Abstract
B lymphocytes exhibit phenotypic differences that correlate with their developmental or functional stages and affect humoral immune responses. One recently described subset of naturally occurring immature transitional type 3 (T3) B lymphocytes is believed to consist of potentially autoimmune cells whose signaling properties have not been studied in detail. This study characterizes intracellular signaling in T3 B cells in wildtype C57BL/6 mice. Protein phosphorylation and Ca2+ responses upon B cell antigen receptor (BCR) engagement were measured by multicolor flow cytometry. We observed high baseline signaling activity and reduced BCR-mediated responses in T3 cells, which confirmed their anergy – a functional state in which lymphocytes recognize chronically present self-antigens but cannot produce immune response due to intrinsic signaling inhibition. Our results also revealed a previously unknown T3-specific phosphorylation pattern of 24 key signaling molecules involved in BCR signal transduction. These characteristics reflect the balance between stimulatory and inhibitory BCR signaling pathways in anergy. Results obtained in the collagen induced arthritis model demonstrate the loss of anergy in T3 B cells during the onset of the disease. Our findings provide rationale for further investigating alterations in B cell signaling patterns as earliest functional biomarkers of changes in the immune tolerance of autoreactive B cells.
Keywords: B cells, tolerance, clonal anergy, BCR signal transduction, protein phosphorylation, Ca2+ signaling
1. INTRODUCTION
Protein phosphorylation and intracellular Ca2+ mobilization upon antigen receptor engagement are among major signal transduction parameters characterizing the activation status of B lymphocytes. A recently recognized concept of the “digital” (on/off) nature of regulation in intracellular signal transduction pathways emphasizes a delicate balance between stimulatory and inhibitory signaling cascades that allows lymphocytes to distinguish between very closely related antigens [1]. This concept, based upon kinetic threshold proofreading model [2] provides rationale for detailed characterization of signaling pathways in potentially autoimmune B cells.
It has been estimated that nearly half of newly produced B cells are autoreactive and must be eliminated or tolerized to prevent autoimmunity [3, 4]. During development, B cells go through consecutive checkpoints to eliminate potential anti-self-specificity. Four major mechanisms are responsible for induction and maintenance of B cell immune tolerance. First, the classical Burnet’s clonal deletion eliminates cells that express self-reactive antigen receptor early in development through activation of FAS and inhibition of the BCL-2 survival pathway. The second mechanism is receptor editing, which through additional V(D)J recombination or somatic hypermutation at later developmental stages generates “corrected” cells with dramatically lower affinity of anti-self receptors (Nemazee and Hogquist, 2003). Up to 30% of mature B cells have undergone receptor editing and are not autoreactive due to changes in receptor specificity [5]. The third mechanism has been termed receptor tuning (also known as clonal anergy) and relies on intrinsic signal transduction events that reduce the ability of B cells to become activated through the self-reactive receptor as well as BCR downregulation (Healy and Goodnow, 1998). Anergic B cells can recognize chronically present antigen but are unable to produce immune response due to intrinsic signaling inhibition. These B cells, though not long-lived, are vulnerable to autoimmune activation, as they may bind cross-reactive autoantigens [6]. The fourth regulatory mechanism is comprised of various microenviromental factors such as intercellular interactions and availability of costimulatory molecules, supply of cytokines, growth factors, inflammatory mediators and other factors (BAFF, IL-7, CD40L, B7) (Kalled, 2005; Lesley et al., 2004; Mackay et al., 2003).
Growing evidence suggests that these tolerogenic mechanisms can be compromised during autoimmune processes [7]. This study focuses on clonal anergy as a means to understand why and how an already established B cell tolerance may be overcome in autoimmunity. Phenotypically distinct subsets of anergic B cells have been identified in both mice and humans [6, 8]. Murine models of autoimmunity demonstrate that autoreactive anergic B cells stay developmentally “arrested” at different (model-specific) immature stages, including the T3 stage. In mice, this B cell subset is identified as B220+/CD93+/IgM−/low/CD23+ [9].
Several important signal transduction characteristics of B cell anergy have been described. One major feature is the failure of the anergic B cell antigen receptor (BCR) to transduce proximal activation signals further to downstream signaling cascades [10, 11]. In addition, in some murine autoimmunity models chronic exposure of B cells to an antigen results in continuously elevated phosphorylation of signaling molecules involved in BCR signaling cascades and is often accompanied by increased intracellular Ca2+ levels [12, 13]. Signaling through co-receptors engaged in the BCR signaling complex is also important as it has been demonstrated that complement-opsonized immunogens that crossreact with autoantigens can break B cell anergy through a BCR/CD21 co-stimulatory mechanism and that the resultant Ab responses are independent of germinal centers [13]. Other factors such as interaction with FAS ligand-expressing CD4+ T cells [14], increased expression of CD86 [15], T-B cooperation [16], failure of anergic B cells to enter the B-cell follicular areas [17] and altered interactions with antigen presenting machrophages and dendritic cells [18, 19] have also been demonstrated to play a role in the maintenance of B cell unresponsiveness towards self-antigens.
Mechanisms involved in averting BCR-triggered signal transduction in anergic B cells are poorly understood and may include ineffective molecular associations within the BCR signaling complex (IgM(IgD)/CD79/CD19/CD21) upon Ag ligation and/or proximal inhibition of the activation signal by phosphatases. Increases in antigen dose and avidity may also contribute to overcoming unresponsiveness to a specific Ag as demonstrated in insulin-specific anergic B cells [20]. This indicates that multiple signaling mechanisms are involved in maintaining the threshold of B cell unresponsiveness and that anergic B cells are not the result of only genetically regulated antigen specificity selection processes. The transient nature of B cell anergy is supported by findings that intact anergic B cells typically express costimulatory molecules at levels comparable to those of normal B cells and are capable of eliciting T cell-mediated responses to antigens presented in the T cell context [20–22]. This suggests that induction of B cell anergy is also influenced by secondary signals/co-stimulants that must be obtained within a certain time period in addition to BCR ligation with antigen and in the absence of such signals B cells can either undergo apoptosis or become anergic. Consistent with this, it has been demonstrated that a prolonged exposure of intact B cells to an antigen may result in transformation into an anergic phenotype [12, 23], whereas signaling features of anergy can be reversed upon withdrawal of the antigen` [18, 24].
