surface markers specific to pluripotent cells. Examples of these latter markers include TRA-1-60, SSEA-3 and SSEA-4; and the developmentally regulated genes, like NANOG and OCT4. These classical markers, however, are not specific enough to be considered markers for stem cells, as other cell types like teratocarcinoma cells also express these same markers (Josephson et al., 2007). Thus, the use of these markers alone is insufficient to characterize iPSCs.
The most widely accepted test of pluripotency is the in vivo demonstration of a stem cell line’s competence to differentiate into the three embryonic germ layers as a teratoma (Müller et al., 2010). Briefly, iPSCs are injected subcutaneously or intramuscularly in an immunocompromised mouse and a few months later teratomas are expected to form. These tumors are resected and sectioned for immunohistochemical staining for markers of endoderm, mesoderm, and ectoderm. This test is both expensive and time consuming, and the methods of performing the test and reporting the results are largely inconsistent (Müller et al., 2010). Moreover, some researchers have demonstrated the ability of partially reprogrammed cells and iPSC lines with genetic and epigenetic aberrancies to pass the test successfully. This failure to distinguish lines exhibiting normal hESC-like pluripotency marker expression has led some to call for new standards of pluripotency (Dolgin, 2010; Williams et al., 2011). Furthermore, the inability of this approach to assess pluripotency in a quantitative and high-throughput manner exacerbates the need for robust methods of selecting lines for further testing. A more simplified approach of in vitro differentiation into embryoid bodies and analysis for generation of the three embryonic germ layers has been suggested as an alternate validation approach (Boulting et al., 2011; Okita et al., 2011). An alternative approach to differentiation has been proposed in which new pluripotent lines can be compared and contrasted to reference expression maps of 12 hiPSC and 20 hESC lines that were generated to enable high-throughput characterization (Bock et al., 2011). This novel approach allows the generation of an expression “deviation scorecard” as an indirect quality control measure of pluripotency by finding gene expression patterns that uniquely identify high-quality pluripotent lines. These authors sought to confirm their findings by comparison to a “lineage scorecard” by assessing lineage-specific expression patterns from embryoid bodies derived from their reference set of pluripotent lines. They found that the “lineage scorecard” could accurately distinguish biases in differentiation propensity between pluripotent lines and identify lines that exhibit impaired differentiation towards neuronal lineages. Such validation has obvious applications in selecting lines for neurotoxicity testing by enabling the collection of phenotypically matched hiPSC lines from different subjects with similar neuronal differentiation propensities, as well as matched epigenetic and transcriptional profiles. Finally, hiPSCs that exhibit a high degree of spontaneous differentiation may need to be excluded – a potential explanation for such a phenotype is an inherent instability in maintaining a normal diploid karyotype.
4.2 Inconsistency and low-efficiency of neuronal differentiation methods
hiPSCs are in many aspects similar to hESCs and can be differentiated in culture into multiple cell types including neurons (Chamberlain et al., 2008; Dimos et al., 2008; Ebert et al., 2009). The availability of robust differentiation protocols to generate a variety of neuronal types from hiPSCs is a necessary requirement to perform neurotoxicological studies. This is not always the case in practice unfortunately, and accurate assessment of the toxicity of a certain compound is frequently hampered by the inconsistency and/or the inefficiency of the differentiation protocol. While this is true for certain neuronal types (e.g. mesencephalic dopaminergic neurons), the problem seems sometimes to pertain to the nature of the hiPSC line used in the differentiation process rather than the differentiation protocol itself (Hu et al., 2010). One of the important factors that causes low efficiency is the persistent reactivation of the latent viruses used in the reprogramming process (Papapetrou et al., 2009). However, subsets of hiPSCs generated by integration-free conditions have been found to also exhibit low neuronal differentiation efficiencies (Hu et al., 2010).
The process of differentiating hiPSCs to neurons is carried out through successive stages that start with forming the neuroectoderm, which marks the early commitment of the stem cells to the neuronal lineage (Figure 1(4.2a)) (Liu and Niswander, 2005; Muñoz-Sanjuán and Brivanlou, 2002). In the absence of external signals hESC-derived neuroectoderm inherently differentiates towards dorsal telencephalic progenitors and eventually to cortical glutamatergic neurons (Li et al., 2009b). In the presence of certain morphogens, however, these neuroectodermal cells are further patterned along the ventro-dorsal and rostro-caudal axes forming region-specific progenitors (Table 1) that give rise later to fully mature neurons (Li et al., 2009b). The time of exposure to these morphogens and their concentration are critical factors that determine the fate of neuroectodermal cells. For example, exposure to FGF8 before SOX1 upregulation results in the generation of mesencephalic dopaminergic progenitors, while forebrain dopaminergic progenitors are typically formed after exposure of SOX1 positive cells to FGF8 (Yan et al., 2005).
Table 1.
Representative protocols for differentiation of hiPSC and hESC to region-specific neuroprogenitors.
| Target Cell | Source Cell | Duration of Differentiation | Comments | References |
|---|---|---|---|---|
| Neuroectoderm (NE) (Pax6+, Sox1+) | iPSC hESC |
1–2 weeks | >80% of stem cells become NE cells. iPSC exhibit variable efficiency | Hu et al. 2010, PNAS.Chambers et al. 2009, Nature Biotechnology. |
| Telencephalic progenitors (Pax6+, FoxG1+) | iPSC hESC |
3–4 weeks | > 90% of NE cells differentiate to this cell type | Zeng et al. 2010, PLoS One. Li et al. 2009, Development. |
| Cortical progenitors (Pax6+, Emx1+) | iPSC hESC |
4 weeks | Important stage in the differentiation to glutamatergic neurons | Li et al. 2009, Development. |
| Medial ganglionic eminence (MGE) progenitors (Pax6−, Nkx2.1+) | iPSC hESC |
4 weeks | Progenitors of GABAergic interneurons and BFCN | Li et al. 2009, Development. |
| Lateral ganglionic eminence (LGE) progenitors (Pax6+, Gsx2+) | iPSC hESC |
4 weeks | Progenitors of GABAergic projection neurons | Li et al. 2009, Development. Aubry et al. 2008, PNAS. |
| Midbrain progenitors (FoxA2+, En1+) | iPSC hESC |
26 days | Early FGF8 treatment is essential | Yan et al. 2005, Stem Cells. |
| Hindbrain/Spinal cord progenitors (Pax6−, Hoxb4+) | iPSC hESC |
17 days | Early treatment with retinoic acid is essential | Zeng et al. 2010, PLoS One. Li et al. 2008, Stem Cells. |
| Multipotent neural crest cells (p75+, Hnk+, Ap2+) | iPSC hESC |
11 days | Cells successfully underwent >25 passages with retention of multipotency | Menendez et al. 2011, PNAS. |
| Ventral spinal cord progenitors (Pax6−, Olig2+) | iPSC hESC |
4 weeks | Retinoic acid for caudalizing, and SHH for ventralizing, are utilized | Zeng et al. 2010, PLoS One. Li et al. 2008, Stem Cells. |
Further maturation of regional specific neuroprogenitors to fully mature neurons follows protocols based on the same principles of the timely treatment with the right concentration of the appropriate morphogen (Figure 1(4.2b)). Many neuronal types have been generated from both hESCs and hiPSCs using several protocols (Table 2) but the efficiency of making the required cell types is generally low. In addition to that, making terminally differentiated neurons consumes a considerable amount of time, and the cost of the different chemicals used in the process is not always affordable for academic researchers.
