The length of the poly(A)-tail that terminates most mRNA can serve as a readout of its metabolism. A long poly(A)-tail is generally associated with actively translating mRNA, whereas a short poly(A)-tail often indicates a translationally repressed state. Here, the authors present a simple new method to measure transcript-specific poly(A)-tail length using standard molecular biology reagents.
Keywords: mRNA polyadenylation, alternative polyadenylation, translational control, LM-PAT assay
Abstract
The addition of a poly(A)-tail to the 3′ termini of RNA molecules influences stability, nuclear export, and efficiency of translation. In the cytoplasm, dynamic changes in the length of the poly(A)-tail have long been recognized as reflective of the switch between translational silence and activation. Thus, measurement of the poly(A)-tail associated with any given mRNA at steady-state can serve as a surrogate readout of its translation-state. Here, we describe a simple new method to 3′-tag adenylated RNA in total RNA samples using the intrinsic property of Escherichia coli DNA polymerase I to extend an RNA primer using a DNA template. This tag can serve as an anchor for cDNA synthesis and subsequent gene-specific PCR to assess poly(A)-tail length. We call this method extension Poly(A) Test (ePAT). The ePAT approach is as efficient as traditional Ligation-Mediated Poly(A) Test (LM-PAT) assays, avoids problems of internal priming associated with oligo-dT-based methods, and allows for the accurate analysis of both the poly(A)-tail length and alternate 3′ UTR usage in 3′ RACE applications.
INTRODUCTION
The poly(A)-tail is involved in all major functions of eukaryotic mRNA, and addition of this tail is the final quality control step that nascent mRNA undergo prior to nuclear export (Proudfoot et al. 2002). In this context, it is thought that the poly(A)-tail promotes circularization of the RNA molecule into a closed-loop configuration that promotes translation initiation (Sachs and Varani 2000). At synthesis, the length of the poly(A)-tail is generally uniform in any given system, with the absolute length being species-dependent; in yeast and mammalian cells, this is ∼90 and 250 adenosine residues, respectively. However, in the cytoplasm, the steady-state length distribution of poly(A)-tails can vary dramatically for transcripts of different functional classes due to differential, transcript-specific deadenylation rates, a disconnect between deadenylation and decapping and/or stabilization during translation (Decker and Parker 1993; Brown and Sachs 1998; Beilharz and Preiss 2007). For example, targeted deadenylation is provoked by microRNA binding (Beilharz et al. 2009; Eulalio et al. 2009; Fabian et al. 2009) or RNA binding proteins. Compensatory and activating cytoplasmic adenylation can further modulate the polyadenylation state of the transcriptomes of many eukaryotes (Rouhana and Wickens 2007; Burns et al. 2011). Finally, eukaryotic cells also employ the ancient, prokaryotic function of RNA adenylation to destabilize mitochondrial, structural, and noncoding RNA (Slomovic et al. 2010; for review, see Eckmann et al. 2011). We have previously shown that the adenylation state of the transcriptome is highly correlated with other gene expression parameters, such as ribosome occupancy and protein abundance (Beilharz and Preiss 2007; Lackner et al. 2007), which has led us to propose that the measurement of mRNA poly(A)-tail length can serve as a surrogate for translation-state measurements.
There are several well-characterized methods for measuring poly(A)-tail length. The most direct method is the combined use of RNase H digest (± Oligo dT) and high-resolution Northern blot (Sippel et al. 1974; Decker and Parker 1993; Sheets et al. 1994). In our own laboratory, we have made extensive use of the Ligation- Mediated Poly(A) Test (LM-PAT) assay developed by the Strickland laboratory (Salles and Strickland 1995; Beilharz and Preiss 2007; Lackner et al. 2007; Beilharz et al. 2009; Traven et al. 2009; Dagley et al. 2011). Finally, several laboratories have used T4 RNA ligase approaches to either circularize mRNA (Couttet et al. 1997) or, more recently, to ligate adaptors to the 3′ end of mRNA (Charlesworth et al. 2004). While each of these assays can serve to measure poly(A)-tail length, each has its own limitations. High-resolution Northern blots are labor-intensive and require a lot of RNA, the LM-PAT assay has low-resolution and a tendency toward exaggerated apparent short-tailed mRNA, and adaptor-ligation approaches are relatively inefficient. We sought to develop an assay that is simple, high-resolution, and efficient for measuring the poly(A)-tail of mRNA and to unambiguously assign the 3′ UTR of specific transcripts. Our assay is based on the use of Klenow polymerase to extend the 3′ termini of specific RNA molecules with dNTPs (Huang and Szostak 1996). We describe how this intrinsic activity (Okazaki and Okazaki 1969) can be harnessed to tag the 3′ end of RNA molecules as an extension-mediated poly(A)-tail length measurement as well as for other applications that require 3′-end labeling.
