Abstract
Objective
Epilepsy is a disease characterized by chronic seizures, but is associated with significant comorbidities between seizures including cognitive impairments, hyperactivity, and depression. To study this interictal state, we characterized the electrical, molecular, and behavior effects of chronic, neocortical interictal spiking in rats.
Methods
A single injection of tetanus toxin into somatosensory cortex generated chronic interictal spiking measured by long-term video EEG monitoring and was correlated with motor activity. The cortical pattern of biomarker activation and the effects of blocking MAPK signaling on interictal spiking and behavior were determined.
Results
Interictal spiking in this model increases in frequency, size, and becomes repetitive over time, but is rarely associated with seizures. Interictal spiking was sufficient to produce the same molecular and cellular pattern of layer 2/3-specific CREB activation and plasticity gene induction as is seen in the human interictal state (Beaumont 2011, submitted). Increasing spike frequency was associated with hyperactivity, demonstrated by increased ambulatory activity and preferential circling toward the spiking hemisphere. Loud noises induced epileptic discharges, identical to spontaneous discharges. Treatment with a selective MAPK inhibitor prevented layer 2/3 CREB activation, reduced the frequency of epileptic discharges, and normalized behavioral abnormalities, but had no effect on seizures induced by electrical kindling.
Interpretation
These results provide insights into the development of interictal epileptic spiking, their relationship to behavior, and suggest that interictal and ictal activities utilize distinct molecular pathways. This model, that parallels recent observations in humans, will be useful to develop therapeutics against interictal spiking and its behavioral comorbidities.
Introduction
Epilepsy is a disease of recurrent seizures which occurs in 0.4–1.0% of the world population (Annegers 1993; Sander 2003; Hirtz et al. 2007). The relationship of interictal spiking to seizures remains poorly understood. Human studies (Lieb et al. 1978; Lange et al. 1983; Gotman 1991) and in vitro preparations (de Curtis & Avanzini 2001) suggest that interictal spikes may be protective against seizures. However, interictal spikes are frequently highest in the same areas of brain that produce seizures and are used to identify epileptic brain regions for resections (Holmes et al. 2000; Asano et al. 2003; Marsh et al. 2009). Removing areas of high spike activity is in fact associated with a reduction of seizures (McBride et al. 1991; Bautista et al. 1999). In addition to seizures, patients with epilepsy experience a variety of comorbid conditions, such as Attention-Deficit Hyperactivity Disorder (ADHD) and other psychiatric conditions (Garcia-Morales et al. 2008). Because ‘ictal’ seizures are relatively brief and infrequent, patients with epilepsy are more often in the ‘interictal’ state. One possibility is that interictal spikes contribute to both epileptogenesis and behavioral comorbidities (Staley et al. 2011). Interictal spikes have been observed in ADHD even without seizures (Richer et al. 2002; Silvestri et al. 2007; Fonseca et al. 2008) and improved behavior is noted in patients with reduced interictal spiking (Pressler et al. 2005).
Recent genome-wide expression studies show a highly consistent pattern of layer 2/3-specific MAPK-CREB signaling and downstream gene activations in the interictal human epileptic neocortex (Rakhade 2005; Beaumont 2011, submitted). Many of these changes in human cortex vary directly in proportion to the degree of interictal spiking rather than seizures (Rakhade et al. 2007), suggesting that interictal spikes, rather than seizures, drive activity-dependent signaling and gene expression necessary for creating and maintaining the epileptic state.
Here we utilize a rat model to characterize the development of interictal spiking, localize these human molecular pathways, and demonstrate significant behavioural comorbidities (Barkmeier & Loeb 2009). We show that interictal spikes start small and grow in size and frequency, spread to involve large areas of cortex, and produce layer 2/3 specific CREB activation and downstream gene expression changes in parallel to those observed in humans. We show that interictal spikes can be induced by environmental stimuli and that interictal spiking is associated with hyperactive behaviours. Finally, we show that MAPK signalling is required for CREB activation, interictal spiking, and hyperactivity, but not for acute seizures, suggesting that therapeutics developed for interictal spiking and possibly epileptogenesis will be distinct from those currently used to treat seizures.
Materials and Methods
Animal model of interictal spiking
All studies were carried out with institutional approval (AIC protocol A01-09-06) on 4-month old male Sprague-Dawley rats kept on a 12-h light/dark cycle and implanted with 6 skull based recording screws (Small Parts, Inc., part#TX00-2-C) after tetanus toxin was stereotactically injected into somatosensory cortex as previously described (Brener et al. 1991; Nilsen et al. 2005) (AP-1mm, L 3.5mm, as measured from bregma,, depth 1.5 mm). Three screws were placed over each hemisphere at AP +4mm, −1mm and −6mm, L3.5mm relative to the bregma. A reference screw was also placed over the nasal sinus. Tetanus toxin (Sigma, catalog# T3194; 1 l at 100ng/μL in 0.01M sodium phosphate) was injected in the left somatosensory cortex. The dose varied from 65–100ng for each batch of toxin based on a dose response study to produce the same level of spiking. Recordings were made using a Stellate Harmonie recording system at 200Hz either for one-hour periods at the same time of day or every other day for 24-hour periods using video EEG monitoring.
Evolution of interictal spiking
A group of tetanus-injected (n=11) and vehicle-injected (n=5) animals were followed by EEG over three weeks and reviewed in a referential montage. Spikes were marked by a blinded reviewer and confirmed by a second. Frequency, duration, amplitude, slope, and field distribution were calculated with Matlab scripts (MathWorks, Natick, MA). Spike clusters were defined as spikes occurring within 500ms of each other. Changing this arbitrary time had little effect. Three-dimensional heatmap plots were generated using software developed by the Graphics and Imaging Lab at the Wayne State University Computer Science Department (J. Hua). The 3-dimensional rat brain model was generated from the LONI rat atlas, and the six electrodes were then added at the approximate locations of our implanted electrodes. Measured numerical values are input for each of the six electrodes, and the software then displays an associated range of colors which blend into neighboring electrodes, where high values are red and low values are green.
