Abstract
Objective
To analyze the prognostic factors thought to be related with survival time after a spinal metastasis operation.
Methods
We retrospectively analyzed 217 patients who underwent spinal metastasis operations in our hospital from 2001 to 2009. Hematological malignancies, such as multiple myeloma and lymphoma, were excluded. The factors thought to be related with postoperative survival time were gender, age (below 55, above 56), primary tumor growth rate (slow, moderate, rapid group), spinal location (cervical, thoracic, and lumbo-sacral spine), the timing of radiation therapy (preoperative, postoperative, no radiation), operation type (decompressive laminectomy with or without posterior fixation, corpectomy with anterior fusion, corpectomy with posterior fixation), preoperative systemic condition (below 5 points, above 6 points classified by Tomita scoring), pre- and postoperative ambulatory function (ambulatory, non-ambulatory), number of spinal metastases (single, multiple), time to spinal metastasis from the primary cancer diagnosis (below 21 months, above 22 months), and postoperative complication.
Results
The study cohort mean age at the time of surgery was 55.5 years. The median survival time after spinal operation and spinal metastasis diagnosis were 6.0 and 9.0 months. In univariate analysis, factors such as gender, primary tumor growth rate, preoperative systemic condition, and preoperative and postoperative ambulatory status were shown to be related to postoperative survival. In multivariate analysis, statistically significant factors were preoperative systemic condition (p=0.048) and postoperative ambulatory status (p<0.001). The other factors had no statistical significance.
Conclusion
The factors predictive for postoperative survival time should be considered in the surgery of spinal metastasis patients.
Keywords: Prognostic factors, Survival time, Spinal metastasis, Surgical management
INTRODUCTION
As diagnostic techniques and treatment options have been improving in cancer patients, their life expectancy has been markedly extended13). Furthermore, the incidence and prevalence of spinal metastasis have been increasing owing to advances in neuroradiological imaging9). Skeletal metastasis is a frequent problem in cancer patients, and spinal metastases account for approximately 50% of bone metastases1). Approximately 10% of cancer patients are diagnosed with symptomatic spinal metastasis during their lifetime, and up to 50% of spinal metastasis patients require treatment due to pain or neurological deficits2,12,30).
Untreated spinal metastasis results in the deterioration of life quality due to severe neurological deficits and intractable pain, which can shorten life expectancy with complications. Treatment options for spinal metastasis include radiation therapy, systemic chemotherapy, bisphosphonates, radioisotopes, and surgical management. Each treatment option has advantages and disadvantages; thus individual therapy should be chosen to provide the maximum effect and minimum morbidity. In most cases of spinal metastasis, non-surgical treatment is considered first, but it is difficult to achieve rapid and direct decompression of neural structures with non-surgical treatment. Surgical management of spinal metastasis is a good treatment method for rapid decompression of neural structures and immediate stabilization of the spine. However, surgical management on spinal metastasis is still controversial6,14,37). General indications for surgery are spinal instability, progressive symptomatic deformities, neurological deficits, and intractable pain resistant to other treatments than surgery. As surgery often requires a significant recovery period and complications can occur, the prediction of postoperative survival time is the most critical factor in making the decision for surgery2). Therefore, several authors have tried to find factors related with postoperative survival time. Such efforts have been proposed by Tomita et al.35), Tokuhashi et al.32,33), North et al.25), Hirabayashi et al.15), Arrigo et al.2), and Moon et al.24).
We reviewed the medical records of 217 patients from 2001 to 2009 in our hospital to find out prognostic factors related to postoperative survival in spinal metastases.