RESULTS AND DISCUSSION
A simple method for 3′-tagging of adenylated RNA
The extension Poly(A) Test (ePAT) approach is a simple two-step assay conducted in one reaction tube that relies on the intrinsic property of the Klenow polymerase to extend RNA molecules with dNTPs from a annealed oligonucleotide DNA template in standard reverse transcription buffers (Fig. 1A). Increasing the temperature to 55°C prior to addition of reverse transcriptase ensures that only the DNA oligos that have annealed to an extended 3′ terminus have a melt-temperature sufficiently high to prime reverse transcription. This eliminates unwanted priming from internal poly(A)-tracts. Subsequent amplification using a gene-specific forward primer and a universal reverse primer results in PCR amplicons that reflect the length distribution of the poly(A)-tail on endogenous mRNA. To calculate the actual length of the poly(A)-tail, a separate “TVN-PAT” reaction can be performed which reports the size of the amplicon with an invariant 12-(A) poly(A)-tail, irrespective of the actual poly(A)-lengths in the sample. The TVN-PAT cDNA is prepared using the identical primer sequence as for ePAT except for the addition of two 3′ variable bases V and N (where V is A, G, or C, and N is any base). These variable bases lock the primer to the polyadenylation site during reverse transcription (Beilharz and Preiss 2009).
FIGURE 1.
(A) Schematic illustration of the ePAT approach. In the first step of the assay, a DNA oligo is annealed to adenylated RNA via an oligo-(dT) stretch. The addition of Klenow polymerase and dNTPs to the reaction results in templated extension of the 3′ terminus of the poly(A) tract to fill in the recessed end. The second step of the assay is performed at 55°C to ensure that the primer used for extension can only remain annealed to end-extended molecules and not internal poly(A) tracts through oligo-(T) alone. PCR amplification of cDNA using a gene-specific primer (primer 1) and a universal reverse primer (primer 2) results in a range of amplicon sizes that reflects the position of the gene-specific primer relative to the poly(A) site and the length distribution of poly(A)-tails in the sample. (B) Serially diluted yeast total RNA, as indicated, was spiked into a fixed concentration (800 ng) of total HeLa RNA and analyzed by ePAT. The 200-ng sample was also used to prepare a TVN-PAT reaction. PCR amplicons from TVN-PAT and ePAT reactions were resolved on 2% high-resolution agarose gels and imaged. The TVN-PAT reaction represents the size of the amplicon with a fixed (A12)-tail. The signal intensity, but not the poly(A)-tail length distribution, of the two yeast genes GAL10 and APQ12 decreases with decreasing total RNA concentration. The human GAPDH adenylation state and concentration remains constant. (C) The signal from the GAL10 and APQ12 transcripts was quantitated by peak-finding and densitometry. The normalized signal (to 100% y-axis) was plotted against concentration (x-axis) and expressed in log2 to show the linear relationship between the signal and the RNA concentration.
To test the fidelity of ePAT in reporting poly(A)-tail length over a range of RNA concentrations, we prepared serial (½) dilutions of total yeast RNA to spike into a fixed concentration of Human (HeLa) total RNA. The yeast total RNA was isolated from cells that had undergone a 10-min incubation with galactose. At this early time point after galactose induction, GAL10 shows a uniform length of ∼40 adenosine residues. APQ12, on the other hand, is not a galactose-responsive gene and shows a “smear” of amplicons reflecting both new and aged transcripts (Fig. 1B). The poly(A)-tail reported by ePAT is invariant across the concentration range tested. Furthermore, densitometry shows that the method is quantitative within the quantitative range of the PCR reaction and the limits of fluorescence detection (Fig. 1C). The signal for APQ12 is linear over the entire dilution range (r2 = 0.9975), whereas the GAL10 signal is quantitative between 100 ng and 6 ng input (r2 = 0.9959). The signal from human GAPDH does not vary in response to a change in the concentration of spike in yeast total RNA.