Table 2.
Representative protocols for differentiation of hiPSCs and hESCs to mature neurons.
| Target Cell | Source Cell | Duration of Differentiation | Comments | References |
|---|---|---|---|---|
| Retinal pigment epithelium (RPE) cells | iPSC hESC |
8–12 weeks | 70–80% of terminal differentiated neurons are RPE | Kokkinaki et al. 2011. Stem Cells. Idelson et al. 2009, Cell Stem Cell. |
| Cortical glutamatergic neurons (VGLUT1+, TBR1+) | iPSC hESC |
6 weeks | 60–70% of stem cells become VGLUT+ even without added morphogens | Zeng et al. 2010, PLoS One. Li et al. 2009, Development. |
| Striatal projection GABAergic neurons (DARPP32+) | hESC | 9 to 10 weeks | The yield is low (22% MAP+, of those 36% are GABA+) | Aubry et al. 2008, PNAS. |
| Basal forebrain cholinergic neurons (BFCN) (ChAT+) | hESC | 35–40 days | SHH is essential in the protocol. Efficiency is high (~90% of final neurons) | Bissonnette et al. 2011, Stem Cells. |
| Midbrain dopamenrgic neurons (TH+, Girk2+) | iPSC hESC |
~40 days | TH+ cells form 30–60% of the terminal neurons. Generated TH+ neurons are functional | Andrezej Swistowski et al. 2010, Stem Cells. Myung Soo Cho et al. 2008, PNAS. |
| Forebrain dopamenergic neurons (TH+, GABA+) | hESC | ~40 days | Protocol yields 30% GABA+ &TH+ neurons in the final culture | Yiping Yan et al. 2005, Stem Cells. |
| Spinal motor neurons (HB9+, ChAT+) | iPSC hESC |
5–8 weeks | ~30% of final neurons exhibit motor neuron markers | Li et al. 2005, Nature Biotechnology. Hu et al. 2009, Nature Protocols. Karumbayaram et al. 2009. Stem Cells. |
| GABAergic interneurons | These cells differentiate alongside glutamatergic, projection GABAergic, and basal forebrain cholinergic neurons | Liu and Zhang 2011. Cell Mol. Life Sci. | ||
| Astrocytes (S100+, GFAP+) | iPSC hESC |
180 days | Almost 90% of final cultures cells are astrocytes in these protocols. | Krencik et. al. 2011, Nature Biotechnology. |
| Oligodendroglial cells (Olig2+, Nkx2.2+, Sox10+, PDGFR+) | iPSC hESC |
4 months | Almost 80% of final cultures cells are oligodendrocytes | Hu et al. 2009, Nature Protocols.Hu et al. 2010, PNAS. |
Recently, fully mature murine neurons have been made by direct conversion of fibroblasts (Vierbuchen et al., 2010). Following that, attempts have been made to make human neurons based on the same principle, using lentiviral vector-based gene delivery system to force the expression of a set of transcription factors peculiar to the final cell type. Indeed, fully mature and functional glutamatergic and dopaminergic neurons were made directly from fibroblasts, circumventing the intermediate step of iPSCs induction (Table 3, Figure 1(4.2c)) (Caiazzo et al., 2011; Qiang et al., 2011). The generated neurons seem to undergo major epigenetic reset expected to occur during such a switch between two terminally differentiated somatic cells. The duration of these protocols is significantly short (18 to 21 days) compared to the iPSC-based protocols, but the efficiency of making the final neuronal type is still low. Clearly though, direct differentiation methods will not be a viable strategy for studies of neurodevelopmental toxicity as the cells bypass the normal neurodevelopmental stages of neuralization, specification and maturation.
Table 3.
Generation of glutamatergic and dopaminergic neurons by direct conversion from fibroblasts.
| Target Cell | Source Cell | Duration of Differentiation | Comments | References |
|---|---|---|---|---|
| Human induced neuronal cells (hiN) | Fibroblasts | 3 weeks | 50–60% of final neuron in culture (~30%) were vGLUT1+. Study limited by the functionally heterogeneous mutations | Qiang et al. 2011, Cell. |
| Mesencephalic dopaminergic neurons | Fetal and adult fibroblasts | 18 days | Very low yield (10% of final culture MAP+ and 6% TH+) | Caiazzo et al. 2011, Nature.Pfisterer et al. 2011. PNAS. |
To improve the efficiency of the current protocols for neural differentiation of iPSCs, certain measures should be considered. Using integration-free iPSC induction methods eliminate the problem of persistent activity of viral genome and its negative effect on the differentiation process. Careful and consistent iPSC culture techniques are necessary to watch for iPSC lines that exhibit aberrant behavior in culture or are found to have abnormal genomes (Neely et al., 2011). Using small molecules inhibitors of Activin/Nodal and BMP signaling pathways significantly increases the efficiency of neural differentiation (Chambers et al., 2009; Morizane et al., 2011). In fact, the variability and inefficiency of iPSCs to differentiate to the early neuroectodermal stage has been effectively overcome by such a regimen (Kim et al., 2010a). As mentioned earlier, optimization of the time of treatment and concentration of the used morphogens is an absolute requirement for an efficient differentiation protocol. Knowledge of such details is greatly enhanced by a parallel understanding of the normal development of the nervous system in human embryos.
Measures to reduce the cost and duration of neural differentiation make it possible to perform more neurotoxicological experiments on neurons that would otherwise be unaffordable. One strategy has been to come up with inexpensive compounds that could substitute for their more expensive counterparts used usually in the differentiation process. An example is the replacement of sonic hedgehog, an important neural morphogen, by the much cheaper puromorphamine, a purine derivative that activate hedgehog pathway (Sinha and Chen, 2006). Maintenance and propagation of hiPSC-generated neuroprogenitors that retain the capability to differentiate to mature neurons is a viable option to shorten the duration of differentiation and to unify the tested neuronal populations (Li et al., 2011). Crypopreservation and later thawing of neurprogenitors could be possible as well, although cell death is observed based on the technique and the used freezing medium (Milosevic et al., 2005).
4.3 Exposure paradigms
Perhaps one of the most powerful applications of iPSC technology in the field of neurotoxicology is the ability to assess the response of neurons and neural progenitors to various toxicants (Figure 1(4.3)). Not only can many different potentially noxious agents be screened, but also the temporal properties of their administration can be used to simulate different patterns of potential human exposure. Many neurotoxicants, methylmercury being a prominent example, show both acute and latent effects. Additionally, they can exhibit heightened toxicity for specific neurodevelopmental stages, cell types, or time points under either acute or chronic exposures (Farina et al., 2011; Ni et al., 2011; Weiss et al., 2002). One advantage of working with iPSCs is that both neural progenitors and terminally differentiated neurons can be assessed. Furthermore, the influence of exposure on developing neurons and the adult phenotype of differentiated neurons can be assessed under a number of exposure paradigms designed to mimic true environmental exposures within a single experimental model. Thus in vitro neuronal differentiation of hiPSCs enables assessment of interactions between early exposure and subsequent risk of neurodegenerative phenotypes in response to acute, chronic, latent and multi-hit toxicant exposure paradigms. A number of toxicants including ethanol, methymercury, PCBs, and cocaine have been shown to elicit neurodevelopmental toxicity by disrupting neural stem cell differentiation (Fritsche et al., 2005; Radio and Mundy, 2008; Tamm et al., 2006; Tateno et al., 2004). Until the advent of iPSCs, these cell types could only be studied through primary culture or ESC derived neural progenitors, which require great effort to obtain and are not patient-specific. Thus, the use of hiPSCs is particularly attractive given that differential susceptibility of iPSC-derived cells from patients of various genetic backgrounds can be studied. However, it is important to note that the in vitro model of iPSC-derived neurons is limited in its capacity to simulate the complex extracellular environment, diversity and organization of various neuronal and glial cell types and the connectivity and activity of the neural circuitry found in vivo. For example, generation of mixed neuronal-glial cultures may be required to accurately model neurotoxicity. This approach would require parallel differentiation of stem cells into neuronal and glial lineages followed by co-culturing both cell types at the desired time point (Haidet-Phillips et al., 2011). However, the methods to obtain consistent mixed or single-lineage neuronal or neuronal-glial cultures from in vitro differentiation protocols of independent hiPSC lines have yet to be achieved.
