Abstract
Background
Patients with severe aortic stenosis and coexisting non-cardiac conditions may be at high risk for surgical replacement of the aortic valve or even be no candidates for surgery. In these patients, transcatheter aortic valve implantation (TAVI) is suggested as an alternative. Results of the PARTNER (Placement of AoRTic TraNscathetER Valve) trial comparing the clinical effectiveness of TAVI with surgical valve replacement and standard therapy were published. The authors assessed the cost-effectiveness of TAVI in Belgium.
Methods
A Markov model of incremental costs, effects (survival and quality of life) and incremental cost-effectiveness of TAVI was developed. The impact on survival, number of events and quality of life was based on the PARTNER trial. Costs per event were context specific.
Results
In high-risk operable patients, even if the minor differences in 30-day and 1-year mortality are taken into account, the incremental cost-effectiveness ratio (ICER) remains on average above €750 000 per quality-adjusted life-year (QALY) gained (incremental cost: €20 400; incremental effect: 0.03 QALYs). In inoperable patients, an ICER of €44 900 per QALY (incremental cost: €33 200; incremental effect: 0.74 QALYs) is calculated, including a life-long extrapolation of the mortality benefit. This result was sensitive to the assumed time horizon. The subgroup of anatomically inoperable patients had better outcomes than medically inoperable patients, with ICERs decreasing more than €10 000/QALY.
Conclusions
It is inappropriate to consider reimbursement of TAVI for high-risk operable patients. Reimbursing TAVI in inoperable patients in essence is a political decision. From an economic perspective, it would be prudent to first target patients that are inoperable because of anatomical prohibitive conditions. In the search for evidence, the authors identified non-published negative results from a randomised controlled TAVI trial. The study sponsor should be more willing to share this information to allow balanced evaluations and policy recommendations. Payers should require these data before taking reimbursement decisions.
Article summary
Article focus
To assess the cost-effectiveness of transcatheter aortic valve implantation (TAVI) for Belgian patients.
Key messages
In high-risk operable patients, surgical aortic valve replacement and TAVI are associated with similar mortality rates at 1 year. However, there is a twice as high rate of stroke after TAVI. From an economic point of view, the less invasive nature of the TAVI procedure does not weigh against the extra costs of about €20 000 per patient.
In inoperable patients, TAVI significantly reduces the rate of death from any cause as compared with a non-surgical approach. The ICER is about €45 000 per QALY gained. Nevertheless, a distinction should be made between inoperability for anatomic versus medical reasons. TAVI offers more value for money in the former patient group with ICERs decreasing more than €10 000 per QALY.
If policy makers are willing/able to pay the relative high price for TAVI, it is advised to focus in the first place on the anatomic inoperable patients.
Strengths and limitations of this study
Hospital billing data of 183 Belgian patients treated with the Edwards SAPIEN valve were at our disposal for cost calculations.
Next to the published pivotal PARTNER (Placement of AoRTic TraNscathetER Valve) trial, a non-published randomised controlled trial (RCT) with TAVI was identified. This Continued Access trial was performed according to the same study protocol of the pivotal PARTNER trial. The mortality outcomes of this Continued Access RCT, disfavouring TAVI, were included in our analysis.
An unbalance in patient characteristics was observed in the inoperable patients of the PARTNER trial, favouring the TAVI group. A subgroup analysis showed a greater improvement for anatomic inoperable patients versus those inoperable for medical reasons.
The most important limitation of our analysis is the non-availability of all relevant study outcomes, especially for the negative non-published Continued Access RCT. The study sponsor should be willing to reveal this information in due time. Payers should require access to these non-published data before deciding on reimbursement.
Background
Degenerative aortic stenosis is a common valvular heart disease, mostly affecting elderly people. Since many of those patients suffer from comorbidities, they might not be amenable to surgical valve replacement. In this context, transcatheter aortic valve implantation (TAVI), a less invasive approach to correct the valvular stenosis, has been developed. Until recently, the assessment of the clinical effectiveness of TAVI had to rely on reports from uncontrolled case series, making it prone to bias. The randomised controlled PARTNER (Placement of AoRTic TraNscathetER Valve) trial (NCT00530894) provided more robust data,1 2 allowing to more reliably estimate the clinical benefit of TAVI as compared with other treatment modalities. After the publication of the pivotal trial results, additional data from the PARTNER trial became available. Among them are non-published negative mortality data from the Continued Access study, related to patients treated according to the randomised protocol for inoperable patients. We used the available trial data to be incorporated in a health economic model in order to calculate TAVI's cost-effectiveness. This is of major importance to support rational reimbursement decisions, taking into account the financial constraints.
Methods
A Markov simulation model is developed in Excel in order to assess the efficiency of TAVI. Having a possible impact on both mortality and quality of life (QoL), both cost-effectiveness (with outcomes expressed in life-years gained (LYG)) and cost-utility analyses (with LYG adjusted for QoL) are performed. The @Risk add-in tool is used for probabilistic modelling and probabilistic sensitivity analyses.
