Abstract
Many veterans chronically ill from the 1991 Gulf War exhibit symptoms of altered sensation, including chronic pain. In this study of 55 veterans of a Construction Battalion previously examined in 1995–1996 and 1997–1998, brain activation to innocuous and noxious heat stimuli was assessed in 2008–2009 with a quantitative sensory testing fMRI protocol in control veterans and groups representing three syndrome variants. Testing outside the scanner revealed no significant differences in warm detection or heat pain threshold among the four groups. In the fMRI study, Syndrome 1 and Syndrome 2, but not Syndrome 3, exhibited hypo-activation to innocuous heat and hyper-activation to noxious heat stimuli compared to controls. The results indicate abnormal central processing of sensory and painful stimuli in 2 of 3 variants of Gulf War illness and call for a more comprehensive study with a larger, representative sample of veterans.
Keywords: functional MRI, neuroimaging, innocuous heat, noxious heat, sensory, pain, Brain Diseases, Gulf War Illness
1 Introduction
An estimated 26% to 32% of 1991 Gulf War veterans experience multisymptom conditions not explained by stress or psychiatric illness (Binns et al., 2004). Although factor analysis has yielded symptom clusters and syndrome classifications of Gulf War Illness (Haley and Kurt, 1997, Iannacchione et al., 2011), objective diagnostic testing has remained elusive. Abnormalities of sensation, including paresthesias, numbness, heat or cold intolerance and chronic pain, are common components of the illness (Binns et al., 2004, Haley, 1999). A study of British Gulf War veterans (Jamal et al., 1996) reported a highly significant two-fold raise in cooling detection threshold (i.e., reduced sensitivity to cold temperature changes) compared to civilian controls during quantitative sensory testing (QST) of right foot. Thermal hyposensitivity in the form of higher cooling detection thresholds (in all extremities) and higher warm detection thresholds (in the hands) were also found in a previous study of American Gulf War veterans (Haley et al., unpublished data by personal communication). A recent study (Cook et al., 2010) revealed similar heat pain thresholds in healthy and ill GW veterans, but increased pain intensity and affect ratings in ill GW veterans, during QST of the thenar eminence of the no-dominant hand.
Blood oxygenation level dependent (BOLD) fMRI has become a highly useful modality to investigate the normal central nervous system (CNS) processing of pain (Apkarian et al., 2005). Event-related fMRI using thermal stimuli has also successfully demonstrated abnormalities in the central processing of pain in a variety of chronic pain conditions of unknown pathophysiology (Chen, 2007).
In the current study, differences in brain activation to innocuous and noxious heat among four groups of Gulf War veterans--Syndrome 1 (Syn1), Syndrome 2 (Syn2), Syndrome 3 (Syn3) (Haley and Kurt, 1997, Iannacchione et al., 2011) and a control group-- all from the same military unit--were assessed with sensory and heat pain fMRI studies. The aims were to examine differences in cerebral processing of innocuous heat and noxious heat between the three syndrome groups and controls, to elucidate the nature of CNS dysfunction in Gulf War illness and to compare the CNS abnormalities in GWI with those of fibromyalgia (Clauw, 2009, Cook et al., 2004, Nebel and Gracely, 2009, Staud et al., 2008, Williams and Gracely, 2006) and of post-traumatic stress disorder (Defrin et al., 2008, Kraus et al., 2009).
2 Methods
2.1 Subjects
Fifty-four right – handed male Gulf War veterans from the 24th Reserve Naval Mobile Construction Battalion participated in a week-long battery of neuroimaging, clinical, neuropsychology, and psychiatry tests, including the innocuous and noxious heat fMRI. Selected as a nested case-control study from a larger survey that defined three primary variants of Gulf War syndrome (Haley and Kurt, 1997, Iannacchione et al., 2011, Sharma, 2011), the subjects (Table 1) included 11 from the Syndrome 1 group (mild cognitive impairment), 17 from Syndrome 2 (more severe confusion-ataxia), 12 from Syndrome 3 (central neuropathic pain, and 14 controls (healthy veterans). The subjects were classified based on the syndromes defined by factor analysis of symptoms they endorsed in an earlier study reported in 1997 (Haley and Kurt, 1997). One Syn2 patient was subsequently removed from the study due to excessive motion during the MRI scan (see Results section 3.2). Diagnoses of psychiatric comorbidities including major depressive disorder, alcohol abuse or dependence, and drug abuse or dependences were made by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 1996) and a diagnosis of PTSD by a score of 40 or more on Clinician-Administered PTSD Scale (CAPS), conducted by a neuropsychologist trained in their administration. Veterans were diagnosed to have PTSD if they had a CAPS score of 40 or more (Weathers et al., 2001). Fibromyalgia (FM) diagnosis was made by the survey definition comprised of the pain distribution criteria of the1990 case definition of the American College of Rheumatology without the tender point examination (Wolfe et al., 1990), and chronic fatigue syndrome (CFS) was diagnosed by Centers for Disease Control and Prevention (CDC) criteria (Fukuda et al., 1994). Differences in age and years of education were assessed with 4-group analysis of variance, and the incidence of clinical conditions (e.g. PTSD, FM, alcohol and drug abuse, etc.), and use of medications of different classes (e.g. opiates) between ill and healthy GW veterans were examined by Fisher exact test (FET).
Table 1.
Demographic and co-morbidity measures in controls and Gulf War syndrome groups
| Characteristic | Controls | Syndrome 1 (Impaired cognition) | Syndrome 2 (Confusion- ataxia) | Syndrome 3 (Central neuro- pathic pain) | P value |
|---|---|---|---|---|---|
| Number of subjects | 14 | 11 | 16 | 12 | |
| Mean years of age (SD) | 61 ( 7) | 51 ( 6) | 63 ( 7) | 57 ( 7) | <0.01 |
| Mean years of education (SD) | 13 ( 2) | 14 (1) | 12 ( 3) | 12 ( 1) | 0.15 |
| Chronic fatigue syndrome, CDC definition (%) | 0 ( 0) | 1 ( 9) | 1 ( 6) | 1 ( 8) | 0.78 |
| Fibromyalgia, survey definition (%) | 1 ( 7) | 2 (18) | 6 (38) | 10 (83) | <0.001 |
| Major depressive disorder, active (%) | 0 ( 0) | 2 (18) | 3 (19) | 0 ( 0) | 0.12 |
| Alcohol abuse or dependence, active (%) | 4 (29) | 4 (36) | 6 (38) | 5 (42) | 0.94 |
| Drug abuse or dependence, active (%) | 2 (14) | 1 (9) | 0 ( 0) | 2 (17) | 0.35 |
| Posttraumatic stress disorder, active (%) | 0 ( 0) | 2 (18) | 4 (25) | 3 (25) | 0.20 |
Both participants and investigators were blinded to group membership during the data acquisition phase and the initial individual data analysis phase of the study. The protocol was approved by the local Institutional Review Board (IRB), and informed consent was acquired from all the subjects prior to their participation.