MATERIALS AND METHODS
We reviewed the medical records and radiological images of 217 patients who underwent spinal metastasis operations from 2001 to 2009 at our hospital. The patients with multiple myeloma and lymphoma were excluded because they had systemic hematological disease, not a metastatic spread of a solid tumor. We followed up spinal metastasis patients until May 2011; survivors comprised 16 patients. We analyzed the prognostic factors we believed were related to postoperative survival times. They were gender, age (below 55 years old or above 56 years old groups based on a mean age of 55.5 years), primary tumor growth rate (the slow growth group involves breast cancer, prostate cancer, and thyroid cancer; moderate growth group involves bladder cancer, cervix cancer, melanoma, liposarcoma, ovarian cancer, osteosarcoma, nasopharyngeal carcinoma, renal cell carcinoma, and tongue cancer; the rapid growth group involves gastric cancer, cholangio-carcinoma, colorectal cancer, esophageal cancer, hepatocellular carcinoma, lung cancer, and masses of unknown origin), involved spinal location (cervical, thoracic, and lumbosacral spine), the timing of radiation therapy (preoperative, postoperative, and no radiation), operation type (decompressive laminectomy with or without posterior fixation, corpectomy with anterior fusion, and corpectomy with posterior fixation), preoperative systemic condition (group with Tomita score ≤5 points, group with Tomita score ≥6 points), pre- and postoperative ambulatory function (ambulatory, non-ambulatory), number of spinal metastases (single, multiple), and time to spinal metastasis from the primary cancer diagnosis (below 21 months or above 22 months, based on a mean value of 21.3 months). The occurrence of postoperative complications (complication, no complication) were also included.
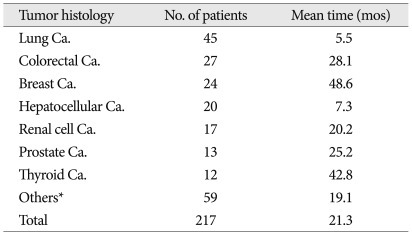
In the study cohort, 128 patients were male, and 89 were female. The below 55 years old group comprised 98 patients, and the above 56 years old group included 119 patients. The slow, moderate, and rapid growth tumor groups included 50, 47, and 120 patients, respectively. With regard to histology of the primary cancer, lung cancer (45 patients) was the most common. Colorectal cancer (27 patients), breast cancer (24 patients), hepatocellular carcinoma (20 patients), renal cell carcinoma (17 patients), prostate cancer (13 patients), and thyroid cancer (12 patients) followed (Table 1). Regarding the spinal level of involvement, thoracic spinal metastasis (100 patients) was the most common, and lumbosacral metastasis (65 patients) and cervical metastasis (32 patients) followed. The remaining 20 patients' cancers involved the cervicothoracic spine or thoracolumbar spine simultaneously. Radiation therapy (RT) was undertaken in 53 patients preoperatively, 104 patients postoperatively and 60 patients had no radiation therapy. In no RT group, some patients received chemotherapy instead of RT and the others did not have RT due to poor systemic condition or postoperative complications.
Table 1.
Mean time to spinal metastasis after primary tumor diagnosis
*MUO, osteosarcoma, bladder, tongue, etc. (tumor groups below 10 patients). Ca : cancer, mos : months
Surgical indications in the present study were as follows : 1) life expectancy longer than 3 months, 2) axial or translational instability due to pathological fractures or tumor invasion, 3) progressed symptomatic spinal metastasis after non-surgical treatment, 4) spinal cord compression with motor weakness (below motor grade IV) or uncontrolled pain.
We classified surgery type into four groups. Decompressive laminectomy was performed in 48 patients, decompressive laminectomy with posterior fixation in 40 patients, corpectomy with anterior fusion in 74 patients, and corpectomy with posterior fixation in 55 patients.