CREB and gene expression
Rats were sacrificed at indicated time points and examined by immunohistochemistry for phosphorylated CREB and in situ hybridization as described (Rakhade et al. 2005). In situ hybridizations were performed using sequence-verified 35S-labeled RNA made from human cDNA and EST probes from Open Biosystems (BDNF: 5193877; DUSP1: 4794895; EGR1: 6188360, truncated 3′ untranslated region; EGR2: 9919; GAPDH: 95132246CA2; NARP: 5198692; TR3: 3921259) and confirmed to share >=87% identity with the rat sequence. Sense controls showed no signal.
Open-field activity
4 tetanus-injected and 4 sham-operated (electrodes implanted, but no injections) animals were followed to measure the effect of interictal spiking on open-field ambulation using simultaneous EEG recording for one hour intervals at the same time each day (ENV-515, MedAssociates, Inc., St. Albans, Vermont). The Activity Monitor software calculated total ambulatory distance, number of ambulatory episodes, rotations, and ambulatory velocity. Other activity measurements, made without tethered EEGs, had increased ambulation compared to tethered animals. 7 tetanus-injected animals without EEGs were compared to 4 sham-operated animals. The ratio of spikes from the left/right hemisphere as well as total spike power (frequency*amplitude) were compared to the ambulatory distance, resting time, and the ratio of counter-clockwise to clockwise rotations. Statistical significance was defined as p<0.05 for Spearman’s correlation coefficient.
Auditory evoked spikes
11 spiking rats and 5 vehicle rats were monitored over time for their EEG responses to a standardized noise: a digital audio recording of a single loud clap, played at the same volume and distance. Extinction was measured using 3 successive noises 10 minutes apart, followed 10 minutes later by a train of three noises one second apart (Fig 8A). Responses were marked on the EEG files and spike amplitude was calculated using Matlab. Response amplitudes were averaged each day for the 10-minute spaced noises, while each spike from the rapid train was considered separately.
MAPK inhibition
4 rats were given the MEK inhibitor SL327 (Sigma-Aldrich, St. Louis, MO, catalog# S4069) in DMSO at 25mg/mL twice daily intraperitoneally for one week following toxin injection (25mg/kg each dose). The concentration and dosing interval was determined by serial serum and brain measurements using mass spectroscopy. Rats were sacrificed and CREB phosphorylation was determined in the brain by immunohistochemical staining. 4 tetanus toxin injected rats were treated with SL327 for one week, were followed with daily EEGs and activity recordings, and compared to tetanus toxin alone. Another group of rats received tetanus toxin and one week of DMSO injections still developed frequent spiking (data not shown). Averaged spike frequencies each week were compared between the groups for each animal for a total of three weeks. Significance was determined using a mixed-model ANOVA assessing the week (within) by group (between).
Kindling
SL327 was given to fully amygdala-kindled rats 30 minutes prior to amygdala stimulation with the same supramaximal stimulation current (500μA, 1s) used for the induction of kindling (Loscher et al. 1986). The behavioral effect of the stimulation (seizure severity score) was scored according to Racine (Racine 1972). The electroencephalographic effect of the stimulation was determined by measuring the duration of the stimulation-induced afterdischarge (afterdischarge duration) defined as an EEG activity with amplitude at least twice the amplitude of the pre-stimulus recording and a frequency greater than 1 Hz. Two days later (day 2), this procedure was repeated with the same rats 30 minutes after intraperitoneal injection of SL327 at 25mg/kg. The proportion of rats protected against secondarily generalized motor seizures (scores 3–5) was determined and used as end point for the anticonvulsant activity. The afterdischarge duration was also compared to the value measured on day 1. For amygdala kindling acquisition experiments, vehicle or SL327 (25 mg/kg) was administered intraperitoneally 30 minutes before each kindling stimulation.
Results
Interictal spikes increase, spread out, and become clustered over time
Tetanus toxin can produce seizures as well as interictal spiking when injected into the hippocampus and motor cortex (Brener et al. 1991; Finnerty & Jefferys 2002; Benke & Swann 2004; Nilsen et al. 2005). However, injection of tetanus toxin into the somatosensory cortex generates interictal discharges often with no associated seizures (Brener et al. 1991). Typically, interictal spikes from somatosensory cortex are first detected by EEG 3–5 days after injection overlying the injection site (Fig 1) and then increase in frequency with time (Fig 2A). Control (vehicle) animals show some, but significantly less spiking. Although spike frequency steadily increases, spike amplitude increases only initially, but then plateaus by 10 days (Fig 2B). The slope of the spike also progressively increases (Fig 2C), while spike duration does not change (Fig 2D). Video EEG monitoring of ten animals with tetanus injections in the somatosensory cortex revealed only a total of four seizures in one animal, consistent with observations that this model primarily produces predominantly interictal rather than ictal activities. Further studies using continuous long-term, 24-hour video EEG monitoring on a larger group of animals also demonstrated the rarity of seizures in this model (Senador et al., data not shown).
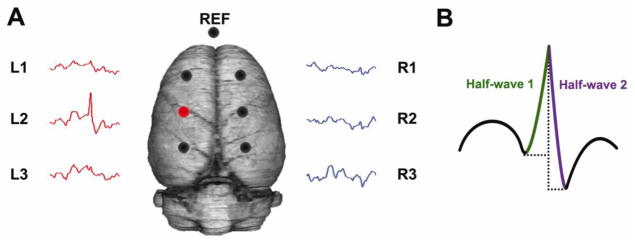
Figure 1. A chronic model of focal interictal spiking.
(A) Three skull electrodes are placed over each hemisphere, and one over the nasal sinus as a reference (circles). Tetanus toxin is injected into the somatosensory cortex under the left, middle electrode (red circle). Spikes with maximal field potentials at the injection site (L2) can be detected in 3–5 days. (B) The amplitude, duration and slope of each interictal spike were quantified by measuring these parameters in each half-wave of the spike and then summing the results.
Figure 2. Interictal spike development over time.