Chronic toxicity is known to play a substantial role in many neuropathologies. Chronic exposure to the heavy metal manganese likely contributes to PD or PD-like pathology in patients (Aschner et al., 2009; Guilarte, 2010). Acute exposures to manganese are known to produce a subclinical and clinical movement disorder collectively referred to as manganism; but despite similarities between manganism and PD, the relationship between chronic manganese exposure and PD remains unclear (Aschner et al., 2009; Guilarte, 2010; Lucchini et al., 2009). iPSC-derived neurons and neural progenitors may permit the study of chronic heavy metal exposures in developing and mature neurons. Such paradigms are useful to study the effect of exposure to a toxicant over a long period of time, possibly mimicking the effect of the cumulative toxicity of environmental toxicants on the CNS throughout the age of the affected individual. Usually the suspected toxicant is applied at low doses whose effect might not be apparent immediately, like the case in acute exposure paradigms, but might eventually exhaust the cellular repertoire of defense mechanisms and eventually manifest as a decrease in the number of viable neuronal subpopulation and/or altered cellular mechanisms that are crucial for normal function. The duration of exposure varies but is often limited by the time required to differentiate stem cells to the desired neuronal type.
This approach confers several advantages given that a wide array of cell types can be assessed via toxicological assays at several time points. Furthermore, the iPSC approach for chronic exposure toxicity testing is consistent with calls to reduce reliance on vertebrate animal models (Buzanska et al., 2009; Harrill et al., 2011). However, a major limitation of hiPSC models for studying chronic neurotoxicants is the lack of endogenous detoxification mechanisms (e.g. peripheral detoxification mechanisms of the liver, or neuroprotective mechanisms within the brain by astrocytes and microglia). The lack of such systems in iPSC-based in vitro differentiation models are extremely difficult to appropriately account for experimentally, although they play a major role in modulating toxicity in vivo. Thus early applications of hiPSC based models may need to focus on toxicants in which the in vivo metabolites are known and their neuronal distribution is understood. As mentioned above, the use of neuronal-glial mixed cultures could be achieved – however even here our basic understanding of regional differences in how toxicological mechanisms and detoxification pathways differ between discrete regions of the brain is insufficient to accurately model the in vivo condition. Nonetheless, cell autonomous mechanisms of vulnerability to and protection against chronic neurotoxicants is presently accessible to investigation. Thus genetic differences between individuals that mechanistically act at the cellular or molecular level of the target will provide insight into patient-specific toxicological risk assessment in a manner not presently addressable by any other model system.
Finally, iPSCs have limitations in evaluating repeated toxicant exposures over the extended lifespan of a given neuronal or glial cell. Although investigators have devised a number of assays to assess pathological processes in iPSC-derived neurons, it may be difficult to demonstrate complex phenomena such as hormesis that act over extended periods of time and rely on undefined cellular functions or interactions. Work has been performed assessing electrophysiological profiles of iPSC-derived neurons, but conclusions regarding repeated exposures at a clinically relevant level may be confounded by a number of variables specific to iPSC reprogramming (Brennand et al., 2011). Despite these caveats, iPSCs may be able to be used to study phenomena, such as preconditioning, in circumstances in which the protective role of an early limited toxicant exposure is not dependent on another cell type. The exploration of preconditioning and hormesis is particularly valuable in the study of neurodevelopmental toxicity. It has been established that low-levels of oxidative stress can lead to hypomethylation and down-regulation of key stress mediators such as heat shock proteins and DNA methyltransferases (Shutoh et al., 2009). Whether such mechanisms occur in iPSC-derived neuronal lineages, and mimic the conditions that give rise to these mechanisms in vivo, remains to be determined. Nonetheless, even if the set point for such mechanisms may differ in vitro from the in vivo condition – human-specific and genetic variation-dependent differences in these mechanisms may still be detectable.
4.4 Coherence of genotype and phenotype
One of the major challenges with hiPSC models is establishing a clear relationship between genotype and phenotype in cells derived from a given patient. It has been previously reported that cell lines derived from individual patients can differ based on DNA methylation pattern and variable gene expression (Boulting et al., 2011; Chin et al., 2009; Doi et al., 2009; Stadtfeld et al., 2010). This variability is believed to underlie variable efficiencies of neuronal differentiation between iPSC lines. Another confounding factor that could disrupt a clear relationship between genotype and phenotype is the presence of reprogramming vectors in virally reprogrammed cells. Variability between lines has been difficult to identify early in the differentiation process. Furthermore, no direct relationship has been noted between expression of pluripotency markers and differentiation capacity (Boulting et al., 2011). Overcoming this difficulty has been addressed by recent work, which was able to successfully predict differentiation potential through analysis of epigenetic and transcriptional differences between lines (Bock et al., 2011; Boulting et al., 2011). In another comparison of 22 human iPSC lines, it was revealed that there were an average of five protein-coding point mutations in sampled regions (Gore et al., 2011). These mutations consisted of splicing, nonsense, and non-synonymous variants that appeared both before and after fibroblast reprogramming (Gore et al., 2011). Similarly, early passage hiPSCs were found to contain a significantly higher frequency and degree of mosaicism for copy number variations compared to late passage hiPSCs or hESCs (Hussein et al., 2011). These de novo mutations, likely stemming from the reprogramming process itself, may obscure genotype-phenotype associations behind this mutational noise.
Genetic and epigenetic abnormalities in hiPSCs can arise either during the process of induction or during the subsequent culture process (Gore et al., 2011; Hussein et al., 2011; Lister et al., 2011). Genetic abnormalities may be at the chromosomal, sub-chromosomal, or single-base levels, and their potential occurrence could have crucial implications for the appropriate use of iPSCs for research purposes. Some studies have suggested a high mutational load present in many hiPSC lines. It has been proposed that whole genome or complete exome sequencing may help identify lines with fewer mutational events and eliminate variability between lines (Gore et al., 2011). The loss of genetic fidelity may be reflected in aberrancies of cell behavior in tissue culture, abnormal morphology, growth rate alteration, and frequent differentiation. While most of the genetic variation involves a small subset of cells, there are recurrent mutational events such as trisomy 12 and trisomy 17 that convey a selective advantage whereby mutant cells replace euploid population after a few passages (Meisner and Johnson, 2008). It is thus reasonable to check the karyotype and genetic integrity of cultured hiPSCs for abnormalities at regular intervals; though at a significant added expense for research laboratories.