The analysis includes direct healthcare costs from the perspective of the healthcare payer.3 Payments out of the public healthcare budget as well as patients' co-payments are included. Since baseline employment rates are expected to be low in the target population, indirect productivity costs are ignored.
Population
The model simulates a hypothetical cohort of 1000 TAVI-eligible patients. The type of participants considered reflects the PARTNER patients. The PARTNER study incorporated two parallel prospective, multicenter, randomised, active-treatment-controlled clinical trials.1 Patients were divided into two cohorts: those who were considered to be candidates for surgery but at high surgical risk (cohort A) and those who were no suitable candidates for surgery (cohort B) (see figure 4 in the supplementary appendix). In the first cohort, TAVI could be performed transfemoral or transapical. The average age was 84 and 83 years in high-risk operable and inoperable patients, respectively. The proportion of men was 57% and 46%, respectively.1 2 This is reflected in the model and taken into account when extrapolating the short-term results into lifetime outcomes. More information on the PARTNER study design is available in the supplementary appendix.
Intervention and comparator
The Edwards SAPIEN heart-valve system (Edwards Lifesciences) is used in the PARTNER study. The comparator is different for the two cohorts. In the high-risk operable patients, this is surgical aortic valve replacement (AVR). In the inoperable patients, the comparator treatment was a so-called standard therapy incorporating a balloon valvuloplasty in most patients.
Time horizon
There was no significant difference in survival after 1 year in the high-risk operable patients. Therefore, in our model, the time horizon is restricted to this period. In inoperable patients, there is a survival advantage after 1 year, and survival data are extrapolated to a lifetime time horizon. Future costs and benefits are discounted at a yearly rate of 3% and 1.5%, respectively.3
Markov model
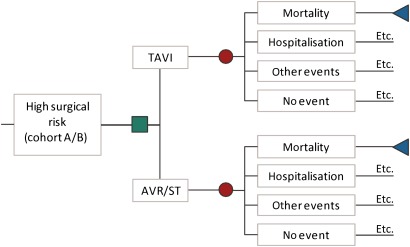
A Markov model with monthly cycles is set up (figure 1). The square node points at the choice between TAVI and the alternative intervention (AVR in high-risk operable patients (cohort A) or standard therapy in inoperable patients (cohort B)). In every cycle, the patient can die (end node), be hospitalised, have an adverse event or no event.
Figure 1.
The TAVI Markov model. AVR, aortic valve replacement; ST, standard therapy; TAVI, transcatheter aortic valve implantation. The green square is a choice node, the red dots are chance nodes and the blue triangles are end nodes. Etc.: this indicates that if the patient survives, he goes to the next cycle in the Markov model. In each monthly cycle, the patient is again at risk of dying, being hospitalised, having other events or no event.
Mortality
The PARTNER study is the first randomised controlled trial (RCT) to assess the safety and efficacy of the Sapien® valve. It was conducted under the auspices of the Food and Drug Administration (FDA) and required to apply for market approval for the valve on the US market. In addition to published study results, information was searched in FDA's Advisory Panel documents, conference documents and press releases, and from the study sponsor.
Table 1 shows the 30-day and 1-year mortality rates in the PARTNER trial. Intention-to-treat results are implemented in the model. In high-risk operable patients, the non-inferiority study showed non-significant differences.2 In inoperable patients, an absolute 20% mortality risk reduction is found after 1 year.1 In contrast, the non-published Continued Access study, following the same protocol, presented an absolute 12.7% higher mortality in the TAVI group.4 The weighted average results in an absolute mortality reduction by TAVI of 12.3%, which is used in the reference case. A validity check was applied to check whether the modelled 1-year mortality rates were consistent with the published rates.
Table 1.
Early and late mortality in the PARTNER trial
| High-risk operable patients |
Inoperable patients |
|||||||||
| Pivotal trial |
Cont. access |
Combined* |
||||||||
| TAVI | AVR | p Value | TAVI | Stand. | p Value | TAVI | Stand. | TAVI | Stand. | |
| N | 348 | 351 | 179 | 179 | 41 | 49 | 220 | 228 | ||
| 30-day mortality | 3.4% | 6.5% | 0.07 | 5.0%† | 2.8% | 0.41 | 9.8% | 2.1% | 5.9% | 2.7% |
| 1-year mortality | 24.2% | 26.8% | 0.44 | 30.7% | 50.7% | <0.001 | 34.3% | 21.6% | 31.4% | 43.7% |
Sources: high-risk operable patients: Smith et al2; inoperable patients: pivotal trial: Leon et al1; Continued Access: FDA.4
The weights are based on the number of participants in the pivotal and Continued Access trials.