The recently described naturally occurring population of B220+/CD23+/CD93+/IgM−/low murine T3 B cells with anergic phenotype [6] has several characteristic features that make this subset a promising candidate to be studied in autoimmunity. These cells are: (a) phenotypically close to mature B cells but have no prior apparent immune experience; (b) express high levels of self-reactive antigen receptors; (c) capable of producing autoreactive Abs (nuclear antigens); (d) bind antigen but do not produce immune response and may exhibit reduced Ca2+ mobilization and protein phosphorylation upon BCR crosslinking.
This study identifies several signaling characteristics in the transitional T3 B cell subset, including the overall reduced signaling response amplitude and subset-specific phosphorylation pattern for key signaling molecules involved in BCR signal transduction. These characteristics reflect anergy-specific balancing of stimulatory/inhibitory BCR signaling cascades and represent an important mechanism that prevents autoimmunity.
2. MATERIALS AND METHODS
2.1. Animals
C57BL/6 and DBA/1J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). 8–12 week old mice were used for experiments. Animals were housed and bred at the University of Colorado Denver School of Medicine animal facility in accordance with institutional guidelines.
2.2. Flow cytometry analysis
Isolated murine splenocytes underwent erythrocyte lysis in the Red blood cell lysing buffer (Sigma, St. Louis, MO). Cells were washed with IMDM supplemented with 2.5% FCS and stained with fluorescently labeled Abs as indicated. For Ca2+ analysis by flow cytometry, cells were incubated with Indo-1AM (Carlsbad, CA) at 5 mM for 30 min at 37°C and co-stained with a cocktail of anti-mouse CD23 FITC, CD24 PerCp-Cy5.5, CD93 PE-Cy7, IgM APC (monovalent Fab), B220 APC-Cy7; anti-mouse CD19, CD1d, CD21 were PE-conjugated. Abs specific to surface markers were obtained from Beckton Dickinson (San Jose, CA) and eBioscience (San Diego, CA). Polyclonal goat F(ab)2 anti-mouse Ig(H+L) (Southern Biotech, Birmingham, AL), referred to in the text as anti-BCR, was used to stimulate cells. One minute after data acquisition was started, anti-BCR Ab (5µg/ml) was added. For intracellular phosphoprotein staining, purified splenocytes were stimulated with anti-BCR for 8min, then rapidly fixed and permeabilized in 20x volume of BD Cytofix/Cytoperm buffer and stained with a cocktail of surface marker Abs (as described above) and with phospho-specific Abs followed by fluorescently labeled secondary Ab. Phospho-specific Abs were obtained from Cell Signaling Technology (Danvers, MA) or Santa Cruz Biotechnology (Santa Cruz, CA). Experiments were performed at room temperature on BD LSR II flow cytometer (Beckton Dickinson, San Jose, CA). B cell subsets of interest were gated upon as indicated and protein phosphorylation intensity (mean fluorescence) or intracellular Ca2+ influx were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). Fluorescence intensity of phospho-specific Ab staining within the subsets was compared between stimulated and nonstimulated cells. Experiments were performed in triplicate for each signaling molecule. For each experiment (i.e. cells obtained from one mouse), triplicates of mean fluorescence intensity (MFI) values for each signaling molecule for each condition (nonstimulated/stimulated with anti-BCR) were averaged. Averaged triplicates from multiple mice were then compiled into a total average for each signaling molecule. Online Supplemental Figure 1A illustrates experimental design and data analysis algorithm. Online Supplemental Figure 1B shows representative examples of the actual flow data (overlay histograms) and corresponding MFI values generated by FlowJo software. n values in figure legends represent the number of mice. Statistical analysis was performed with Prism software (GraphPad, San Diego, CA).
2.3. Western blot analysis
Intact B cells were isolated from splenic leukocyte homogenates with MACS B cell isolation kit (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s protocol. Purified B cells (2×106/ml) were treated with anti-BCR (5µg/ml) for 8min and then immediately lysed in cold 0.5% CHAPS buffer (150mM NaCl, 10 mM Tris, 10 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1 mM PMSF, 10 mM NaF, 0.4 mM EDTA, 1 mM aprotinin, 1 mM antitrypsin and 1 mM leupeptin, (Sigma, St. Louis, MO), pH 7.5). Lysateswere kept on ice for 30 min, and then centrifuged at 10,000 rpm for 10 min at 4°C. Supernatants were mixed with Laemmli SDS reducing sample buffer (Sigma, St. Louis, MO) and heated for 5 min at 95°C. Proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes and visualized using phospho-specific Abs in conjunction with ECL (PerkinElmer, Boston, MA). Loading controls were done with Ponceau S (BioRad Laboratories, Hercules, CA) staining as follows: membranes were washed from ECL reagent, incubated with Ponceau S for 10 min, washed again and scanned for digital densitometry analysis (whole lanes were boxed and average density was compared between the lanes; obtained values differed by <5%). Densitometry analysis was performed with NIH Image software.
2.4 Experimentally induced arthritis
Collagen-induced arthritis was induced as described [25]. Briefly, 8–10 week old DBA/1J mice were immunized subcutaneously with bovine type II collagen (Elastin Products, MO) at 4 mg/mouse and given repeated immunization at day 21. Animals developed visible sign of arthritic joint inflammation after day 28 (data not shown), at which time splenocytes were harvested for flow cytometry analysis. There were no significant phenotypical differences between T3 B cells from C57BL/6 and DBA/1J mice.
3. RESULTS
3.1. Transitional T3 B cells exhibit elevated baseline pTyr levels and reduced protein phosphorylation in response to BCR ligation
Freshly isolated live murine splenocytes were stained with a cocktail of fluorescently labeled Abs specific to B cell surface molecules (B220, CD93, IgM, CD23). Transitional B220+/CD93+ B cells were gated upon and then transitional type T3 B cells were further differentiated based on CD23+ and IgMlow expression [6] (Figure 1A). The ungated IgMhigh/CD23+/− B cells consist of transitional type 1 and 2 cells [6] whose signaling characteristics are beyond the scope of this study. In conjunction with the surface staining, total tyrosine phosphorylation was measured in fixed/permeabilized cells co-stained intracellularly with fluorescently labeled anti-pTyr mAb (PY20). Transitional T3 cells exhibited elevated baseline pTyr levels (Figure 1B) and a reduction in protein phosphorylation in response to BCR ligation (Figure 1C) as compared to mature naïve cells comprising the bulk of B220+ population.
Figure 1.
