Abstract
Our understanding of immunity to fungal pathogens has advanced considerably in recent years. Particularly significant have been the parallel discoveries in the C-type lectin receptor family and the Th effector arms of immunity, especially Th17 cells and their signature cytokine IL-17. Many of these studies have focused on the most common human fungal pathogen Candida albicans, which is typically a commensal microbe in healthy individuals but causes various disease manifestations in immunocompromised hosts, ranging from mild mucosal infections to lethal disseminated disease. Here, we discuss emerging fundamental discoveries with C. albicans that have informed our overall molecular understanding of fungal immunity. In particular, we focus on the importance of pattern recognition receptor-mediated fungal recognition and subsequent IL-17 responses in host defense against mucosal candidiasis. In light of these recent advances, we also discuss the implications for anti-cytokine biologic therapy and vaccine development.
Introduction
It is estimated that 1.5 million fungal species populate the planet, but only a few hundred establish infection in humans and an even smaller number reside as commensals (Hube, 2009). However, in the rare situations where they cause disease, fungal infections are associated with significant morbidity and mortality, and can be difficult to diagnose in a clinically relevant time frame. To date, there are no vaccines against any fungal organisms, so it is imperative to understand the intricate host-pathogen relationships between humans and fungi.
Until recently, little was known about the mechanisms by which the innate immune system recognizes fungal pathogens, or the subsequent development of pathogen-specific adaptive immune responses. Two major concepts in recent years have significantly impacted our understanding of fungal immunity. First, the discovery of C-type lectin receptors (CLRs) as recognition elements for fungi shed light on the innate mechanisms of rapid antifungal responses. Second, the discovery of Th17 cells as a distinct T helper cell population set the stage for discoveries revealing a key role for this new T cell subset in antifungal immunity. In this review we will discuss CLRs and other relevant pattern recognition receptors (PRRs) in innate fungal recognition, and the subsequent activation of Th17-based adaptive immunity. We will focus on these responses primarily in the context of the most common and best-characterized human fungal pathogen, Candida albicans, although lessons learned from this organism may well be applicable to other fungal pathogens.
Pattern recognition of Candida albicans: TLRs and Beyond
As attested by the 2011 Nobel Prize in Medicine or Physiology, the concept of “pattern recognition” by the innate immune system fundamentally altered our view of how microbes are recognized at the molecular level, and ultimately how adaptive responses are shaped by this recognition. Toll-like receptors (TLRs) were the first such PRRs to be recognized, but new studies have shed valuable light on how other molecules such CLRs and the inflammasome detect microbes and subsequently direct skewing of Th cell responses.
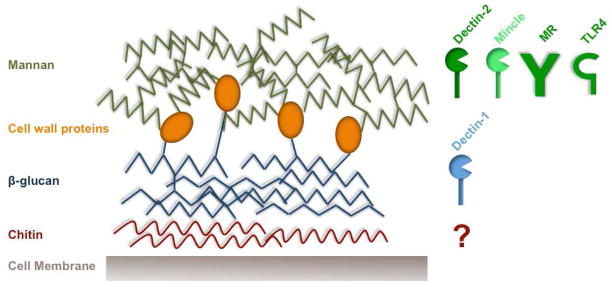
Most fungal PRRs recognize components of the C. albicans cell wall, which is a complex array of layered proteins and carbohydrates (Gow et al., 2011) (Figure 1). Candida albicans is a dimorphic fungus, existing in yeast (conidia) or hyphal (filamentous) forms. The outer portion of the Candida cell wall is largely composed of mannan and manoproteins, and the inner layer is composed of β-(1,3)-glucan and chitin moieties. Expression of cell wall proteins and carbohydrates is significantly altered during the yeast to hyphal transition, which occurs when the fungus invades target organs. The immune system, by virtue of distinct PRRs, can distinguish these fungal forms, in ways that are beginning to be unraveled. Accumulating evidence demonstrates that PRR engagement by C. albicans in antigen presenting cells (APCs) results in secretion of specific cytokines including IL-1β, IL-23 and IL-6 (Gow et al., 2011; Netea et al., 2008a; Romani, 2011). These cytokines in turn promote skewing of activated CD4+ T cells into the Th17 lineage, which express IL-17 (also known as IL-17A), IL-17F and IL-22. IL-17 and IL-17F are closely related cytokines that signal through a common receptor (composed of the IL-17RA and IL-17RC subunits), and IL-17R signaling is clearly crucial for effective anti-Candida immunity (Conti and Gaffen, 2010) (Figure 2). The importance of the IL-17/Th17 pathway is also borne out in humans, as discussed in more detail in subsequent sections (see Table 1).
