Abstract
We report here two cases of primary intraosseous meningioma with aggressive behavior. A 68-year-old man presented with a one year history of a soft, enlarging mass in the right parietal region. Magnetic resonance image (MRI) revealed a 6 cm sized, heterogeneously-enhancing, bony expansile mass in the right parietal bone, and computed tomograph (CT) showed a bony, destructive lesion. The tumor, including the surrounding normal bone, was totally resected. Dural invasion was not apparent. Diagnosis was atypical meningioma, which extensively metastasized within the skull one year later. A 74-year-old woman presented with a 5-month history of a soft mass on the left frontal area. MRI revealed a 4 cm sized, multilobulated, strongly-enhancing lesion on the left frontal bone, and CT showed a destructive lesion. The mass was adhered tightly to the scalp and dura mater. The lesion was totally removed. Biopsy showed a papillary meningioma. The patient refused adjuvant radiation therapy and later underwent two reoperations for recurred lesions, at 19 and at 45 months postoperative. The patient experienced back pain 5 years later, and MRI showed an osteolytic lesion on the 11th thoracic vertebra. After her operation, a metastatic papillary meningioma was diagnosed. These osteolytic intraosseous meningiomas had atypical/malignant pathologies, which metastasized to whole skull and the spine.
Keywords: Intraosseous, Meningioma, Metastasis, Osteolysis
INTRODUCTION
Primary meningiomas arising outside the intracranial compartment account for 1% to 2% of all meningiomas. Such neoplasms are called primary extradural meningiomas. Reportedly, these meningiomas occur in the calvaria, scalp, orbit, paranasal sinuses, oropharynx, nasopharynx, neck, and skin10). The subset of extradural meningiomas that arise in bone are called primary intraosseous meningiomas15). This type of meningioma represents approximately two-thirds of all extradural meningiomas10). The vast majority of intraosseous meningiomas arise in cranial bones, although there are a few reported cases in which the tumor originated in the mandible12). These have a wider range of presenting symptoms and appear more prone to develop malignant features than do intracranial meningiomas as reported 2% incidence of intracranial meningiomas with malignant features, compared to 11% of extradural meningiomas11,16). Our two cases of primary intraosseous meningioma represent 0.3% of the 504 meningioma cases diagnosed at our department from 2004 to 2009. We report here two cases of primary osteolytic intraosseous meningioma with atypical/malignant pathology, which metastasized to whole skull and the spine.
CASE REPORT
Case 1
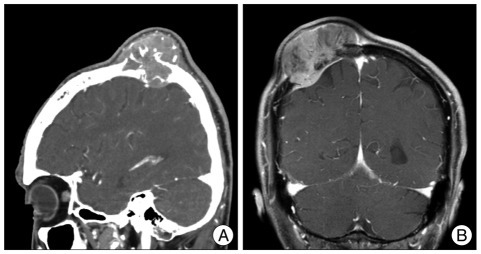
A 68-year-old man presented with a 1-year history of a soft, enlarging mass in the right parietal region. On admission, his neurological examination was within normal ranges. Skull X-rays and a computed tomogram (CT) of the head showed a bony, destructive lesion abutting the scalp and dura in the right parietal bone (Fig. 1A). Magnetic resonance imaging (MRI) revealed a heterogeneously-enhancing, bony expansile mass in the right parietal bone, extending into intracranial epidural space (Fig. 1B). Its greatest diameter was about 6 centimeters. The patient underwent surgery for resection of the mass. The scalp and subcutaneous tissues were normal and the outer table of the skull was destroyed by the well-encapsulated, soft mass. The tumor appeared to arise extradurally. Peeling off the outer layer of the dura and inspecting the dura's inner surface did not reveal the tumor. The tumor, including the surrounding normal bone, was totally resected. The permanent section of the specimen showed cellular whorl formation, frequent mitosis (5 mitoses per 10 high-power fields), and necrosis. The biopsy confirmed the tumor as an atypical (WHO grade II) meningioma (Fig. 2). The adjuvant treatment was not done because the mass was totally removed. One year later, he complaint palpable mass on previous operation site. Follow-up brain MRI revealed multiple variable sized nodules and masses in whole skull, causing epidural and subgaleal extraosseous mass formations (Fig. 3). Biopsy was confirmed as the same pathology and the patient did not want further treatment for these lesions.
Fig. 1.