We compared the efficacy of ePAT directly to the standard LM-PAT method that relies on serial ligation of p(dT)12–18 and an anchor primer to generate cDNA that encompasses the full poly(A)-tail (Salles and Strickland 1995). First, we compared the methods by generating a series of in vitro synthesized and adenylated TOM5 transcripts (Fig. 3A, see below; Supplemental Fig. A). The adenylated transcripts were spiked into total HeLa RNA to asses the accuracy of ePAT in length calling. By this approach, there was a good overall correlation between the average tail length of in vitro transcripts and the observed size of the ePAT amplification products. Specifically, in vitro transcripts with an estimated average poly(A)-tail length of ∼15, ∼45, ∼65, and ∼115 adenosine residues (see Supplemental Fig. A) showed an estimated poly(A)-tail length of ∼20, ∼40, ∼65, and ∼95 by ePAT. The estimation of the average tail length of both the in vitro transcripts and the PCR products is based on migration relative to RNA and DNA ladders, respectively, and is accurate to approximately ±10 nt. Both assays reflect the length of the in vitro synthesized poly(A)-tail. However, the LM-PAT assay is slightly biased toward specific sizes, preferentially reporting the ∼45-A transcripts and artifactually reporting a tail of this length in mRNA having longer poly(A)-tails.
FIGURE 3.
Alternate polyadenylation sites are revealed by ePAT. (A) Schematic representation of the time course of galactose induction and glucose repression. The arrows indicate the time points at which cells were harvested. (B) HXK1 transcription is rapidly repressed by glucose. During this repression phase, the ratio between HXK1-(long) and HXK1-(short) changes as the short 3′ UTR form is transiently induced without a significant increase in poly(A)-tail length. GCV1 is transiently induced with a long poly(A)-tail by glucose addition. The faster migrating GCV1 amplicon, indicated by **, is likely a product of internal priming in the TVN reaction. The GAL10 and APQ12 panels are included as pulse-chase and ePAT assay controls, respectively.
Next, we monitored the adenylation state of specific endogenous transcripts in response to a transcriptional pulse chase regimen in Saccharomyces cerevisiae involving activation of gene expression by galactose followed by repression by the addition of glucose (Decker and Parker 1993; Beilharz and Preiss 2007). Yeast cells were harvested 10 min after galactose induction and at the indicated time points in the pulse period after glucose repression (Fig. 2B). At the point of transcriptional shutdown (glucose addition), the two 3′ UTR isoforms of the GAL1 transcript had a long (∼50 A) poly(A)-tail that shortened over time (Fig. 2C). Again, the two assays were equally efficient at generating cDNA that reflected the adenylation state of the transcripts. However, the ePAT assay provided a better reflection of the distribution of poly(A) tails in the sample. Because the LM-PAT assay depends on serial annealing of fixed length oligo-(pdT) oligonucleotides, only limited resolution can be achieved (see also Beilharz and Preiss [2009] for further discussion). We monitored the kinetics of deadenylation of both GAL1 3′ UTR isoforms after transcriptional repression by densitometry and peak-identification (Fig. 1D). Linear deadenylation rates for both the GAL1 transcript isoforms were calculated from ePAT data. Such decay curves were previously only possible by high-resolution Northern blots (Decker and Parker 1993). Therefore, compared to LM-PAT, the ePAT method provided a more accurate measurement of the poly(A)-tail length distribution and more detailed estimation of the deadenylation kinetics. It is important to note that the GAL1 transcript has previously been shown to undergo an additional nonstandard post-transcriptional cleavage and adenylation step to generate the GAL1-(short) transcript ∼10 min after glucose repression (Vodala et al. 2008). However, the similar initial length and decay kinetics of the poly(A)-tail at the start of the chase period in this experiment suggests that both forms were generated by the canonical transcript cleavage and adenylation machinery. Any additional cleavage products would remain invisible to the assay until they became adenylated.
FIGURE 2.
