Abstract
The treatment of bilateral vertebral artery dissecting aneurysms (VADAs) presenting with subarachnoid hemorrhage (SAH) is still challenging. The authors report a rare case of bilateral VADA treated with coil trapping of ruptured VADA and covered stents implantation after multiple unsuccessful stent assisted coiling of the contralateral unruptured VADA. A 44-year-old woman was admitted to our hospital because of severe headache and sudden stuporous consciousness. Brain CT showed thick SAH and intraventricular hemorrhage. Cerebral angiography demonstrated bilateral VADA. Based on the SAH pattern and aneurysm configurations, the right VADA was considered ruptured. This was trapped with endovascular coils without difficulty. One month later, the contralateral unruptured VADA was protected using a stent-within-a-stent technique, but marked enlargement of the left VADA was detected by 8-months follow-up angiography. Subsequently two times coil packing for pseudosacs resulted in near complete occlusion of left VADA. However, it continued to grow. Covered stents graft below the posterior inferior cerebellar artery (PICA) origin and a coronary stent implantation across the origin of the PICA resulted in near complete obliteration of the VADA. Covered stent graft can be used as a last therapeutic option for the management of VADA, which requires absolute preservation of VA flow.
Keywords: Vertebral artery dissecting aneurysm, SAH, Stent assisted coiling, Trapping, Covered stent graft, Endovascular embolization
INTRODUCTION
Bilateral vertebral artery dissecting aneurysms (VADAs) presenting with subarachnoid hemorrhage (SAH) are rare and their management is still challenging4,6). Successful results with staged bilateral VA occlusion for vertebro-basilar DA have been reported4,14,15), but in many cases bilateral VA occlusion cannot be tolerated if the patients do not pass the balloon test occlusion. In this situation, reconstructive treatment is required. Of these, stent assisted coiling (SAC) or the stent-within-a-stent (SWS) technique has been performed with relatively good results1,18). And recently, covered stent grafts for VADA has been reported with good outcomes3).
After occlusion of a unilateral VA either by surgically or endovascularly, contralateral VADA enlargement or even rupture has been reported in the literature4,5,14,16). Increased hemodynamic stress to contralateral VADA might precipitate the growth of preexisting VADA.
The authors report a rare case of bilateral VADAs presenting with SAH, which was treated with internal trapping of the ruptured dissecting segment and covered stent graft after SWS and subsequent multiple session of SAC of the contralateral unruptured VADA.
CASE REPORT
A 44-year-old woman was transferred to our hospital because of headache and sudden mental deterioration. She was stuporous, but had no motor weakness. She had no history of hypertension, diabetes mellitus, or head injury. A physical examination revealed severe neck stiffness.
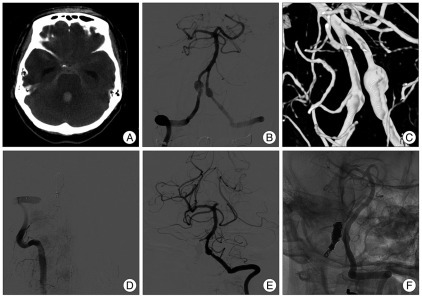
Brain computed tomography (CT) scans showed a thick SAH on the basal, prepontine, both Sylvian, ambient, and cerebellopontine angle cisterns, predominantly on the right side and acute hydrocephalus due to intraventricular hemorrhage on all ventricles (Fig. 1A). CT angiograms demonstrated bilateral VADAs, which were larger on the right side (not shown). An external ventricular drainage catheter was inserted urgently before the endovascular procedure. Digital subtraction angiography (DSA) and 3-dimensional rotational angiograms demonstrated a 6×12 mm sized right VADA [the right posterior inferior cerebellar artery (PICA) was not visible], and a 5×7 mm sized left VADA, which was incorporated with the PICA origin (In-PICA type) (Fig. 1B, C). The right VADA was considered ruptured because the SAH predominated on the right side and the right VADA was larger than the contralateral VADA. Endovascular coil trapping of the ruptured right VADA was performed on the same day using 19 coils. Postoperative angiography showed complete obliteration of right VADA with sufficient flow to the basilar artery via left VA (Fig. 1D, E).
Fig. 1.