(A) Gating strategy to distinguish transitional type 3 (T3) B cells: B220+/CD93hi/IgMlo/CD23+. (B) Total phosphotyrosine (pTyr) baseline (filled bars) and after stimulation with polyclonal anti-BCR (superimposed clear bars) in B220+ and T3 B cell subsets; mean fluorescence intensity (MFI) of intracellular staining with anti-pTyr mAb PY20. (C) pTyr responses to anti-BCR in B220+ and T3 B cell subsets normalized as % increase over corresponding unstimulated controls (n=44). Statistically significant differences (Student’s T-test) marked with *; data shown as mean +/− SEM with p values.
3.2. Anergic pattern of BCR-induced protein phosphorylation in T3 B cells
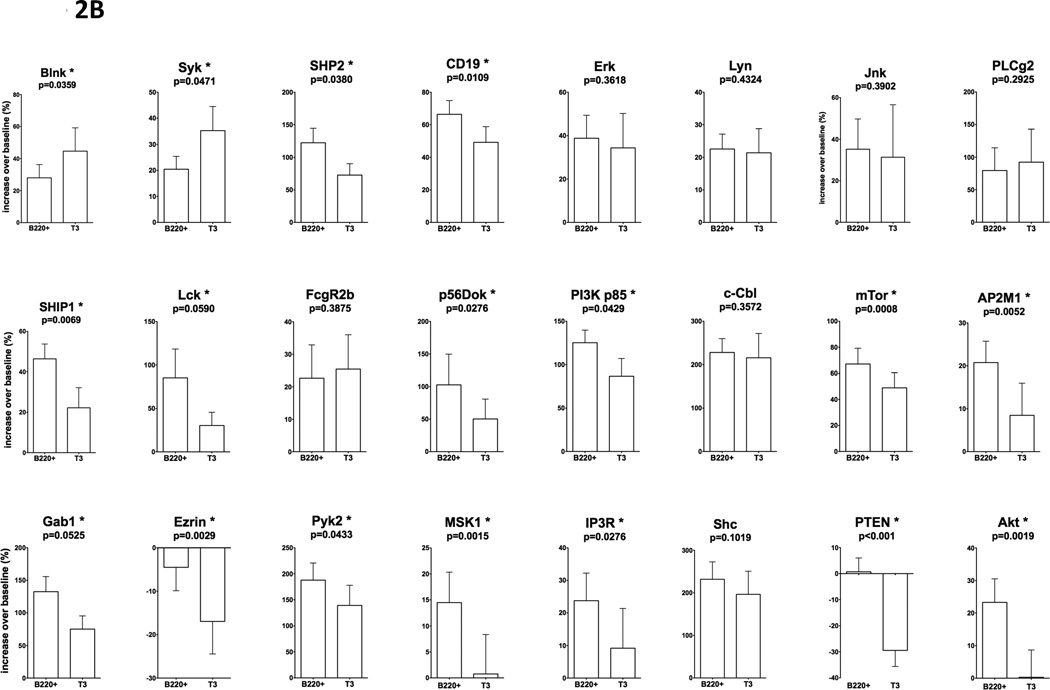
In order to characterize signaling pathways that determine signaling features of transitional T3 B cells described above, we examined phosphorylation of key signaling molecules involved in BCR-mediated pathways. We focused on a panel of 24 signal transduction proteins involved in pathways leading to several major outcomes of lymphocyte activation: Ca2+ mobilization, lipid raft aggregation, BCR internalization, integrin-dependent cytoskeletal rearrangements, glucose uptake and generation of ATP, transcription factors activation, protein synthesis, growth arrest and apoptosis (highlighted in yellow in Figure 6). We compared phosphorylation levels of individual signaling molecules in B220+ and T3 B cells (baseline and in response to BCR ligation). Results are shown in Figure 2. For each signaling molecule filled bars indicate baseline phosphorylation levels in unstimulated cells (Figure 2A), and in clear bars - phosphorylation in response to BCR ligation normalized as % increase over corresponding unstimulated controls (Figure 2B). Significant differences between pairs are indicated for each molecule. Statistical analysis (Student’s T-test) was based on sample sizes ranging from 7 to 21 independent experiments for each molecule. Several signaling characteristics were identified in T3 B cells that distinguish this subset from mature B220+ B cells. In intact (unstimulated) T3 cells baseline phosphorylation levels of Blnk, Syk, Lck, Cbl, Pyk2, Shc, PTEN tended to be higher with p values within 90% confidence (p<0.1).
Figure 6.
Schematic representation of signaling pathways alterations in transitional T3 B cells as compared B220+ cells.
Figure 2.
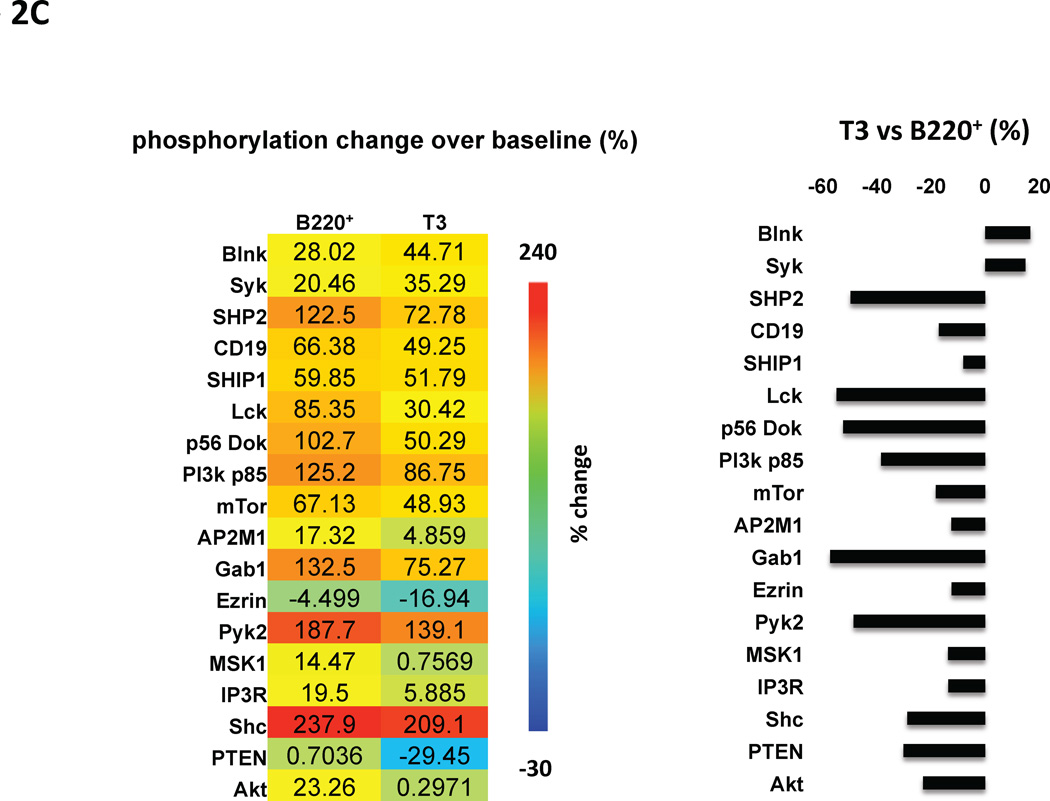
Phosphorylation levels of major signaling molecules in intact or anti-BCR stimulated B220+ or T3 B cell subsets (C57BL/6). For each signaling molecule filled bars indicate baseline phosphorylation levels (MFI) in unstimulated cells (A), and clear bars – phosphorylation in response to BCR ligation normalized as % increase over corresponding unstimulated controls (B). Statistically significant differences between pairs are marked. Independent experiments were averaged: Blnk n=8; Syk n=9; SHP2 n=21; CD19 n=23; Erk n=9; Lyn n=11; Jnk n=7; PLCγ2 n=9; SHIP1 n=17; Lck n=6; FcγRIIb n=6; Dok2 p56 n=4; PI3K p85 n=12; c-Cbl n=12; mTor n=12; AP2M1 n=12; Gab1 n=10; Ezrin n=12; Pyk2 n=11; MSK1 n=12; IP3R n=12; Shc n=11; PTEN n=10; Akt n=12). Student’s T-test statistical analysis; data shown as mean +/− SEM with p values for B220+ vs. T3. Heatmap representation of statistically significant changes in the phosphorylation of signaling molecules calculated as % increase over corresponding unstimulated controls (C, left panel). Relative comparison of statistically significant phosphorylation changes in response to anti-BCR between B220+ and transitional T3 B cell subset (C, right panel).