(A) Four in vitro synthesized and adenylated transcripts with the indicated average poly(A)-tail length were spiked into a fixed amount of total HeLa RNA (800 ng) and analyzed by ePAT and LM-PAT (see Supplemental Material for details of in vitro transcripts). (B) Schematic representation of the time course of galactose induction and glucose repression. The arrows indicate the time points at which cells were harvested. (C) The 10-min time point represents newly synthesized GAL1 transcript with a long poly(A)-tail. The GAL1-(long) and GAL1-(short) transcripts are generated by alternate poly(A) site usage. (D) The time-dependent shortening of the two GAL1 amplicons was quantified relative to the 100-bp ladder and the migration of the TVN product. This is only really possible using the ePAT assay, since laddering in the LM-PAT assay results in two peaks of similar intensity at the last time point of this assay (20 min).
Alternate poly(A) site usage is revealed by ePAT
Recent evidence has shown that the use of alternate poly(A) sites is widespread and dynamic in eukaryotic transcriptomes (Mangone et al. 2010; Ozsolak et al. 2010; Yoon and Brem 2010; Jan et al. 2011; Shepard et al. 2011; Wu et al. 2011). The use of shorter 3′ UTRs often provides a mechanism to remove the influence of post-transcriptional regulatory modules within 3′ UTRs, such as microRNA and regulatory protein binding sites (Sandberg et al. 2008). Since cDNA priming for ePAT requires extension of the 3′ end of the RNA molecule, internal poly(A) stretches are avoided. HXK1 is one of a number of genes, including GAL1 above, that change their subnuclear position in response to activation by galactose in a 3′ UTR-dependent manner, suggesting a role for 3′ processing in gene activation and/or repression (Abruzzi et al. 2006; Taddei et al. 2006; Vodala et al. 2008). In this study, we performed a transcriptional pulse chase (Fig. 3A) and found evidence for alternative polyadenylation of the HXK1 transcript. The HXK1-(long) transcript followed normal decay kinetics of deadenylation followed by decay of the transcript body after repression by glucose. The compaction of the PCR amplicons from a smear to a tight, short-tailed band explains the apparent increase of the HXK1-(long) transcript at 12.5 and 15 min. In contrast, the abundance of HXK1-(short) transcript transiently increased at 17.5 min. This was similar to the observation of GAL1-(short) accumulation by a nonstandard cleavage and polyadenylation mechanism reported by Vodala et al. (2008). Sequencing of the two HXK1 PCR amplicons allowed for precise mapping of the alternate poly(A)-sites, located 65 and 175 bases after the stop codon for the short and long UTRs, respectively. This result suggests that a switch to the shorter UTR removes two of three canonical UGUA pumilio consensus sites previously identified as candidate Puf1 sites in the 3′ UTR of HXK1 (Ulbricht and Olivas 2008). However, how this might influence Hxk1 protein expression remains unexplored. It is important to note that, in contrast to GAL1 and HXK1, the apparent shorter alternate 3′ UTR of GCV1 suggested by the TVN-PAT reaction (see ** in Fig 3B) is likely an artifact, having a size consistent with internal priming to a 7-adenosine internal stretch within the GCV1 transcript. Thus, in contrast to oligo-dT-based priming methods, the ePAT approach allows identification of bona fide alternate poly(A) sites.
3′-tagging by ePAT is efficient
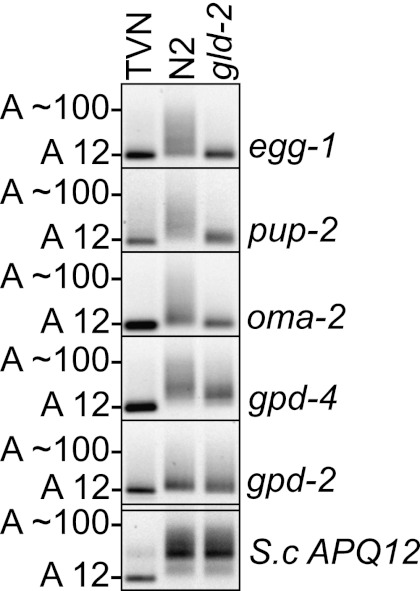
An alternative method for measuring poly(A)-tail length has been the use of T4-ligase to append preadenylated linkers to the 3′ end of mRNA using methods analogous to those applied to the cloning of microRNA (Charlesworth et al. 2004; Kim et al. 2009) or circularization of mRNA (Couttet et al. 1997; Rissland and Norbury 2009). These approaches are conceptually appealing in that they do not require knowledge of the bases that terminate the RNA of interest and can, therefore, be used to identify 3′ uridilation and other heteropolymeric 3′ extensions on RNA. The disadvantage of these methods is that they are inefficient and often require high PCR cycle numbers (commonly 40 cycles) and/or the use of radiolabeling to detect a product. To assess the performance of ePAT in applications where a T4-RNA ligation-based assay had been previously applied, we replicated the results presented by Kim et al. (2009), who elegantly demonstrated the dependence of several transcripts on the cytoplasmic deadenylase Gld-2 in the Caenorhabditis elegans germline. Using 10-fold less RNA input, twofold less cDNA, and 12 fewer cycles of PCR amplification (28 versus 40), the ePAT assay replicated and extended the data provided by Kim et al. (2009) (Fig. 4). RNA was isolated from wild-type Bristol Normal (N2) and gld-2 mutant worms. Two hundred ng of this was spiked into yeast total RNA and subject to ePAT. The adenylation state of egg-1, pup-2, and oma-2 were confirmed to depend on Gld-2, as does the germline specific gpd-4 (GAPDH). The steady-state poly(A)-tail length of somatic gpd-2 (GAPDH) and eft-3 were not significantly altered (data not shown).