4.5 Appropriate modeling of epigenetic influence and ‘residual epigenetic marks’
Epigentic state is known to have a powerful influence on chemical toxicity (Fragou et al., 2011; LeBaron et al., 2010; Perera and Herbstman, 2011). Thus an important consideration in the application of iPSC technology in the study of neurotoxicology is the status of epigenetic modifications such as genomic DNA methylation and histone modifications (Figure 1(4.5)). Given the critical role of epigentic state in development and differentiation, understanding the epigenetic profiles of hiPSCs is an important component to validating patient-specific experimental findings. The induction of somatic cells to iPSCs is known to alter their epigenetic profile (Guenther et al., 2010). The iPSC epigenetic profile, while similar to that of ESCs, has notable characteristic features. These epigenetic modifications can have a significant influence on the ability of iPSCs to differentiate into various cellular subtypes (Kim et al., 2010b). In order to generate a greater understanding of the nature of the unique epigenetic profile of iPSCs, a recent study generated a whole-genome single-base resolution DNA “methylome” via bisulphite sequencing (Lister et al., 2011). Investigators found that iPSC lines were methylated at CG dinucleotide pairs at a higher frequency than somatic cells, in a manner similar to that of ESCs (Lister et al., 2011). Furthermore, upon comparison of the methylomes of iPSCs with that of ESCs, notable unique methylation loci were identified between cell types. One challenge when analyzing iPSC epigenetics is distinguishing between methylated regions that are vestiges of the somatic cell from which they are derived and those that result directly from reprogramming. It has been established that low-passage number iPSCs contain DNA methylation signatures derived from the tissue of origin (Kim et al., 2010b). Many of these methylated regions are shared between individual iPSC lines, suggesting certain loci have an increased susceptibility to de novo methylation (Lister et al., 2011). Potentially, these high susceptibility regions can be monitored for stochastic methylation, a valuable tool when characterizing the efficiency of reprogramming and differentiation of a given line (Lister et al., 2011). Interestingly, a recent study has noted that iPSCs methylation patterns become similar to ESCs with each sequential passage number (Nishino et al., 2011). The mechanism of this methylation is thought to be the result of the convergence of stochastic de novo hyper-methylation in adjacent genomic regions (Nishino et al., 2011). As the mechanisms of de novo methylation become revealed, interventions with chromatin-modifying drugs have the potential to address these concerns. Furthermore, given that fibroblasts are a common somatic tissue source, the methylation profile of iPSCs used in neurotoxicological assays may need to be standardized before disease modeling of neurological disease can be directly comparable between studies.
4.6 Undefined modifiers of toxicity
Perhaps one of the greatest challenges in applying iPSC technology to the field of neurotoxicology is replicating the toxicological conditions that reflect the insults arising in vivo from actual environmental exposures. Influences due to the specific route of exposure, interactions with other toxicants and environmental modifiers, as well as previous exposures, nutritional state, or chronic illness of the individual are currently difficult to account for using in vitro models, including differentiated neuronal lineages of hiPSCs. Furthermore, the actions of such undefined modifiers of toxicity may occur across diverse temporal and spatial axes (Figure 1(4.6)). Thus the environmental, anatomical and developmental history of the primary target cell of a toxicant may be influenced by previous exposures, nutritional state, and status of support tissues or neuronal connectivity that is challenging to model in vitro. Furthermore, this issue is complicated by potential differences in the epigenetic status of iPSC derived cells that may not reflect the in vivo epigenetic state of the intended neuronal populations being modeled from the individual or human population being studied. The multi-factorial and synergistic potential of unknown modifiers to influence both the type and magnitude of toxicological outcomes are likely critical to actual disease etiology. Furthermore, it is often of particular interest to examine the role of neurotoxicants under specific disease states. Similar spatial, temporal, and anatomic constraints impact neurological disease modeling by hiPSCs as well (Saha and Jaenisch, 2009). Thus undefined modifiers of neurotoxicity are layered upon the additional complexities of in vitro disease modeling. However, it should be pointed out that similar challenges in interpreting experimental data apply to most model systems.
At the present state, effort should be directed toward standardization of iPSC phenotypes and development of adequate controls and appropriate validation of experimental results. For example, iPSCs derived from unaffected siblings are valuable controls as they generally have a similar environmental and genetic background as the patient. Thus, experimental differences in the sensitivity of phenotypic outcomes to toxicant exposure of differentiated or differentiating neuronal cells are more likely due to known genetic or epigenetic differences between the subjects. Furthermore, multiple lines should be generated from each subject to account for the inherent variability between individual lines.
Aging and senescence is also an important concern when generating iPSCs. It has been established that fibroblasts derived from older mice have lower efficiency of reprogramming (Li et al., 2009a). This may be mediated by expressions of genes related to senescence such as the Ink4b/Arf locus, which is upregulated in biological aging. It has been demonstrated that the generation of iPSC lines from older patients can be improved by inhibiting senescence with siRNAs, treating somatic cells with antioxidants, or reprogramming in low-oxygen conditions (Banito and Gil, 2010; Esteban et al., 2010; Utikal et al., 2009; Yoshida et al., 2009). An important question, particularly in the study of neurodegenerative diseases, is whether aging can be reproduced in iPSC-derived cells. Reprogramming of fibroblasts into iPSCs is thought to reset markers of cellular age such as telomere length (Suhr et al., 2010). This change in telomere length is transient however, as telomere shortening was observed in iPSC-derived cells after differentiation (Suhr et al., 2010). Similarly, it has been established that the “energetic capacity” of cells can be reset through reprogramming, by reestablishing mitochondrial compliment to a neonatal-like state (Suhr et al., 2010). As mitochondrial capacity decreases with aging largely due to oxidative stress, these findings suggest that future studies utilizing iPSCs may focus on replicating the cellular state of older subjects through prior exposure to reactive oxygen species. Nutritional variables could also be addressed with a similar approach; simultaneous treatment with naturally occurring antioxidants or chronic alcohol exposure could give insight into neuroprotective or neurotoxic roles of various substances. Upon further standardization of patient derived iPSCs, disease-modifying variables such as developmental history, early-life environmental exposures, epigenetic status, nutritional state, and age may be able to be accounted for in toxicological studies.
4.7 Challenges of scale
In the area of neurotoxicology, iPSCs can help address questions regarding why individuals with similar genetic backgrounds demonstrate differential susceptibility to toxicants. But before hiPSC-based neurotoxicological approaches can be broadly applied for toxicological risk assessment and in depth patient-based mechanistic and neuroprotective studies the critical hurdles of high cost and poor scalability associated with maintenance and analysis of multiple hiPSC lines simultaneously must be addressed. Significant person-hours are required to reprogram, validate, differentiate, and toxicologically assess multiple cell lines from just a single patient. Consequently, a seemingly straightforward study seeking to explore differences between patients with a given mutation and controls can easily become overwhelming for even a moderately sized research group. Thus, studies using hiPSCs remain quite low-throughput at present time, with recent published data comparing simple measures in no more than 10–20 lines, and even fewer lines for more complex phenotypic analysis. However, significant improvements may be within reach by use of robotic automation and more efficient methodology (Dolmetsch and Geschwind, 2011). Perhaps the two disciplines that would benefit most from high-throughput hiPSC development are drug development and toxicology. For example, drug screening would require sufficient cells and sample size such that thousands of compounds could be screened (Ellis and Bhatia, 2011).