In anti-BCR stimulated B cells differences in phosphorylation of individual signaling molecules were significantly more pronounced (p<0.05) with increased phosphorylation of Syk and Blnk and decreased phosphorylation of CD19, Akt, mTor, IP3R, AP2M1, PTEN, p56 Dok, SHIP1, Ezrin, MSK1, Lck, SHP2, PI3K p85, Gab1, Pyk2, Shc. These findings are summarized in Figure 2C (left panel) with the heatmap representing changes in the phosphorylation of signaling molecules in each cell subset calculated as % increase over corresponding unstimulated controls. Figure 2C (right 1 panel) outlines a comparison of signaling patterns in the T3 B cell subset normalized to the B220+ population. The results in the context of BCR signaling pathways are illustrated in Figure 6.
3.3. T3 B cells have increased baseline cytoplasmic Ca2+ levels and reduced amplitude of Ca2+ response to BCR stimulation
We examined baseline intracellular Ca2+ levels and amplitudes of BCR-mediated responses in T3 vs. mature B220+ B cell subsets. In these experiments B cells were labeled with the same surface staining Ab cocktail as above, and with cell-permeable Ca2+ probe Indo-1AM. T3 B cells had significantly increased overall baseline cytoplasmic Ca2+ levels (Figure 3 left panel; statistical analysis as above). Amplitudes (% change from baseline) of Ca2+ responses to anti-BCR stimulation were also significantly reduced in T3 cells (Figure 3, right panel).
Figure 3.
(A) Intracellular Ca2+ levels in B220+ and T3 B cells (C57BL/6). Filled bars represent baseline in intact cells and superimposed clear bars – increases in response to anti-BCR stimulation. (B) Amplitude of Ca2+ responses in BCR-stimulated B220+ and T3 B cells normalized as % increase over corresponding baseline in intact cells. Statistically significant differences between pairs (Student’s T-test) are marked with * /**. Data in each graph is shown as mean +/− SEM with p values for B220+ vs. T3; n=40. n values represent the number of mice and samples from each mouse were analyzed in triplicate.
3.4. Phenotypical heterogeneity of B cells exhibiting high, intermediate and low amplitudes of BCR-mediated Ca2+ responses
We evaluated surface marker expression profiles in B cells exhibiting a range of amplitudes of BCR-mediated Ca2+ responses (Figure 4A). This approach allowed us to discern similarities between low responding B220+ cells and specifically T3 B cells as well as address the presence of additional B cell subsets that are hyporesponsive to BCR stimulation. Results are shown in Figure 4B (repeated measures ANOVA analysis followed by Bonferroni's Multiple Pairs Comparison Test was based on the following sample sizes: IgM n=41; B220 n=44; CD23 n=45; CD19 n=10; CD21 n=15; CD1d n=9; CD24 n=25; CD93 n=44; average Ca2+ level for cells in each gate, n=44). Surprisingly, the amplitude of Ca2+ response inversely correlated with surface IgM expression. Lower responses strongly correlated with upregulation of CD1d, CD24 and CD93 (p<0.05). Low responding B cells also tended to have lower CD19 expression, although in our data set this was not statistically significant (p=0.11). B220 B cell surface marker served as an internal control as no significant differences in its expression were expected. Noteworthy, the combination of higher levels of CD1d and CD24 in the low responding subset is indicative of immature/transitional B cell phenotype, which is consistent with increased expression of CD93 in this subset.
Figure 4.
(A) Flow cytometry analysis separating C57BL/6 B220+ B cells that produce high, intermediate and low responses to anti-BCR. (B) Surface marker expression profiles in these subsets: IgM n=41; B220 n=44; CD23 n=45; CD19 n=10; CD21 n=15; CD1d n=9; CD5 n=3; CD24 n=25; CD93 n=44; Ca2+ n=44; statistically significant differences between pairs (ANOVA analysis with Bonferroni's Multiple Pairs Comparison Test p<0.05 are marked with *. Data shown as mean +/− SEM.