Uncertainty surrounding mortality is modelled with β distributions with the same mean. The α parameter of these distributions equals the number of events in the PARTNER randomised controlled trial. For example: 5% mortality on a total of 179 patients is reflected with a β distribution with the α parameter being 9 (ie, 5% of 179=9 patients) and the β parameter being 170 (ie, 179−9).
AVR, aortic valve replacement; Cont., continued; Stand., standard therapy; TAVI, transcatheter aortic valve implantation.
The monthly mortality rate between the second month and 1 year is deducted from the 1-month and 1-year mortality and is assumed to be constant over this period. This monthly mortality rate increases yearly according to the age- and sex-specific mortality rates of the total Belgian population to extrapolate trial results to the lifetime time horizon for inoperable patients.
Utilities
The PARTNER study protocol mentions QoL was measured with the EQ-5D questionnaire in both the high-risk operable and inoperable patients. This generic utility measure was applied both at baseline and after 1, 6 and 12 months. Results were not published but provided by the study sponsor for inoperable patients and included in the model (details are provided in table 3 in the supplementary appendix). An adjustment for baseline QoL differences was included. We assumed a linear QoL evolution between baseline, 1, 6 and 12 months and a constant QoL afterwards.
Unfortunately, although requested from the study sponsor, no EQ-5D results were provided for high-risk operable patients. At 30 days, more patients in the transcatheter group than in the surgical group had a reduction in symptoms to NYHA class II or lower (p<0.001). Among patients who could perform a 6 min walk test, patients in the transcatheter group walked farther than those in the surgical group (p=0.002).2 Therefore, a similar difference as observed during the first month in inoperable patients was assumed. The NYHA functional class was no longer different at 6 months. At 1 year, there were no significant between-group differences in cardiac symptoms and the 6 min walk distance.2 Therefore, in the base case, no further differences in QoL were included in the model for high-risk operable patients. This assumption was altered in a sensitivity analysis.
Costs
To calculate incremental costs (ICs), the initial cost differences between TAVI and AVR during hospitalisation (high-risk operable patients) and between TAVI and standard therapy (inoperable patients) are taken into account. Second, events with a possible incremental impact were selected from the published PARTNER trial. Included events are repeat hospitalisations, minor/major stroke and TIA, and cardiac reintervention. Vascular complications and major bleedings are not separately included to avoid double counting since these mainly occur during the initial or repeat hospitalisation. The percentage of events observed in the PARTNER trial is provided in the supplementary appendix (table 4). Only results for the pivotal trial were published and thus implemented in our model. In the Markov model with monthly cycles, events between 30 days and 1 year are transformed to monthly percentages, which are also used in the lifetime extrapolation in the model for inoperable patients. In this cohort of inoperable patients, cardiac reinterventions were considered being balloon aortic valvuloplasty, which was performed in 84% of patients, repeat TAVI in the TAVI group (1.7%) and AVR which occurred in almost 10% of the standard therapy group (see table 4 in the supplementary appendix).
Aggregated cost data of 183 consecutive patients treated in Belgium between June 2006 and June 2010 with the Edwards SAPIEN® valve (99 transfemoral and 84 transapical) were obtained. The interventional procedure and the device cost not (yet) being reimbursed, a cost of €18 000 and €1500, for the device and the procedure, respectively, was assigned. This eventually resulted in a cost of about €41 000 and €50 000 for transfemoral and transapical TAVI (table 2). In the inoperable cohort, not all patients assigned to TAVI actually underwent TAVI and some patients in the standard therapy group received AVR and/or balloon valvuloplasty. The cost of the initial procedure was adjusted for this.
Table 2.
Overview of cost data
| Variable | Mean | Uncertainty | Source |
| TAVI (high-risk operable patients) | |||
| TF | €40 917 | Normal (mean: 40 917; SD mean 1204) | Gov. Health Ins. Database* |
| TA | €49 799 | Normal (mean: 49 799; SD mean 1994) | Gov. Health Ins. Database* |
| All | €43 571† | ||
| TAVI (inoperable patients) | €40 057‡ | ||
| Standard therapy | €3170§ | ||
| AVR | €23 749¶ | Normal (mean: 23 749; SD mean 191) | Gov. Health Ins. Database** |
| Balloon aortic valvuloplasty | €489†† | Fixed fee | Belgian Nomenclature code |
| Repeat hospitalisation | €5983 | Gamma (mean: 5983; P5: 1339; P95: 15 596) | TCT, APR-DRG 194 (Heart failure) |
| Stroke | TCT, APR-DRG 045 (CVA with stroke) | ||
| Minor | €4679‡‡ | Gamma (mean: 3292; P5: 932; P95: 6842) | Minor |
| Gamma (mean: 6066; P5: 1574; P95: 17 285) | Moderate | ||
| Major | €12 493‡‡ | Gamma (mean: 9593; P5: 1630; P95: 27 526) | Major |
| Gamma (mean: 15 392; P5: 2631; P95: 40 079) | Extreme | ||
| TIA | €3946 | Gamma (mean: 3946; P5: 974; P95: 9942) | TCT, APR-DRG 047 (TIA) |
| Follow-up fees | €43.2/month§§ | Uniform (±50%) | Expert opinion |
| Follow-up drugs | €20.5/month¶¶ | Uniform (±50%) | Gov. Health Ins. Database* |
IMA data.