FIGURE 4.
The cytoplasmic poly(A)-polymerase Gld-2 is required for normal adenylation of transcripts in the C. elegans germline. The adenylation state of four maternal mRNA egg-1, Pup-2, oma-2, and gpd-4 was analyzed in Bristol Normal (N2) and gld-2 mutant worms. In each case, the mRNA is short-tailed in the mutant, which reflects the inactive state. The adenylation state of the somatic transcript gpd-2 is not affected in a major way by the loss of Gld-2. Yeast total RNA (800 ng) from a deadenylase-deficient mutant (pan2Δ/ccr4-1) was spiked into the assay as ballast for the low concentration of worm RNA (200 ng). The yeast APQ12 panel is included to demonstrate equal ePAT efficiency across samples.
To infer the efficiency of ePAT, we designed a PCR calculator that uses the PCR cycle number, an estimated PCR efficiency, and the amplicon length and mass in combination with the molecular weight of a DNA base pair to estimate the number of cDNA input molecules that contribute to the final PCR product (Supplemental File 2). For example, at least 5000 template egg-1 cDNA molecules in the PCR reaction mix were required to generate 110 ng of the 120-bp PCR amplicon as measured by densitometry (Fig. 4, gld-2). If 40 cycles of amplification had been required to generate this amount of product, then the number of contributing cDNA molecules would reduce to approximately two. Thus, with every additional PCR cycle necessary to produce detectable product, there is an increased risk of biased sampling. Interestingly, using ePAT, we observed a tail-length distribution of ∼12–100 A-residues for most transcripts in wild-type (N2) worms, which was a striking difference to that observed by Kim et al. (2009). Using the ligation approach and 40 cycles of amplification, Kim et al. (2009) reported a uniform tail length of ∼40 bases for oma-2. The length was significantly reduced in gld-2 mutant worms as measured by both studies. Taken together, we have shown that the ePAT approach returns similar and possibly more detailed information on poly(A) status than T4-ligase-based approaches and at significantly higher efficiency.
CONCLUSION
In this study, we describe a simple method to tag adenylated RNA within a population and demonstrate its use for the identification of alternate 3′ UTRs and measurement of the poly(A)-tail length of mRNA. The method is quantitative within the linear range of amplification and can be applied to detect transcripts from very low concentrations of RNA. This approach is the closest method described to date that matches the sensitivity observed by high-resolution Northern blot but avoids radiolabeled probes, requires a fraction of the time and RNA input, and generates enough cDNA for analysis of over 20 specific transcripts from a single ePAT reaction. Moreover, all of the necessary components are standard in any molecular biology laboratory.
MATERIALS AND METHODS
Saccharomyces cerevisiae
The By4741 yeast strain (MATa his3Δ0 leu2Δ0 met15Δ0 ura3Δ0) was grown to exponential phase (OD600 ∼0.6) in rich medium (2% peptone and 1% yeast extract) with 2% raffinose as the sole carbon source. Transcription of GAL genes were transiently induced by addition of 2% galactose and then repressed after 10 min by the addition of 2% glucose. At each indicated time point, 5 mL of culture was removed into 15 mL tubes containing 50 μL 10% sodium azide prechilled in an ice bath. Once all samples were collected, the cells were harvested by centrifugation (4000g for 2 min), washed once in ice-cold water containing sodium azide (0.1%), snap frozen, and stored at −80°C.