Preoperative systemic condition was based on the Tomita scoring system which evaluates the systemic extent of cancer with primary tumor growth rate, condition of visceral metastasis, and bone metastasis35). The Tomita scores range from 2 to 10 points and higher score means poorer systemic status. We classified all the patients into 2 groups; unfavorable vs. favorable. The unfavorable group comprised 137 patients (≥6 points), and the favorable group comprised 80 patients (≤5 points). Pre- and postoperative ambulatory function was divided into 2 groups; ambulatory and non-ambulatory. We defined ambulatory status as Nurick grade 1 to 4. Non-ambulatory status means Nurick grade 5. The preoperative ambulatory group contained 64 patients, and the non-ambulatory group comprised 153 patients. The postoperative ambulatory group included 179 patients, and the non-ambulatory group included 38 patients. The single spinal metastasis group included 106 patients, and the multiple level involvement group included 111 patients. The number of patients who had postoperative complication was 46, and that of no complication group was 171.
The time interval from primary cancer diagnosis to spinal metastasis was divided into 2 groups. Based on a mean value of 21.3 months, the number of patients whose period was less than 21 months was 148, and the number of patients whose period was instalonger than 22 months was 69. We performed Kaplan-Meier analysis to estimate event-time distributions, made comparisons using Log-Rank statistics (Mantel-Cox with SPSS, version 14.0) and tested a Cox proportional hazards model to determine which independent factors prognosticated for postoperative survival. p-values of <0.05 were considered significant.
RESULTS
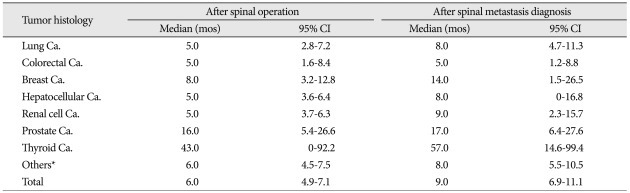
The mean age of the study cohort at the time of spine surgery was 55.5 years (range 17-83 years). The mean time interval to spinal metastasis after primary cancer diagnosis was 21.3 months (range 0-216 months). The time interval between diagnosis of primary cancer and spinal metastasis was a mean of 5.5 months (range 0-70 months) in lung cancer patients. However, the breast cancer group showed a mean 48.6 months (range 0-216 months) (Table 1). The median values of overall survival times after spinal operation and spinal metastasis diagnosis were 6.0 months [95% confidence interval (CI) : 4.9-7.1 months] and 9.0 months (95% CI : 6.9-11.1 months), respectively. Thyroid cancer patients had the longest values, with 43.0 months (95% CI : 0-92.2 months) after spinal operation and 57.0 months (95% CI : 21.7-99.4 months) after spinal metastasis diagnosis. The group with the shortest durations was the colorectal cancer group, with 5.0 months (95% CI : 1.6-8.4 months) and 8.0 months (95% CI : 1.2-8.8 months) (Table 2) after spinal metastasis diagnosis.