2.2 Tasks
Warm detection and heat pain thresholds were first determined outside the scanner with a Medoc Advanced Thermal Stimulator (Cary, NC). This stimulator has a 3 cm × 3 cm surface area non-magnetic Peltier thermode with computer-controlled temperature program and filtered cables for use with the MRI system. Subjects were familiarized with the methods before the start of assessments using a standard set of instructions and procedures. Warm detection threshold was always obtained before the heat pain threshold. The thermode was placed against the ventral inner forearm of the right arm and the warm detection and heat pain thresholds were determined by the method of limits (Heldestad et al., 2010, Yarnitsky, 1997), using the median of 10 successive threshold measurements (inter-stimulus interval ~ 10 sec). From a baseline of 32°C, probe temperature increased at a rate of 2°C /s until the subjects responded that they sensed the stimulus (for warm detection) or found the stimulus painful and barely tolerable (for heat pain threshold) by pressing a button on a handheld device, after which the temperature fell to baseline at 8°C/s. The subjects subsequently underwent fMRI in the scanner with first 3 runs with the innocuous heat stimuli and then 3 runs with the noxious heat stimuli.
The subject-specific thermode test temperature for innocuous heat fMRI runs was the average of the warm detection and heat pain thresholds determined outside the scanner. Each stimulus epoch consisted of 8 º C/s ramp-up from 32°C baseline to the predetermined test temperature ( the average of subject-specific warm detection and heat pain thresholds ) followed by a 3 second plateau at the test temperature and 8º C/s ramp-down to baseline temperature of 32°C. There were 10 such identical stimulus epochs in each run and the inter-stimulus interval (ISI) durations between stimuli were comprised of thermal stimulation at baseline temperature, with durations adjusted so that the time between onset of consecutive epochs was varied between 22, 24, and 26 seconds. There were 3 innocuous heat fMRI runs, each with duration of: 4 min and 26 sec.
The subject-specific thermode test temperature for the noxious heat fMRI runs was the heat pain threshold temperature determined outside the scanner. Each stimulus epoch consisted of 8 º C/s ramp-up from 32°C baseline to the pre-determined heat pain test temperature (heat pain threshold) followed by a 3 second plateau at the threshold temperature and 8º C/s ramp-down to baseline temperature of 32°C. There were 10 such identical stimulus epochs in each run and the inter-stimulus interval (ISI) durations between stimuli were comprised of thermal stimulation at baseline temperature, with durations adjusted so that the time between onset of consecutive epochs was varied between 26, 28, 30 and 32 seconds. The subjects were administered 3 such noxious heat stimulus epochs outside the MRI scanner to ensure they could tolerate the task inside the scanner. The noxious heat fMRI runs were administered after completion of the innocuous heat fMRI runs. There were 3 noxious heat fMRI runs, each with duration of 5 min and 6 s. To minimize sensitization, there were 5–7 minute gaps between consecutive noxious heat fMRI runs, during which other MRI scans were acquired.
2.3 Image Acquisition
MR images were acquired on a 3T Siemens Magnetom Trio TIM scanner with a 12-channel receiver array head coil. BOLD fMRI scans were acquired using a conventional EPI sequence with FOV = 220 mm, TR/TE/FA = 2000 ms/24 ms/90°; forty 3.4 mm sagittal slices; 3 mm x 3 mm in-plane resolution, and band width of 2414 Hz/pixel. There were 150 (130) measurements in each of 3 noxious (innocuous) heat fMRI scans. Prospective real-time motion correction (Thesen et al., 2000) was employed with all EPI fMRI scans to minimize motion artifacts. A time-of-flight MR angiogram (TR/TE/FA = 26 ms/6 ms/40°; 0.9 mm x 0.9 mm in-plane resolution) with the same FOV and slice prescriptions as the EPI fMRI scans was acquired for angiographic reference. A whole-brain 3D T1-weighted MPRAGE sequence (FOV = 230 mm; TR/TI/TE/FA = 2250 ms/900 ms/3 ms/9°; 0.9 mm x 0.9 mm x 1 mm resolution) provided anatomic detail. All scans were acquired with parallel imaging (GRAPPA; acceleration factor = 2; 24–36 phase encoding reference lines). Cardiac and respiration physiological responses were monitored and recorded with a BIOPAC (Goleta, CA) finger pulse-oximeter and respiration belt during all fMRI scans.
2.4 Data Analysis
Image analysis was conducted with AFNI, FSL and Matlab. BOLD EPI images from the scanner were volume registered to account for global rigid motion with an intensity-based, iterative, linearized, weighted least squares algorithm (Cox and Jesmanowicz, 1999). For each voxel, the signal was modeled as the convolution of a stimulus vector, comprised of the onset-times of heat stimulus epochs, with their corresponding voxel impulse response functions (IRFs), plus 3rd order polynomial drifts and constant baseline. IRFs were deconvolved for each voxel from the knowledge of the observed voxel fMRI intensity time-series and the stimulus vectors, under a multiple linear regression framework. The voxel time-series were also orthogonalized to the estimated motion parameters from the volume registration algorithm within the same regression model, and image volumes exhibiting more than 3 mm (~1 voxel) maximal displacement were excluded from analysis. The linear regression F-statistic was used to quantify activation at the individual subject level. The fractional (normalized to the regression-estimate baseline) amplitude of the hemodynamic response (HDR) was also calculated at each voxel. Each subject’s high resolution anatomic image was spatially normalized to the AFNI Talairach template. The resultant transformation matrix was used to warp the maps of voxel-wise fractional HDR amplitude for each run and each condition (noxious and innocuous heat) to Talairach space. Two-way mixed effects analyses of variance (ANOVA: Group x Runs; random subject factor nested within the levels of the Group factor) were employed to obtain individual group activation maps and between-group differences in activation for the innocuous and noxious heat conditions. The amplitude data were co-varied for age, since heat perception varies with age (Lariviere et al., 2007, Lautenbacher et al., 2005, Lin et al., 2005). Thus age-effects, if any, have been removed from the fMRI activation results described below. The treatment-mean two-tailed t-tests from the ANOVA served as individual group activation maps for noxious and innocuous heat. Inter-group differences in noxious (and innocuous) heat for all three runs combined were obtained with appropriate t-contrasts. The influences of PTSD and FM on the results were examined separately by employing factorial Group x PTSD and Group x FM ANOVA models, with both PTSD and FM being categorical factors taking values “Yes” or “No”. Overall significances of resultant statistic maps were obtained by means of Monte-Carlo simulation of the process of image generation, spatial correlation of voxels, intensity thresholding, masking (whole-brain) and cluster identification (Forman et al., 1995) through the AlphaSim program implemented in AFNI. The p-values for different condition brain activation as well as contrasts reported in the results refer to cluster-level significance after multiple comparison correction implemented with AlphaSim.
3 Results
3.1 Demographic & Clinical Characteristics of the GW Veterans Cohort
Analysis of demographic characteristics indicated that the comparison groups differed on age, with the syndrome 1 group being younger than the other 3 groups (Table 1), but not on education. Since chronic pain is a variable component of GWI, the 4 groups differed significantly on the survey definition of FM, but there were no group differences on the CDC definition of CFS, active depression, alcohol or drug abuse/dependence, or PTSD (Table 1).