Pre- and post-operative radiologic images in a 44-year-old woman presenting with SAH. Brain CT (A) shows thick SAH on basal, prepontine, and both cerebellopontine angle cisterns, and acute hydrocephalus due to intraventricular hemorrhage. Preoperative digital subtraction angiography (B) and 3-dimensional rotational angiogram (C) demonstrate a 6×12 mm sized right VADA (the right PICA is not visualized), and a 5×7 mm sized left VADA, which is incorporated with PICA origin. Postoperative vertebral angiograms after packing of 19 coils for right VADA show complete trapping of the right VADA with sufficient flow to the basilar artery via the left vertebral artery (D and E). Vertebral angiogram after double stent implantation for the left VADA demonstrates slightly reduced blood flow to the aneurysmal sac (F). SAH : subarachnoid hemorrhage, VADA : vertebral artery dissecting aneurysm, PICA : posterior inferior cerebellar artery.
One month later, two overlapping stents (SWS) implantation (Enterprise 4.5×22 mm, and 4.5×28 mm, Cordis Neurovascular, Miami, FL, USA) was performed for the unruptured left VADA to prevent possible growth due to increased hemodynamic stress to the left VA caused by contralateral VA sacrifice. After double stent implantation for the left VADA, blood flow to aneurysmal sac was slightly reduced (Fig. 1F). Subsequently, the patient was alert with dull mentality. She was discharged to a rehabilitation hospital at 6 weeks after initial SAH.
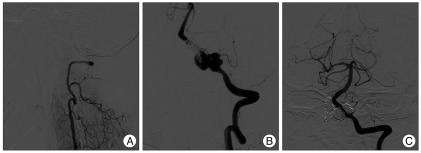
Eight months follow-up angiograms demonstrated stable occlusion of the right VADA (Fig. 2A) and marked growth of the left VADA (Fig. 2B). At least two pseudosacs were identified, and these were separately treated with coil packing though the interstices of stent strut. Postoperative angiograms showed near complete obliteration of the left VADA and sparing of left PICA flow (Fig. 2C).
Fig. 2.
Follow-up vertebral angiograms at 8 months after double overlapping stenting on the left VADA showing stable occlusion of right VADA (A) and marked growth of the left VADA (B). Postoperative left vertebral angiogram after coil packing into the two different pseudosacs shows near complete obliteration of left VADA, while sparing left PICA flow (C). VADA : vertebral artery dissecting aneurysm, PICA : posterior inferior cerebellar artery.
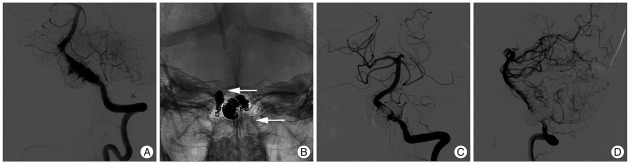
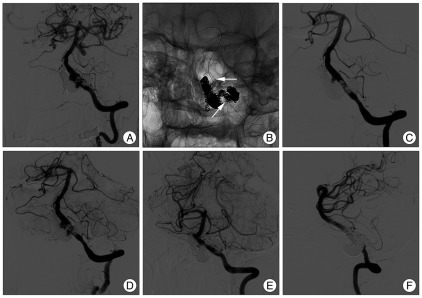
Angiography conducted at 5 months after the left VADA procedure demonstrated further growth of left VADA (Fig. 3A), which was re-treated with further coil packing into the growing pseudosacs and bailout stenting using a 4.5×28 mm Enterprise stent to maintain left VA flow (Fig. 3B). Postoperative angiograms showed near complete obliteration of the VADA, and sparing of left PICA flow (Fig. 3C, D). However, follow-up angiograms 4 months later showed regrowth of the left VADA (Fig. 4A). Further coil packing was considered ineffective in this patient, and more definitive treatment, such as, occipital artery-PICA bypass surgery followed by endovascular reconstruction of the VA using a covered stent was considered, but the PICA diameter was too small. Thus, we planned covered stent implantation for the dissection segment below the PICA origin, and coverage of the remaining part of the dissection segment with balloon expandable coronary stent to induce spontaneous occlusion of the pseudosac as a result of flow stagnation and diversion, sparing the PICA flow. Telescopic stenting with two covered stents (Jostent Graftmaster 3.5×12 mm, 3.5×9 mm, Abbott vascular, IL, USA) was successfully performed on left VA just below the PICA origin. Control angiography showed marked reduction of blood flow to the VADA, but retrograde flow was still visible near the PICA origin. A third stent (Driver stent 3.5×12 mm; Medtronic Vascular, Santa Rosa, CA, USA) was then implanted across the PICA origin, covering the distal part of the dissecting segment (Fig. 4B). Final angiography still demonstrated slow retrograde flow to the VADA even though flow velocity into the aneurysmal sac was markedly reduced (Fig. 4C, D). Follow up DSA at 2 months showed near complete occlusion of the left VADA (Fig. 4E, F). Follow-up angiography will be performed to confirm complete occlusion of the VADA in the near future.