Table 2.
Median survival time after spinal operation and spinal metastasis diagnosis
*MUO, osteosarcoma, bladder, tongue, etc. (tumor groups below 10 patients). Ca. : cancer, CI : confidence interval, mos : months
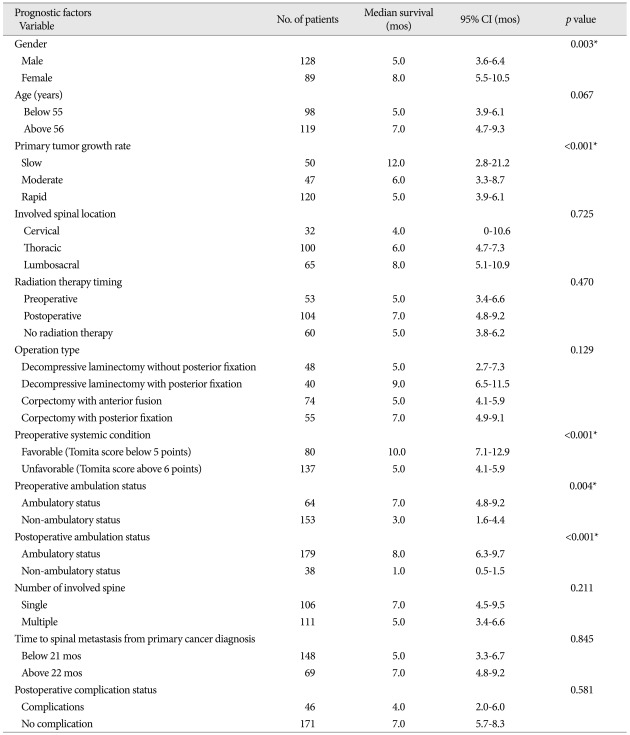
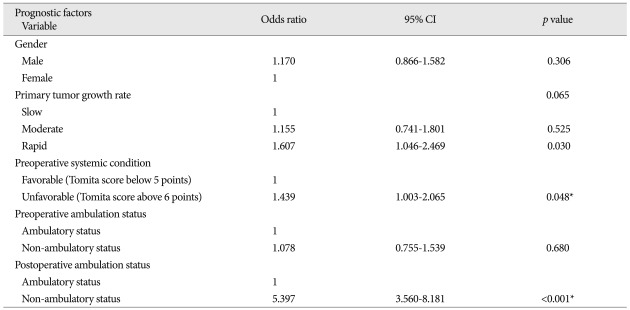
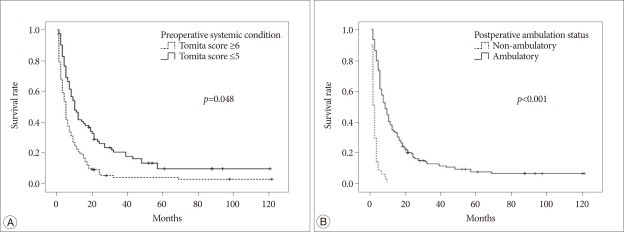
In univariate analysis, statistically significant factors related with longer postoperative survival were gender, primary tumor growth rate, preoperative systemic condition, and pre- and postoperative ambulatory status (p<0.05) (Table 3). In males, the median survival after surgery was 5.0 months (95% CI : 3.6-6.4 months) and 8.0 months in females (95% CI : 5.5-10.5 months). The median survival of the slow growing tumor group was 12.0 months (95% CI : 2.8-21.2 months), and those of the moderate and rapid growth groups were 6.0 and 5.0 months (95% CIs : 3.3-8.7 months and 3.9-6.1 months), respectively. Regarding preoperative systemic condition, the median survival in patients whose Tomita score was lower than 5 points was 10.0 months (95% CI : 7.1-12.9 months), and that in patients with a Tomita score higher than 6 points was 5.0 months (95% CI : 4.1-5.9 months). Preoperative ambulatory status also was a significant prognostic factor; the median survival time of the walking group was 7.0 months (95% CI : 4.8-9.2 months), and that of the non-ambulatory group was 3.0 months (95% CI : 1.6-4.4 months). Maintained ambulatory function after surgery also was a good prognostic factor. The median survival time was 8.0 months (95% CI : 6.3-9.7 months) in the ambulatory group and 1.0 month (95% CI : 0.5-1.5 months) in the non-ambulatory group. Age, involved spinal location, radiotherapy timing, operation type, number of spinal metastases, time to spinal metastasis from the primary cancer diagnosis, and the occurrence of postoperative complication had no statistical significance (p>0.05) (Table 3). We performed logistic regressive analysis of the multivariate Cox proportional hazard model with significant factors in univariate analysis (Table 4) (Fig. 1). It showed that preoperative systemic condition [odds ratio (OR) : 1.439, 95% CI : 1.003-2.065, p=0.048] and postoperative ambulation status (OR : 5.397, 95% CI : 3.560-8.181, p<0.001) were prognostic factors associated with longer postoperative survival.
Table 3.
Univariate analysis of factors related to postoperative survival time
*Significant at p<0.05. CI : confidence interval, mos : months
Table 4.
Multivariate analysis of factors related to postoperative survival time
*Significant at p<0.05. CI : confidence interval
Fig. 1.
A : Survival curve of the groups with different preoperative systemic conditions. B : Survival curve of the groups with different postoperative ambulatory statuses.