Case 1 : Preoperative radiologic findings. A : CT image shows a bony, destructive lesion, in the right parietal bone abutting the scalp and dura. B : Coronal MRI revealing a heterogeneously-enhancing, bony, expansile mass in the right parietal bone, extending into intracranial epidural space.
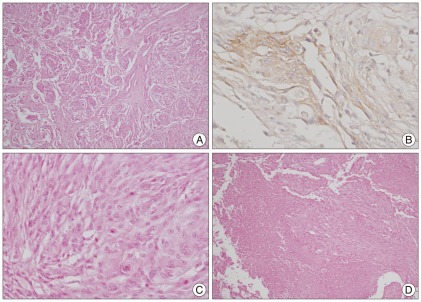
Fig. 2.
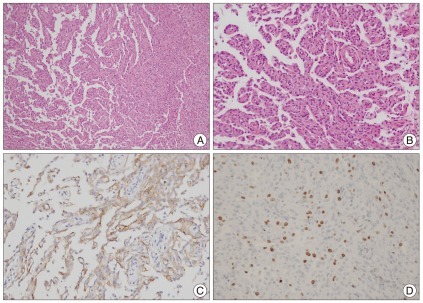
Case 1 : Pathologic findings (atypical meningioma). A : Cellular whorl formation (H&E, original magnification ×100). B : Immunopositive for epithelial membrane antigen (original magnification ×400). C : Frequent mitosis, (H&E, original magnification ×400). D : Necrosis (H&E, original magnification ×100)
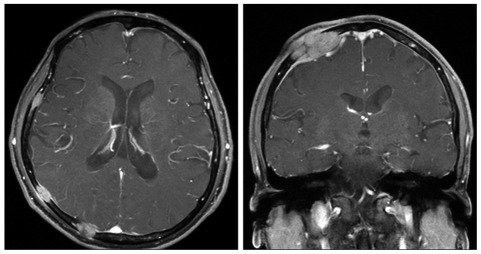
Fig. 3.
Case 1 : Follow-up radiologic findings. One year later, follow-up brain MRI shows multiple variable sized nodules and masses in whole skull, causing epidural and subgaleal extraosseous mass formations.
Case 2
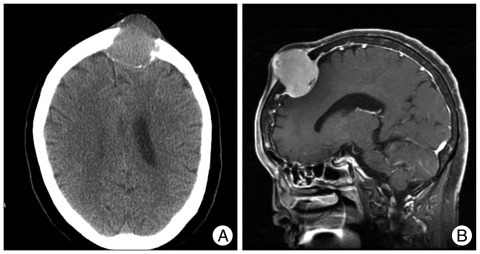
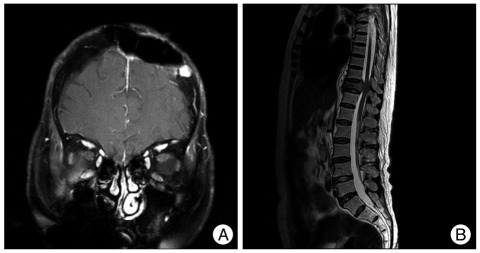
A 74-year-old woman presented with a 5-month history of a soft mass on the left frontal area. Skull X-rays and head CT showed a bony, destructive lesion extending to the scalp and displacing the dura at the left frontal bone (Fig. 4A). MRI revealed a 4-centimeter, multilobulated, strongly-enhancing mass on the left frontal bone. The mass was located intra- and extra-cranially, with thickening of the adjacent scalp and meninges (Fig. 4B). The tumor had invaded the scalp. The skull was destroyed by the well-encapsulated, soft mass. The mass was adhered tightly at the scalp and dura mater. The lesion was totally removed, including surrounding normal bone. The tumor showed no involvement on the inner surface of the dura. Biopsy showed a papillary (WHO grade III) meningioma with papillary pagrowth pattern and the immunohistochemistry for epithelial membrane antigen was positive (Fig. 5). Ki-67 labeling index was 3%. The patient refused adjuvant radiation therapy. Subsequently, at 19- and again at 45-months later, the patient underwent reoperations for recurrent lesions on scalp and skull. Follow-up brain MRI showed a 1 cm, homogenously-enhancing, recurred lesion on her skull, adjacent to the previous lesion (Fig. 6A). Five years later after the first surgery, the patient experienced back pain that slowly progressed for 4 months. Spine MRI showed an osteolytic lesion on the body and posterior element of the 11th thoracic vertebra (Fig. 6B). The patient received corpectomy and posterior fusion, with a diagnosis of metastatic papillary meningioma.
Fig. 4.