3.5. Reversal of T3 B cell anergic signaling features in experimentally induced arthritis
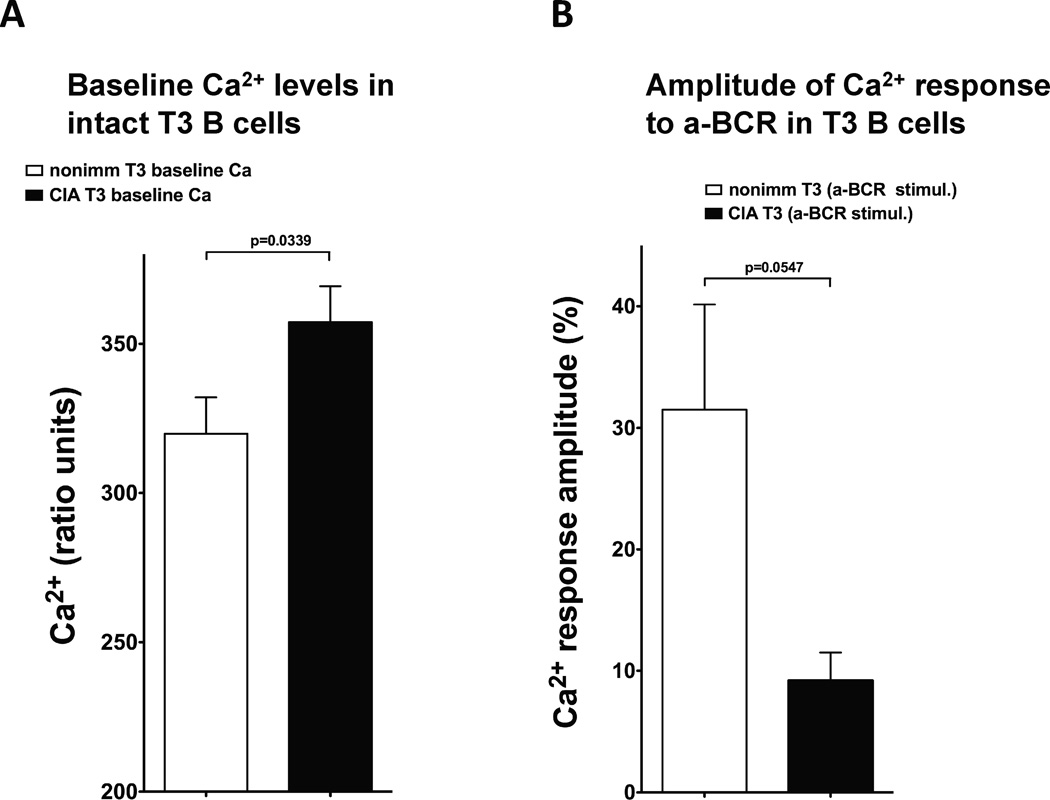
In order to compare signaling properties of naturally occurring T3 B cells under normal physiological conditions to pathological autoimmune environment, we utilized a well-established murine model of collagen-induced arthritis (CIA) whereby DBA/1J animals are immunized with type II collagen and develop arthritis within 4 weeks [25]. T3 B cells from arthritic mice exhibited a reversal of major anergic signaling features (total pTyr and Ca2+ signaling). A reduction in baseline total protein phosphorylation in unstimulated T3 vs. B220+ B cells from arthritic animals (compare Fig. 7A to Fig. 1B) was accompanied by an increase in pTyr response after anti-BCR stimulation (compare Fig. 7B to Fig. 1C). Side-by-side comparison of T3 B cell pTyr responses to anti-BCR between arthritic and control (nonimmunized DBA/1J) mice revealed an increase in the amplitude of response to anti-BCR stimulation in cells from arthritic animals (Figure 7C). This is consistent with the loss of anergy in the T3 B cell subset in autoimmunity and strongly supports our hypothesis. Furthermore, as compared to control animals, T3 B cells from arthritic mice had significantly increased baseline intracellular Ca2+ levels (Fig. 8A) and reduced amplitude of Ca2+ signaling responses to anti-BCR stimulation (Fig. 8B). The latter characteristic of Ca2+ response in arthritic T3 B cells is consistent with our previously published observations [13] in Ars/A1 transgenic murine anergy model whereby anergic B cells have elevated baseline but decreased amplitude of Ca2+ response to the anergy-breaking stumulus.
Figure 7.
Total protein phosphorylation (pTyr) in intact or anti-BCR stimulated B220+ or T3 B cell subsets in DBA/1J mice with collagen-induced arthritis. (A) pTyr baseline in nonstimulated cells (filled bars) and after stimulation with polyclonal anti-BCR (superimposed clear bars) in B220+ and T3 subsets; mean fluorescence intensity (MFI) of intracellular staining with anti-pTyr mAb PY20. (B) pTyr responses to anti-BCR in B220+ and T3 B cell subsets normalized as % increase over corresponding unstimulated controls. (C) Comparison between T3 responses to anti-BCR in nonimmunized controls vs. CIA mice. Statistically significant differences (Student’s T-test) marked with *; data shown as mean +/− SEM with p values; CIA group n=20, nonimmunized group n=9.
Figure 8.
(A) Baseline intracellular Ca2+ levels in B220+ and T3 B cells in arthritic (CIA) and control DBA/1J mice. (B) Amplitude of Ca2+ responses in BCR-stimulated B220+ and T3 B cells normalized as % increase over corresponding baseline in intact cells. Statistically significant differences between pairs (Student’s T-test) are marked. Data in each graph is shown as mean +/− SEM with p values for B220+ vs. T3; CIA group n=20, nonimmunized group n=9.
3.6. Validation of phospho-flow results by Western blot
In order to rule out any potential artifacts that can be introduced by simultaneous multicolor flow cytometry analysis of cell surface markers and intracellular phospho-proteins, we confirmed the results by Western blotting of purified B cell lysates with the same phospho-specific Abs (Figure 5). Phosphorylation patterns of individual signaling molecules (and total pTyr) corresponded to those observed in the B220+ cell subset analyzed by flow cytometry (Fig. 2A), including molecules like Ezrin and Akt that exhibited a decrease in phosphorylation after BCR stimulation.
Figure 5.
Western blots of total pTyr (PY20 mAb) and major BCR-triggered signaling molecules phosphorylation (Blnk, p56Dok, Ezrin, AP2M1, CD19, Erk, FcgRIIb, Jnk, Lck, Lyn, PI3k p85, PLCg2, Pyk2, Shc, SHIP1, SHP2, Syk, PTEN) in response to anti-IgM in B220+ cells (C57BL/6). Densitometry charts below each panel illustrate quantified results.
Western blot results also confirmed phospho-flow results for signaling proteins that have not been well characterized in BCR-mediated signaling. These include: AP2M1, which was implicated in clathrin-mediated receptor internalization of MHCII and other BCR co-receptors on B cell surface [26, 27] involved in CD19-CD22 regulation [28]; Lck, a well known T cell signaling molecule also expressed in B2 and B1 cells [29, 30] and associated with CD19 [31], although its role in B2 signaling is still under study; MSK1, a nuclear kinase activated by the ERK1/2 and p38 MAPK signaling cascades and involved in Nf-kB signaling [32–35] associated with apoptotic responses in B cells [36]. Correlation between Erk1/2 and MSK1 phosphorylation suggests the involvement of the latter in BCR signaling regulation, which has not been described before.