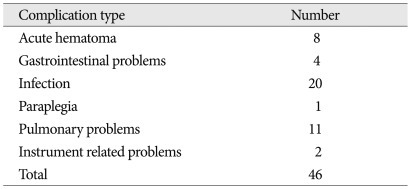
Postoperative complications were found in 46 patients (21.2%). These included acute hematoma in 8 patients, gastrointestinal problems in 4 patients, infection in 20 patients, paraplegia in 1 patient, pulmonary complications in 11 patients, and instrument-related problems in 2 patients (Table 5). The one-month mortality rate was 14.3% (31 patients). Among 46 patients with postoperative complications, 12 patients belonged to the postoperative non-ambulatory group.
Table 5.
Postoperative complications
DISCUSSION
As diagnostic methods and treatment options for cancer patients have improved, their survival has been extended. Therefore, spinal metastasis can be seen in cancer patients more frequently than in the past9). As a result, management of spinal metastasis has become an important issue. In spinal metastasis, the effectiveness of surgical treatment is still controversial6,14,37). Progressive and untreated spinal metastasis may lead to compression fracture of the vertebral body, vertebral column instability, pain due to the nerve root and spinal cord, and significant neurological deficits19). In severe cases, it may cause quadriplegia, paraplegia, and urinary incontinence and can also shorten survival with complications. Non-surgical treatment methods are suitable for patients who are ambulatory, who have minimal neurological deficits, or who have pain. However, it is difficult to expect a rapid and direct decompression of neural structures with non-surgical treatment methods. Surgery is undoubtedly the best treatment option for rapid decompression of spinal neural structures and immediate stabilization of the spine31). In recent studies, the effectiveness of surgical treatment has been validated 7,8,11,16,18,21,27). These articles assert that surgery could improve quality of remaining life with maintained ambulatory function, and en bloc resection of a solitary spinal metastasis increases the survival rate. However, surgery can cause dangerous complications leading to significant morbidity and mortality. Furthermore, some complications involve prolonged recovery time and could delay adjuvant therapy. Therefore, we have to consider life expectancy after spinal metastasis surgery in the selection of surgical candidates.
Several studies have attempted to investigate prognostic factors for survival outcome after spinal metastasis surgery2,15,24,25,32,33,35). Popular scoring systems for the evaluation of preoperative systemic condition were suggested by Tomita and Tokuhashi. The Tomita scoring system is scored by primary tumor growth rate, condition of visceral metastasis, and bone metastasis35). In this report, these factors had statistical significance and different weight. It also suggested treatment strategies for spinal metastasis ranging from wide or marginal excision to supportive care according to Tomita's score and the treatment goal. Tokuhashi et al.32,33) suggested another scoring system and subsequently issued a revised system. Six factors, such as general condition, extra-spinal metastasis, number of vertebral body metastases, grade of major internal organ metastasis, primary cancer type, and level of palsy comprise their scoring system. The factors which comprise the Tomita scoring system are also included in the Tokuhashi scoring system. However, several differences are present between the two scoring systems. Tomita emphasized the grade of primary tumor growth rate and visceral metastasis; however, Tokuhashi placed more weight on the primary tumor growth rate. Colorectal cancer belongs to the rapid tumor growth group in Tomita's classification, but it is included in the good prognosis group in Tokuhashi's classification. North et al.25) also analyzed prognostic factors related with postoperative survival time. They evaluated multiple factors, such as tumor type, gender, age, preoperative ambulatory grade, continence, radiotherapy, type of surgery, and number of compressed spinal levels, etc. In their study, statistically significant prognostic factors for reduced survival were surgical intervention extending over two or more spinal segments, recurrent or persistent disease after primary radiotherapy involving the operative site, diagnosis other than breast cancer, and a cervical spinal procedure. Hirabayashi et al.15) identified anatomic sites of primary cancer and postoperative ambulation were associated with longer survival after palliative spinal surgery. Arrigo et al.2) confirmed that primary tumor type, radio-sensitivity of the tumor and preoperative ambulatory status were significant predictors of survival. Moon et al.24) found significant factors related with survival were preoperative Eastern Cooperative Oncology Group performance status and Tomita score.