Fig. 3.
Follow-up left vertebral angiogram (A) at 5 months after coil packing of the left VADA demonstrating its further growth. Postoperative skull radiograph (B) shows triple overlapping stents and coil mass on the left vertebral artery after further coil packing of the two growing pseudosacs and bailout stenting using an Enterprise stent. White arrows indicate proximal and distal end of the triple stents. Postoperative angiograms (C and D) show near complete obliteration of the VADA and sparing of left PICA flow. VADA : vertebral artery dissecting aneurysm, PICA : posterior inferior cerebellar artery.
Fig. 4.
Follow-up angiograms at 4 months after final embolization also showing left VADA regrowth (A). Postoperative skull radiograph after telescopic stenting with two covered stents graft (Graftmaster 3.5×12 mm, 3.5×9 mm) on the left vertebral artery just below the PICA origin and a third stent (Driver 3.5×12 mm) across the PICA origin (B). Arrows indicate the proximal end of covered stent and distal end of Driver stent. Final vertebral angiograms at early arterial (C) and late arterial phase (D) still demonstrate slow retrograde flow to VADA, although flow velocity into the VADA is markedly decreased. Follow-up angiograms 2 month later show near complete occlusion of the left VADA (E and F). VADA : vertebral artery dissecting aneurysm, PICA : posterior inferior cerebellar artery.
DISCUSSION
Bilateral VADA presenting with SAH is extremely rare6,9-11), and no established treatment is exists for this type of VADA. Even though there has been a successful report with conservative treatment under strict sedation and blood pressure control during the acute stage10), this is not a reasonable option for a ruptured VADA because of the high risk of rebleeding during the acute stage. Bilateral VA occlusion combined with surgical vascular reconstruction may be the treatment of choice for this type of lesion16), but in many cases, vascular reconstruction of the posterior circulation is difficult or not possible. Another treatment option might be unilateral VA occlusion of a ruptured VADA followed by stent only therapy or SAC for a contralateral unruptured VADA. Successful reports with stent only therapy for VADA have been published8,12). Park et al.12) treated 29 vertebro-basilar DAs in 27 patients by stent only therapy. Complete or partial obliteration was achieved in 88.8%, and angiographic improvement was more common in patients with multiple overlapping stents. Stent-induced hemodynamic changes, such as decreases in inflow momentum, velocity, and wall shear stress, could help prevent aneurysm growth and rupture7,20).
To treat bilateral VADA presenting with SAH, it is important to determine which aneurysm has ruptured. The possible clues of side of rupture are SAH distribution and the shape and size of the DA4). If the SAH predominates in one side or one aneurysm is larger, or has a bleb, then there is a high likelihood of rupture of that side. In our case, the right VADA was considered ruptured because SAH predominates on the right side and the right VADA was larger. Furthermore, no important branch was visible on the right VADA, indicating that the right VADA could be trapped completely using coils.
After unilateral VA sacrifice, enlargement or even rupture of contralateral VADA has been reported5,16). Increased hemodynamic stress to the contralateral VA may induce aneurysm growth. In our case, to prevent aneurysm growth or rupture after unilateral trapping of the right VADA, two overlapping stents were implanted into the contralateral VADA. However, even though the immediate postoperative angiogram showed a slight decrease in aneurysm filling, the unruptured VADA enlarged over time, and subsequent, overlapping stenting using two Enterprise stents did not prevent aneurysm growth. The possible reasons why our result was disappointing are related to the degree of apposition of stent and DA and the metal covered area within the vessel wall. Park et al.12) used coronary stents to treat all DAs, except five. Coronary stents are balloon expandable stents, and thus there is a greater chance of good apposition of the DA wall and stent strut during ballooning. On the other hand, the Enterprise stent is a self expandable stent, and although radial force is enough to prevent stent movement after deployment, the pressure applied to the DA wall may not be sufficient to occlude the DA inlet or outlet. Coronary stent has a smaller cell size than Enterprise stent. Thus, the porosity of coronary stent is lower than that of Enterprise stent. The relatively higher porosity of Enterprise stent might have been responsible for aneurysm growth because of less flow diversion effect.