Modern tissue culture protocols are both time consuming and have tremendous batch-batch variability that may influence downstream assays. One recently proposed approach is the wide-scale utilization of stirred suspension bioreactors, which dramatically reduce cost and increase yield of expansion 58-fold (Shafa et al., 2011). iPSCs generated in stirred suspension bioreactors retain all pluripotency markers and are able to differentiate into all germ layers. However the aggregation into multicellular structures that occur under this approach will require adaptation to neuronal differentiation and neural specification methods that use monolayer iPSC cultures as their starting point. This approach has yet to be widely adapted in research laboratories owing to its high costs and specialized equipment, but may represent an important step toward high-throughput assays and commercialization of iPSCs.
Issues regarding scalability are an active area of discussion as it hinders collaboration between research groups. These issues include: reproducibility of protocols, cell line nomenclature, intellectual property issues, and lack of a detailed and centralized database of available hiPSC lines (Luong et al., 2011). There is a strong demand within the field to establish an iPSC library in conjunction with a clinical database, tissue bank, and genome wide association studies (GWAS) (Hankowski et al., 2011). Such a database would address concerns regarding reproducibility and nomenclature, making data directly comparable across studies.
The rapid development of hiPSC technology has benefited tremendously from the tailwind of hESC research. Aside from the unique reprogramming method, the reagents, differentiation protocols, and media are near identical to that of hESCs. However, the patient-specific nature of hiPSCs has generated a new demand for scaling up these protocols in a high-throughput manner. Thus, current cost-constraints and difficulties in cross-institutional collaboration will likely spur rapid innovation in scalability in the near future. This positive outlook of high-throughput screening utilizing iPSCs should incentivize investigators interested in toxicological assessment of neurological disease to adapt this technology as soon as possible.
5. The potential hiPSC technology for personalized medicine and risk assessment
As discussed above, hiPSCs have a wide-range of potential clinical applications, particularly in the field of personalized medicine. These applications include generation of cells for transplantation therapy and as a model of human disease (Hankowski et al., 2011). The major advantage of iPSCs over other disease models is that it has the potential to model any disease and toxicological phenotype, especially those that do not currently have an animal model. Furthermore, hiPSCs permit the study of diseases that are both genetic and environmental in nature. Thus, for the first time, the study of gene-environment interactions utilizing cellular subtypes derived from patients afflicted with a given illness is possible. Data derived from such studies in the future may provide a critical translational link between model organism-based, computational, and in vitro toxicological data to epidemiological and other human population studies. Furthermore, this approach is not solely for learning more about disease pathogenesis, but may be directly utilized in clinical practice. For example, by using patient-specific iPSC-derived neurons to customize neuroprotective strategies and environmental safety information for individuals. These future applications may also include direct transplantation of patient derived cells, toxicity studies, and drug efficacy and safety evaluations.
A lofty but often stated goal for hiPSC technology is its potential to provide personalized neurotoxicological risk assessment. For example, iPSC lines generated from an individual may enable assessment of individualized sensitivities to common environmental pollutants enabling targeted prophylactic measures. Likewise, assessment of heightened sensitivity to particular common toxicological insults (e.g. oxidative stress, mitochondrial dysfunction, protein misfolding, calcium handling deficits) may enable personalized neuroprotective strategies to be derived and prescribed. However, a measure of skepticism is warranted given the novelty of iPSC-associated approaches.
hiPSC technology has the potential to accelerate the pace of drug discovery and improve understanding of disease pathogenesis. Clinical, genetic and exposure history data of patients for which hiPSC are generated is especially relevant and should be collected as thoroughly as possible, abiding by IRB and HIPPA regulations to protect patients’ privacy (Hankowski et al., 2011). Disease modeling using hiPSCs has already shown promise given recent success modeling Rett syndrome, Alzheimer’s disease, neuronal lysosomal disorders, Amyotrophic lateral sclerosis, schizophrenia, Parkinson’s Disease and others (Brennand et al., 2011; Lemonnier et al., 2011; Marchetto et al., 2011; Marchetto et al., 2010a; Seibler et al., 2011; Yagi et al., 2011). In the field of drug discovery, iPSCs have the ability to fill the information not covered by genome-wide association studies and databases. For example, novel compounds can be screened on mature cells derived from iPSCs generated from patients with a given illness. Through this approach, both a toxicity and efficacy profile for a given therapeutic agent can be generated (Hankowski et al., 2011).
Similarly, toxicity phenotypes can be studied in vitro by exposing iPSC-derived neurons to various toxicants and inflammatory factors (Inoue and Yamanaka, 2011; Marchetto et al., 2010b; Wichterle and Przedborski, 2010). Utilization of hiPSC-based toxicity testing for drug development, includes the promise of developing high-throughput assays utilizing iPSC-derived hepatocytes to examine the metabolism of novel compounds (Inoue and Yamanaka, 2011; Laustriat et al., 2010). The effects of aging on disease-toxicity relationships may be challenging to model given that iPSC-derived cells present from the early stages of development. Future efforts can be directed towards inducing cellular injury and metabolic changes that simulate the effects of aging in this process. Although iPSCs show promise in the study of neurological disease, one must acknowledge the difficulty of validating iPSC models of psychiatric illness. Since disorders such as schizophrenia, autism, and depression have a poorly understood molecular and cellular basis, investigators will face difficulty defining which iPSC-derived neuronal lines demonstrate the disease-specific phenotype (Dolmetsch and Geschwind, 2011).
6. Conclusions
The ability of iPSC lines to be isolated from patients with any neurological disease provides an important tool for characterization of susceptibility to various toxicants. Neurotoxicological studies of iPSC-derived cells are readily adaptable to existing toxicity assays, which allows experiments to be controlled against primary neuronal cultures. Validation of iPSC-based findings will likely be dependent on appropriate validation of the iPSCs themselves. For example, expressions of surface markers of pluripotency markers after reprogramming and lineage specific markers during differentiation by immunocytochemistry or flow cytometry are critical validation steps (Vojnits and Bremer, 2010). One of the foremost challenges facing neurotoxicological application is residual epigenetic signatures from reprogramming. Another challenge is the heterogeneous nature of cell types generated during differentiation (Anson et al., 2011). Ultimately, these concerns regarding the quality of the iPSC models necessitate that resulting neurotoxicological findings be validated in other model systems. Should a differential susceptibility to a neurotoxicant be identified in iPSC-derived cells, these results will need to be supported by epidemiological, animal, and human studies for the conclusion to be deemed valid.
The translational value of iPSC-based studies may be helped by identification of the key determinants and markers of the experimentally introduced genetic and epigenetic differences that contribute to measurable toxicological and phenotypic variation between iPSC lines and their derivatives. Controlling for these differences in the iPSC lines used for studies should minimize experimental noise and increase the coherence of human genotype – phenotype correlations. At present, since the impact of such experimental variation is currently unmeasured – it remains to be seen the numbers of iPSC lines per subject or the numbers of subjects needed to have sufficient statistical power to draw meaningful conclusions in neurotoxicological studies. However, as more studies are reported in the literature, a meta-analysis could be performed to evaluate these features and inform experimental design. One important caveat of iPSC-based neurotoxicological assays is the inability to completely model the in vivo neuronal environment, including the spectrum of neuronal network connections and glial interactions. Thus at present only toxicological phenotypes that can be modeled in a cell autonomous manner, or perhaps simplistic mutlicellular models, are likely to provide meaningful information. Toxicities that depend on multi-regional interactions between brain regions or the periphery are not likely to be captured by iPSC-based modeling. However, when suitable iPSC-based neurotoxicological assays can be designed such systems may be uniquely powered as a translatable screening tool to inform and guide resources for appropriate human and animal model in vivo studies.