3.2 Sensory Thresholds and fMRI Data Quality
Sensory testing conducted outside the MRI scanner (see Supplementary Materials: Table S1) demonstrated no significant differences in the warm detection threshold (F1,3 = 0.35, p > 0.8) and heat pain threshold (F1,3 = 1.5, p > 0.2) among the four groups. There were no significant (regression F-stat p > 0.2) age-related trends in the warm detection and heat pain thresholds across the cohort. However, when examined individually, Syn1 exhibited a significantly (p < 0.05) increasing trend and Syn3 exhibited a significantly (p < 0.01) decreasing trend in heat pain threshold with age. As a result the Group x Age interaction effect in heat pain threshold was significant (p < 0.003). There were no significant differences between the groups in warm detection threshold (Group x Age F-stat p > 0.4).
In the fMRI paradigms, one veteran from the Syn2 group was excluded from the study due to excessive Parkinson-like tremors which made it difficult to get useful MRI data. One veteran from the Syn2 group declined to stay in the scanner longer than 30 minutes and hence was not examined with the innocuous heat fMRI paradigm. One veteran from the Syn3 group performed only two runs of the noxious heat fMRI task and declined to be scanned for the third run. Only 6 of the 53 veterans exhibited large motions (> 3 mm) during the fMRI runs which required that the images in the motion-corrupted epochs be ignored in subsequent analyses. Of these, four exhibited large motion in less than 5% of image volumes in time-series; one subject in 10%; and one in 20%. Volumes that exhibited large motions were excluded from the analysis.
3.3 Age-related Effects on Brain Activation to Noxious and Innocuous Heat
The Group x Age interaction effect in fMRI activation to innocuous heat was significant (Group x Age F-stat p < 0.05) in bilateral SMA, PPC, and right hemisphere insula, S2 and thalamus. For the noxious heat fMRI paradigm, the Group x Age interaction effect was significant (Group x Age F-stat p < 0.05) in right hemisphere insula, S2 and DLPFC. The results shown in the sections below have been obtained with age as a covariate.
3.4 Brain Activation to Innocuous Heat in Controls
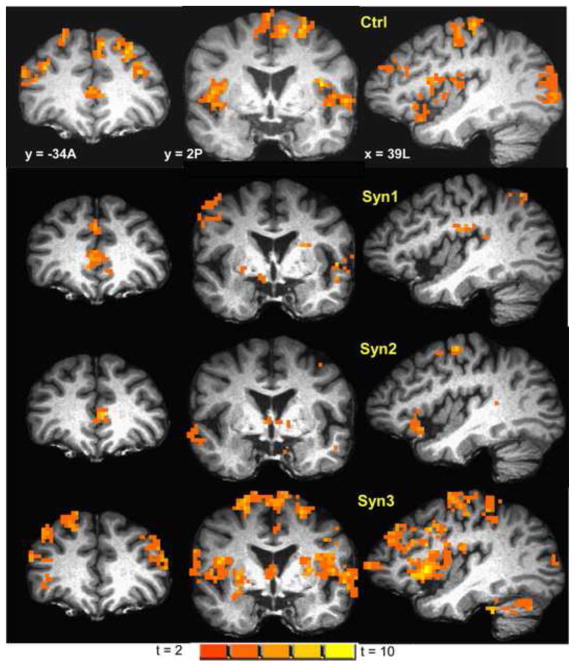
In the control group innocuous heat stimuli (collapsed across all three runs) produced significant (cluster-level p < 0.001) activation in a number of regions involved in heat perception (see Table 2 and Figure 1) including bilateral S1, S2, insula, and inferior parietal lobule.
Table 2.
Brain areasa activated by innocuous heat stimulation of ventrum of the right forearm in controls, along with maximum activation intensity (1-sample t-scores; cluster-level p < 0.001) and location of maxima within those areas. Clusters of activation spanned more than one listed area.
| Area | Maximum activation intensity group t- score | Talairach co-ordinates RAI (mm) of maxima | Area | Maximum activation intensity group t- score | Talairach co-ordinates RAI (mm) of maxima |
|---|---|---|---|---|---|
| L S1 | 5.4 | 41, 24, 57 | R S1 | 6.1 | −51, 24, 43 |
| L S2 | 6.1 | −51, 24, 43 | R S2 | 5.4 | −35, 11, 20 |
| L Insula | 8.3 | 38, 0, 13 | R Insula | 6.5 | −31, −8, 11 |
| L medial PPC | 4.6 | 23, 64, 34 | R medial PPC | 4.6 | −10, 56, 50 |
| L SMA/BA6 | 5.6 | 9, −2, 53 | R SMA/BA6 | 3.9 | −7, 5, 53 |
| L premotor | 8.3 | 20, 25, 65 | R premotor | 6.1 | −35, 7, 37 |
| L Cingulate | 7.8 | 5, −14, 25 | R Cingulate | 5.8 | −2, −12, 22 |
| L IPL | 6 | 50, 47, 27 | R IPL | 6.3 | −61, 34, 36 |
| L Mid. Temp. Gyrus | 5.7 | 49, 27, −9 | R Mid. Temp. Gyrus | 5.8 | −46, 18, 13 |
| L Ant. STG/BA 38 | 6.7 | 47, −14, −7 | R Ant. STG/BA 38 | 4.8 | −31, −8, −28 |
| L Parahipp. Gyrus | - | - | R Parahipp. Gyrus | 5.2 | −36, 23, 12 |
| L Thalamus | 3.2 | 7, 18, −1 | R Thalamus | 3.3 | −17, 15, 5 |
| L Caudate | - | - | R Caudate | 3.6 | −14, 7, 19 |
| LPutamen | 5.2 | 19, −4, -4 | R Putamen | 4.5 | −26, −7, 4 |
| L Cerebellum | R Cerebellum | 2.6 | −30, 55, −35 | ||
| L DLPFC | 5 | 34, −33, 16 | R DLPFC | 6 | −38, −33, 17 |
| L Sup. Front. Gyrus | 5.1 | 10, −37, 36 | R Sup. Front. Gyrus | 7.8 | −23, −29, 52 |
| DMPFC | 6.2 | 4, −48, 20 |
L S1 = Left hemisphere S1; R S1 = Right Hemisphere S1; PPC = posterior parietal cortex; SMA = supplementary motor area; BA = Brodmann Area; IPL = inferior parietal lobule; Mid. Temp. = Middle Temporal; Ant. STG = Anterior Superior Temporal Gyrus; Parahipp. = Parahippocampal; Sup. Front. = Superior Frontal; DLPFC = dorsolateral prefrontal cortex; DMPFC = dorsomedial prefrontal cortex; RAI = Right-Left, Anterior-Posterior, Inferior-Superior.
Figure 1.
Map of BOLD fMRI brain activation from innocuous heat stimulation of the ventrum of the right forearm in controls (1-sample two-tailed t-test map; cluster-level p < 0.001), overlaid on a representative subject’s spatially normalized high-resolution anatomic. Slice locations in RAI Talairach co-ordinates. Left hemisphere is on the right side of the coronal slices. Figure shows innocuous heat activation in a number of areas including DLPFC, S1, S2, insula and SMA. Activation maps for Syn1, Syn2 and Syn3, at the same slice locations and with same color-scale as controls are also displayed.