Figure 1.
The Candida cell wall and PRRs that recognize subcomponents thereof. The yeast cell wall is composed of a variety of proteins and carbohydrates that serve as pathogen associated molecular patterns (PAMPs). These are recognized by PRRs in host cells and consequently induce inflammatory immune responses. The skeleton or the inner portion of the cell wall is mainly composed of chitin and β-(1,3)-glucan. Whereas β-(1,3)-glucan is recognized by Dectin-1, the receptor for chitin remains to be identified. The outer portion of the cell wall is comprised of mannoproteins and mannan, which are recognized by TLR4 and CLRs such as dectin-2, mincle and the mannose receptor. Engagement of appropriate PRRs by these cell wall molecular moieties results in the production of cytokines that shape anti-fungal immune responses.
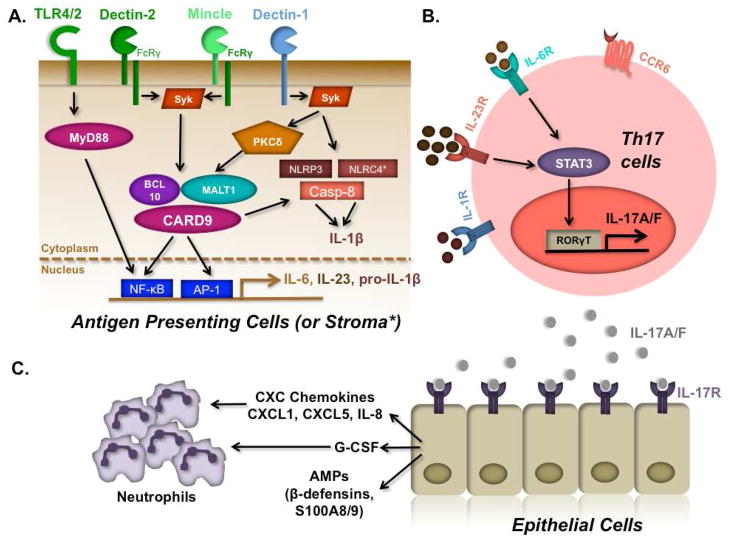
Figure 2.
PRR and Th17-based immunity to Candida albicans. A. PRRs including CLRs (dectin-1, dectin-2, mincle), TLRs (TLR2, 4) and inflammasomes (NLRP3, NLRC4, Caspase-8) respond to Candida PAMPs by inducing the NF-κB and MAPK pathways. B. PRRs in turn trigger expression and secretion of IL-6, IL-23 and IL-1β, which induce Th17 differentiation via the transcription factors STAT3 and RORγt. C. IL-17A and IL-17F produced by Th17 cells act on epithelial and mesenchymal cells to induce expression of neutrophil attracting chemokines (IL-8, CXCL1, CXCL5) and G-CSF, as well as AMPs such as defensins and S100 proteins.
Table 1.
Human genetic deficiencies associated with candidiasis and IL-17.