Acknowledgments
K.K.K. acknowledges support by the Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical Scientist Training Program. This work was supported by grants from the NIH P30 ES000267 and NIH RO1 ES016931.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anson BD, Kolaja KL, Kamp TJ. Opportunities for use of human iPS cells in predictive toxicology. Clin Pharmacol Ther. 2011;89:754–8. doi: 10.1038/clpt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Erikson KM, Herrero Hernandez E, Tjalkens R. Manganese and its role in Parkinson’s disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–66. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry L, Bugi A, Lefort N, Rousseau F, Peschanski M, Perrier AL. Striatal progenitors derived from human ES cells mature into DARPP32 neurons in vitro and in quinolinic acid-lesioned rats. Proc Natl Acad Sci USA. 2008;105:16707–12. doi: 10.1073/pnas.0808488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banito A, Gil J. Induced pluripotent stem cells and senescence: learning the biology to improve the technology. EMBO Reports. 2010;11:353–9. doi: 10.1038/embor.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette CJ, Lyass L, Bhattacharyya BJ, Belmadani A, Miller RJ, Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29:802–11. doi: 10.1002/stem.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, Gnirke A, Eggan K, Meissner A. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144:439–52. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulting GL, Kiskinis E, Croft GF, Amoroso MW, Oakley DH, Wainger BJ, Williams DJ, Kahler DJ, Yamaki M, Davidow L, Rodolfa CT, Dimos JT, Mikkilineni S, MacDermott AB, Woolf CJ, Henderson CE, Wichterle H, Eggan K. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279–86. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008;2:151–9. doi: 10.1016/j.stem.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JM, Gassmann K, Kayser R, Stegeman H, De Groot D, Fritsche E, Shafer TJ. Neural progenitor cells as models for high-throughput screens of developmental neurotoxicity: state of the science. Neurotoxicol Teratol. 2010;32:4–15. doi: 10.1016/j.ntt.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Breier JM, Radio NM, Mundy WR, Shafer TJ. Development of a high-throughput screening assay for chemical effects on proliferation and viability of immortalized human neural progenitor cells. Toxicol Sci. 2008;105:119–33. doi: 10.1093/toxsci/kfn115. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–5. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzanska L, Sypecka J, Nerini-Molteni S, Compagnoni A, Hogberg HT, del Torchio R, Domanska-Janik K, Zimmer J, Coecke S. A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells. 2009;27:2591–601. doi: 10.1002/stem.179. [DOI] [PubMed] [Google Scholar]
- Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011 doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Chamberlain SJ, Li X-J, Lalande M. Induced pluripotent stem (iPS) cells as in vitro models of human neurogenetic disorders. Neurogenetics. 2008;9:227–35. doi: 10.1007/s10048-008-0147-z. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–23. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MS, Lee Y-E, Kim JY, Chung S, Cho YH, Kim D-S, Kang S-M, Lee H, Kim M-H, Kim J-H, Leem JW, Oh SK, Choi YM, Hwang D-Y, Chang JW, Kim D-W. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:3392–7. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon S, Stark A, Peterson E, Gloi A, Kortsha G, Pounds J, Chettle D, Gorell J. Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ Health Perspect. 2006;114:1872–6. doi: 10.1289/ehp.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FD, De Palma G, Ahmadi A, Osborne A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Soderkvist P, Felice A group Gs. Gene-environment interactions in parkinsonism and Parkinson’s disease: the Geoparkinson study. Occupational and Environmental Medicine. 2007a;64:673–80. doi: 10.1136/oem.2006.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S, Counsell C, Mozzoni P, Haites N, Wettinger SB, Mutti A, Otelea M, Seaton A, Söderkvist P, Felice A group Gs. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occupational and Environmental Medicine. 2007b;64:666–72. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Doi A, Park I-H, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, Ladd-Acosta C, Rho J, Loewer S, Miller J, Schlaeger T, Daley GQ, Feinberg AP. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature Genetics. 2009;41:1350–3. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. Putting stem cells to the test. Nat Med. 2010;16:1354–7. doi: 10.1038/nm1210-1354. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R, Geschwind DH. The human brain in a dish: the promise of iPSC-derived neurons. Cell. 2011;145:831–4. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Moisan F. Update in the epidemiology of Parkinson’s disease. Curr Opin Neurol. 2008;21:454–60. doi: 10.1097/WCO.0b013e3283050461. [DOI] [PubMed] [Google Scholar]
- Ellis J, Bhatia M. iPSC technology: platform for drug discovery. Point. Clinical pharmacology and therapeutics. 2011;89:639–41. doi: 10.1038/clpt.2011.22. [DOI] [PubMed] [Google Scholar]
- Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71–9. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Farina M, Rocha JBT, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011;89:555–63. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ Res. 2007;104:420–32. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Fragou D, Fragou A, Kouidou S, Njau S, Kovatsi L. Epigenetic mechanisms in metal toxicity. Toxicology Mechanisms and Methods. 2011;21:343–52. doi: 10.3109/15376516.2011.557878. [DOI] [PubMed] [Google Scholar]
- Frigerio R, Fujishiro H, Maraganore DM, Klos KJ, DelleDonne A, Heckman MG, Crook JE, Josephs KA, Parisi JE, Boeve BF, Dickson DW, Ahlskog JE. Comparison of risk factor profiles in incidental Lewy body disease and Parkinson disease. Arch Neurol. 2009;66:1114–9. doi: 10.1001/archneurol.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio R, Sanft KR, Grossardt BR, Peterson BJ, Elbaz A, Bower JH, Ahlskog JE, de Andrade M, Maraganore DM, Rocca WA. Chemical exposures and Parkinson’s disease: a population-based case-control study. Mov Disord. 2006;21:1688–92. doi: 10.1002/mds.21009. [DOI] [PubMed] [Google Scholar]
- Fritsche E, Cline JE, Nguyen N-H, Scanlan TS, Abel J. Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environmental Health Perspectives. 2005;113:871–6. doi: 10.1289/ehp.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Tan X, Luo Y, Kanda H. Relationship between Blood Levels of Heavy Metals and Parkinson’s Disease in China. Neuroepidemiology. 2009;34:18–24. doi: 10.1159/000255462. [DOI] [PubMed] [Google Scholar]
- Genschow E, Spielmann H, Scholz G, Pohl I, Seiler A, Clemann N, Bremer S, Becker K. Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. Altern Lab Anim. 2004;32:209–44. doi: 10.1177/026119290403200305. [DOI] [PubMed] [Google Scholar]
- Gore A, Li Z, Fung H-L, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee J-H, Loh Y-H, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LSB, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther MG, Frampton GM, Soldner F, Hockemeyer D, Mitalipova M, Jaenisch R, Young RA. Chromatin structure and gene expression programs of human embryonic and induced pluripotent stem cells. Cell Stem Cell. 2010;7:249–57. doi: 10.1016/j.stem.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson’s disease: a critical review and new findings. Environ Health Perspect. 2010;118:1071–80. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–8. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankowski KE, Hamazaki T, Umezawa A, Terada N. Induced pluripotent stem cells as a next-generation biomedical interface. Lab Invest. 2011;91:972–7. doi: 10.1038/labinvest.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill JA, Freudenrich TM, Machacek DW, Stice SL, Mundy WR. Quantitative assessment of neurite outgrowth in human embryonic stem cell-derived hN2 cells using automated high-content image analysis. Neurotoxicology. 2010;31:277–90. doi: 10.1016/j.neuro.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Harrill JA, Freudenrich TM, Robinette BL, Mundy WR. Comparative sensitivity of human and rat neural cultures to chemical-induced inhibition of neurite outgrowth. Toxicol Appl Pharmacol. 2011 doi: 10.1016/j.taap.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heng BC, Richards M, Shu Y, Gribbon P. Induced pluripotent stem cells: a new tool for toxicology screening? Archives of Toxicology. 2009;83:641–4. doi: 10.1007/s00204-009-0414-2. [DOI] [PubMed] [Google Scholar]