However, most of these studies had a small sample design (less than 100 patients) except for the studies by Arrigo and Moon et al. In our study cohort of 217 patients, the median survival time after spinal operation was 6.0 months. This value is shorter than that of other published reports, which ranged from 7 to 14 months7,10,15,20,25,34,36). In the present study, patients with Tomita scores from 8 to 10 comprised 25.3% (55 patients), and 55.3% (120 patients) belonged to the rapid growth tumor group. Patients with poor general condition and rapid growth tumors were associated with short postoperative survival.
In multivariate analysis, preoperative systemic condition and postoperative ambulation status were significant factors. Between these two factors, postoperative ambulatory function was the more powerful prognostic factor (p<0.001)4,11,15). For this reason, if some patients have a chance to recover their ambulatory function, more aggressive surgical management is recommended. In recent studies on spinal metastasis treatment, it is known that direct decompressive surgery with postoperative radiotherapy is superior to treatment with radiotherapy alone in terms of post-treatment survival18,27). According to the results with regard to postoperative ambulation, the surgery with or without postoperative radiotherapy group (84%) had better results compared with the radiotherapy only group (57-64%). Furthermore, the chance to regain ambulatory function was higher in the surgery with or without postoperative radiotherapy group (54-62%) compared with the radiotherapy only group (19-26%)18,27).
In the present study, 115 preoperative non-ambulatory patients (75.2%) regained ambulatory function after surgery. Factors related to postoperative ambulatory function were investigated in some reports18,27). North et al.25) found risk factors related with the loss of postoperative ambulation, including loss of preoperative ambulatory ability, recurrent or persistent disease after primary radiotherapy of the operative site, a procedure other than corpectomy, and tumor type other than breast cancer18,27). Patchell et al.27) reported surgery and the pretreatment Frankel score to be associated with longer ambulatory time18).
Gender, which was not significant in other reports, was a significant factor for postoperative survival in univariate analysis2,15,25). The reason may be the difference in the composition of primary cancer histologies between male and female patients. The proportion of the rapid growth tumor group was higher in male patients (65.6%, 84/128 patients) than in the female (43.8%, 36/89 patients). In multivariate analysis, gender proved to be statistically insignificant.
Primary cancer histology can affect postoperative survival in spinal metastasis because it determines the aggressiveness of spinal metastasis and response to treatment3,15). Thyroid cancer, prostate cancer, and breast cancer are included in the slow growing tumor groups2,15,25,32,33,35). Median survival times of spinal metastasis according to tumor histology were reported as 2.9-4.7 months in lung cancer, 4.1-6.9 months in colorectal cancer, 21.0-27.1 months in breast cancer, and 6.2 months in hepatocellular carcinoma, in previous articles2,5,17,26,29). In the present study, postoperative survival was different according to the primary tumor growth rate, but statistical significance was not found.
Regarding the spinal level of involvement, the median survival after surgery of the cervical spinal metastasis group was 4.0 months. The median survival times of the thoracic spinal metastasis and lumbosacral metastasis groups were 6.0 and 8.0 months, respectively. In general, metastases to the cervical spine are known as having worse prognoses than thoracic and lumbosacral metastases because many anatomical structures like the esophagus, trachea, vessels, and nerves are located around the cervical spine and frequently injured intraoperatively22,23,28). However, the involved spinal location had no statistical significance in our series.
The median survival time after surgery of the postoperative RT group (7.0 months) was shown to be longer than the no RT or preoperative RT groups (5.0 months, respectively). The patients in the preoperative RT group were in a more progressed state at the time of surgery than the patients in the postoperative RT group because the surgically removed metastasis had been already irradiated as an initial treatment.