3.5 Group Differences in Brain Activation to Innocuous Heat
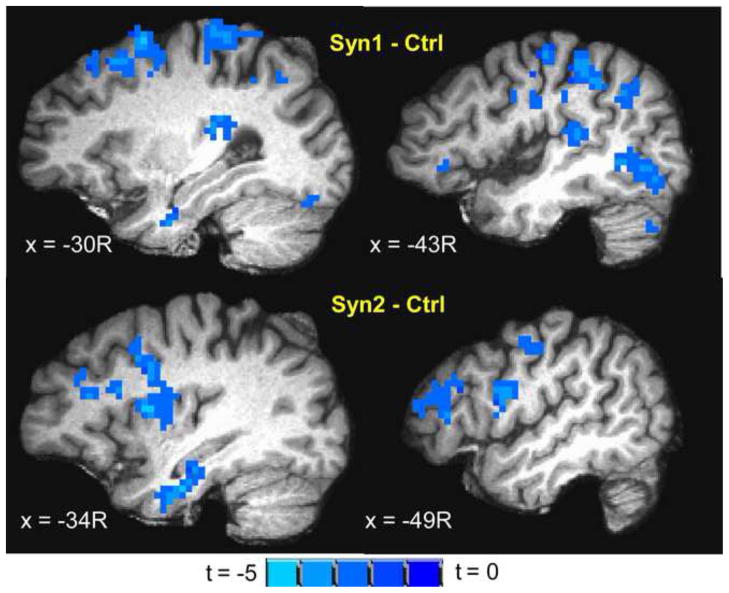
Syn1 and Syn2 exhibited significant (cluster-level p < 0.05) activation to innocuous heat in similar areas as controls (Figure 1), but much reduced in extent and intensity in all areas except ventral anterior cingulate. Both Syn1 and Syn2 exhibited significantly less (ANOVA t-contrast cluster-level p < 0.05) activation in a number of areas involved in heat perception including bilateral S1, S2, insula, SMA, medial PPC, IPL, premotor cortex and DMPFC compared to controls (Table 3, Figure 2). In contrast, Syn1 and Syn2 exhibited significantly (cluster-level p < 0.05) greater activation to innocuous heat compared to controls in ventral anterior cingulate. No significant differences were observed in the intensity and extent of innocuous heat activation between Syn3 and controls, except for greater Syn3 activation in left DLPFC.
Table 3.
Brain areasa exhibiting significant differences in activation to innocuous heat stimulation of ventrum of the right forearm between ill GWI veterans (with Syn1/Syn2)b and controls, along with maximum difference in activation (Syn1/Syn2–Ctrl t-contrast; cluster-level p < 0.05) and location of maxima within those areas. Clusters spanned more than one listed area.
| Syn1– Ctrl | Syn2– Ctrl | |||
|---|---|---|---|---|
| Area | Maximum activation difference Syn1 – Ctrl t-contrast | Talairach co-ordinates RAI (mm) of maxima | Maximum activation difference Syn2 Ctrl t-contrast | Talairach co-ordinates RAI (mm) of maxima |
| L S1 | −3.3 | 38, 34, 38 | −3.7 | 32, 32, 40 |
| R S1 | −3.7 | −47, 35, 46 | −3.4 | −20, 27, 47 |
| L S2 | −2.5 | 46, 5, 4 | −3.5 | 46, 7, 3 |
| R S2 | −4.1 | −41, 31, 13 | −3.8 | −64, 26, 23 |
| L Insula | −3.8 | 37, −11, 1 | −3.5 | 34, −8, 13 |
| R Insula | −3.4 | −34, −2, 17 | −4.1 | −35, −5, 10 |
| L medial PPC | −3.5 | 6, 53, 50 | −3.5 | 22, 65, 34 |
| R medial PPC | −3.5 | −18, 53, 35 | −3.3 | −17, 52, 62 |
| L SMA/BA6 | −3.5 | 1, 17, 47 | −3.3 | 10, 16, 53 |
| R SMA/BA6 | −4 | −4, 8, 51 | −3.6 | −5, 31, 62 |
| L premotor | −2.8 | 22, 23, 65 | −2.4 | 17, 21, 64 |
| R premotor | −3 | −31, 25, 53 | −2.8 | −22, 21, 64 |
| L IPL | −3.2 | 34, 39, 38 | - | - |
| R IPL | −3.7 | −44, 34, 46 | - | - |
| L Uncus | −3.5 | 15, 1, −22 | - | - |
| R Uncus | −2.9 | −20, 0, −34 | −4.1 | −34, 14, −28 |
| R Parahipp. Gyrus | - | - | -3.5 | −25, 14, −12 |
| R Caudate | - | - | −3.2 | −17, −11, 19 |
| L Putamen | −2.2 | 27, −8, −2 | - | - |
| R Putamen | - | - | −4 | −30, 2, 8 |
| L DLPFC | - | - | −3 | 47, −42, 16 |
| R DLPFC | - | - | −3.7 | −52, −35, 8 |
| L Sup. Front. Gyrus | −3.15 | 16, −27, 54 | - | - |
| R Sup. Front. Gyrus | −3.15 | −32, −29, 47 | - | - |
| DMPFC | −3.7 | −4, −47, 31 | −3.6 | 2, −43, 19 |
| Vent. Ant. Cing. | 4.4 | 5, −18, −7 | 3.4 | 9, −44, −6 |
L S1 = Left hemisphere S1; R S1 = Right Hemisphere S1; PPC = posterior parietal cortex; SMA = supplementary motor area; BA = Brodmann Area; IPL = inferior parietal lobule; Parahipp. = Parahippocampal; Sup. Front. = Superior Frontal; DLPFC = dorsolateral prefrontal cortex; DMPFC = dorsomedial prefrontal cortex; Vent. Ant. Cing. = ventral anterior cingulate; RAI = Right-Left, Anterior-Posterior, Inferior-Superior
Only L DLPFC (Maximum Syn3–Ctrl t-score 3.4; RAI Talairach Co-ordinates: 46, -24, 9) exhibited significant difference in activation between Ctrl and Syn3.
Figure 2.
Inter group differences (ANOVA t-contrast; cluster-level p < 0.05) in brain activation to innocuous heat stimulation of ventrum of the right forearm overlaid on a representative subject’s spatially normalized high-resolution anatomic. Slice locations in RAI Talairach co-ordinates. (top) Syn1 – Ctrl showing decreased activation to innocuous heat in Syn1 in S1, S2, insula, DLPFC, precentral gyrus and IPL; (bottom) Syn2 – Ctrl showing decreased activation to innocuous heat in Syn2 in S2, insula, DLPFC and precentral gyrus.