The weighted average of TF and TA TAVI cost. The weight is determined by the number of TF and TA patients in the PARTNER trial, being 244 and 104, respectively.
In the PARTNER trial, of the 179 patients assigned to TAVI, six (3.4%) did not receive a transcatheter heart valve: two patients died before the scheduled implantation, transfemoral access was unsuccessful in two patients and the intraprocedural annulus measurement was too large in two patients.1 Similarly, in our model, the complete TAVI procedure cost was assigned to 96.6% (173/179) of patients, 1.1% were assigned no costs because they died before the procedure and 2.2% was assigned the procedure cost without the TAVI device cost of €18 000. This results in an average cost of €40 057.
In the PARTNER trial, 12 of the patients who were assigned to standard therapy (6.7%) underwent aortic-valve replacement, five (2.8%) underwent placement of a conduit from the left ventricular apex to the descending aorta plus aortic valve replacement (AVR) and four (2.2%) underwent TAVI at a non-participating site outside the USA.1 Therefore, the cost of AVR is assigned to 9.5% (6.7% + 2.8%) of the patients and the TAVI cost to 2.2%. This results in an additional cost of €3170 in the standard therapy group.
The cost was calculated for patients (N=4811) older than 70 and an SOI index of 3 or 4. If this was restricted to patients (N=1506) older than 80 with an SOI index of 3 or 4, the cost remained the same (€23 772).
TCT data. Year of pricing: 2006–2010 for TAVI and standard therapy; 2008 for all TCT-based APR-DRG prices; 2004–2007 for AVR. Due to the incremental character of the analysis, price adjustments would have no substantial influence on results. Furthermore, the analysis in high-risk operable patients showed that the result is determined by the (lack of) incremental effect of TAVI versus AVR.
The fee is included explicitly in the control group. If this procedure would include a hospitalisation, then these costs are already included in the model as ‘repeat hospitalisation’. In the TAVI group, to avoid double counting, this cost is not included separately since it is part of the TAVI procedure, and thus, the cost is already included in the analysis.
For stroke, the TCT makes a distinction between four categories (minor, moderate, major and extreme). The former two categories were reclassified as minor stroke and the latter two as major stroke.
The follow-up costs included the following: four consultations cardiologist (€34.5/unit), four ECG (€17.18/unit); one echo, full transthoracal ultrasound bilan of the heart (€67.16/unit); three echo, repetition within the calendar year of full transthoracal ultrasound bilan of the heart (€67.16/unit) or limited transthoracal ultrasound bilan of the heart (€38.75); one full transoesophageal ultrasound bilan of the heart (€113.01/unit) or limited transoesophageal ultrasound bilan of the heart (€58.12/unit).
The selected drugs were the following: acenocoumarol, aspirin, clopidogrel, dalteparin, danaparoid, enoxaparin, fenprocoumon, heparin, nadroparine, ticlopidine, tirofiban and warfarin. The real-world expenditures in the Belgian Edwards TF TAVI group was €246 per year or €20.5 per month. For more details, we refer to the full HTA report.6
APR-DRG, All Patient Refined Diagnosis Related Groups; AVR, aortic valve replacement; Gov. Health Ins. Database, Governmental Health Insurance Database; IMA, Intermutualistic Agency; SOI, severity of illness; TAVI, transcatheter aortic valve implantation; TCT, Belgian Technical Cell; TIA, transient ischaemic attack.
To calculate the cost of a surgical AVR, we obtained data from 9213 hospital stays between 2004 and 2007. This sample contains both older and younger patients with a different severity of illness (grades 1–4). Since the PARTNER study mainly includes older patients with multiple comorbidities, older patients (>70) with a higher severity of illness index (3 or 4) were selected, resulting in an average AVR cost of €23 749.
For the cost of balloon aortic valvuloplasty, the fee of €488.75 is taken into account. Costs for repeat hospitalisations, minor or major stroke and TIA are based on the APR-DRG costs for these categories as published (publicly available at http://www.tct.fgov.be). It is assumed that the cost of a specific event is the same across all treatment groups. Finally, a theoretical follow-up cost of about €43 per month and a drug cost of €21 per month (table 2), attributable to the number of surviving patients, is included in the model. We assume these monthly costs to be the same for all survivors in the model.