(A) A single injection of tetanus toxin causes interictal spike development which increases in frequency with time (n=11). Vehicle animals injected only with the carrier buffer (n=5) show very little spiking and do not change with time. Tetanus-injected animals have higher spike frequency than vehicles by the first week (p=0.029, one-tailed t-test, Bonferroni correction; 79.1±18.1 and 9.4±2.9 spikes/hour, ± sem), which persists at two weeks (p=0.020, one-tailed t-test, Bonferroni correction; 101.3±19.5 and 9.5±2.9 spikes/hour, ± sem) and three weeks (p=0.040, one-tailed t-test, Bonferroni correction; 178.33±45.3 and 8.6±4.2 spikes/hour±sem). (B) Tetanus-induced spikes initially increase in amplitude, but then plateau by 10 days (Best fit model: y=a*xb+c; R2=0.667). (C) Spike slope steadily increases with time (R2=0.530; Linear regression slope=0.307μV/day, p=0.0004), while (D) duration does not change significantly (R2=0.148; Linear regression slope=−0.299ms/day, p=0.104). Error bars are standard error of the mean.
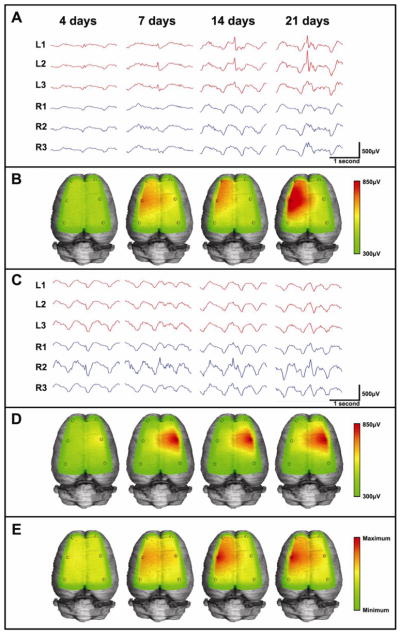
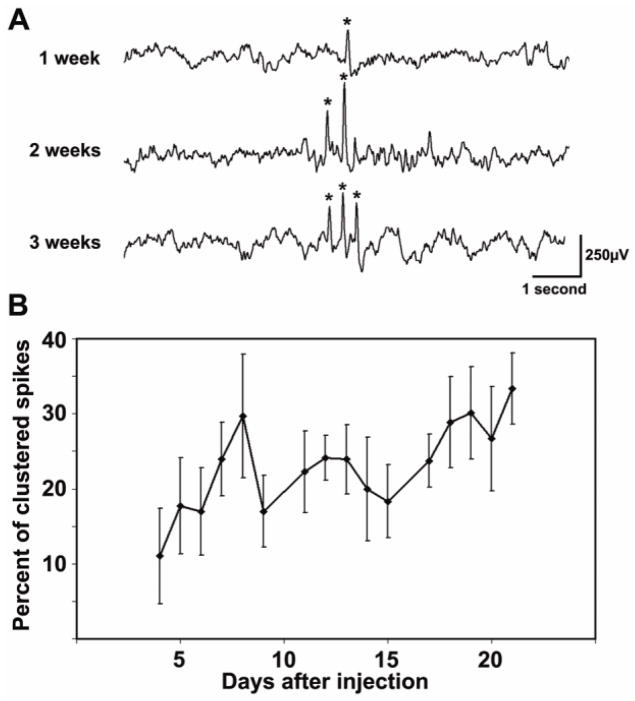
These changes in spike parameters were also accompanied by spread of the field size across the neocortex, as detected by the six recording electrodes (Fig 3). A majority of rats showed a stereotypical pattern of spike expansion that spread over time to involve the neocortex both at the injection site and anterior to it, but not posterior to it (Fig 3A–B). A second pattern of spread developed as a ‘mirror focus’ on the opposite (right) hemisphere as shown in Fig 3C–D. This mirror effect has also been reported with hippocampal injections (Brener et al. 1991; Jefferys et al. 1992). Pooling data from all rats shows the most common expansion pattern was from the injection site to the left anterior and the right middle electrodes (Fig 3E). In some animals, interictal spikes were also observed in clusters lasting at most 1–2 seconds (Fig 4). Doublets often appeared by one week after injection, followed by longer spike runs, so that by three weeks over 30% of the spikes in a recording were clustered when averaging all recorded animals. No clinical seizure activities were ever observed with these clusters.
Figure 3. Lateral expansion of spike field potentials.
(A) Sample EEG traces and (B) 3-dimensional heatmap plots are shown of a spike focus from a rat which develops spikes predominantly in the left middle (L2) and anterior (L1) channels. The heatmaps, calculated from the average amplitudes of spikes in each recording channel over time, show the spread to involve nearby electrodes. Similarly, sample EEG traces (C) and heatmaps (D) show one of the rats which developed right-sided spikes, despite injection of tetanus toxin on the left. Averaging the field distribution of 11 rats over time (E), shows that spikes begin initially at the injection site, but soon spread anterior and sometimes contralateral. No rats showed spread to posterior channels.
Figure 4. Spikes become repetitive.
(A) An EEG sample is shown from a rat that initially developed isolated spikes. By the second week, ‘doublets’ were seen, and by the third week, spikes (indicated by *) occurred repetitively with 3–6 in a row. (B) Quantifying this increase by calculating the percent of spikes that were clustered with other spikes shows a progressive increase in clustering with time (n=11; R2=0.492; Linear regression slope=0.75%/day, p=0.0025).