3.6 Brain Activation to Noxious Heat in Controls
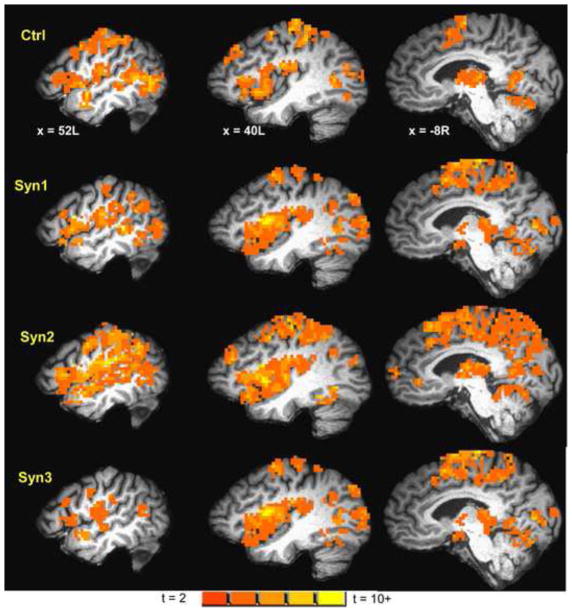
The heat pain condition invoked strong (cluster-level p < 0.0001) activation in a number of heat pain processing areas (see Table 4, Figure 3) including bilateral S1, S2, insula, inferior parietal lobule, medial PPC, SMA, dorsal anterior cingulate and thalamus in the controls.
Table 4.
Brain areasa activated by noxious heat stimulation of ventrum of the right forearm in controls, with maximum activation intensity (1-sample t-scores; cluster-level p < 0.0001) and location of maxima within those areas. Clusters of activation spanned more than one listed area.
| Area | Maximum activation intensity group t- score | Talairach co-ordinates RAI (mm) of maxima | Area | Maximum activation intensity group t- score | Talairach co-ordinates RAI (mm) of maxima |
|---|---|---|---|---|---|
| L S1 | 10.1 | 55, 16, 28 | R S1 | 6.1 | −54, 20, 35 |
| L S2 | 6.9 | 43, 14, 16 | R S2 | 8.5 | −52, 22, 23 |
| L Insula | 8.3 | 35, −17, −6 | R Insula | 9.6 | −32, −25, 2 |
| L medial PPC | 8.5 | 24, 59, 30 | R medial PPC | 7.6 | −5, 65, 51 |
| L SMA/BA6 | 7.4 | 4, 22, 62 | R SMA/BA6 | 7.4 | −9, 8, 59 |
| L premotor | 12.1 | 27, 14, 53 | R premotor | 9.2 | −19, 11, 57 |
| L Cingulate | 6.5 | 4, −14, 29 | R Cingulate | 5.9 | −11, 4, 35 |
| L IPL | 7.7 | 35, 39, 51 | R IPL | 6.0 | −62, 38, 28 |
| L Ant. STG/BA 38 | 7.2 | 47, −7, −7 | R Ant. STG/BA 38 | 6.8 | −40, −21, −30 |
| L Amygdala | 4.5 | 19, 8, −15 | R Amygdala | - | - |
| L Parahipp. Gyrus | 6 | 23, 13, −22 | R Parahipp. Gyrus | 6.9 | −28, 31, −15 |
| L MD Thalamus | 11.1 | 8, 20, 4 | R MD Thalamus | 6.6 | −7, 23, 8 |
| L VPL Thalamus | 7.7 | 16, 17, 4 | R Pulvinar | 6.4 | −18, 24, 4 |
| L Caudate | 7.2 | 17, −20, −1 | R Caudate | 6.6 | −19, −17, 8 |
| LPutamen | 9.1 | 19, −8, −7 | R Putamen | 6.3 | −20, −15, 1 |
| L Cerebellum | 9.1 | 11, 46, −16 | R Cerebellum | 8.6 | −25, 59, −24 |
| DMPFC | 6 | 4, −46, 23 | Brainstem | 7.5 | −3, 31, −32 |
L S1 = Left hemisphere S1; R S1 = Right Hemisphere S1; PPC = posterior parietal cortex; SMA = supplementary motor area; BA = Brodmann Area; IPL = inferior parietal lobule; Ant. STG = Anterior Superior Temporal Gyrus; Parahipp. = Parahippocampal; Sup. Front. = Superior Frontal; DMPFC = dorsomedial prefrontal cortex; MD = medial dorsal; VPL = ventroposteriolateral; RAI = Right-Left, Anterior-Posterior, Inferior-Superior.
Figure 3.
Map of BOLD fMRI brain activation from noxious heat stimulation of the ventrum of the right forearm in controls (1-sample two-tailed t-test map; cluster-level p < 0.0001), heat overlaid on a representative subject’s spatially normalized high-resolution anatomic. Slice locations in RAI Talairach co-ordinates. Figure shows activation to noxious heat in a number of areas including S1, S2, insula, STG, thalamus, and cerebellum. Activation maps for Syn1, Syn2 and Syn3, at the same slice locations and with same color-scale as controls are also displayed.
3.7 Group Differences in Brain Activation to Noxious Heat
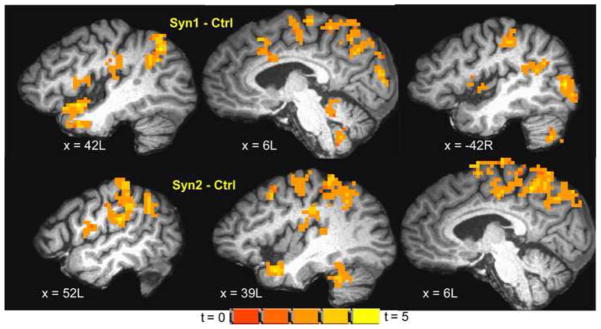
Syn1, Syn2 and Syn3 exhibited significant (cluster-level p < 0.0001) activation to noxious heat in similar areas as controls (Figure 3). However, the noxious heat activation was increased in intensity and spatial extent in Syn1 and Syn2: both Syn1 and Syn2 groups showed significant (ANOVA t-contrast cluster-level p < 0.05) hyper-activation compared to controls in a number of regions involved in pain processing (see Table 5; Figure 4) including bilateral S1, S2, insula, IPL, SMA, BA 38 and cingulate gyrus, and brainstem. In addition Syn1 (though not Syn2) also exhibited higher noxious heat activation in left amygdala, parahippocampal gyrus, and thalamus and right basal ganglia (Table 5). Syn3 showed slightly greater activation to noxious heat compared to controls in similar brain areas as Syn1 and Syn2, but the differences did not survive the p < 0.05 threshold (ANOVA t-contrast cluster-level p > 0.05).
Table 5.
Brain areasa exhibiting significant differences in activation to noxious heat stimulation of ventrum of the right forearm between ill GWI veterans (with Syn1/Syn2)b and controls, along with maximum difference in activation (Syn1/Syn2 Ctrl t-contrast; cluster-level p < 0.05) and location of maxima within those areas. Clusters spanned more than one listed area.