CONCLUSION
As diagnostic tools and treatment methods have improved, the number of spinal metastasis patients has increased. The management of spinal metastasis patients is a very difficult problem. Predicting postoperative life expectancy is an important factor for selecting the appropriate treatment method. In the present study cohort of 217 patients, the overall median survival was 6.0 months following the spine metastasis operation. Preoperative systemic condition and postoperative ambulatory status were statistically significant for postoperative survival time.
References
- 1.Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12(Suppl 2):S202–S213. doi: 10.1007/s00586-003-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrigo RT, Kalanithi P, Cheng I, Alamin T, Carragee EJ, Mindea SA, et al. Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery. 2011;68:674–681. doi: 10.1227/NEU.0b013e318207780c. discussion 681. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky MH, Lis E, Raizer J, Lee H, Boland P. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459–469. [PubMed] [Google Scholar]
- 4.Black P. Spinal metastasis : current status and recommended guidelines for management. Neurosurgery. 1979;5:726–746. doi: 10.1227/00006123-197912000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Stafford SL, Schild SE, Martenson JA, Schiff D. Metastatic spinal cord compression in patients with colorectal cancer. J Neurooncol. 1999;44:175–180. doi: 10.1023/a:1006312306713. [DOI] [PubMed] [Google Scholar]
- 6.Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327:614–619. doi: 10.1056/NEJM199208273270907. [DOI] [PubMed] [Google Scholar]
- 7.Chaichana KL, Pendleton C, Sciubba DM, Wolinsky JP, Gokaslan ZL. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. Clinical article. J Neurosurg Spine. 2009;11:56–63. doi: 10.3171/2009.1.SPINE08657. [DOI] [PubMed] [Google Scholar]
- 8.Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62:683–692. doi: 10.1227/01.neu.0000317317.33365.15. discussion 683-692. [DOI] [PubMed] [Google Scholar]
- 9.Cho DC, Sung JK. Palliative surgery for metastatic thoracic and lumbar tumors using posterolateral transpedicular approach with posterior instrumentation. Surg Neurol. 2009;71:424–433. doi: 10.1016/j.surneu.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 10.Finkelstein JA, Zaveri G, Wai E, Vidmar M, Kreder H, Chow E. A population-based study of surgery for spinal metastases. Survival rates and complications. J Bone Joint Surg Br. 2003;85:1045–1050. doi: 10.1302/0301-620x.85b7.14201. [DOI] [PubMed] [Google Scholar]
- 11.Gerszten PC, Welch WC. Current surgical management of metastatic spinal disease. Oncology (Williston Park) 2000;14:1013–1024. discussion 1024, 1029-1030. [PubMed] [Google Scholar]
- 12.Harel R, Angelov L. Spine metastases : current treatments and future directions. Eur J Cancer. 2010;46:2696–2707. doi: 10.1016/j.ejca.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 14.Heidecke V, Rainov NG, Burkert W. Results and outcome of neurosurgical treatment for extradural metastases in the cervical spine. Acta Neurochir (Wien) 2003;145:873–880. doi: 10.1007/s00701-003-0107-1. discussion 880-881. [DOI] [PubMed] [Google Scholar]
- 15.Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, et al. Clinical outcome and survival after palliative surgery for spinal metastases : palliative surgery in spinal metastases. Cancer. 2003;97:476–484. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 16.Ibrahim A, Crockard A, Antonietti P, Boriani S, Bünger C, Gasbarrini A, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8:271–278. doi: 10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 17.Kim SU, Kim do Y, Park JY, Ahn SH, Nah HJ, Chon CY, et al. Hepatocellular carcinoma presenting with bone metastasis : clinical characteristics and prognostic factors. J Cancer Res Clin Oncol. 2008;134:1377–1384. doi: 10.1007/s00432-008-0410-6. [DOI] [PubMed] [Google Scholar]