Interictal spiking is sufficient to induce layer-specific CREB and gene activations
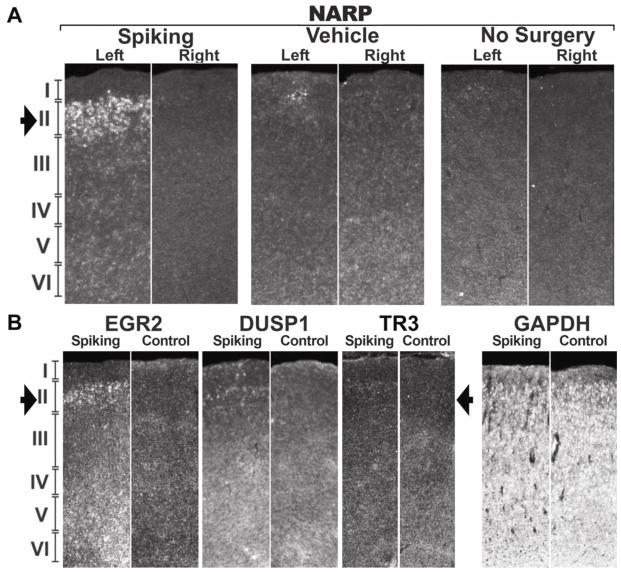
Recent observations in human neocortex have shown that interictal spiking is associated with layer-specific signaling and gene activations (Rakhade 2005, 2007; Beaumont et al. 2011, submitted). Figure 5 shows that unilateral spiking for one week is sufficient to produce a similar pattern of sustained CREB activation restricted to layer 2/3. Serial sectioned in situ hybridizations on an animal with large field potential over the left hemisphere at two weeks of spiking (Fig 5C), showed unilateral activation of activity dependent genes (Fig 5D), also similar to those observed in human epileptic cortex. None of these changes were seen in normal, surgery-naïve rats or in rats with vehicle injections and no interictal spiking (Fig 6A). While some genes are induced diffusely through all cortical layers, such as EGR1, others including NARP, EGR2, DUSP1, TR3 and BDNF are restricted to layer 2/3 like CREB (Fig 5D, 6B).
Figure 5. Lateralized and layer-specific activation of CREB and downstream plasticity genes.
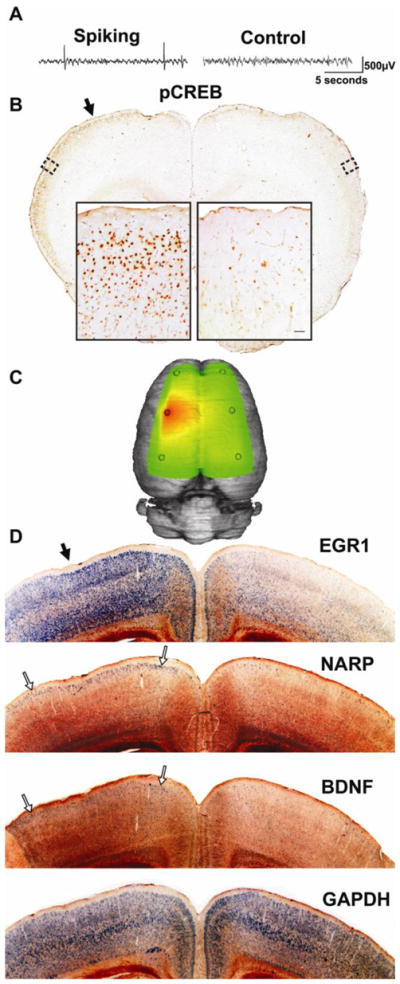
(A) A sample recording at one week after tetanus toxin injection shows interictal spikes confined to the left hemisphere. (B) At this time, most rats (n=5/7) show selective CREB phosphorylation in layer 2/3 of the spiking hemisphere relative to the contralateral, non-spiking hemisphere. This phosphorylation extends far beyond the injection site (solid arrow; slice approximately 1mm anterior to injection site). (C) A three-dimensional heatmap shows the interictal spike field size at two weeks after tetanus toxin that is associated with left-sided gene inductions shown in (D) within 1mm of the injection site (solid arrow). By two weeks after tetanus toxin injection, lateralized gene expression can be seen in 3 of 8 tetanus-injected rats, while in others it was seen bilaterally, suggesting further spread. Using radioactive in situ hybridizations, EGR1 shows robust signal throughout the cortex, but is especially strong in the superficial part of layer 2/3. NARP and BNDF, however, show relatively restricted expression in layer 2/3 of the spiking hemisphere (open arrows), while the non-spiking hemisphere shows comparatively sparse signal. The housekeeping gene GAPDH was used as a control and shows an even signal across both hemispheres in layers 2–6. This was also seen in surgery-naïve animals (n=3) for both GAPDH and all the other probes shown in this figure (data not shown). In situ hybridization images were inverted from dark-field images to improve visibility.
Figure 6. Layer-specific gene expression in interictally-spiking rats.
(A) Rats injected with tetanus toxin in the left somatosensory cortex showed layer-specific gene induction in the spiking hemisphere, while the contralateral hemisphere, vehicle-injected animals, and surgery- naïve animals did not show this increase in gene expression, as shown in this example with NARP (NPTX2). (B) Additional CREB-regulated genes show increased expression predominantly in layers 2/3, including EGR2, DUSP1 and to a lesser extent, TR3 (NR4A1). All of these genes were found to be upregulated in human epileptic neocortex and to contain conserved Cyclic AMP Response Elements.
Hyperactive behavior and environmental induction of interictal spiking
Daily measurements of ambulatory mobility showed a significant increase in ambulatory activity as interictal spiking increased in contrast to sham-treated rats (with EEG recording electrodes but no brain injection) that became less active, and more accustomed to the activity chamber over time (Fig 7). Spiking and non-spiking rats also diverged in the number of ambulatory episodes per recording session (Fig 7B), but did not differ in ambulatory velocity (Fig 7C). Total spike power correlated positively with ambulatory distance (R=0.350, p=0.039) and negatively with resting time (R=−0.410, p=0.008). Therefore, not only are interictal spiking rats hyperactive relative to control animals, but the level of their activity varies with their level of spiking.
Figure 7. Interictal spikes alter rat motility behavior.
(A) Open field activity measurements show that tetanus-treated spiking rats are consistently hyperactive relative to sham-operated controls and diverged significantly by the third week of observation (n=4 tetanus, 4 sham; 1 week: p=0.054, 8068.1±1139.0cm versus 5568.7±565.5cm; 2 weeks: p=0.098, 8146.0±1832.1cm versus 5501.3±1078.1cm; 3 weeks: p=7.68x10−5, 8641.7±338.2cm versus 4680.2±236.7cm). (B) Similarly, spiking animals also showed an increase in the number of discrete ambulatory episodes per recording session by the third week (1 week: p=0.116, 296.1±54.7 versus 215.6±42. episodes; 2 weeks: p=0.223, 277.4±49.4 versus 219.9±50.8 episodes; 3 weeks: p=0.00110, 307.0±21.3 versus 169.1±18.3 episodes). (C) The two groups did not differ in the speed of ambulation, however (1 week: p=0.824, 18.7±0.9 versus 18.4±1.3 cm/s; 2 weeks: p=0.613, 18.4±0.6 versus 18.7±0.6 cm/s; 3 weeks: p=0.402, 19.3±0.6 versus 18.7±0.8 cm/s).