| Syn1–Ctrl | Syn2–Ctrl | |||
|---|---|---|---|---|
| Area | Maximum activation difference Syn1–Ctrl t-contrast | Talairach co-ordinates RAI (mm) of maxima | Maximum activation difference Syn1–Ctrl t-contrast | Talairach co-ordinates RAI (mm) of maxima |
| L S1 | 2.8 | 28, 38, 55 | 4.15 | 29, 35, 49 |
| R S1 | 3.5 | −37, 26, 42 | 4.5 | −31, 29, 28 |
| L S2 | 3.1 | 47, 19, 16 | 4.3 | 52, 27, 19 |
| R S2 | 3.0 | −38, 26, 22 | 3.4 | −58, 13, 19 |
| L Insula | 3.6 | 37, 1, 8 | 3.4 | 41, 7, 16 |
| R Insula | 3.5 | −38, −7, 8 | 3.4 | −41, 38, 19 |
| L medial PPC | 4.6 | 11, 62, 35 | 4.2 | 20, 46, 59 |
| R medial PPC | 3.5 | −17, 48, 44 | 4.1 | −21, 46, 44 |
| L SMA/BA6 | 3.4 | 2, 27, 59 | 4.0 | 7, −17, 44 |
| R SMA/BA6 | 4.1 | −4, −11, 62 | 3.6 | −6, 25, 62 |
| L premotor | 3.3 | 24, 22, 61 | 4.2 | 25, 22, 59 |
| R premotor | 3.9 | −26, 21, 55 | 3.9 | −40, 14, 46 |
| L Cingulate | 3.6 | 5, −3, 36 | 3.3 | 6, 16, 41 |
| R Cingulate | 3.3 | −8, 23, 38 | 3.4 | −7, 20, 33 |
| L IPL | 4.0 | 40, 59, 44 | 4.1 | 55, 34, 32 |
| R IPL | 3.0 | −44, 44, 44 | 3.7 | −35, 56, 38 |
| L Amygdala | 3.3 | 25, 1, −18 | - | - |
| R Amygdala | 3.5 | −25, 4, −15 | 3.2 | −25, 1, −18 |
| L Ant. STG/BA 38 | 4.0 | 43, −7, −12 | 4.0 | 41, −8, −25 |
| R Ant. STG/BA 38 | 3.7 | −46, −14, −12 | 4.0 | −54, −7, −11 |
| L Parahipp. Gyrus | 3.3 | 19, 22, −17 | - | - |
| R Parahipp. Gyrus | 2.7 | −26, 31, −13 | 2.6 | −21, 27, −9 |
| L Thalamus | 2.8 | 16, 22, 2 | - | - |
| R Caudate | 3.5 | −16, −17, 12 | - | - |
| R Putamen | 2.8 | −22, 1, 2 | - | - |
| L Cerebellum | 3.2 | 45, 46, −29 | 3.9 | 38, 46, −28 |
| R Cerebellum | 3.5 | −20, 47, −27 | 3.0 | −25, 77, −30 |
| Brainstem | 3.6 | −5, 32, −24 | 2.6 | −4, 27, −16 |
L S1 = Left hemisphere S1; R S1 = Right Hemisphere S1; PPC = posterior parietal cortex; SMA = supplementary motor area; BA = Brodmann Area; IPL = inferior parietal lobule; Parahipp. = Parahippocampal; Ant. STG = Anterior Superior Temporal Gyrus; RAI = Right-Left, Anterior-Posterior, Inferior-Superior
No significant difference in activation to noxious heat observed between Ctrl and Syn3.
Figure 4.
Inter group differences (ANOVA t-contrast; cluster-level p < 0.05) in brain activation to noxious heat stimulation of ventrum of the right forearm overlaid on a representative subject’s spatially normalized high-resolution anatomic. Slice locations in RAI Talairach co-ordinates. (top) Syn1 – Ctrl showing increased activation to noxious heat in Syn1 in S1, S2, insula, IPL, SMA, PCL, BA7 and cerebellum; (bottom) Syn2 – Ctrl showing increased activation to noxious heat in Syn2 in similar regions.
3.8 Influence of PTSD
Nine out of 39 GWI patients (2/11 from Syn1, 4/16 from Syn2 and 3/12 from Syn3) used in final analysis met the SCID diagnostic criteria for PTSD, indicating no significant difference in the incidence of PTSD (FET p > 0.2) between ill veterans and controls. The Group x PTSD interaction was not significant (Group x PTSD F-statistic cluster-level p > 0.05) for both the noxious and innocuous heat conditions. Correspondingly reanalyzing the ANOVA after excluding the PTSD patients did not change the significance of between-group differences in noxious or innocuous heat activation.
3.9 Influence of Fibromyalgia
Eighteen of 39 GWI patients (2/11 from Syn1, 6/16 from Syn2 and 10/12 from Syn3) as well as one subject from the battalion control group met the ACR survey criteria for fibromyalgia (FM), a significantly (FET p < 0.001) greater incidence in the GW-ill veterans as a whole than in the controls. However, the Group x FM interaction was not significant (Group x FM F-statistic cluster-level p > 0.05) for both the noxious and innocuous heat conditions. The Group x FM interaction remained not significant (Group x FM F-statistic cluster-level p > 0.05) even after the one control subject with FM was not included in the analyses.
3.10 Influence of Medications
There were no significant differences (FET p > 0.1) in the use of medications between controls and GWI patients for any of the medicine classes listed in Table S2 (Supplementary Materials). Further, none of the medications significantly affected the brain activation fMRI results, when analyzed with factorial ANOVA models (Group x Medication F-statistic cluster-level p > 0.05).
4 Discussion
The central finding of our study was that two variants of Gulf War syndrome, Syn1 and Syn2, showed diverse abnormalities of brain processing of sensory stimuli compared with normal brain processing in the Syn3 and control groups. The Syn1 and Syn2 groups demonstrated hypo-activation to innocuous heat in sensory perception areas and hyper-activation to noxious heat stimuli in brain areas normally serving sensory perception and threat and arousal as well as in thalamocortical circuits and cerebellum.
Patterns of activation in the Syn3 and control groups were in general agreement with results from previous studies on thermal sensation fMRI of innocuous heat stimuli (Moulton et al., 2005, Porro et al., 2004, Rolls et al., 2008, Tseng et al., 2009) and noxious heat stimuli (Apkarian et al., 2005, Borsook et al., 2008, Chen, 2007, Price, 2000, Tracey et al., 2000, Tseng et al., 2009), with strong activation observed in areas related to pain perception (S1, S2, insula), perceiving intrusion (insular cortex, PPC), arousal (SMA, amygdala) and unpleasantness (anterior cingulate). Strong activation in medial dorsal and ventroposteriolateral nuclei of thalamus, basal ganglia and cerebellum were also observed. There was considerable overlap between noxious and innocuous heat activation, but with noxious heat evoking consistently stronger activation in all areas.
Both Syn1 and Syn2 exhibited less activation to innocuous heat than controls in S1, S2, insula, premotor cortex and SMA (Figure 2, Table 3), indicating decreased sensory perception in ill Gulf War veterans belonging to those syndrome groups. Syn2 also exhibited hypo-activation in DMPFC and DLPFC compared to controls, which could result from deficiency in attending to the heat stimuli. Studies (Tracey et al., 2002, Villemure and Bushnell, 2009) have shown a correlation between somatosensory perception and attention to sensory stimuli.
Both Syn1 and Syn2 exhibited greater activation to noxious heat than controls in bilateral S1, S2, premotor cortex, insula, medial posterior parietal cortex, IPL, SMA, BA 38 and cingulate gyrus, cerebellum and brainstem (Figure 4, Table 5), indicating increased pain perception in the ill Gulf War veterans belonging to those syndrome groups.