Caenorhabditis elegans
C. elegans wild-type Bristol N2 and gld-2(q497) strains were maintained at 20°C using standard methods (Brenner 1974).
RNA extraction
Total RNA from yeast cells was prepared according to the hot phenol method. Total C. elegans RNA was prepared by suspending between 50 and 100 snap frozen worms in 1 mL of Trizol (Life Technologies). After addition of ∼200 μL ziconia beads (Biospec Products), the sample was homogenized for 30 sec using a Mini-Beadbeater 8 (Biospec Products). Trizol extraction was performed according to the manufacturer's instructions, except that 2 μL glycogen was added prior to precipitation with isopropanol. To improve the generally poor Nano-drop QC A260/A230 ratios that result from Trizol purifications, we resuspended the resulting pellet in 100 μL dH2O and reprecipitated the RNA with 1/10 volume of 3 M NaOac [pH 5.2] and 2.5 volumes of ethanol. RNA quantification was performed with a Nano-Drop 1000 (Thermo Scientific).
The ePAT method and product detection
The ePAT approach uses the same PAT-anchor primer that has been described for the LM-PAT method (PAT anchor 5′-GCGAGCTCCGCGGCCGCGTTTTTTTTTTTT), which was stored as small 100-μM aliquots at −20°C. Of note, alternative anchor sequences were equally efficient in our laboratory. The incubation steps of the assay were most conveniently performed in a thermocycler with an accessible lid programmed with a series of temperature hold/pause settings, where the pause maintains temperature while allowing access to the tubes.
To assemble the ePAT reaction, 1 μg of total RNA (or less) and 1 μL of PAT-anchor primer were combined and brought to a volume of 8 μL with dH2O in a 200-μL PCR tube. The mixture was incubated at 80°C for 5 min and then cooled to room temperature. Once cooled, the sample was flash-centrifuged, and 12 μL of a master mix was added that contained 4 μL dH2O, 4 μL 5× Superscript III buffer (Life Technologies), 1 μL 100 mM DTT, 1 μL 10 mM dNTPs, 1 μL RNaseOUT (Life Technologies), and 1 μL (5 U) Klenow polymerase (New England Biolabs) per reaction. The sample was mixed thoroughly by inversion, flash-centrifuged, and then incubated at 25°C for 1 h. Importantly, 37°C worked equally well in our laboratory and may improve selectivity. The polymerase was then inactivated by increasing the temperature to 80°C for 10 min prior to cooling the reaction to 55°C for 1 min. While maintaining the tubes at that temperature in the block, 1 μL (200 U) of Superscript III (Life Technologies) was added to the tubes. The tubes were closed and mixed rapidly by flick-inversion. Incubation was then resumed at 55°C for 1 h, followed by inactivation of the reverse transcriptase by increasing the temperature to 80°C for 10 min. It is critical to maintain the temperature during the reverse transcription step because internal priming can occur if the temperature drops. At 55°C, only priming from an end-extended RNA molecule is possible. It can also be useful to include spiked-in RNA from an unrelated organism as ballast for dilute RNA reactions and to control for equal assay efficiency across samples. We routinely spike total HeLa RNA in yeast samples and RNA from a deadenylase deficient yeast strain in metazoan RNA samples.
For the PCR reactions, cDNA was diluted 1:6 by the addition of 120 μL dH2O. The PCR reactions were typically conducted in a total volume of 20 μL using 5 μL of diluted cDNA input and a fast-start polymerase, such as Fast-Start (Roche) or Amplitaq Gold 360 master (Life Technologies). It can also be useful to include a TVN-PAT reaction as a size control for the size of the amplicon with a fixed A12 poly(A)-tail. Both the LM-PAT and the TVN-PAT reactions were performed exactly as described previously (Beilharz and Preiss 2009). The cycle number was dependent on the abundance of the transcript of interest in the sample but normally ranges between 23–33 cycles.