Sensitivity and scenario analyses
The impact of uncertainty around all the model's input parameters on the results was modelled probabilistically. The applied distribution depends on the type of variable5: transition probabilities (mortality or chance for another event) and utilities are modelled as β distributions. This distribution is limited to the 0–1 scale and reflects the possible outcomes for these variables. The α parameter of this distribution equals the number of events in the PARTNER RCT. The β parameter is adjusted to equal the published percentage of events. Strict correlation is imposed between the modelled probabilities at 30 days and 1 year to avoid irrational modelled outcomes. Relying on the central limit theorem, TAVI and AVR costs are modelled as normal distribution around the mean. Due to the large uncertainty around follow-up costs, a uniform distribution (±50%) is applied. Finally, publicly available TCT cost data are published with P5 and P95 values. For these cost variables, γ distributions reflecting the same mean, P5 and P95 values are modelled.
One thousand Latin Hypercube simulations are performed. Outcomes with their surrounding uncertainty are presented for IC, incremental effects (IE) and the incremental cost-effectiveness ratio (ICER). Results are shown on the cost-effectiveness plane and cost-effectiveness acceptability curves. In our probabilistic sensitivity analysis, rank correlation coefficients are calculated between the output values (the ICERs) and the sampled input values to indicate the relative importance of variables and their uncertainty on the uncertainty surrounding the outcomes.
One-way sensitivity analysis is performed on several input variables. In this case, we start from the published pivotal trial data because no full details of the Continued Access trial were provided by the study sponsor. Including the results on mortality from the Continued Access trial is modelled as one of the scenarios and is considered as base case since there is no good reason to exclude the results from this trial with the same protocol as the pivotal trial. The following scenarios are modelled for high-risk operable patients: TAVI device cost of €10 000 and an optimistic QoL improvement of 0.1 during the whole first year. In the model for inoperable patients, the scenarios are restricted 3-year time horizon, no discounting or discount rate of 5% for both costs and effects, no adjustment of TAVI and standard therapy costs for actually performed treatment, inclusion or exclusion of events in the model (major/minor stroke or TIA) and TAVI device cost of €10 000. One specific subgroup scenario analysis is added for mortality risk reduction in medically versus anatomically inoperable patients. Although the reduction in mortality is observed in both subgroups in the pivotal trial, it is larger in the latter subgroup (absolute 27.9%) than in the former subgroup (absolute 17%). Results of these analyses are presented on a tornado graph.
Results
Based on the results of the PARTNER trial in high-risk operable patients, the incremental survival benefit of TAVI in comparison with AVR is minimal, on average 0.03 (95% CI −0.01 to− 0.07) quality-adjusted life-years (QALYs). In combination with substantial ICs (on average €20 397, 95% CI 18 278 to 22 617), this results in very high ICERs of about €750 000 per QALY gained. This contrast between minimal gains and substantial expenses is shown on the cost-effectiveness plane in the supplementary appendix (figure 5). Even the scenario analyses with a price reduction of the TAVI device cost to €10 000 or an assumed QoL improvement of 0.1 during the whole first year results in relatively high ICERs, that is, on average €455 000 and €205 000 per QALY gained, respectively.
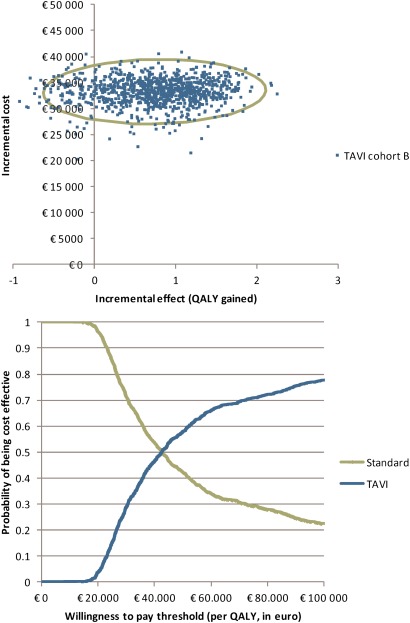
For inoperable patients, the IC of TAVI compared with standard therapy is about €33 200 with an IE on survival of 0.88 LYG or 0.74 QALYs gained. As such, the ICER becomes about €42 600 per LYG or on average €44 900 per QALY gained (see details on IC, IE and ICERs in table 5 in the supplementary appendix and figure 2, top). The cost-effectiveness acceptability curve (figure 2, bottom) indicates that a willingness-to-pay for a QALY of more than €42 000 is needed to have about 50% chance the intervention is considered cost-effective in this cohort of patients.
Figure 2.
Cost-effectiveness plane (top) and cost-effectiveness acceptability curve (bottom) for TAVI in inoperable patients. The green curve on the cost-effectiveness plane is the 95% confidence ellipse.