| Gene Product | Disease | Impact on Th17/IL−17 | References |
|---|---|---|---|
| Dectin-1 | CMC | Reduced frequency of Th17 cells | Ferwerda et al., 2009 |
| CARD9 | CMC Disseminated candidiasis |
Impaired dectin-1 [and dectin-2/mincle?] signaling Reduced frequency of Th17 cells |
Glocker et al., 2009 |
| STAT3 | Autosomal dominant Hyper IgE (Job’s) Syndrome (CMC) | Impaired IL-6/21/22/23 signaling Reduced frequency of Th17 cells |
Ma et al., 2008 Milner et al., 2008 |
| TYK2 | Autosomal recessive Hyper IgE (Job’s) Syndrome (CMC) | Defective IL-23 signaling Reduced Th17 frequency |
Minegishi et al., 2006 |
| IL-17RA | CMC | Complete IL-17RA deficiency Abolished responses to IL-17A/F |
Puel et al., 2011 |
| IL-17F | CMC | Impaired signaling through IL-17A/F | Puel et al., 2011 |
| STAT1 | CMC | Increased responses to IFNα/β, IFNγ and IL-27 Reduced Th17 frequency |
Liu et al., 2011, van der Veerdonk et al., 2011b |
| AIRE | Autoimmune Polyendocrinopathy Syndrome-1 (CMC) | Neutralizing auto-antibodies against IL-17A, IL-17F and IL-22 |
Kisand et al., 2010 Puel et al., 2010 |
Toll-like Receptors
Of the Toll-like receptors, TLR2 and TLR4 are the major participants in C. albicans recognition. TLR2 binds to phospholipomannans and β-glucan (the major component of yeast zymosan), and acts in combination with dectin-1 to induce pro-inflammatory responses in a variety of Candida infection settings (Hise et al., 2009; Netea, 2006; Villamon et al., 2004; Yuan and Wilhelmus, 2010) (Figure 1, 2A). TLR2 has also been shown to suppress inflammatory responses to Candida via production of IL-10 and enhanced Treg survival. Accordingly, TLR2−/− mice are more resistant to disseminated candidiasis than WT, supporting a detrimental rather than protective role for this receptor (Netea et al., 2004). On the other hand, TLR4 recognizes C. albicans O-linked mannan and stimulates production of the inflammatory cytokine TNFα in human mononuclear cells and murine macrophages. TLR4−/− mice exhibited increased susceptibility to disseminated candidiasis (Netea et al., 2002). Although TLR9, TLR1 and TLR6 are also implicated in recognizing Candida pathogen associated molecular patterns (PAMPs), none are required for immunity to this organism (Netea et al., 2008b; van de Veerdonk et al., 2008). Mice deficient in MyD88, an adaptor central to TLR signaling, are sensitive to Candida infection, which supports a role for either TLRs or alternatively IL-1 family cytokines (Bellocchio et al., 2004). However, humans with MyD88 defects do not appear to be particularly prone to fungal infections (von Bernuth et al., 2008). Thus, other pathways of pattern recognition are important in C. albicans immune sensing and the subsequent priming of T helper cell responses. Alternatively, this paradox may indicate a difference in how mice and humans sense this organism.
C-type lectin receptors
The CLRs have garnered considerable attention in the context of Candida, and appear to be comparatively more critical than TLRs for immunity in both mouse models and humans (Saijo and Iwakura, 2011; Vautier et al., 2010). The best-characterized CLRs with respect to Candida are dectin-1, dectin-2 and mincle (Figure 1, 2A). Although details of their respective signaling pathways are still being elucidated, they all appear to mediate signaling through the kinase Syk1, the adaptors CARD9/Bcl-10/MALT1 and the NF-κB and Ras/Raf-1 pathways (Gow et al., 2011; Willment and Brown, 2008). Dectin-2 and Mincle form heteromeric complexes with the FcγR, whereas Dectin-1 does not engage this subunit. Recently, the kinase PKCδ was shown to be activated by dectin-1 and induce phosphorylation of CARD9, Both CARD9−/− and PKCδ−/− mice are susceptible to disseminated candidiasis (LeibundGut-Landmann et al., 2007; Strasser et al., 2012). The CLR DC-SIGN in human dendritic cells (DCs) is involved in mediating Candida uptake via recognition of N-linked mannan (Cambi et al., 2008; Takahara et al., 2012). Although the role of DC-SIGN in the generation of Th17 responses during C. albicans infection is unclear, it appears to inhibit dectin-1-dependent Th17 generation and instead favor Th1 responses in a model of tuberculosis (Zenaro et al., 2009). Similarly, the lectin Galectin 3 also recognizes Candida, but its specific contributions are still not well defined (reviewed in (Brown, 2010; Gow et al., 2011; Netea et al., 2008a). Although the specific contribution of this receptor to antifungal immunity is not well defined, a recent study showed that Galectin 3 associates with dectin-1 and contributes to TNFα production in macrophages (Esteban et al., 2011). Exactly how signals from these disparate CLRs are integrated is still not fully understood.
Dectin-1 recognizes the β-(1,3)-glucan, which is usually buried underneath a layer of cell wall proteins and mannan moieties, posing an issue of accessibility for innate immune cells. Nevertheless, β-glucan is exposed in bud scars that are revealed during the process of hyphal transition, which facilitates its recognition and may be the essential signal that alerts the host of a transition from fungal colonization to infection (Netea et al., 2008a). Consistently, dectin-1-deficient mice exhibit impaired production of inflammatory cytokines such as IL-6 and granulocyte-colony stimulating factor (G-CSF) (Taylor et al., 2007), which drive Th17 differentiation and are induced upon IL-17 signaling (Gaffen, 2009) (Figure 2B). Furthermore, β-glucan stimulation of bone marrow dendritic cells (BMDCs) promotes skewing of Th17 cell differentiation, thus linking CLR signaling and activation of a Th17 response (LeibundGut-Landmann et al., 2007).