The laterality of the interictal spiking correlated with the direction of movement. Video observations suggest that rats with left-sided spiking rotated more counterclockwise, while right-sided spiking rotated clockwise. Quantitative activity monitoring confirmed that the ratio between the number of spikes originating in the left hemisphere versus the number originating in the right hemisphere correlated significantly with the ratio between the number of counterclockwise and clockwise rotations (R=0.396, p=0.013).
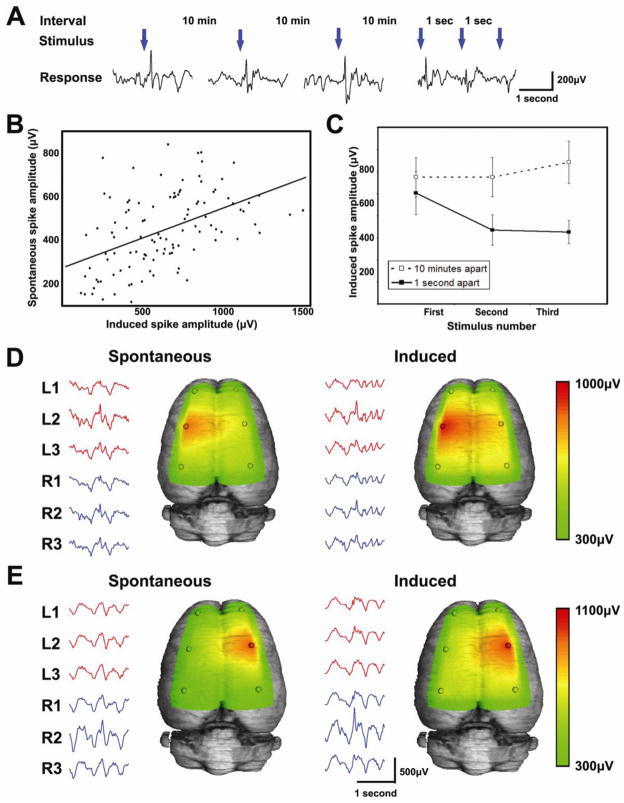
While epileptic patients and certain animal strains have seizures in response to environmental stimuli, little is known about the effect of environmental stimuli on interictal spiking. We found that rats with spontaneous interictal spiking develop evoked interictal spikes in response to a loud noise or when being physically startled (Fig 8). This only occurred in rats with spontaneous epileptic spikes and produced an electrical field potential identical to the spontaneous spike field that enlarged over time in parallel with the rat’s spontaneous spikes (Fig 8B). Figures 8A and C show that when auditory stimuli are presented every 10 minutes, they consistently produce an induced spike; however, when presented every second, the response extinguishes rapidly.
Figure 8. Induction and rapid extinguishment of interictal spikes by loud noises.
(A) Following the development of spontaneous interictal spiking, all of our rats developed inducible spikes in response to a loud noise. This was measured by presenting each rat with 3 successive noises daily, first at 10 minutes apart and later at 1 second intervals. (B) The amplitude of the induced spikes increased over time as each rat’s spontaneous spikes increased in amplitude. The scatter plot shows the correlation between the amplitude of induced spikes versus the amplitude of the rat’s spontaneous spikes (n=11; Spearman R=0.481, p=1.21x10−7). (C) When the sounds are played 10 minutes apart, response amplitude does not change. However, when the train of sounds are played at 1-second intervals, the evoked EEG response to the stimulus extinguishes rapidly (First versus second sound: 503.0±98μV versus 334.6±69.2μV; p=0.017; amplitude±sem. Second versus third: 334.6±69.2μV versus 326.0±52.4μV; p=0.874; amplitude±sem). (D, E) Rats with spontaneous left-sided spiking have induced spikes (shown by the heatmaps) that appear in the same field pattern on the left, while rats with spontaneous right-sided spiking have induced spikes on the right. All rats (n=11/11) showed induced spikes in the same field distribution as their spontaneous spikes.
MAPK-CREB signaling is required for interictal spiking but not for acute seizures
While the results in Figure 5 show activation of CREB in layer 2/3 of interictal spiking rats which parallels that seen in human epileptic neocortex, it is unclear whether the MAPK-CREB pathway is required. Figure 9A shows that the selective MAPK (MEK) inhibitor SL327 (Atkins et al. 1998; Davis et al. 2000) given twice daily for one week following tetanus toxin injection into somatosensory cortex is sufficient to block chronic CREB phosphorylation in layer 2/3. Long term EEG monitoring of rats treated with SL327 for one week showed a significant reduction in the development of interictal spiking that lasted for several weeks after the drug was discontinued (Fig 9B). Preventing the development of interictal spiking with SL327 also normalized the increased ambulatory behavior seen in spiking rats (Fig 9C). Vehicle animals showed no increase over time and ambulated almost exactly as much as the SL327-treated animals (729.4±93.2cm).
Figure 9. MAPK-CREB inhibition reduces interictal spike development, normalizes behavior, but has no acute effects on seizures.