- Heuer J, Bremer S, Pohl I, Spielmann H. Development of an in vitro embryotoxicity test using murine embryonic stem cell cultures. Toxicol In Vitro. 1993;7:551–6. doi: 10.1016/0887-2333(93)90064-c. [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B-Y, Weick JP, Yu J, Ma L-X, Zhang X-Q, Thomson JA, Zhang S-C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107:4335–40. doi: 10.1073/pnas.0910012107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B-Y, Zhang S-C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nature Protocols. 2009;4:1295–304. doi: 10.1038/nprot.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Närvä E, Ng S, Sourour M, Hämäläinen R, Olsson C, Lundin K, Mikkola M, Trokovic R, Peitz M, Brüstle O, Bazett-Jones DP, Alitalo K, Lahesmaa R, Nagy A, Otonkoski T. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Initiative ISC, Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo ABH, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz-Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RDG, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O’Brien CM, Oh SKW, Olsson C, Otonkoski T, Park K-Y, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RAR, Reubinoff BE, Robins AJ, Rossant J, Rugg-Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, van den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–16. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- Inoue H, Yamanaka S. The use of induced pluripotent stem cells in drug development. Clinical pharmacology and therapeutics. 2011;89:655–61. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- Josephson R, Ording CJ, Liu Y, Shin S, Lakshmipathy U, Toumadje A, Love B, Chesnut JD, Andrews PW, Rao MS, Auerbach JM. Qualification of embryonal carcinoma 2102Ep as a reference for human embryonic stem cell research. Stem Cells. 2007;25:437–46. doi: 10.1634/stemcells.2006-0236. [DOI] [PubMed] [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, Comyns K, Goldman S, Korell M, Langston J, Ross G, Sandler D. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165:364–74. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, Wiedau-Pazos M, Kornblum HI, Lowry WE. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–11. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-S, Lee JS, Leem JW, Huh YJ, Kim JY, Kim H-S, Park I-H, Daley GQ, Hwang D-Y, Kim D-W. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Reviews. 2010a;6:270–81. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LIR, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010b;467:285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011;29:825–35. doi: 10.1002/stem.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang Z-J, Zhang S-C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–34. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuegler PB, Zimmer B, Waldmann T, Baudis B, Ilmjarv S, Hescheler J, Gaughwin P, Brundin P, Mundy W, Bal-Price AK, Schrattenholz A, Krause KH, van Thriel C, Rao MS, Kadereit S, Leist M. Markers of murine embryonic and neural stem cells, neurons and astrocytes: reference points for developmental neurotoxicity testing. ALTEX. 2010;27:17–42. doi: 10.14573/altex.2010.1.16. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Laustriat D, Gide J, Peschanski M. Human pluripotent stem cells in drug discovery and predictive toxicology. Biochem Soc Trans. 2010;38:1051–7. doi: 10.1042/BST0381051. [DOI] [PubMed] [Google Scholar]
- LeBaron MJ, Rasoulpour RJ, Klapacz J, Ellis-Hutchings RG, Hollnagel HM, Gollapudi BB. Epigenetics and chemical safety assessment. Mutation Research. 2010;705:83–95. doi: 10.1016/j.mrrev.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Leist M, Bremer S, Brundin P, Hescheler J, Kirkeby A, Krause K-H, Poerzgen P, Puceat M, Schmidt M, Schrattenholz A, Zak NB, Hentze H. The biological and ethical basis of the use of human embryonic stem cells for in vitro test systems or cell therapy. ALTEX. 2008;25:163–90. [PubMed] [Google Scholar]
- Lemonnier T, Blanchard S, Toli D, Roy E, Bigou S, Froissart R, Rouvet I, Vitry S, Heard JM, Bohl D. Modeling neuronal defects associated with a lysosomal disorder using patient-derived induced pluripotent stem cells. Human Molecular Genetics. 2011;20:3653–66. doi: 10.1093/hmg/ddr285. [DOI] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Cañamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009a;460:1136–9. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sun W, Zhang Y, Wei W, Ambasudhan R, Xia P, Talantova M, Lin T, Kim J, Wang X, Kim WR, Lipton SA, Zhang K, Ding S. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci USA. 2011;108:8299–304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-J, Hu B-Y, Jones SA, Zhang Y-S, Lavaute T, Du Z-W, Zhang S-C. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–93. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-J, Zhang X, Johnson MA, Wang Z-B, Lavaute T, Zhang S-C. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009b;136:4055–63. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, Lin X, Hahm HS, Hao E, Hayek A, Ding S. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–8. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Kida YS, Hawkins RD, Nery JR, Hon G, Antosiewicz-Bourget J, O’Malley R, Castanon R, Klugman S, Downes M, Yu R, Stewart R, Ren B, Thomson JA, Evans RM, Ecker JR. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–54. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhang S-C. Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci. 2011;68:3995–4008. doi: 10.1007/s00018-011-0770-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini RG, Martin CJ, Doney BC. From manganism to manganese-induced parkinsonism: a conceptual model based on the evolution of exposure. Neuromolecular Med. 2009;11:311–21. doi: 10.1007/s12017-009-8108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong MX, Auerbach J, Crook JM, Daheron L, Hei D, Lomax G, Loring JF, Ludwig T, Schlaeger TM, Smith KP, Stacey G, Xu R-H, Zeng F. A call for standardized naming and reporting of human ESC and iPSC lines. Cell Stem Cell. 2011;8:357–9. doi: 10.1016/j.stem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Human Molecular Genetics. 2011;20:R109–15. doi: 10.1093/hmg/ddr336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MCN, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010a;143:527–39. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MCN, Winner B, Gage FH. Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Human Molecular Genetics. 2010b doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner LF, Johnson JA. Protocols for cytogenetic studies of human embryonic stem cells. Methods. 2008;45:133–41. doi: 10.1016/j.ymeth.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240–5. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic J, Storch A, Schwarz J. Cryopreservation does not affect proliferation and multipotency of murine neural precursor cells. Stem Cells. 2005;23:681–8. doi: 10.1634/stemcells.2004-0135. [DOI] [PubMed] [Google Scholar]
- Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, Schuwald J, Weinmann N, Fritsche E. Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect. 2009;117:1131–8. doi: 10.1289/ehp.0800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J. Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res. 2011;89:117–26. doi: 10.1002/jnr.22547. [DOI] [PubMed] [Google Scholar]
- Müller F-J, Goldmann J, Löser P, Loring JF. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell. 2010;6:412–4. doi: 10.1016/j.stem.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Muñoz-Sanjuán I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–80. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Neely M, Tidball A, Aboud A, Ess K, Bowman A. Induced pluripotent stem cells (iPSCs) - an emerging model system for the study of human neurotoxicology. In: Aschner M, Sunol C, Price A, editors. Neuromethods - Springer Protocols: Cell Culture Techniques. New York, NY: Humana Press; 2011. [Google Scholar]