However, the role of dectin-1 in fungal host defense remains a topic of debate. Whereas one study showed that dectin-1−/− mice showed increased susceptibility to disseminated candidiasis (Taylor et al., 2007) another report found that dectin-1−/− mice were resistant (Saijo et al., 2007). Intriguingly, in the study by Saijo et al., dectin-1−/− mice were susceptible to Pneumocystis carinii, suggesting that the role of dectin-1 in antifungal immunity may be pathogen-specific. In addition to promoting Th17 responses, dectin-1 signaling appears to play a role in balancing the frequencies of Th1 and Th17 cells. Specifically, the absence of dectin-1 during lung infection with Aspergillus fumigatus causes reduced production of IFNγ and T-bet, a transcription factor that controls Th1 differentiation, resulting in decreased Th1 responses and correspondingly enhanced Th17 differentiation (Rivera, 2011). Therefore, β-glucan recognition by dectin-1 shapes the overall nature of antifungal CD4+ T helper responses. As described in detail below, dectin-1-deficient humans with chronic mucocutaneous candidiasis (CMC) have been identified, supporting the idea that dectin-1 is a bona fide recognition element for Candida (Table 1). However, disease in these patients was generally mild, and the mutation was later found to be a fairly common polymorphism linked to Candida colonization in transplant recipients (Plantinga et al., 2009). Thus, PRRs other than dectin-1 probably play central roles in immunity to Candida.
Dectin-2 recognizes N-linked mannan sugars, which are localized in the exterior layer of the yeast cell wall (Robinson et al., 2009), and appears to be especially important in recognition of hyphae (Bi et al., 2010). Similar to dectin-1, stimulation of BMDCs via dectin-2 triggers Th17 differentiation. C. albicans-specific Th17 cells are diminished more dramatically in the absence of dectin-2 than in the absence of dectin-1 (Robinson et al., 2009). However, not all studies of dectin-2 in antifungal host defense are consistent. One report showed that antibody blockade of dectin-2 in dectin-1−/− mice led to reduced IL-17 production during systemic Candida infections. Surprisingly, however, diminished IL-17 production did not correlate with disseminated disease as assessed by kidney fungal burden (Robinson et al., 2009). In contrast, dectin 2−/− mice were found to be more susceptible to disseminated candidiasis than WT mice (Saijo et al., 2010). The basis for these discrepancies was attributed to different observation periods and disease kinetics, but may also indicate that additional elements are involved in host immunity to Candida. While dectin-1 and dectin-2 recognize different components of the Candida cell wall and thus may preferentially “see” yeast versus hyphal forms of the microbe, in vivo studies have not yet fully clarified which CLR is more important.. Ultimately, it is likely that signaling through a multiplicity of PRRs is needed to develop an optimal immune response.
In addition to dectins, mincle and the mannose receptor (MR) recognize mannan carbohydrates from Candida (Figure 1). In human peripheral blood mononuclear cells (PBMCs), mannan was found to induce more IL-17 than other fungal PAMPs such as β-glucan and chitin (van de Veerdonk et al., 2009), perhaps indicating a dominant role for MR in this process. Interestingly, this study also demonstrated MR-dependent production of IL-17 in cells from dectin-1-deficient patients, supporting the idea that stimulation of MR by C. albicans is potentially a primary pathway for the generation of antigen-specific Th17 responses. Mincle binds to the mycobacterial component Trehalose-6,6-dimycolate (TDM) and its synthetic analogue Trehalose-6, 6-dibehenate (TDB), and induces Th1 and Th17 responses in a Syk- and CARD9-dependent manner (Ishikawa et al., 2009; Schoenen et al., 2010; Werninghaus et al., 2009). This suggests that fungal cell wall components such as α-mannans may induce Th17 responses via mincle. Interestingly, whereas mincle-deficient mice are susceptible to disseminated candidiasis (Wells et al., 2008), MR-deficient mice are resistant (Lee, 2003), arguing that the MR is redundant with CLRs in terms of mediating anti-Candida immunity.