Case 2 : Preoperative radiologic findings. A : CT image shows a bony, destructive lesion in the left frontal bone. B : MRI revealing a 4-centimeter, multilobulated, strong-enhancing mass on the left frontal bone, with thickening of adjacent scalp and meninges.
Fig. 5.
Case 2 : Pathologic findings (papillary meningioma). A : Papillary structures mixed with meningothelial sheets (H&E, original magnification ×100). B : Tumor cells in papillary pattern (original magnification ×200). C : Immunopositive for epithelial membrane antigen (original magnification ×200). D : Three percent of Ki-67 labeling index (H&E, original magnification ×100).
Fig. 6.
Case 2 : Follow-up radiologic findings. A : Follow-up MRI demonstrates a 1 cm, homogenously-enhancing, recurred lesion on the skull, adjacent to the previous lesion. B : Spinal MRI shows the osteolytic lesion on the body and posterior element of the 11th thoracic vertebra.
DISCUSSION
By definition, in a primary extradural meningioma, the tumor's growth either proceeds from outside the dura to inside or demonstrates exclusive involvement of the dura's outer layer with an extracranial main mass10). These tumors are classified according to calvarial involvement and location in relation to the base of the skull. The most common skull base sites are in the middle ear/temporal bone region (18%), the paranasal sinuses (15%), the sphenoid bone (9%), and the infratemporal fossa (4%). Lang et al.10) proposed a classification system for primary extradural meningioma, as follows : tumors that are purely extra-calvarial are Type I, purely calvarial tumors are Type II, and calvarial tumors with extracranial extensions are Type III.
The majority of intraosseous meningiomas are of the osteoblastic subtype1,2). Osteoblastic intraosseous meningiomas may induce hyperostosis3-5). Crawford et al.3), in a review of the literature, found that radiographic evidence of hyperostosis appeared in 59% of these meningiomas, whereas 32% showed osteolytic changes in the surrounding bone, and 6% revealed mixed features of both osteolysis and hyperostosis. The bone expansion and hyperdense skull lesions of this type of intraosseous meningioma may appear radiologically similar to en plaque meningioma, osteoma, osteosarcoma, Paget's disease, and fibrous dysplasia4,9). These lesions may present as osteolytic skull lesions6). Differential diagnosis of an osteolytic skull lesion includes chondroma, epidermoid cyst, osteogenic sarcoma, myeloma, and metastatic cancer6,13). Diagnosing intraosseous meningioma preoperatively seems difficult to clinicians even if the patient's symptoms include a gradually expanding mass. Although meningioma is rare, differential diagnosis of any intraosseous osteolytic lesion should be included.
Although, in general, intraosseous meningiomas are reportedly slow-growing, histologically benign lesions, there have been frequent reports of atypical and malignant histological subtypes10). Recent studies indicate that intraosseous meningiomas have a higher incidence of malignant features than intradural meningiomas have7,14,17). One report reviewed 65 published cases of intraosseous meningioma, assessed during the CT era, and found that 17 (26%) had atypical or malignant histological features10). Osteolytic lesions, although a more rare form of intraosseous meningioma, have a greater likelihood of atypical or malignant features compared to osteoblastic tumors. Our two cases had osteolytic lesions that showed atypical/malignant pathologies.
Wide surgical excision of an intraosseous meningioma is the treatment of choice and is potentially curative, if the surgery is even possible, because 26% of these tumors show evidence of atypical or malignant changes3,8). Clinicians may consider patients with such lesions as stronger candidates for adjuvant therapy, depending on the clinical circumstances. Some authors recommend adjuvant radiation therapy for patients with lesions that are not completely resectable, if the residual lesion is symptomatic or shows radiographic evidence of progression3,10,14).
Of the cases of benign meningiomas found in the literature, 22% experienced recurrence, and basally-located, primary extradural meningiomas showed a higher rate of recurrence than primary extradural meningiomas located along the convexity10). This study reviewed atypical and malignant tumor death rates (29%) as compared to benign tumors (4.8%). Out of total 9 patients, 3 harbored atypical or malignant tumors, 2 of which recurred (67%) and metastasized to a distant site; despite aggressive therapy, both patients died. This study did not mention the location of these metastases. Our one case with papillary meningioma showed frequent recurrences and distant metastasis to the spine, and the other showed the metastasis to the whole skull.
CONCLUSION
Two cases of osteolytic intraosseous meningiomas had atypical/malignant pathologies, which metastasized to whole skull and the spine.