The probabilistic sensitivity analysis shows that the most important stochastic variables in the model are the mortality and utility at 12 months in the TAVI group (correlation coefficients of 0.21 and −0.48, respectively).6 The one-way sensitivity analyses indicate the importance of the extrapolation assumption. With a 3-year time horizon, the ICER of the pivotal PARTNER trial almost doubled (from €37 400 per QALY to €71 600 per QALY). Finally, the anatomically inoperable patients have a more favourable ICER than the medically inoperable patients (figure 6 in the supplementary appendix).
Discussion
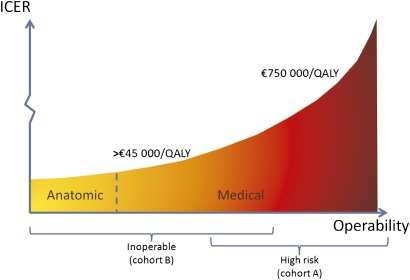
This is the first non-industry-based study that calculates the cost-effectiveness of TAVI, based on the results of a randomised controlled trial. The cost-effectiveness of TAVI is highly dependent on the patient population under consideration. In high-risk operable patients, TAVI and surgery are associated with similar mortality rates at 1 year and produce similar improvements in cardiac symptoms. However, the twice as high rate of stroke after TAVI as compared with surgery (8.3% vs 4.3% at 1 year) is problematic. From a medical point of view, TAVI might be considered as an alternative to surgery for high-risk patients who are willing to accept this higher risk of stroke.7 From an economic point of view, the less invasive nature of the TAVI procedure does not weigh against the extra costs. With an average ICER of about €750 000 per QALY (figure 3), it is hard to defend a reimbursement for TAVI in high-risk operable patients as an efficient use of limited resources. This can be altered if TAVI costs become similar to those of AVR. The long-term consequences of stroke on both QoL and costs should also not be underestimated and would probably worsen the ICER of TAVI.
Figure 3.
TAVI's cost-effectiveness. ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year. The x-axis indicates the operability of patients. There is an overlap between medically inoperable and high-risk operable patients. Anatomically inoperable patients are readily identifiable. For high-risk operable patients, the ICERs are very high (€750 000 per QALY). For inoperable patients, the ICER was on average €45 000 per QALY. Within the latter category, ICERs are better for anatomic inoperable patients and worse for medical inoperable patients.
In inoperable patients, TAVI significantly reduces the rate of death from any cause as compared with a non-surgical approach (absolute risk reduction of 12.3% in combined pivotal and Continued Access trial). TAVI also significantly improves cardiac symptoms and reduces the need for repeat hospitalisations. Stroke rate at 1 year, however, was twice as high in TAVI patients compared with those treated non-surgically (10.6% vs 4.5%). From a medical point of view, the overall benefit of TAVI in those patients seems to outweigh the risks, and therefore, it may be appropriate to consider and discuss TAVI with patients who cannot be operated.
For inoperable patients, combining the mortality data from both the pivotal and Continued Access trial results in an ICER of about €45 000 per QALY (figure 3). In most countries, as well as in Belgium, there is no explicit ICER threshold. Only NICE (National Institute for Health and Clinical Excellence) has explicitly mentioned this in their Guide to the Methods of Technology Appraisal.8 Applying NICE's threshold values of £20 000 (£1=€1.14, 25 August 2011) and £30 000 per QALY, results in a 9.2% and 36.7% chance, respectively, that TAVI is considered as being a cost-effective intervention. This rather is an optimistic estimate due to several factors: the unbalanced patient characteristics in the PARTNER trial in favour of the TAVI group (see next paragraph), the lifelong extrapolation assumption on survival (see FDA remark in the supplementary appendix (figure 7)), keeping QoL at a high level in the long term in this ageing population, and the possible long-term consequences and costs of stroke.
In the PARTNER trial, baseline characteristics of inoperable patients were unevenly distributed among the two study groups, most if not all imbalances favouring survival in the TAVI-treated patients. The logistic EuroSCORE was higher in the control arm (30.4 vs 26.4, p=0.04), and both chronic obstructive pulmonary disease (52.5% vs 41.3%) and atrial fibrillation (48.8% vs 32.9%) were statistically significantly (p<0.05) more prevalent. Frail patients (28% vs 18.1%, p=0.09) were also over-represented. In general, patients who are inoperable due to comorbidities were over-represented in the control arm. The PARTNER study protocol stipulates that both medical and anatomic conditions may lead to the surgeons' conclusion of inoperability and that the surgeons' consult notes shall specify the medical or anatomic factors.9 Patients with coexisting anatomic conditions (extensively calcified aorta, deleterious effects of chest-wall irradiation and chest-wall deformity) were better represented in the TAVI group than in the control group (29.6% vs 20.7%, p=0.05). These anatomically inoperable patients probably have a better prognosis and QoL after remediating the aortic valve stenosis. Because of the imbalance in patient characteristics and the possibly better prognosis, this one subgroup analysis for this specific patient characteristic was asked to the study sponsor.