The inflammasome
The intracellular inflammasome has emerged as another PRR in antifungal host defense. Composed of cytosolic Nod-like receptors (NLRs), notably NLRP1, NLRP3 and NLRC4, complexed with several adaptors and proteases, the inflammasome activates caspase-1 and thereby permits cleavage and secretion of the proinflammatory cytokines IL-1β and IL-18. While neither the cytosolic receptors NOD1 nor NOD2 are required for Candida recognition (van der Graaf et al., 2006), inflammasomes are involved. Mice deficient in the IL-1 receptor (IL-1R) or apoptosis associated speck-like protein (ASC), an essential subunit of the inflammasome, exhibit increased oral fungal burdens in a model of oral mucosal candidiasis (Hise et al., 2009). Fungal dissemination in this model was even more pronounced than mucosal infection. Consistent with this concept, NLRP3−/− mice are susceptible to disseminated candidiasis as well as mucosal disease (Gross et al., 2009). Elegant bone marrow chimera experiments confirmed the role of the NLRP3 inflammasome in preventing Candida dissemination, and further demonstrated that the NLRC4 inflammasome compartment was also involved in oral C. albicans host infections (Tomalka et al., 2011). Notably, NLRP3 is required in hematopoietic cells, whereas NLRC4 functions at the level of the mucosal stroma. Moreover, both NLRP3 and NLRC4 deficiencies resulted in decreased expression of IL-17, IL-17F and also one of the IL-17 receptor subunits (IL-17RA), directly linking the inflammasomes and IL-17 (Tomalka et al., 2011). Furthermore, BMDCs from ASC−/− and caspase-1−/− mice exhibited impaired production of IL-1β and IL-18 following Candida exposure, which are important for Th17 and Th1 development, respectively. Indeed, splenocytes from IL-1β- and IL-18-deficient mice exhibited impaired production of IL-17 and IFNγ in response to stimulation from Candida (van de Veerdonk et al., 2011a). The development of protective Th17 responses via inflammasome activation resulted from the recognition of C. albicans hyphae by human macrophages. The yeast form did not activate the inflammasome, demonstrating that this pathway is likely important for discriminating between colonizing yeast and invasive hyphae (Cheng et al., 2011).
In addition to the role of the NLRP3 and NLRC4 inflammasomes in processing pro-IL-1β, a recent study identified dectin-1-dependent activation of a non-canonical caspase-8 inflammasome (Gringhuis et al., 2012). Dectin-1 engagement resulted in the activation of Syk and the transcription of the IL-1β gene through the CARD9-Bcl10-MALT1 complex (Figure 2B). Recruitment of MALT1-caspase-8 and ASC to this scaffold resulted in processing of pro-IL-1β to mature IL-1β. The activation of this non-canonical pathway did not require C. albicans internalization, whereas activation of NLRP3 inflammasome was completely dependent on internalization. Interestingly, some C. albicans strains trigger IL-1β production primarily via caspase-8 while others also activate the NLRP3 inflammasome, suggesting that the ligand for NLRP3 is not present in all fungi. It would be of interest to determine the role of the caspase-8 inflammasome in the development of Th17 responses, but that was not addressed in this study.
Taken together, the priming of host-protective Th17 responses is likely a combined effort of multiple PRR pathways that recognize different components of C. albicans, and trigger the production of cytokines that predominantly direct Th17 differentiation. Nuances of how different fungal PAMPS trigger different PRRs is an area that requires more development, and subtle alterations of the host response may ensue depending on the specific substrain of Candida that is involved and the anatomic site of infection.
Antifungal cell-mediated immunity: Life before Th17
A dramatic illustration of the importance of cell mediated immunity to C. albicans came from the AIDS pandemic. HIV+ individuals are exquisitely susceptible to many opportunistic fungal pathogens, including oral thrush caused mainly by C. albicans (Klatt and Brenchley, 2010). Development of opportunistic infections in AIDS tracks with loss of CD4+ T lymphocytes, implicating that cytokines produced by CD4+ T cells are important in immunity to Candida (Glocker and Grimbacher, 2010). Studies in 1995 to probe CD4 immunity in mucosal candidiasis employed a mouse model of gastric Candida infection. In this setting, both IFNγ- and IL-5-producing CD4+ T cells, indicative of Th1 and Th2, respectively, were found in Peyer’s Patches and mesenteric lymph nodes, and corresponded with fungal clearance. Furthermore, neutralization of IL-4 resulted in increased IFNγ production and enhanced yeast clearance (Cenci et al., 1995). These data were interpreted to mean that protective antifungal immunity is attributable to Th1 cells, even though hallmark cytokines of both Th1 and Th2 lineages were produced during infection (Cenci et al., 1998; Cenci et al., 1995). Since these studies occurred prior to the recognition of the Th17 population, the alternative interpretation that non-Th1/Th2 lineages might be involved was not considered.