Eighteen of 39 GWI patients (2/11 from Syn1, 6/16 from Syn2 and 10/12 from Syn3) as well as one subject from the battalion control group met the ACR survey criteria for FM based on the survey (Table 1), indicating a significantly greater rate in the ill veterans. However, although the increased activation to noxious heat in Syn1 and Syn2 is similar to increased brain activation to heat pain observed in FM (Clauw, 2009, Cook et al., 2004, Nebel and Gracely, 2009), the pattern of hypoactivity to innocuous heat in Syn1 and Syn2 is inconsistent with allodynia observed in studies of FM. Further, although 10 of 12 Syn3 patients met criteria for FM, the differences between the Syn3 and Ctrl groups did not differ significantly on activation to noxious heat. Quantitative examination of the influence of the FM symptom criteria with a factorial model also revealed no significant Group x FM interaction affect on both innocuous and noxious heat conditions. Thus the sensory fMRI activation differences between the syndrome groups and controls cannot be attributed to FM. In fact, when differences between subjects meeting and not meeting the FM criteria were examined (ignoring GWI syndrome group membership), FM-classified subjects exhibited decreased activation to noxious heat in a number of brain areas associated with pain processing (see Supplementary Materials), a result discordant with the FM FMRI studies (Clauw, 2009, Cook et al., 2004, Nebel and Gracely, 2009). This discordance is explained by our findings that the Syn1 and Syn2 subjects, who constituted 19/34 of the non-FM sample, showed hyperactivation to noxious heat compared to the normal activation of the Syn3 or Ctrl groups, whose subjects constituted the majority (11/19) of those with FM. This adds weight to the conclusion that brain responses to noxious and innocuous heat in GWI veterans are not determined by their FM classification.
Nine out of 39 GWI patients (2/11 from Syn1, 4/16 from Syn2 and 3/12 from Syn3) used in final analysis met the SCID criteria for PTSD (Table 1), indicating a marginally greater incidence of PTSD in the ill veterans. QST studies of patients with PTSD have revealed hyposensitivity to pain (i.e., higher heat pain thresholds) accompanied by hyper-reactivity to suprathreshold painful heat stimuli (Defrin et al., 2008). In the GWI fMRI study, the Syn1 and Syn2 groups exhibited hyperactivity to noxious heat stimuli, but their QST heat pain thresholds were normal and not elevated as reported for PTSD (Defrin et al., 2008). Quantitative examination of the PTSD influences with a factorial model also revealed no significant Group x PTSD interaction affect for both innocuous and noxious heat conditions. Thus the sensory fMRI activation differences between the syndrome groups and controls cannot be attributed to PTSD. A recent study of hippocampal rCBF with and without infusion of the reversible acetylcholinesterase inhibitor physostigmine in this same cohort also found that hippocampal rCBF differences between syndrome groups and controls could not be attributed to PTSD (Li et al., 2011).
Our finding no significant differences between Syn3 and controls in brain activation to noxious heat and innocuous heat was surprising since Syn3 is the group most characterized by chronic pain symptoms, which are known to cause hyperalgesia in many disease models (Clauw, 2009, Verne et al., 2004). This finding of normal processing of sensory stimuli in brain suggests that the chronic pain problems of the Syn3 group may be due to abnormal control mechanisms lower down, such as abnormalities of gating in the spinal cord (Bradesi, 2010, Svensson and Brodin, 2010). More research incorporating a larger sample size is warranted to investigate this phenomenon.
A number of deep brain regions found to respond abnormally to heat stimuli in this fMRI study (e.g., caudate, putamen, thalamus, hippocampus, pons) were previously reported (based on studies conducted in 1997–1998) to have abnormal metabolite concentrations on MR spectroscopy (Haley et al., 2000a, Haley et al., 2000b) and to show abnormal responses of rCBF (measured with SPECT) to cholinergic stimulation (Haley et al., 2009) in members of the same battalion studied here. MR studies conducted in 2008–2009 have also shown abnormalities of baseline rCBF and responses of rCBF to cholinergic challenge in hippocampus and in amygdala and caudate (Li et al., 2011, Liu et al., 2011) in ill Gulf War veterans from this same cohort. The abnormal brain responses to heat stimuli found in this study (in veterans with Syn1 and Syn2) and data from other recent neuroimaging studies (Calley et al., 2010, Li et al., 2011, Liu et al., 2011) underscore that GWI is not a single homogeneous illness and lend support to the validity of the classification of three primary syndrome groups from factor analysis of symptoms (Haley and Kurt, 1997, Iannacchione, et al., 2011). Furthermore, the war-related brain damage in these ill veterans from the Persian Gulf War appears distinct from more general effects of PTSD (Li et al., 2011) and of chronic pain symptomatology as in FM. Since, however, our sample sizes were small, particularly in the Syn1 and Syn3 groups, and the sample was drawn from a single military unit, a more comprehensive study with a larger, more representative sample of Gulf War veterans is needed.
Conclusion
Two (Syn1 and Syn2) of three syndrome variants of Gulf War illness had significantly different brain responses to innocuous and noxious heat stimuli in an objective quantitative sensory fMRI study. Brain responses in the third syndrome variant (Syn3), which has the most pain clinically, did not differ significantly from controls, suggesting a brainstem or spinal cord gating mechanism, rather than deficits in brain activation to heat perception in Syn3. These findings form a hypothesis to be tested in larger, representative samples of Gulf War veterans.
Supplementary Material
Highlights.
Syn1 and Syn2 GWI patients exhibited hypo-activation during innocuous heat fMRI.
Syn1 and Syn2 GWI patients exhibited hyper-activation during noxious heat fMRI.
No significant differences in warming or heat pain threshold among the groups.
Abnormal sensory processing in GWI was not consistent with fibromyalgia or PTSD.
Acknowledgments
This study was supported by IDIQ contract VA549-P-0027 (Robert W. Haley, M.D., PI), awarded and administered by the Department of Veterans Affairs Medical Center, Dallas, TX, and by Grant Number UL1RR024982, titled North and Central Texas Clinical and Translational Science Initiative (Milton Packer, M.D., PI), from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. The content does not necessarily reflect the position or the policy of the Federal government or the sponsoring agencies, and no official endorsement should be inferred. The authors wish to thank Munro Cullum, Ph.D., John Hart, M.D., and Andrea Hester Ph.D., for assistance with clinical diagnoses.