(A) Animals given the selective MEK inhibitor SL327 for one week following tetanus toxin injection show less CREB phosphorylation relative to control animals given equivalent volumes of DMSO (Scalebar = 10μm. Qualitatively seen in n=3). (B) Animals treated with SL327 for one week after tetanus injection, but then monitored for an additional three weeks (n=4) showed significantly reduced spike frequency compared to animals that received only tetanus toxin (n=11), and were similar to animals injected with vehicle solution (n=5). A mixed model ANOVA assessing the week (within) by group (between) was significant (F(4,32) = 2.95, p < .05). Follow-up simple effects tests were significant at each of the weeks (F(2,17) = 8.27, p < .01, F(2, 17) = 8.15, p < .01, and F(2,16) = 5.50, p < .05, for weeks 1, 2 and 3, respectively). Contrast tests were all significant (F(1,17) = 16.52, p < .01, F(1, 17) = 15.82, p < .01, and F(1,16) = 10.11, p < .01, for weeks 1, 2 and 3, respectively). This effect persisted for the entire recording period, suggesting that MAPK-CREB activation is required for the development of interictal discharges. (C) Open field ambulatory distance was reduced in SL327 treated animals that displayed less spiking than untreated tetanus toxin rats (1 week: p=0.502, t-test, Bonferroni correction; 1095.2±229.5cm and 812.1±370.2cm; 2 weeks p=0.101, t-test, Bonferroni correction; 1256.3±137.1cm and 814.3±283.0cm; 3 weeks: p=0.0002, t-test, Bonferroni correction; 1720.7±187.3cm and 496.9±109.3cm). (D) During the acquisition of kindling, animals treated with SL327 (25mg/kg i.p.) 30 minutes prior to daily stimulation (500 μA, 1ms monophasic square wave pulse, 50Hz for 1s) showed minimal differences compared to vehicle-treated controls (n=9 per group). A mild proconvulsant effect was noted at stimulation #2 (p=0.032; two-tailed Mann-Whitney; n=9 per group) with an anticonvulsant effect following the sixth stimulation (p= 0.136; two-tailed Mann-Whitney; n=9 per group).
Given this effect on interictal spikes, we asked whether MAPK blockade had a similar effect on seizures. SL327 versus the DMSO vehicle were given to fully amygdala-kindled rats at the same dose used to block interictal spiking, 30 minutes prior to supramaximal amygdala stimulation (500μA, 1s). There was no reduction in acute seizures in SL327-treated animals compared to vehicle, with all animals exhibiting stage 5 seizures, and no difference in the afterdischarge duration (p=0.294; two-tailed t-test; 102±7.0 and 114±8.3 seconds, ± sem, n=9 per group). This is consistent with reports demonstrating that SL327 has no effect on pilocarpine-induced seizures in hippocampal slices (Berkeley et al. 2002). SL327 also had only minimal effects on kindling acquisition shown in Figure 9D, suggesting that MAPK signaling is not required for electrical kindling. Taken together, these data suggest that the molecular pathways and potential therapeutic targets for interictal spikes and seizures may be distinct.
Discussion
Interictal spikes: Generation of layer-specific neuronal hypersynchrony
A fundamental neocortical function is to coordinate the firing of large populations of neurons in one or more distinct brain regions. In the epileptic state, however, neuronal hypersynchrony goes too far, resulting in the unintended firing of large populations of neurons. Recent functional genomic studies identified the induction of a highly consistent pattern of genes and signaling pathways in human seizure onset zones during the interictal state (Rakhade 2005; Beaumont 2011, submitted). The most prominent of these pathways, MAPK-CREB, was highly localized to layer 2/3 and associated with a significant increase in synaptic terminals. Close correlations between gene expression levels and interictal spiking suggests either that interictal spikes are caused by or result in these layer-specific molecular changes (Rakhade et al. 2007). Here, we developed a ‘non-lesional’ rat model of chronic, neocortical interictal spiking to understand the electrical, molecular, and behavioral consequences of interictal spiking that may parallel the development of interictal spiking in human epilepsy (French et al. 1993; Mathern et al. 1994; Lhatoo et al. 2001; Mikaeloff et al. 2006). Tetanus toxin has the advantage of being cleared from the brain within a few days (Mellanby 1989) and does not cause the extensive neuronal loss associated with other models (Jefferys 1996; Sharma et al. 2007). While we cannot rule out subtle pathological changes as a result of either the tetanus toxin or the development of interictal spiking, cresyl violet staining did not show any clear histological changes in tetanus-injected animals (Barkmeier & Loeb 2009).
We demonstrate that, after an initial latent period, interictal spikes grow in amplitude, frequency, and field size over several weeks in a process that is self-sustaining and self-expanding. While the spikes in this study occur in the absence of seizures and are thus not technically “interictal”, they share the same shape, size, and duration as interictal spikes seen in seizure models, even before spontaneous seizures occur. In addition, in human epilepsies there are often regions of the brain that show “interictal” spiking, yet no seizures, suggesting that there may be different mechanisms underlying interictal spikes versus seizures. As the goal of this study was to evaluate the effects of interictal spikes specifically, the dose of tetanus toxin used was calibrated such that only a single animal had an observed seizure, thereby isolating the effects of spiking alone. While seizures are extremely rare, spikes can become clustered in a pattern suggesting they may be a precursor to seizure development (Staley et al. 2005). This is supported by a recent study showing the frequency and clustering of spikes prior to the development of seizures can predict which animals will go on to develop spontaneous seizures following kainic acid-induced status epilepticus (White et al. 2010). It is thus intriguing to speculate that therapeutics that block the propagation or clustering of interictal spikes could be antiepileptogenic (Loeb 2011).
Interictal spiking was sufficient to induce both CREB phosphorylation and downstream activity-dependent gene expression in a layer-specific pattern that closely parallels those seen in chronically active human epileptic neocortex (Rakhade 2005; Beaumont 2011, submitted). Activated CREB and downstream plasticity genes were highest in layer 2/3 brain regions showing the largest epileptic field potentials, suggesting that ongoing interictal activity drives layer-specific changes in plasticity resulting in the progressive enlargement of regions with increased neuronal hypersynchrony. Consistently, rats where a mirror focus developed in the right hemisphere, despite left-sided tetanus toxin injection, CREB and downstream gene activations were present both on the left and right sides relative to naive controls, suggesting a more widespread pattern of neuronal synchrony across both hemispheres. These spatially-restricted and laminar-specific activation patterns parallel those we have seen in human neocortical epilepsy, suggesting that this rat model is a valid model of the human disease that could be useful for pharmacological interventions.