To detect the PCR amplicons from TVN-PAT, ePAT, and LM-PAT PCR reactions, 50% of a 20-μL PCR reaction was loaded per lane into a 2% high-resolution agarose gel (Ultra pure 1000; Life Technologies) that was prestained with SYBR safe (Life Technologies). The primers used in this study are supplied (Supplemental File 1). To estimate the PCR product sizes and to quantify the mass of PCR amplicons from such gels, the band intensity and migration was determined relative to a 100-bp ladder (New England Biolabs) using an LAS 3000 imager and multigauge software (Fujifilm). To track deadenylation kinetics, the migration of the highest peak intensity of each band was determined for each lane and expressed relative to the migration of the TVN-PAT peak. The length of poly(A) at time zero was then normalized to 100%, and the peaks of subsequent time points were expressed relative to the normalized control. Using this approach in Figure 2, highly linear [R2 = 0.9997 and 0.9682 for GAL1-(long) and GAL1-(short), respectively] deadenylation curves were possible from ePAT samples but not LM-PAT samples [R2 = 0.1751 and 0.4049 for GAL1-(long) and GAL1-(short), respectively]. The graphs and statistical analyses were prepared using GraphPad Prism software.
Efficiency calculations used to estimate the number of cDNA input molecules were based on a calculated mass of 110 ng and an amplicon length of 120-bp at an estimated efficiency of 98% as user inputs for oma-2 in the PCR calculator (Supplemental File 2).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank members of the Beilharz, Traven, and Boag labs for critical reading of the manuscript and Trevor Lithgow for the TOM5 in vitro transcription plasmid. T.B. is supported by an Australian Research Council Fellowship (DP0878224). Work on C. elegans germline development in the P.B. laboratory is supported by NHMRC project grant (606575). Work on the PUF RNA binding proteins in the A.T. laboratory is supported by an ARC Discovery project (DP1092850).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.031898.111.
REFERENCES
- Abruzzi KC, Belostotsky DA, Chekanova JA, Dower K, Rosbash M 2006. 3′-end formation signals modulate the association of genes with the nuclear periphery as well as mRNP dot formation. EMBO J 25: 4253–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz TH, Preiss T 2007. Widespread use of poly(A) tail length control to accentuate expression of the yeast transcriptome. RNA 13: 982–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz TH, Preiss T 2009. Transcriptome-wide measurement of mRNA polyadenylation state. Methods 48: 294–300 [DOI] [PubMed] [Google Scholar]
- Beilharz TH, Humphreys DT, Clancy JL, Thermann R, Martin DI, Hentze MW, Preiss T 2009. microRNA-mediated messenger RNA deadenylation contributes to translational repression in mammalian cells. PLoS ONE 4: e6783 doi: 10.1371/journal.pone.0006783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Sachs AB 1998. Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation. Mol Cell Biol 18: 6548–6559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns DM, D'Ambrogio A, Nottrott S, Richter JD 2011. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature 473: 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth A, Cox LL, MacNicol AM 2004. Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J Biol Chem 279: 17650–17659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couttet P, Fromont-Racine M, Steel D, Pictet R, Grange T 1997. Messenger RNA deadenylylation precedes decapping in mammalian cells. Proc Natl Acad Sci 94: 5628–5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley MJ, Gentle IE, Beilharz TH, Pettolino FA, Djordjevic JT, Lo TL, Uwamahoro N, Rupasinghe T, Tull DL, McConville M, et al. 2011. Cell wall integrity is linked to mitochondria and phospholipid homeostasis in Candida albicans through the activity of the post-transcriptional regulator Ccr4-Pop2. Mol Microbiol 79: 968–989 [DOI] [PubMed] [Google Scholar]
- Decker CJ, Parker R 1993. A turnover pathway for both stable and unstable mRNAs in yeast: Evidence for a requirement for deadenylation. Genes Dev 7: 1632–1643 [DOI] [PubMed] [Google Scholar]