- 18.Klimo P, Jr, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee IW, Ha YS, Lee SW, Jo TH, Baik MW, Yoon SH, et al. Clinical analysis of 27 cases of metastatic spine tumors. J Korean Neurosurg Soc. 1988;17:63–72. [Google Scholar]
- 20.Leithner A, Radl R, Gruber G, Hochegger M, Leithner K, Welkerling H, et al. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J. 2008;17:1488–1495. doi: 10.1007/s00586-008-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liljenqvist U, Lerner T, Halm H, Buerger H, Gosheger G, Winkelmann W. En bloc spondylectomy in malignant tumors of the spine. Eur Spine J. 2008;17:600–609. doi: 10.1007/s00586-008-0599-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JK, Rosenberg WS, Schmidt MH. Titanium cage-assisted polymethylmethacrylate reconstruction for cervical spinal metastasis : technical note. Neurosurgery. 2005;56:E207. doi: 10.1227/01.neu.0000144494.12738.81. discussion E207. [DOI] [PubMed] [Google Scholar]
- 23.Mazel C, Balabaud L, Bennis S, Hansen S. Cervical and thoracic spine tumor management : surgical indications, techniques, and outcomes. Orthop Clin North Am. 2009;40:75–92. doi: 10.1016/j.ocl.2008.09.008. vi-vii. [DOI] [PubMed] [Google Scholar]
- 24.Moon KY, Chung CK, Jahng TA, Kim HJ, Kim CH. Postoperative survival and ambulatory outcome in metastatic spinal tumors : prognostic factor analysis. J Korean Neurosurg Soc. 2011;50:216–223. doi: 10.3340/jkns.2011.50.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.North RB, LaRocca VR, Schwartz J, North CA, Zahurak M, Davis RF, et al. Surgical management of spinal metastases : analysis of prognostic factors during a 10-year experience. J Neurosurg Spine. 2005;2:564–573. doi: 10.3171/spi.2005.2.5.0564. [DOI] [PubMed] [Google Scholar]
- 26.Ogihara S, Seichi A, Hozumi T, Oka H, Ieki R, Nakamura K, et al. Prognostic factors for patients with spinal metastases from lung cancer. Spine (Phila Pa 1976) 2006;31:1585–1590. doi: 10.1097/01.brs.0000222146.91398.c9. [DOI] [PubMed] [Google Scholar]
- 27.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer : a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 28.Riegel T, Schilling T, Sitter H, Benes L, Wilke A, Gross MW, et al. [Analysis of factors affecting the prognosis of vertebral metastases] Zentralbl Neurochir. 2002;63:2–6. doi: 10.1055/s-2002-31578. [DOI] [PubMed] [Google Scholar]
- 29.Sciubba DM, Gokaslan ZL, Suk I, Suki D, Maldaun MV, McCutcheon IE, et al. Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J. 2007;16:1659–1667. doi: 10.1007/s00586-007-0380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciubba DM, Petteys RJ, Dekutoski MB, Fisher CG, Fehlings MG, Ondra SL, et al. Diagnosis and management of metastatic spine disease. J Neurosurg Spine. 2010;13:94–108. doi: 10.3171/2010.3.SPINE09202. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz MP, Mekhail A, Benzel EC. Management of metastatic tumors of the spine : strategies and operative indications. Neurosurg Focus. 2001;11:e2. doi: 10.3171/foc.2001.11.6.3. [DOI] [PubMed] [Google Scholar]
- 32.Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976) 2009;34:69–73. doi: 10.1097/BRS.0b013e3181913f19. [DOI] [PubMed] [Google Scholar]
- 33.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 34.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 36.Ulmar B, Huch K, Naumann U, Catalkaya S, Cakir B, Gerstner S, et al. Evaluation of the Tokuhashi prognosis score and its modifications in 217 patients with vertebral metastases. Eur J Surg Oncol. 2007;33:914–919. doi: 10.1016/j.ejso.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741–748. doi: 10.3171/jns.1980.53.6.0741. [DOI] [PubMed] [Google Scholar]