MAPK-CREB inhibition prevents interictal spiking
Given the strong, layer-specific activation of the MAPK-CREB pathway in both human neocortical epilepsy and this animal model, we determined the effect of a targeted MAPK inhibitor. The selective MEK inhibitor, SL327, not only prevented layer 2/3 CREB phosphorylation, but markedly reduced interictal spiking. Because the interictal spiking focus continues to increase in size and frequency over time, well after the initiating tetanus toxin has been cleared from the brain, one potential explanation for the long-lasting effects of SL327 is that MAPK is required for both the initial synaptic reorganization as well as a positive feedback loop that leads to the lateral spread of aberrant synaptic connections associated with the generation and growth of epileptic potentials. These findings raise the possibility that blocking this pathway within a well-defined therapeutic window after an insult to the brain could prevent the synaptic reorganization required for interictal spiking and possibly seizures. The observation that this same drug had no effect on acute seizures and kindling suggests that therapeutics directed at interictal spiking may not work on seizures and vice versa (Loeb 2011). While the current study is limited by sample size and a single dose and treatment strategy, larger scale studies that explore the effects of MAPK inhibition at different time points and using a variety of other interictal and ictal models will be needed to fully characterize the effects of MAPK and CREB activation on both ictal and interictal development.
Behavioral consequences of interictal spiking
Not only did MAPK inhibition reduce interictal spiking, it also normalized ambulatory behavior, suggesting that some of the hyperactive behavioral changes seen in these animals could be directly due to interictal spiking. This is interesting in the context of reports showing that ADHD is frequently associated with epilepsy (Garcia-Morales et al. 2008) and that interictal discharges are more common in children with ADHD than in normal children, even in the absence of clinical seizures (Richer et al. 2002; Holtmann et al. 2003; Becker et al. 2004; Boutros et al. 2005; Silvestri et al. 2007; Fonseca et al. 2008). Further evidence that interictal spiking has a direct effect on behavior comes from the observation that lateralization of interictal spiking was associated with asymmetric ambulation (rotations). It is possible that activation of the ipsilateral somatosensory cortex produces an uncomfortable sensation contralaterally resulting in movements away from the sensation. Behavioral effects of interictal spiking appear to be region-dependent, as hippocampal spikes are associated with cognitive disruption (Kleen et al. 2010) and spikes during neonatal development impair reference memory and long-term potentiation in these rats as adults (Khan et al. 2010).
Finally, we show that interictal spikes can be induced by the environment only in spontaneously-spiking rats. These induced spikes occur in the same field distribution as each rat’s spontaneous spikes, implying that the same population of neurons is being activated. This is perhaps the first observation that a common environmental stimulus such as sound or startle can alter interictal spiking activity. Taken together, these observations have clinical importance for patients with neuropsychiatric disorders, as they suggest that environmental stimuli can modulate interictal spiking, which, in turn, can modulate behavior.
Highlights.
A model of isolated interictal spiking in the rat neocortex was characterized
Interictal spiking develops gradually and spreads to larger cortical areas
Interictal spiking leads to layer specific gene and pathway activation that can be blocked with a MAPK inhibitor
Interictal spiking produces hyperactive behaviors
Acknowledgments
This work was funded by NIH/NINDS R01NS045207 and R01NS058802 (JAL), and also supported in part by the State of Michigan Joe Young Funds to the Department of Psychiatry and Behavioral Neurosciences (NNB). We thank Dr. Jing Li of the Karmanos Cancer Institute for helping with pharmacokinetic studies, Dr. Darren Fuerst for statistical analysis, and Dr. Ruggero Serafini for reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annegers JF. The Epidemiology of Epilepsy. In: Wyllie E, editor. The Treatment of Epilepsy: Principles and Practices. Lea & Febiger; Philadelphia: 1993. pp. 157–64. [Google Scholar]
- Asano E, Muzik O, Shah A, Juhasz C, Chugani DC, Sood S, Janisse J, Ergun EL, Ahn-Ewing J, Shen C, Gotman J, Chugani HT. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia. 2003;44:425–34. doi: 10.1046/j.1528-1157.2003.38902.x. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–9. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Barkmeier DT, Loeb JA. An animal model to study the clinical significance of interictal spiking. Clin EEG Neurosci. 2009;40:234–8. doi: 10.1177/155005940904000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40:880–90. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Becker K, Sinzig JK, Holtmann M. Attention deficits and subclinical epileptiform discharges: are EEG diagnostics in ADHD optional or essential? Dev Med Child Neurol. 2004;46:431–2. doi: 10.1017/s0012162204230705. [DOI] [PubMed] [Google Scholar]
- Benke TA, Swann J. The tetanus toxin model of chronic epilepsy. Adv Exp Med Biol. 2004;548:226–38. doi: 10.1007/978-1-4757-6376-8_16. [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82:192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Boutros N, Fraenkel L, Feingold A. A four-step approach for developing diagnostic tests in psychiatry: EEG in ADHD as a test case. J Neuropsychiatry Clin Neurosci. 2005;17:455–64. doi: 10.1176/jnp.17.4.455. [DOI] [PubMed] [Google Scholar]
- Brener K, Amitai Y, Jefferys JG, Gutnick MJ. Chronic Epileptic Foci in Neocortex: In Vivo and In Vitro Effects of Tetanus Toxin. Eur J Neurosci. 1991;3:47–54. doi: 10.1111/j.1460-9568.1991.tb00810.x. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–72. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–67. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Jefferys JG. Investigation of the neuronal aggregate generating seizures in the rat tetanus toxin model of epilepsy. J Neurophysiol. 2002;88:2919–27. doi: 10.1152/jn.00211.2002. [DOI] [PubMed] [Google Scholar]