4. DISCUSSION
This study identifies unique intracellular signaling properties in the subset of naturally occurring T3 B cells isolated from wild type mice and distinguished by the expression of B220, CD93, CD23 and IgM. The results revealed previously uncharacterized signaling profile, which is distinctive for anergic B cells (Figure 6). This specific phosphorylation pattern of key signaling molecules involved in BCR signal transduction is the major finding of this study. Several other distinguishing signaling characteristics were identified in T3 cells as compared to B220+ mature B cells. These include: (a) high baseline protein phosphorylation activity in intact T3 cells and (b) significantly reduced overall protein phosphorylation in response to BCR-mediated stimulation as well as (c) increased baseline cytoplasmic Ca2+ levels in intact T3 cells and (d) reduced amplitude of Ca2+ response to BCR ligation. These signaling properties have been described previously in some transgenic models of autoimmunity and are indicative of functional anergy, a state in which B cells can recognize chronically present antigen but are unable to produce immune response due to intrinsic signaling inhibition (reviewed in [37]). These characteristics reflect unique subset-specific balancing of stimulatory and inhibitory BCR signaling cascades in intact anergic T3 B cells.
As shown in Figure 1B, transitional T3 B cells had increased baseline total pTyr levels compared to mature naïve B220+ cells, which is consistent with elevated signaling activity and distinguishes this subset from mature B cells that have completed their development and antigen specificity maturation. The fact that T3 cells also exhibited a reduction in total protein phosphorylation in response to BCR ligation (in addition to the elevated baseline activity) determines their diminished responsiveness and can me attributed to “receptor tuning” signaling mechanisms that inhibit signals elicited by the antigen receptor (Figure 1C) [24]. However, the phosphorylation of inhibitory phosphatases SHIP and SHP was not increased. Instead, we observed a reduction in the phosphorylation of these molecules, which suggests the involvement of other inhibitory pathways. Overall, T3 cells had a pronounced overall reduction in BCR-induced phosphorylation of the majority signaling molecules included in our assessment panel, with the exception of Blnk and Syk (Figure 2C). Downregulation of membrane IgM in T3 cells should also be considered a contributing factor to these signaling characteristics.
T3 B cells have a unique protein phosphorylation pattern when stimulated via BCR, which distinguishes this subset from the general B220+ B cell population (Figure 6, Figure 2C). Major differences lie in the decreased activation of CD19 and the associated downstream signaling cascades that include Lyn recruitment by the CD19/Igα/β/IgM complex, PI3K(p85)-mediated activation of PiP3 as well as subsequent downregulation of PIP3 via FcγRIIb–initiated SHIP/Dok/PTEN inhibitory loop. At a minimum, these signaling events must have further inhibitory effects on the involvement of Akt and mTor-mediated pathways that play major roles in protein synthesis, glucose uptake for ATP generation and growth arrest/apoptotic cellular responses, which is confirmed by diminished phosphorylation of these molecules. Decreased activation of Igα/β-proximal inhibitory phosphatase SHP also adds to these effects as it can result in the inhibition of Syk, although this effect appears to be counterbalanced by other influences as Syk phosphorylation was not reduced.
IP3R is a key regulator of receptor-induced Ca2+ influx via store-operated membrane ion channels (CRAC) and its decreased phosphorylation in T3 cells is consistent with lower amplitudes of Ca2+ responses in this subset. Finally, hypophosphorylation of Ezrin and AP2M1 in T3 cells is indicative of alterations in the processing of BCR/antigen membrane complexes as these proteins are involved in lipid raft formation and clathrin-dependent BCR internalization. These effects are in accord with observed significant decrease in the phosphorylation of Pyk2, which further diminishes integrin-mediated cytoskeletal rearrangements in this B cell subset during antigen processing. Taken together, this pattern of individual signaling molecules phosphorylation can be a new characteristic feature of anergic B cells.
Analysis of intracellular Ca2+ signaling revealed that T3 B cells have increased baseline and decreased BCR-triggered Ca2+ signaling activity, both statistically significant (Figure 3). This is in agreement with the total pTyr data (Figure 1) and further supports the hypothesis that these cells are hyporesponsive to BCR stimulation due to intrinsic signaling activity that arises from constant antigen exposure and supports the argument for their anergic status.
Although the main focus of this study is on unique and previously unknown signaling properties of naturally occurring T3 B cells in wildtype mice under normal physiological conditions, a comparison to pathological autoimmune environment provides important evidence for the role of this cell subset in autoimmunity. After encounter of autoimmunogenic antigen in vivo (immunization with type II collagen leading to the development collagen induced arthritis [25]), T3 B cells isolated from DBA/1J mice exhibited major alterations in the anergic signaling profile. These include significant reduction of baseline protein phosphporylation activity and increase in the amplitude of response to BCR stimulation (Figure 7). These results demonstrate the role of this B cell subset in the development of autoimmunity and are consistent with the loss of anergy in the T3 B cell subset, which is characterized by the opposite general pTyr signaling features – increased baseline and reduced response to anti-BCR. Furthermore, anergy-specific Ca2+ signaling properties of wildtype T3 B cells described in Figure 3 are consistent with our previous results obtained in transgenic Ars/A1 antigen-specific model of B cell anergy [13] in that in both cases anergic cells have elevated baseline and reduced amplitude of Ca2+ signaling upon BCR engagement, as compared to non-anergic B cells.
Phenotypic analysis of the bulk of wildtype C57BL/6 B220+ cells arbitrarily separated into low, intermediate and high responders based on intracellular Ca2+ levels (Figure 5) revealed that lower responses correlated with upregulation of IgM, CD1d, CD24 and CD93 and a tendency for decreased CD19 expression. High levels of CD93 and CD24 in low responders indicate that cells in this group are transitional, although this population is heterogeneous and does not phenotypically exactly match the T3 subset. Overall, transitional B cells were less sensitive to multiclonal BCR stimulation than mature B cell population. Increased levels of IgM are indicative of transitional T1 and T2 B cells as well as phenotype-switched mature B cells that have had antigen exposure. In addition, increased levels of CD1d imply that this subset also includes marginal zone B cells [38]. It is therefore difficult to determine whether the lower responses observed in these experiments are due to anergy or a combination of factors, but the heterogeneous population of low responding B cells must include T3 cells. Low IgM expression is a characteristic feature of the T3 phenotype and therefore the inverse correlation between the strength of BCR-induced Ca2+ response and surface IgM levels appears counterintuitive. However, low level of IgM expression in itself cannot be considered as the major cause of unresponsiveness as it has been demonstrated that ligation of only ~10% of surface BCR is sufficient to induce B cell responses [12]. Another consideration is that T3 B cells are a very minor population [9] and their relative quantity within the low responding subset needs to be taken into account. A more detailed characterization of different B cell types comprising the low responding subset is currently under study in our laboratory.