The absolute mortality gain after TAVI was an absolute 17 percentages in the non-anatomical inoperable group versus an absolute 27.9 percentages in the anatomical inoperable group. This results in an improved ICER for the latter group (about €11 000 per QALY lower) and vice versa for the other group (about €5000 per QALY higher). Results of this subgroup analysis were only provided for the pivotal trial and for the mortality end point. Further refinement of the cost-effectiveness calculation is only possible if further details for the whole population (pivotal and Continued Access) and for all relevant outcomes (mortality, events and QoL) are made available by the study sponsor. With currently best available data, the performed analyses show that, if one is willing to pay the considerable amount for the QALYs gained, reimbursement of TAVI could in the first place focus on this subgroup of anatomical inoperable patients.
The clinical differentiation of PARTNER high-risk operable from inoperable patients based on objective parameters is not straightforward and essentially based on the clinical feeling of the physicians involved. Comparing the patient characteristics between high-risk operable and inoperable patients in the PARTNER trial reveals there are no clear differences in the clinical baseline characteristics of the two populations, with exception of anatomical prohibitive conditions.6 While high-risk operable patients should be excluded from TAVI treatment on medico-economic grounds, physicians can, however, subjectively label them as inoperable patients. Belgian registry data showed that a substantial part of the Belgian TAVI patients have better characteristics (lower NYHA class and less coronary artery disease and previous CABG) than PARTNER high-risk operable and inoperable patients.6 Also the German registry has shown that many patients received TAVI, although their valves could also have been replaced by open surgery.10 Broadening of the population to lower risk populations might result in worse outcomes. To avoid waste of resources, artificially including patients should be avoided at all times. Anatomically inoperable patients, who benefit most from the intervention, can easily be distinguished from other patients (figure 3).
It should be noted that the evidence from the PARTNER trial for inoperable patients only applies to the transfemoral approach. Although the FDA proposed to do so, the PARTNER study sponsor did not include a transapical arm in inoperable patients (see figure 4 in the supplementary appendix).4 In high-risk operable patients, a subgroup analysis suggests that the transapical approach is not inferior to surgery but doubles the stroke risk. Recently, the results from the STACCATO trial, an independent RCT of transapical TAVI in operable elderly patients have been presented.11 The primary end point (30-day all-cause mortality, major stroke and/or renal failure) was reached in 5/34 TAVI and 1/36 surgically treated patients, and the study was prematurely terminated after advice from the Data Safety Monitoring Board. Given the fact that operative risk estimation is a highly subjective matter, it is as yet unclear to what extent the operative risk of patients enrolled in STACCATO is different from that of PARTNER patients or from patients currently treated all over Europe. These observations once more put into question the appropriateness for the European regulators granting the transapical Sapien valve a CE label in 2007.12 It also shows the importance of performing high-quality randomised trials with clinically relevant endpoints prior to granting marketing approval of innovative high-risk devices.12
The contrast between the USA and EU regulation is striking. Evidence of clinical efficacy is required before market entry in the USA but not in Europe.12 This is also the case with TAVI. In the USA, the PARTNER-US study design is an RCT to demonstrate efficacy. In contrast, in Europe, the PARTNER-EU and other studies are mere registries with no control group. Despite European data from thousands of patients, it remains unclear from these registries for whom the intervention is beneficial due to a lack of a proper research design. For innovative high-risk devices, the future EU Device Directive should move away from requiring clinical safety and ‘performance’ data only to also require pre-market data that demonstrate ‘clinical efficacy’.12
Not publishing negative or prematurely terminated studies is problematic for assessing clinical efficacy and supporting rational decision making. Manufacturers should reveal all relevant or requested information to health technology assessment bodies. If this does not work on a voluntary basis, then, policy makers should have the courage to take more drastic measures. For example, government could refuse to take a reimbursement decision as long as not all relevant data are provided. The German Institute for Quality and Efficiency in Health Care (IQWiG) assessment report on the antidepressant reboxetine has shown this can be a very effective measure: Pfizer did not submit a complete list of unpublished trials as requested by IQWiG. IQWiG therefore issued the preliminary conclusion that because of the high risk of publication bias, no meaningful assessment of reboxetine was possible and thus no benefit of the drug could be proved.13 Pfizer then decided to provide most of the missing data and the subsequent assessment showed that, overall, reboxetine had no benefit.14–16 Unfortunately, several other examples of publication bias exist that could have influenced policy recommendations and decisions if full information was available. The non-availability of complete information is, as mentioned in a BMJ editorial, ‘a disservice to research participants, patients, health systems, and the whole endeavour of clinical medicine’.17
Conclusions
Based on currently available data, it is not recommended to reimburse TAVI for high-risk operable patients. Although the intervention is less invasive, patients have no survival benefit after 1 year, the risk of stroke is doubled and the costs are significantly higher. In inoperable patients, a distinction should be made between medically and anatomically inoperable patients. In the former group, TAVI treatment is palliative and the patient's life expectancy and QoL remain limited due to comorbidities that persist after correction of the aortic stenosis. Anatomically inoperable patients are likely to benefit most from TAVI since they not necessarily have debilitating comorbid conditions. In those patients, the ICER is also the most favourable. If decision makers are willing to reimburse the relatively high price for TAVI, then it is recommended to focus in the first place on this clinically readily identifiable subgroup.