- Ni M, Li X, Yin Z, Sidoryk-Węgrzynowicz M, Jiang H, Farina M, Rocha JBT, Syversen T, Aschner M. Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia. 2011;59:810–20. doi: 10.1002/glia.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino K, Toyoda M, Yamazaki-Inoue M, Fukawatase Y, Chikazawa E, Sakaguchi H, Akutsu H, Umezawa A. DNA methylation dynamics in human induced pluripotent stem cells over time. PLoS Genetics. 2011;7:e1002085. doi: 10.1371/journal.pgen.1002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K-I, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nature Methods. 2011;8:409–12. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Pal R, Mamidi MK, Das AK, Bhonde R. Human embryonic stem cell proliferation and differentiation as parameters to evaluate developmental toxicity. J Cell Physiol. 2011;226:1583–95. doi: 10.1002/jcp.22484. [DOI] [PubMed] [Google Scholar]
- Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J, Tabar V, Mo Q, Studer L, Sadelain M. Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12759–64. doi: 10.1073/pnas.0904825106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I-H, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reproductive Toxicology. 2011;31:363–73. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Björklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10343–8. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, Moreno H, Abeliovich A. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–71. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rada-Iglesias A, Wysocka J. Epigenomics of human embryonic stem cells and induced pluripotent stem cells: insights into pluripotency and implications for disease. Genome Med. 2011;3:36. doi: 10.1186/gm252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radio NM, Mundy WR. Developmental neurotoxicity testing in vitro: models for assessing chemical effects on neurite outgrowth. Neurotoxicology. 2008;29:361–76. doi: 10.1016/j.neuro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Robinson JF, Theunissen PT, van Dartel DA, Pennings JL, Faustman EM, Piersma AH. Comparison of MeHg-induced toxicogenomic responses across in vivo and in vitro models used in developmental toxicology. Reprod Toxicol. 2011;32:180–8. doi: 10.1016/j.reprotox.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–95. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz G, Pohl I, Genschow E, Klemm M, Spielmann H. Embryotoxicity screening using embryonic stem cells in vitro: correlation to in vivo teratogenicity. Cells Tissues Organs. 1999;165:203–11. doi: 10.1159/000016700. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Gassmann K, Götz C, Hübenthal U, Moors M, Krause G, Merk HF, Nguyen N-H, Scanlan TS, Abel J, Rose CR, Fritsche E. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environ Health Perspect. 2010;118:572–8. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 2011;31:5970–6. doi: 10.1523/JNEUROSCI.4441-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler AE, Spielmann H. The validated embryonic stem cell test to predict embryotoxicity in vitro. Nat Protoc. 2011;6:961–78. doi: 10.1038/nprot.2011.348. [DOI] [PubMed] [Google Scholar]
- Shafa M, Sjonnesen K, Yamashita A, Liu S, Michalak M, Kallos MS, Rancourt DE. Expansion and long-term maintenance of induced pluripotent stem cells in stirred suspension bioreactors. Journal of Tissue Engineering and Regenerative Medicine. 2011 doi: 10.1002/term.450. [DOI] [PubMed] [Google Scholar]
- Shutoh Y, Takeda M, Ohtsuka R, Haishima A, Yamaguchi S, Fujie H, Komatsu Y, Maita K, Harada T. Low dose effects of dichlorodiphenyltrichloroethane (DDT) on gene transcription and DNA methylation in the hypothalamus of young male rats: implication of hormesis-like effects. The Journal of Toxicological Sciences. 2009;34:469–82. doi: 10.2131/jts.34.469. [DOI] [PubMed] [Google Scholar]
- Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Spielmann H, Pohl I, Doering B, Liebsch M, Moldenhauer F. The embryonic stem cell test, an in vitro embryotoxicity test using two permanent mouse cell lines: 3T3 fibroblasts and embryonic stem cells. Toxicol In Vitro. 1997;10:119–27. [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–81. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–9. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummann T, Hareng L, Bremer S. Hazard assessment of methylmercury toxicity to neuronal induction in embryogenesis using human embryonic stem cells. Toxicology. 2009;257:117–26. doi: 10.1016/j.tox.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Suhr ST, Chang EA, Tjong J, Alcasid N, Perkins GA, Goissis MD, Ellisman MH, Perez GI, Cibelli JB. Mitochondrial rejuvenation after induced pluripotency. PLoS ONE. 2010;5:e14095. doi: 10.1371/journal.pone.0014095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28:1893–904. doi: 10.1002/stem.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. Journal of Neurochemistry. 2006;97:69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Tateno M, Ukai W, Ozawa H, Yamamoto M, Toki S, Ikeda H, Saito T. Ethanol inhibition of neural stem cell differentiation is reduced by neurotrophic factors. Alcoholism, Clinical and Experimental Research. 2004;28:134S–8S. doi: 10.1097/01.alc.0000133538.40841.36. [DOI] [PubMed] [Google Scholar]
- Theunissen PT, Pennings JLA, Robinson JF, Claessen SMH, Kleinjans JCS, Piersma AH. Time-response evaluation by transcriptomics of methylmercury effects on neural differentiation of murine embryonic stem cells. Toxicol Sci. 2011;122:437–47. doi: 10.1093/toxsci/kfr134. [DOI] [PubMed] [Google Scholar]
- Tofighi R, Moors M, Bose R, Ibrahim WNW, Ceccatelli S. Neural stem cells for developmental neurotoxicity studies. Methods Mol Biol. 2011a;758:67–80. doi: 10.1007/978-1-61779-170-3_5. [DOI] [PubMed] [Google Scholar]
- Tofighi R, Wan Ibrahim WN, Rebellato P, Andersson PL, Uhlen P, Ceccatelli S. Non-dioxin like polychlorinated biphenyls interfere with neuronal differentiation of embryonic neural stem cells. Toxicol Sci. 2011b:192–201. doi: 10.1093/toxsci/kfr221. [DOI] [PubMed] [Google Scholar]
- Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460:1145–8. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojnits K, Bremer S. Challenges of using pluripotent stem cells for safety assessments of substances. Toxicology. 2010;270:10–7. doi: 10.1016/j.tox.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environmental Health Perspectives. 2002;110 (Suppl 5):851–4. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Przedborski S. What can pluripotent stem cells teach us about neurodegenerative diseases? Nature Neuroscience. 2010;13:800–4. doi: 10.1038/nn.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Schuldt B, Müller F-J. A guide to stem cell identification: Progress and challenges in system-wide predictive testing with complex biomarkers. BioEssays. 2011;33:880–90. doi: 10.1002/bies.201100073. [DOI] [PubMed] [Google Scholar]
- Winkler J, Sotiriadou I, Chen S, Hescheler J, Sachinidis A. The potential of embryonic stem cells combined with -omics technologies as model systems for toxicology. Curr Med Chem. 2009;16:4814–27. doi: 10.2174/092986709789909657. [DOI] [PubMed] [Google Scholar]
- Wobus AM, Loser P. Present state and future perspectives of using pluripotent stem cells in toxicology research. Arch Toxicol. 2011;85:79–117. doi: 10.1007/s00204-010-0641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Human Molecular Genetics. 2011;20:4530–9. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang S-C. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–90. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–41. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yusa K, Rad R, Takeda J, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6:363–9. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu H-X, Chen F-P, Yue L, Li X-J, Xu R-H. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS ONE. 2010;5:e11853. doi: 10.1371/journal.pone.0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–74. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]