Oveall, our results suggest that potentially autoreactive T3 B cells are subjected to immunoregulatory mechanisms that, under normal conditions, prevent this subset from activation and production of autoreactive antibodies via intracellular signal inhibitory mechanisms and possibly additional microenviromental factors such as intercellular interactions and availability of costimulatory molecules, supply of cytokines, growth factors, inflammatory mediators and other factors. These inhibitory mechanisms become disrupted in the onset of autoimmunity.
5. CONCLUSIONS
In summary, this study has identified a distinct phosphorylation pattern for key signaling molecules involved in BCR-triggered signal transduction in wildtype T3 B cells, which is characteristic of their anergic state. This pattern (Figure 6) features increased phosphorylation of Syk and Blnk and decreased phosphorylation of CD19, Akt, mTor, IP3R, AP2M1, PTEN, p56 Dok, SHIP1, Ezrin, MSK1, Lck, SHP2, PI3K p85, Gab1, Pyk2, Shc. These characteristics reflect anergy-specific balancing of stimulatory/inhibitory BCR signaling cascades and represent an important mechanism that prevents autoimmunity. Our results also confirmed in wild type mice that naturally occuring T3 B cells are indeed anergic as they exhibit two major anergy-specific signaling properties previously described in some (but not all) transgenic models of autoimmunity - elevated baseline and reduced amplitude of total pTyr and Ca2+ responses to BCR stimulation. Results obtained in the collagen induced arthritis model with DBA/1J mice demonstrate the role of T3 B cell subset in the development of autoimmunity. Signaling features of T3 B cells in arthritis are consistent with the loss of anergy during the onset of the disease.
Our findings provide further rationale for investigating T3 B cell signaling patterns as earliest functional biomarkers of changes in the immune tolerance of potentially autoreactive B cells that precede detectable phenotypical changes and may provide novel insights into the role of B cell anergy in the pathology and early diagnostics of autoimmunity.
HIGHLIGHTS.
Anergic B cells recognize chronically present self-antigens but cannot produce immune response
Naturally occurring immature transitional type 3 (T3) B cells have anergic signaling phenotype
Discovered T3 subset-specific pattern in the phosphorylation of 24 major signaling proteins
Balancing of stimulatory/inhibitory signaling cascades maintains immune tolerance in T3 B cells
Supplementary Material
online Supplemental Figure 1. Phospho-flow cytometry experimental design and data analysis algorithm. After stimulation with anti-BCR (no stimulation for controls), fixed/permeabilized cells were stained with a combination of surface markers and phospho-Abs specific to intracellular signaling molecules. Cell populations of interest (B220+ and T3) were gated upon based on surface markers expression (see article Fig. 1A). (A) Fluorescence intensity of phospho-specific Ab staining within the subsets was compared between stimulated and nonstimulated cells. Experiments were performed in triplicate for each signaling molecule. For each experiment (i.e. cells obtained from one mouse), triplicates of mean fluorescence intensity (MFI) values for each signaling molecule for each condition (nonstimulated/stimulated with anti-BCR) were averaged. Averaged triplicates from multiple mice were then compiled into a total average for each signaling molecule. (B) representative examples of the actual flow data (overlay histograms) and corresponding MFI values generated by FlowJo software.
ACKNOWLEDGEMENTS
Authors would like to thank Janet Siebert (CytoAnalytics, Denver, CO) for helpful discussions on statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS Biol. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankovic M, Casellas R, Yannoutsos N, Wardemann H, Nussenzweig MC. RAGs and regulation of autoantibodies. Annu Rev Immunol. 2004;22:485–501. doi: 10.1146/annurev.immunol.22.012703.104707. [DOI] [PubMed] [Google Scholar]
- 4.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 5.Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, et al. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 6.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, et al. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Current opinion in immunology. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, et al. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 10.Cooke MP, Heath AW, Shokat KM, Zeng Y, Finkelman FD, Linsley PS, et al. Immunoglobulin signal transduction guides the specificity of B cell-T cell interactions and is blocked in tolerant self-reactive B cells. J Exp Med. 1994;179:425–438. doi: 10.1084/jem.179.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintraub BC, Jun JE, Bishop AC, Shokat KM, Thomas ML, Goodnow CC. Entry of B cell receptor into signaling domains is inhibited in tolerant B cells. J Exp Med. 2000;191:1443–1448. doi: 10.1084/jem.191.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilen BJ, Burke KM, Sleater M, Cambier JC. Transmodulation of BCR signaling by transduction-incompetent antigen receptors: implications for impaired signaling in anergic B cells. J Immunol. 2002;168:4344–4351. doi: 10.4049/jimmunol.168.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyubchenko T, Dal Porto JM, Holers VM, Cambier JC. Cutting edge: Complement (C3d)-linked antigens break B cell anergy. J Immunol. 2007;179:2695–2699. doi: 10.4049/jimmunol.179.5.2695. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub JP, Godfrey V, Wolthusen PA, Cheek RL, Eisenberg RA, Cohen PL. Immunological and pathological consequences of mutations in both Fas and Fas ligand. Cell Immunol. 1998;186:8–17. doi: 10.1006/cimm.1998.1290. [DOI] [PubMed] [Google Scholar]
- 15.Rathmell JC, Fournier S, Weintraub BC, Allison JP, Goodnow CC. Repression of B7.2 on self-reactive B cells is essential to prevent proliferation and allow Fas-mediated deletion by CD4(+) T cells. J Exp Med. 1998;188:651–659. doi: 10.1084/jem.188.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feuerstein N, Chen F, Madaio M, Maldonado M, Eisenberg RA. Induction of autoimmunity in a transgenic model of B cell receptor peripheral tolerance: changes in coreceptors and B cell receptor-induced tyrosine-phosphoproteins. J Immunol. 1999;163:5287–5297. [PubMed] [Google Scholar]