References
- 1.Agrawal V, Ludwig N, Agrawal A, Bulsara KR. Intraosseous intracranial meningioma. AJNR Am J Neuroradiol. 2007;28:314–315. [PMC free article] [PubMed] [Google Scholar]
- 2.Arana E, Menor F, Lloret RM. Intraosseous meningioma. J Neurosurg. 1996;85:362–363. doi: 10.3171/jns.1996.85.2.0362a. [DOI] [PubMed] [Google Scholar]
- 3.Crawford TS, Kleinschmidt-DeMasters BK, Lillehei KO. Primary intraosseous meningioma. Case report. J Neurosurg. 1995;83:912–915. doi: 10.3171/jns.1995.83.5.0912. [DOI] [PubMed] [Google Scholar]
- 4.Daffner RH, Yakulis R, Maroon JC. Intraosseous meningioma. Skeletal Radiol. 1998;27:108–111. doi: 10.1007/s002560050347. [DOI] [PubMed] [Google Scholar]
- 5.Devi B, Bhat D, Madhusudhan H, Santhosh V, Shankar S. Primary intraosseous meningioma of orbit and anterior cranial fossa : a case report and literature review. Australas Radiol. 2001;45:211–214. doi: 10.1046/j.1440-1673.2001.00904.x. [DOI] [PubMed] [Google Scholar]
- 6.Elder JB, Atkinson R, Zee CS, Chen TC. Primary intraosseous meningioma. Neurosurg Focus. 2007;23:E13. doi: 10.3171/FOC-07/10/E13. [DOI] [PubMed] [Google Scholar]
- 7.Husaini TA. An unusual osteolytic meningioma. J Pathol. 1970;101:57–58. doi: 10.1002/path.1711010107. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki K, Otsuka F, Matsui T, Ogura T, Makino H. Effect of etidronate on intraosseous meningioma. Endocr J. 2004;51:389–390. doi: 10.1507/endocrj.51.389. [DOI] [PubMed] [Google Scholar]
- 9.Jayaraj K, Martinez S, Freeman A, Lyles KW. Intraosseous meningioma--a mimicry of Paget's disease? J Bone Miner Res. 2001;16:1154–1156. doi: 10.1359/jbmr.2001.16.6.1154. [DOI] [PubMed] [Google Scholar]
- 10.Lang FF, Macdonald OK, Fuller GN, DeMonte F. Primary extradural meningiomas : a report on nine cases and review of the literature from the era of computerized tomography scanning. J Neurosurg. 2000;93:940–950. doi: 10.3171/jns.2000.93.6.0940. [DOI] [PubMed] [Google Scholar]
- 11.Lapresle J, Netsky MG, Zimmerman HM. [The pathology of meningiomas; a study of 121 cases] Am J Pathol. 1952;28:757–791. [PMC free article] [PubMed] [Google Scholar]
- 12.Lell M, Tudor C, Aigner T, Kessler P. Primary intraosseous meningioma of the mandible : CT and MR imaging features. AJNR Am J Neuroradiol. 2007;28:129–131. [PMC free article] [PubMed] [Google Scholar]
- 13.Marwah N, Gupta S, Marwah S, Singh S, Kalra R, Arora B. Primary intraosseous meningioma. Indian J Pathol Microbiol. 2008;51:51–52. doi: 10.4103/0377-4929.40396. [DOI] [PubMed] [Google Scholar]
- 14.Partington MD, Scheithauer BW, Piepgras DG. Carcinoembryonic antigen production associated with an osteolytic meningioma. Case report. J Neurosurg. 1995;82:489–492. doi: 10.3171/jns.1995.82.3.0489. [DOI] [PubMed] [Google Scholar]
- 15.Politi M, Romeike BF, Papanagiotou P, Nabhan A, Struffert T, Feiden W, et al. Intraosseous hemangioma of the skull with dural tail sign : radiologic features with pathologic correlation. AJNR Am J Neuroradiol. 2005;26:2049–2052. [PMC free article] [PubMed] [Google Scholar]
- 16.Shuangshoti S. Primary meningiomas outside the central nervous system. In: Al-Mefty O, editor. Meningiomas. New York: Raven Press; 1991. pp. 107–128. [Google Scholar]
- 17.Younis G, Sawaya R. Intracranial osteolytic malignant meningiomas appearing as extracranial soft-tissue masses. Neurosurgery. 1992;30:932–935. doi: 10.1227/00006123-199206000-00022. [DOI] [PubMed] [Google Scholar]