Finally, the study sponsor should be more willing to share all relevant data in due time, that is, before important policy decisions are taken. Both positive and negative study results providing details on all relevant outcomes (mortality, adverse events and QoL) for the most important subgroups should be revealed. This will enable more balanced evaluations and well-founded policy recommendations. Payers should insist to have full information before taking reimbursement decisions.
Supplementary Material
Footnotes
To cite: Neyt M, Van Brabandt H, Devriese S, et al. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open 2012;2:e001032. doi:10.1136/bmjopen-2012-001032
Contributors: MN carried out the economic analysis and drafted the paper. HVB provided medical advice and performed a medical literature review in search for relevant data. SD and SVDS analysed the Belgian data. All authors participated in revising the draft paper and approved the version to be published. MN is guarantor for the paper.
Funding: There was no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Individual patient data are not available. Aggregated data are published in ‘Neyt M, Van Brabandt H, Van de Sande S, Devriese S. Health Technology Assessment. Transcatheter Aortic Valve Implantation (TAVI): a Health Technology Assessment Update. Health Technology Assessment (HTA). Brussels: Belgian Health Care Knowledge Centre (KCE), 2011.’ which is publicly available at http://www.kce.fgov.be/publication/report/transcatheter-aortic-valve-implantation-tavi-a-health-technology-assessment-updat
References
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607 [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98 [DOI] [PubMed] [Google Scholar]
- 3.Cleemput I, Van Wilder P, Vrijens F, et al. Guidelines for Pharmacoeconomic Evaluations in Belgium. Health Technology Assessment (HTA). Brussels: Belgian Health Care Knowledge Centre (KCE), 2008 [Google Scholar]
- 4.FDA Meeting Materials of the Circulatory System Devices Panel. 2011. Department of Health and Human Services, Center for Devices and Radiological Health, Medical Devices Advisory Committee, Gaithersburg, Maryland, Washington DC, 20 July 2011. Annapolis, MD, USA: Free State Reporting, Inc., 2011 [Google Scholar]
- 5.Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press, 2006 [Google Scholar]
- 6.Neyt M, Van Brabandt H, Van de Sande S, et al. Health Technology Assessment. Transcatheter Aortic Valve Implantation (TAVI): A Health Technology Assessment Update. Health Technology Assessment (HTA). Brussels: Belgian Health Care Knowledge Centre (KCE), 2011 [Google Scholar]
- 7.Schaff HV. Transcatheter aortic-valve implantation—at what price? N Engl J Med 2011;364:2256–8 [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence Guide to the Methods of Technology Appraisal. London, UK: NICE, 2007 [PubMed] [Google Scholar]
- 9.Edwards Lifesciences The PARTNER trial: Placement of AoRTic TraNscathetER Valves Trial (version 3.2) (protocol for the PARTNER trial). 2009 [Google Scholar]
- 10.Mohr F. The German aortic valve registry [Oral presentation]. 40th Annual Meeting of the German Society for Thoracic and Cardiovascular Surgery, Stuttgart, Germany, 2011 [Google Scholar]
- 11.Nielsen H, Klaaborg K, Nissen H, et al. A prospective, randomized trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis. The STACCATO Trial. Transcatheter Cardiovascular Therapeutics (TCT) congress, San Francisco, USA: 2011 [DOI] [PubMed] [Google Scholar]
- 12.Hulstaert F, Neyt M, Vinck I, et al. The Pre-market Clinical Evaluation of Innovative High-risk Medical Devices. Brussels: Belgian Health Care Knowledge Centre (KCE), 2011 [Google Scholar]
- 13.Stafford N. German agency refuses to rule on drug's benefits until Pfizer discloses all trial results. BMJ 2009;338:b2521. [DOI] [PubMed] [Google Scholar]
- 14.Institute for Quality and Efficiency in Health Care (IQWIG) Bupropion, Mirtazapine, and Reboxetine in the Treatment of Depression: Final Report; Commission A05–20C (In German). Cologne, Germany: Institute for Quality and Efficiency in Health Care, 2009 [PubMed] [Google Scholar]
- 15.Wieseler B, McGauran N, Kaiser T. Finding studies on reboxetine: a tale of hide and seek. BMJ 2010;341:c4942. [DOI] [PubMed] [Google Scholar]
- 16.Eyding D, Lelgemann M, Grouven U, et al. Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials. BMJ 2010;341:c4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehman R, Loder E. Missing clinical trial data. BMJ 2012;344:d8158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.