Abbreviations
- ANOVA
ANalysis Of VAriance
- BA
Brodmann Area
- BOLD
Blood Oxygenation Level Dependent
- CAPS
Clinician-Administered PTSD Scale
- CNS
central nervous system
- Ctrl
Controls
- DLPFC
DorsoLateral Prefrontal Cortex
- DMPFC
DorsoMedial Prefrontal Cortex
- FA
Flip Angle
- FM
fibromyalgia
- fMRI
functional Magnetic Resonance Imaging
- GRAPPA
GeneRalized Autocalibrating Partially Parallel Acquisitions
- GWI
Gulf War Illness
- HDR
hemodynamic response
- IPL
Inferior Parietal Lobule
- ISI
Inter-Stimulus Interval
- MPRAGE
Magnetization Prepared Rapid Acquisition Gradient Echo
- PPC
Posterior Parietal Cortex
- PTSD
Post-Traumatic Stress Disorder
- QST
Quantitative Sensory Testing
- ROI
region of interest
- rCBF
regional Cerebral Blood Flow
- S1
Primary somatosensory cortex
- S2
Secondary somatosensory cortex
- SCID
Structured Clinical Interview for DSM-IV Axis I Disorders
- SMA
Supplementary Motor Area
- SPECT
Single-Photon Emission Computed
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Binns JH, Cherry N, Golomb BA, Graves JC, Haley RW, Knox ML, et al. In: Report of Research Advisory Committee on Gulf War Veterans’ Illnesses. Affairs V, editor. Topeka, KS: 2004. [Google Scholar]
- Borsook D, Moulton EA, Tully S, Schmahmann JD, Becerra L. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum. 2008;7:252–72. doi: 10.1007/s12311-008-0011-6. [DOI] [PubMed] [Google Scholar]
- Bradesi S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol Motil. 2010;22:499–511. doi: 10.1111/j.1365-2982.2010.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calley CS, Kraut MA, Spence JS, Briggs RW, Haley RW, Hart J., Jr The neuroanatomic correlates of semantic memory deficits in patients with Gulf War illnesses: a pilot study. Brain Imaging Behav. 2010;4:248–55. doi: 10.1007/s11682-010-9103-2. [DOI] [PubMed] [Google Scholar]
- Chen LM. Imaging of pain. Int Anesthesiol Clin. 2007;45:39–57. doi: 10.1097/AIA.0b013e31803419d3. [DOI] [PubMed] [Google Scholar]
- Clauw DJ. Fibromyalgia: an overview. Am J Med. 2009;122:S3–S13. doi: 10.1016/j.amjmed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–78. [PubMed] [Google Scholar]
- Cook DB, Stegner AJ, Ellingson LD. Exercise alters pain sensitivity in Gulf War veterans with chronic musculoskeletal pain. J Pain. 2010;11:764–72. doi: 10.1016/j.jpain.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–8. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Defrin R, Ginzburg K, Solomon Z, Polad E, Bloch M, Govezensky M, et al. Quantitative testing of pain perception in subjects with PTSD--implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450–9. doi: 10.1016/j.pain.2008.05.006. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis 1 Disorders: SCID I/P. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Haley RW. Chronic multisystem illness among Gulf War veterans. JAMA. 1999;282:327. author reply 8–9. [PubMed] [Google Scholar]
- Haley RW, Fleckenstein JL, Marshall WW, McDonald GG, Kramer GL, Petty F. Effect of basal ganglia injury on central dopamine activity in Gulf War syndrome: correlation of proton magnetic resonance spectroscopy and plasma homovanillic acid levels. Arch Neurol. 2000a;57:1280–5. doi: 10.1001/archneur.57.9.1280. [DOI] [PubMed] [Google Scholar]
- Haley RW, Kurt TL. Self-reported exposure to neurotoxic chemical combinations in the Gulf War. A cross-sectional epidemiologic study. JAMA. 1997;277:231–7. [PubMed] [Google Scholar]
- Haley RW, Marshall WW, McDonald GG, Daugherty MA, Petty F, Fleckenstein JL. Brain abnormalities in Gulf War syndrome: evaluation with 1H MR spectroscopy. Radiology. 2000b;215:807–17. doi: 10.1148/radiology.215.3.r00jn48807. [DOI] [PubMed] [Google Scholar]
- Haley RW, Spence JS, Carmack PS, Gunst RF, Schucany WR, Petty F, et al. Abnormal brain response to cholinergic challenge in chronic encephalopathy from the 1991 Gulf War. Psychiatry Res. 2009;171:207–20. doi: 10.1016/j.pscychresns.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Heldestad V, Linder J, Sellersjo L, Nordh E. Reproducibility and influence of test modality order on thermal perception and thermal pain thresholds in quantitative sensory testing. Clin Neurophysiol. 2010 doi: 10.1016/j.clinph.2010.03.055. [DOI] [PubMed] [Google Scholar]
- Iannacchione VG, Dever JA, Bann CM, Considine KA, Creel D, Best H, et al. Validation of a research case definition of Gulf War illness in the 1991 U.S. military population. Neuroepidemiology. 2011 doi: 10.1159/000331478. (in press) [DOI] [PubMed] [Google Scholar]
- Jamal GA, Hansen S, Apartopoulos F, Peden A. The "Gulf War syndrome". Is there evidence of dysfunction in the nervous system? J Neurol Neurosurg Psychiatry. 1996;60:449–51. doi: 10.1136/jnnp.60.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A, Geuze E, Schmahl C, Greffrath W, Treede RD, Bohus M, et al. Differentiation of pain ratings in combat-related posttraumatic stress disorder. Pain. 2009;143:179–85. doi: 10.1016/j.pain.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Lariviere M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–10. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–8. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Li X, Spence J, Buhner DM, Haley R, Briggs R. Investigation of hippocampal dysfunction in Gulf War veterans using ASL perfusion imaging with physostigmine challenge. Radiology. 2011 doi: 10.1148/radiol.11101715. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Hsieh SC, Chao CC, Chang YC, Hsieh ST. Influence of aging on thermal and vibratory thresholds of quantitative sensory testing. J Peripher Nerv Syst. 2005;10:269–81. doi: 10.1111/j.1085-9489.2005.10305.x. [DOI] [PubMed] [Google Scholar]
- Liu P, Aslan S, Li X, Buhner DM, Spence JS, Briggs RW, et al. Perfusion deficit to cholinergic challenge in veterans with Gulf War Illness. Neurotoxicology. 2011;32:242–6. doi: 10.1016/j.neuro.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol. 2005;93:2183–93. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- Nebel MB, Gracely RH. Neuroimaging of fibromyalgia. Rheum Dis Clin North Am. 2009;35:313–27. doi: 10.1016/j.rdc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Porro CA, Lui F, Facchin P, Maieron M, Baraldi P. Percept-related activity in the human somatosensory system: functional magnetic resonance imaging studies. Magnetic Resonance Imaging. 2004;22:1539–48. doi: 10.1016/j.mri.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. Neuroimage. 2008;41:1504–13. doi: 10.1016/j.neuroimage.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Sharma SK. Importance of Case Definition in Epidemiological Studies. Neuroepidemiology. 2011;37:141–2. doi: 10.1159/000332609. [DOI] [PubMed] [Google Scholar]
- Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–89. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Brodin E. Spinal astrocytes in pain processing: non-neuronal cells as therapeutic targets. Mol Interv. 2010;10:25–38. doi: 10.1124/mi.10.1.6. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–65. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, et al. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288:159–62. doi: 10.1016/s0304-3940(00)01224-6. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748–52. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng MT, Tseng WY, Chao CC, Lin HE, Hsieh ST. Distinct and shared cerebral activations in processing innocuous versus noxious contact heat revealed by functional magnetic resonance imaging. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Representations of pain in the brain. Curr Rheumatol Rep. 2004;6:261–5. doi: 10.1007/s11926-004-0033-0. [DOI] [PubMed] [Google Scholar]
- Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci. 2009;29:705–15. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Williams DA, Gracely RH. Biology and therapy of fibromyalgia. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res Ther. 2006;8:224. doi: 10.1186/ar2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20:198–204. doi: 10.1002/(sici)1097-4598(199702)20:2<198::aid-mus10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.