For the management of VADA not amenable to internal trapping, the SAC followed by the SWS technique have been reported1,18,19). Suh et al.18) treated 11 VADA patients successfully in this manner without any treatment-related complications. Follow-up angiography at 6 to 12 months on the surviving 10 patients showed complete DA obliteration in 9. They used 10 Neuroform stents, and 1 balloon expandable coronary stent, and during the procedure they used in-stent balloon assistance to ensure tight packing. However, in our case, marked regrowth of the left VADA was identified after double overlapping stenting. If additional overlapping stenting results in failure, coil packing into the DA might be impossible because triple or quadruple stenting will probably prevent microcatheter selection. Thus, we performed SAC twice before covered stents graft. Covered stent graft below the origin of the PICA can occlude inlet of the VADA and preserve left PICA flow. However, covered stents are very stiff and hard to navigate tortuous cerebral vessel. Thus, we used short length of two covered stents to navigate the VA easily.
Covered stent grafts for intracranial aneurysms were first described by Redekop and colleagues in 200113). Since their report, these stent grafts have infrequently been used for the management of carotid cavernous fistulas, internal carotid artery aneurysms, and VA aneurysms2,3). To our knowledge, only 11 patients with VADA have been treated with a covered stent graft successfully2,3,13). Theoretically, a covered stent graft can obliterate the dissecting aneurysm while maintaining the patency of the parent vessel, and it represents a good treatment option for VADA. However, some considerations should be borne in mind. First, covered stents should not be implanted for vessels with important perforators because they occlude any branch of the parent vessel along the length of the stent. For instance, a covered stent graft for In-PICA type of VADA can result in Wallenberg syndrome or even death due to PICA occlusion. He et al.3) treated 5 cases of VADA successfully with a covered stent graft. Of these, VADA was located at proximal to the PICA in 4. To avoid accidental PICA occlusion, they selected patients with dissection distant from important vessels, such as, the PICA, by at least 2 mm. In our case we also deployed the covered stents just proximal to the PICA origin to prevent accidental occlusion of the PICA. The second consideration is the navigability of the stent to the intracranial VA. Covered stents are relatively stiff compared with other stent systems. Thus, if the proximal VA is tortuous or the V3 segment angle is very acute, a covered stent graft may not be possible. To overcome the navigability issue a shorter length of stent can be used to improve navigability. The third consideration is the restenosis issue. In one study, after coronary stenting restenosis occurred in 25% of patients17). A covered stent, such as, the Jostent Graftmaster, is a stainless steel stent with the PTFE membrane placed between 2 layers of stent struts. It has been associated with an increased risk of delayed restenosis/occlusion of VA17). However, fortunately no restenosis or occlusion of parent artery after covered stent graft in VADA has been reported yet.
Recently, newly designed stents with low porosity, such as, the Pipeline stent have been introduced to create a flow-diversion effect and to induce spontaneous thrombosis in large/giant or fusiform aneurysm. The pipeline stent may help to overcome the limitation of highly porous stents for the management of this challenging aneurysm in the near future.
CONCLUSION
Bilateral VADA presenting with SAH is rare. In terms of its management, contralateral VADA enlargement due to the increased hemodynamic stress after ipsilateral VA flow sacrifice must be kept in mind. Covered stent graft for the involved VADA can be a valuable therapeutic option, especially in patients that require absolute preservation of VA flow. Early follow-up angiography is mandatory.
References
- 1.Ahn JY, Han IB, Kim TG, Yoon PH, Lee YJ, Lee BH, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol. 2006;27:1514–1520. [PMC free article] [PubMed] [Google Scholar]
- 2.Ding H, He M, You C, Deng L. [Application of endovascular covered stent for treating vertebral dissecting aneurysm and carotid-cavernous fistula] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:215–218. [PubMed] [Google Scholar]