- Fonseca LC, Tedrus GM, Moraes C, Vicente Machado A, Almeida MP, Oliveira DO. Epileptiform abnormalities and quantitative EEG in children with attention-deficit/hyperactivity disorder. Arq Neuropsiquiatr. 2008;66:462–7. doi: 10.1590/s0004-282x2008000400004. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–80. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Garcia-Morales I, de la Pena Mayor P, Kanner AM. Psychiatric comorbidities in epilepsy: identification and treatment. Neurologist. 2008;14:S15–25. doi: 10.1097/01.nrl.0000340788.07672.51. [DOI] [PubMed] [Google Scholar]
- Gotman J. Relationships between interictal spiking and seizures: human and experimental evidence. Can J Neurol Sci. 1991;18:573–6. doi: 10.1017/s031716710003273x. [DOI] [PubMed] [Google Scholar]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–37. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure. 2000;9:407–11. doi: 10.1053/seiz.2000.0423. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Becker K, Kentner-Figura B, Schmidt MH. Increased frequency of rolandic spikes in ADHD children. Epilepsia. 2003;44:1241–4. doi: 10.1046/j.1528-1157.2003.13403.x. [DOI] [PubMed] [Google Scholar]
- Jefferys JG. Chronic epileptic foci induced by intracranial tetanus toxin. Epilepsy Res Suppl. 1996;12:111–7. [PubMed] [Google Scholar]
- Jefferys JG, Evans BJ, Hughes SA, Williams SF. Neuropathology of the chronic epileptic syndrome induced by intrahippocampal tetanus toxin in rat: preservation of pyramidal cells and incidence of dark cells. Neuropathol Appl Neurobiol. 1992;18:53–70. doi: 10.1111/j.1365-2990.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- Khan OI, Zhao Q, Miller F, Holmes GL. Interictal spikes in developing rats cause long-standing cognitive deficits. Neurobiol Dis. 2010 doi: 10.1016/j.nbd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010;67:250–7. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange HH, Lieb JP, Engel J, Jr, Crandall PH. Temporo-spatial patterns of pre-ictal spike activity in human temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1983;56:543–55. doi: 10.1016/0013-4694(83)90022-6. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Sander JW, Fish D. Temporal lobe epilepsy following febrile seizures: unusually prolonged latent periods. Eur Neurol. 2001;46:165–6. doi: 10.1159/000050796. [DOI] [PubMed] [Google Scholar]
- Lieb JP, Woods SC, Siccardi A, Crandall PH, Walter DO, Leake B. Quantitative analysis of depth spiking in relation to seizure foci in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1978;44:641–63. doi: 10.1016/0013-4694(78)90130-x. [DOI] [PubMed] [Google Scholar]
- Loeb JA. Identifying targets for preventing epilepsy using systems biology. Neuroscience Letters. 2011;497:205–12. doi: 10.1016/j.neulet.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Jackel R, Czuczwar SJ. Is amygdala kindling in rats a model for drug-resistant partial epilepsy? Experimental neurology. 1986;93:211–26. doi: 10.1016/0014-4886(86)90160-3. [DOI] [PubMed] [Google Scholar]
- Marsh ED, Peltzer B, Brown MW, Iii, Wusthoff C, Storm PB, Jr, Litt B, Porter BE. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. Traumatic compared to non-traumatic clinical-pathologic associations in temporal lobe epilepsy. Epilepsy Res. 1994;19:129–39. doi: 10.1016/0920-1211(94)90023-x. [DOI] [PubMed] [Google Scholar]
- McBride MC, Binnie CD, Janota I, Polkey CE. Predictive value of intraoperative electrocorticograms in resective epilepsy surgery. Ann Neurol. 1991;30:526–32. doi: 10.1002/ana.410300404. [DOI] [PubMed] [Google Scholar]
- Mellanby JH. Elimination of 125I from rat brain after injection of small doses of 125I-labelled tetanus toxin into the hippocampus. Neurosci Lett. 1989;(Suppl 36):S55. [Google Scholar]
- Mikaeloff Y, Jambaque I, Hertz-Pannier L, Zamfirescu A, Adamsbaum C, Plouin P, Dulac O, Chiron C. Devastating epileptic encephalopathy in school-aged children (DESC): a pseudo encephalitis. Epilepsy Res. 2006;69:67–79. doi: 10.1016/j.eplepsyres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Nilsen KE, Walker MC, Cock HR. Characterization of the tetanus toxin model of refractory focal neocortical epilepsy in the rat. Epilepsia. 2005;46:179–87. doi: 10.1111/j.0013-9580.2005.26004.x. [DOI] [PubMed] [Google Scholar]
- Pressler RM, Robinson RO, Wilson GA, Binnie CD. Treatment of interictal epileptiform discharges can improve behavior in children with behavioral problems and epilepsy. J Pediatr. 2005;146:112–7. doi: 10.1016/j.jpeds.2004.08.084. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Shah AK, Agarwal R, Yao B, Asano E, Loeb JA. Activity-Dependent Gene Expression Correlates with Interictal Spiking in Human Neocortical Epilepsy. Epilepsia. 2007;48:86–95. doi: 10.1111/j.1528-1167.2007.01294.x. [DOI] [PubMed] [Google Scholar]
- Rakhade SN, Yao B, Ahmed S, Asano E, Beaumont TL, Shah AK, Draghici S, Krauss R, Chugani HT, Sood S, Loeb JA. A common pattern of persistent gene activation in human neocortical epileptic foci. Ann Neurol. 2005;58:736–47. doi: 10.1002/ana.20633. [DOI] [PubMed] [Google Scholar]
- Richer LP, Shevell MI, Rosenblatt BR. Epileptiform abnormalities in children with attention-deficit-hyperactivity disorder. Pediatr Neurol. 2002;26:125–9. doi: 10.1016/s0887-8994(01)00370-8. [DOI] [PubMed] [Google Scholar]
- Sander JW. The epidemiology of epilepsy revisited. Current opinion in neurology. 2003;16:165–70. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicologic pathology. 2007;35:984–99. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- Silvestri R, Gagliano A, Calarese T, Arico I, Cedro C, Condurso R, Germano E, Vita G, Tortorella G. Ictal and interictal EEG abnormalities in ADHD children recorded over night by video-polysomnography. Epilepsy Res. 2007;75:130–7. doi: 10.1016/j.eplepsyres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–6. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Staley K, White A, Dudek FE. Interictal spikes: Harbingers or causes of epilepsy? Neuroscience Letters. 2011;497:247–50. doi: 10.1016/j.neulet.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Williams PA, Hellier JL, Clark S, Edward Dudek F, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2010;51:371–83. doi: 10.1111/j.1528-1167.2009.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]