- Eckmann CR, Rammelt C, Wahle E 2011. Control of poly(A) tail length. Wiley Interdiscip Rev RNA 2: 348–361 [DOI] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E 2009. Deadenylation is a widespread effect of miRNA regulation. RNA 15: 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian MR, Mathonnet G, Sundermeier T, Mathys H, Zipprich JT, Svitkin YV, Rivas F, Jinek M, Wohlschlegel J, Doudna JA, et al. 2009. Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol Cell 35: 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Szostak JW 1996. A simple method for 3′-labeling of RNA. Nucleic Acids Res 24: 4360–4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan CH, Friedman RC, Ruby JG, Bartel DP 2011. Formation, regulation and evolution of Caenorhabditis elegans 3′UTRs. Nature 469: 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Nykamp K, Suh N, Bachorik JL, Wang L, Kimble J 2009. Antagonism between GLD-2 binding partners controls gamete sex. Dev Cell 16: 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner DH, Beilharz TH, Marguerat S, Mata J, Watt S, Schubert F, Preiss T, Bahler J 2007. A network of multiple regulatory layers shapes gene expression in fission yeast. Mol Cell 26: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangone M, Manoharan AP, Thierry-Mieg D, Thierry-Mieg J, Han T, Mackowiak SD, Mis E, Zegar C, Gutwein MR, Khivansara V, et al. 2010. The landscape of C. elegans 3′UTRs. Science 329: 432–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Okazaki R 1969. Mechanism of DNA chain growth. IV. Direction of synthesis of T4 short DNA chains as revealed by exonucleolytic degradation. Proc Natl Acad Sci 64: 1242–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsolak F, Kapranov P, Foissac S, Kim SW, Fishilevich E, Monaghan AP, John B, Milos PM 2010. Comprehensive polyadenylation site maps in yeast and human reveal pervasive alternative polyadenylation. Cell 143: 1018–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot NJ, Furger A, Dye MJ 2002. Integrating mRNA processing with transcription. Cell 108: 501–512 [DOI] [PubMed] [Google Scholar]
- Rissland OS, Norbury CJ 2009. Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nat Struct Mol Biol 16: 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhana L, Wickens M 2007. Autoregulation of GLD-2 cytoplasmic poly(A) polymerase. RNA 13: 188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs AB, Varani G 2000. Eukaryotic translation initiation: There are (at least) two sides to every story. Nat Struct Biol 7: 356–361 [DOI] [PubMed] [Google Scholar]
- Salles FJ, Strickland S 1995. Rapid and sensitive analysis of mRNA polyadenylation states by PCR. PCR Methods Appl 4: 317–321 [DOI] [PubMed] [Google Scholar]
- Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB 2008. Proliferating cells express mRNAs with shortened 3′ untranslated regions and fewer microRNA target sites. Science 320: 1643–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets MD, Fox CA, Hunt T, Vande Woude G, Wickens M 1994. The 3′-untranslated regions of c-mos and cyclin mRNAs stimulate translation by regulating cytoplasmic polyadenylation. Genes Dev 8: 926–938 [DOI] [PubMed] [Google Scholar]
- Shepard PJ, Choi EA, Lu J, Flanagan LA, Hertel KJ, Shi Y 2011. Complex and dynamic landscape of RNA polyadenylation revealed by PAS-Seq. RNA 17: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel AE, Stavrianopoulos JG, Schutz G, Feigelson P 1974. Translational properties of rabbit globin mRNA after specific removal of poly(A) with ribonuclease H. Proc Natl Acad Sci 71: 4635–4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomovic S, Fremder E, Staals RH, Pruijn GJ, Schuster G 2010. Addition of poly(A) and poly(A)-rich tails during RNA degradation in the cytoplasm of human cells. Proc Natl Acad Sci 107: 7407–7412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM 2006. Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441: 774–778 [DOI] [PubMed] [Google Scholar]
- Traven A, Beilharz TH, Lo TL, Lueder F, Preiss T, Heierhorst J 2009. The Ccr4-Pop2-NOT mRNA deadenylase contributes to septin organization in Saccharomyces cerevisiae. Genetics 182: 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht RJ, Olivas WM 2008. Puf1p acts in combination with other yeast Puf proteins to control mRNA stability. RNA 14: 246–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodala S, Abruzzi KC, Rosbash M 2008. The nuclear exosome and adenylation regulate posttranscriptional tethering of yeast GAL genes to the nuclear periphery. Mol Cell 31: 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liu M, Downie B, Liang C, Ji G, Li QQ, Hunt AG 2011. Genome-wide landscape of polyadenylation in Arabidopsis provides evidence for extensive alternative polyadenylation. Proc Natl Acad Sci 108: 12533–12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon OK, Brem RB 2010. Noncanonical transcript forms in yeast and their regulation during environmental stress. RNA 16: 1256–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]