- 3.He M, Zhang H, Lei D, Mao BY, You C, Xie XD, et al. Application of covered stent grafts for intracranial vertebral artery dissecting aneurysms. J Neurosurg. 2009;110:418–426. doi: 10.3171/2008.3.17470. [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Kohno K, Takechi A, Kohno K, Matsushige T, Takeda T. Bilateral vertebral artery dissecting aneurysm with subarachnoid hemorrhage treated with staged bilateral vertebral artery coil occlusion : a case report. Surg Neurol. 2008;70:319–322. doi: 10.1016/j.surneu.2007.04.019. discussion 322. [DOI] [PubMed] [Google Scholar]
- 5.Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A. Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery : case report. Neurol Med Chir (Tokyo) 2009;49:468–470. doi: 10.2176/nmc.49.468. [DOI] [PubMed] [Google Scholar]
- 6.Koh JS, Ryu CW, Lee SH, Bang JS, Kim GK. Bilateral vertebral-artery-dissecting aneurysm causing subarachnoid hemorrhage cured by staged endovascular reconstruction after occlusion. Cerebrovasc Dis. 2009;27:202–204. doi: 10.1159/000193464. [DOI] [PubMed] [Google Scholar]
- 7.Lieber BB, Stancampiano AP, Wakhloo AK. Alteration of hemodynamics in aneurysm models by stenting : influence of stent porosity. Ann Biomed Eng. 1997;25:460–469. doi: 10.1007/BF02684187. [DOI] [PubMed] [Google Scholar]
- 8.Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg. 2001;94:427–432. doi: 10.3171/jns.2001.94.3.0427. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K, Akagi K, Abekura M, Sakaguchi T. Obliteration of bilateral dissecting aneurysms of the vertebral arteries following repeated subarachnoid hemorrhage : a case report. Neurol Res. 2002;24:837–841. doi: 10.1179/016164102101200825. [DOI] [PubMed] [Google Scholar]
- 10.Nashimoto T, Komata T, Honma J, Yamashita S, Seki Y, Kurashima A, et al. Successful treatment of bilateral vertebral artery dissecting aneurysms with subarachnoid hemorrhage : report of three cases. J Stroke Cerebrovasc Dis. 2010 doi: 10.1016/j.jstrokecerebrovasdis.2010.10.003. in press. [DOI] [PubMed] [Google Scholar]
- 11.Otawara Y, Ogasawara K, Ogawa A, Kogure T. Dissecting aneurysms of the bilateral vertebral arteries with subarachnoid hemorrhage : report of three cases. Neurosurgery. 2002;50:1372–1374. doi: 10.1097/00006123-200206000-00033. discussion 1374-1375. [DOI] [PubMed] [Google Scholar]
- 12.Park SI, Kim BM, Kim DI, Shin YS, Suh SH, Chung EC, et al. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2009;30:1351–1356. doi: 10.3174/ajnr.A1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base by using endovascular stents. J Neurosurg. 2001;95:412–419. doi: 10.3171/jns.2001.95.3.0412. [DOI] [PubMed] [Google Scholar]
- 14.Redekop G, TerBrugge K, Willinsky R. Subarachnoid hemorrhage from vertebrobasilar dissecting aneurysm treated with staged bilateral vertebral artery occlusion : the importance of early follow-up angiography : technical case report. Neurosurgery. 1999;45:1258–1262. doi: 10.1097/00006123-199911000-00056. discussion 1262-1263. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto S, Ohba S, Shibukawa M, Kiura Y, Okazaki T, Arita K, et al. Staged bilateral vertebral artery occlusion for ruptured dissecting aneurysms of the basilar artery : a report of 2 cases. Surg Neurol. 2005;64:456–461. doi: 10.1016/j.surneu.2005.01.021. discussion 461. [DOI] [PubMed] [Google Scholar]
- 16.Somekawa K, Nagata K, Kawamoto S, Furuya H, Tanioka D, Isoo A. [Treatment for the ruptured bilateral vertebral dissecting aneurysms] No Shinkei Geka. 2002;30:321–325. [PubMed] [Google Scholar]
- 17.Stone GW, Goldberg S, O'Shaughnessy C, Midei M, Siegel RM, Cristea E, et al. 5-year follow-up of polytetrafluoroethylene-covered stents compared with bare-metal stents in aortocoronary saphenous vein grafts the randomized (BARRICADE barrier approach to restenosis : restrict intima to curtail adverse events) trial. JACC Cardiovasc Interv. 2011;4:300–309. doi: 10.1016/j.jcin.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Suh SH, Kim BM, Park SI, Kim DI, Shin YS, Kim EJ, et al. Stent-assisted coil embolization followed by a stent-within-a-stent technique for ruptured dissecting aneurysms of the intracranial vertebrobasilar artery. Clinical article. J Neurosurg. 2009;111:48–52. doi: 10.3171/2009.2.JNS081418. [DOI] [PubMed] [Google Scholar]
- 19.Yoon WK, Kim YW, Kim SR, Park IS, Kim SD, Jo KW, et al. Angiographic and clinical outcomes of stent-alone treatment for spontaneous vertebrobasilar dissecting aneurysm. Acta Neurochir (Wien) 2010;152:1477–1486. doi: 10.1007/s00701-010-0693-7. discussion 1486. [DOI] [PubMed] [Google Scholar]
- 20.Zenteno MA, Santos-Franco JA, Freitas-Modenesi JM, Gómez C, Murillo-Bonilla L, Aburto-Murrieta Y, et al. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J Neurosurg. 2008;108:1104–1118. doi: 10.3171/JNS/2008/108/6/1104. [DOI] [PubMed] [Google Scholar]