- 17.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 18.Kilmon MA, Rutan JA, Clarke SH, Vilen BJ. Low-affinity, Smith antigen-specific B cells are tolerized by dendritic cells and macrophages. J Immunol. 2005;175:37–41. doi: 10.4049/jimmunol.175.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert MR, Carnathan DG, Cogswell PC, Lin L, Baldwin AS, Jr, Vilen BJ. Dendritic cells from lupus-prone mice are defective in repressing immunoglobulin secretion. J Immunol. 2007;178:4803–4810. doi: 10.4049/jimmunol.178.8.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acevedo-Suarez CA, Kilkenny DM, Reich MB, Thomas JW. Impaired intracellular calcium mobilization and NFATc1 availability in tolerant anti-insulin B cells. J Immunol. 2006;177:2234–2241. doi: 10.4049/jimmunol.177.4.2234. [DOI] [PubMed] [Google Scholar]
- 21.Eris JM, Basten A, Brink R, Doherty K, Kehry MR, Hodgkin PD. Anergic self-reactive B cells present self antigen and respond normally to CD40-dependent T-cell signals but are defective in antigen-receptor-mediated functions. Proc Natl Acad Sci U S A. 1994;91:4392–4396. doi: 10.1073/pnas.91.10.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noorchashm H, Bui A, Li HL, Eaton A, Mandik-Nayak L, Sokol C, et al. Characterization of anergic anti-DNA B cells: B cell anergy is a T cell-independent and potentially reversible process. Int Immunol. 1999;11:765–776. doi: 10.1093/intimm/11.5.765. [DOI] [PubMed] [Google Scholar]
- 23.Vilen BJ, Famiglietti SJ, Carbone AM, Kay BK, Cambier JC. B cell antigen receptor desensitization: disruption of receptor coupling to tyrosine kinase activation. J Immunol. 1997;159:231–243. [PMC free article] [PubMed] [Google Scholar]
- 24.Gauld SB, Benschop RJ, Merrell KT, Cambier JC. Maintenance of B cell anergy requires constant antigen receptor occupancy and signaling. Nat Immunol. 2005;6:1160–1167. doi: 10.1038/ni1256. [DOI] [PubMed] [Google Scholar]
- 25.Moore AR. Collagen-induced arthritis. Methods Mol Biol. 2003;225:175–179. doi: 10.1385/1-59259-374-7:175. [DOI] [PubMed] [Google Scholar]
- 26.Reid PA, Watts C. Constitutive endocytosis and recycling of major histocompatibility complex class II glycoproteins in human B-lymphoblastoid cells. Immunology. 1992;77:539–542. [PMC free article] [PubMed] [Google Scholar]
- 27.Lu X, Axtell RC, Collawn JF, Gibson A, Justement LB, Raman C. AP2 adaptor complex-dependent internalization of CD5: differential regulation in T and B cells. J Immunol. 2002;168:5612–5620. doi: 10.4049/jimmunol.168.11.5612. [DOI] [PubMed] [Google Scholar]
- 28.John B, Herrin BR, Raman C, Wang YN, Bobbitt KR, Brody BA, et al. The B cell coreceptor CD22 associates with AP50, a clathrin-coated pit adapter protein, via tyrosine-dependent interaction. J Immunol. 2003;170:3534–3543. doi: 10.4049/jimmunol.170.7.3534. [DOI] [PubMed] [Google Scholar]
- 29.Dal Porto JM, Burke K, Cambier JC. Regulation of BCR signal transduction in B-1 cells requires the expression of the Src family kinase Lck. Immunity. 2004;21:443–453. doi: 10.1016/j.immuni.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Frances R, Tumang JR, Rothstein TL. B-1 cells are deficient in Lck: defective B cell receptor signal transduction in B-1 cells occurs in the absence of elevated Lck expression. J Immunol. 2005;175:27–31. doi: 10.4049/jimmunol.175.1.27. [DOI] [PubMed] [Google Scholar]
- 31.Waddick KG, Chae HP, Tuel-Ahlgren L, Jarvis LJ, Dibirdik I, Myers DE, et al. Engagement of the CD19 receptor on human B-lineage leukemia cells activates LCK tyrosine kinase and facilitates radiation-induced apoptosis. Radiat Res. 1993;136:313–319. [PubMed] [Google Scholar]
- 32.Jacks KA, Koch CA. Differential regulation of mitogen- and stress-activated protein kinase-1 and -2 (MSK1 and MSK2) by CK2 following UV radiation. J Biol Chem. 2010;285:1661–1670. doi: 10.1074/jbc.M109.083808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darragh J, Ananieva O, Courtney A, Elcombe S, Arthur JS. MSK1 regulates the transcription of IL-1ra in response to TLR activation in macrophages. Biochem J. 2010;425:595–602. doi: 10.1042/BJ20091062. [DOI] [PubMed] [Google Scholar]
- 34.Reber L, Vermeulen L, Haegeman G, Frossard N. Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS One. 2009;4:e4393. doi: 10.1371/journal.pone.0004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9:1028–1036. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 36.El Mchichi B, Hadji A, Vazquez A, Leca G. p38 MAPK and MSK1 mediate caspase-8 activation in manganese-induced mitochondria-dependent cell death. Cell Death Differ. 2007;14:1826–1836. doi: 10.1038/sj.cdd.4402187. [DOI] [PubMed] [Google Scholar]
- 37.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyubchenko T, dal Porto J, Cambier JC, Holers VM. Coligation of the B cell receptor with complement receptor type 2 (CR2/CD21) using its natural ligand C3dg: activation without engagement of an inhibitory signaling pathway. J Immunol. 2005;174:3264–3272. doi: 10.4049/jimmunol.174.6.3264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
online Supplemental Figure 1. Phospho-flow cytometry experimental design and data analysis algorithm. After stimulation with anti-BCR (no stimulation for controls), fixed/permeabilized cells were stained with a combination of surface markers and phospho-Abs specific to intracellular signaling molecules. Cell populations of interest (B220+ and T3) were gated upon based on surface markers expression (see article Fig. 1A). (A) Fluorescence intensity of phospho-specific Ab staining within the subsets was compared between stimulated and nonstimulated cells. Experiments were performed in triplicate for each signaling molecule. For each experiment (i.e. cells obtained from one mouse), triplicates of mean fluorescence intensity (MFI) values for each signaling molecule for each condition (nonstimulated/stimulated with anti-BCR) were averaged. Averaged triplicates from multiple mice were then compiled into a total average for each signaling molecule. (B) representative examples of the actual flow data (overlay histograms) and corresponding MFI values generated by FlowJo software.