Studies in a mouse model of oropharyngeal candidiasis (OPC, “thrush”) showed that nude (T cell deficient) mice were susceptible to infection, but could be protected by transfer of CD4+ T cells (Farah et al., 2002). Th1-related cytokines, including IFNγ and TNFα, but not Th2 cytokines were expressed in the oral tissue, consistent with a Th1-biased response. Interestingly, however, depletion of CD4 or CD8 T cells in a susceptible mouse strain did not exacerbate oral colonization with Candida (Ashman et al., 2003), although adoptive transfer of Candida-specific T cells did induce protection from a disseminated challenge (Sieck et al., 1993). Thus, both innate cells and CD4 cells were implicated in immunity to OPC, although the specific subset and cytokines were still not well clarified.
Antifungal cell-mediated immunity: Life after Th17
The Th1/Th2 paradigm was first described in 1986, and immune responses, whether infectious or autoimmune, were pigeonholed into these categories for nearly two decades. Over time it became clear that this model was fraught with discrepancies. A central paradox was that the signature Th1 effector cytokine, IFNγ, was significantly less important in various disease settings than was IL-12, the key Th1 inductive cytokine (Steinman, 2007). A significant overhaul in the prevailing paradigm of CD4-mediated immunity occurred with the discovery of Th17 cells, which reconciled many of these discrepancies. Of particular importance were the findings that (i) IL-23 promotes IL-17 expression in T cells (Aggarwal et al., 2002), and (ii) the p40 subunit of IL-12 is shared with IL-23 and the IL-12Rβ1 is a subunit of the IL-23R (Ghilardi and Ouyang, 2007). Thus, mice lacking either IL-12p40 or IL-12Rβ1 are deficient not only in IL-12 (hence, lacking Th1 cells), but also IL-23 (hence, lacking Th17 cells). Subsequent studies showed that Th17 cells arise from inductive signals from TGFβ, IL-6 and IL-1β, while IL-23 is an essential maintenance and pathogenic factor for Th17 cells (McGeachy and Cua, 2007; Stockinger and Veldhoen, 2007).
The application of this concept to Candida albicans immunity has developed over the last several years. A role for the IL-17 axis in antifungal host defense was first shown in 2004, in which IL-17 receptor (IL-17R)-deficient mice inoculated i.v. with C. albicans exhibited decreased survival and increased kidney fungal burden compared to WT counterparts (Huang et al., 2004). Although disputed in one report (De Luca et al., 2010a), most data are consistent with a protective role for IL-17 in systemic candidemia (Huang et al., 2004; van de Veerdonk et al., 2010). Furthermore, an experimental vaccine containing the C. albicans adhesin protein Als3p and aluminium hydroxide as an adjuvant conferred protection against systemic candidiasis via induction of both Th17 and Th1 responses (Lin, 2009).
In a model of OPC, mice lacking Th1 effector cytokines IFNγ or TNFα were resistant to oral infection, yet mice lacking IL-12p40 were found to be susceptible (Farah et al., 2006). Since IL-12p40 is shared by IL-12 (p40/p35) and IL-23 (p40/p19) (Oppmann et al., 2000), this finding hinted at a role for protective IL-23-dependent rather than IL-12-dependent pathways. In a direct test of this hypothesis, IL-23p19−/− mice subjected to OPC exhibited overt thrush lesions and elevated fungal burdens whereas IL-12p35−/− mice did not. High susceptibility was also observed in IL-17RA−/− and IL-17RC−/− mice, implicating the IL-17 pathway directly (Conti et al., 2009; Ho et al., 2010) (Figure 2C). Furthermore, in extended time courses, IL-17R−/− mice never recovered from Candida infection, whereas IL-12p35−/− mice fully cleared the microbe, albeit delayed compared to WT mice.
Parallel findings were made in mouse models of dermal candidiasis, in which IL-17 and IL-23 but not IL-12 were essential for immunity to Candida (Kagami et al., 2010). A detailed study of skin-resident DC subsets showed that Th17 cells against Candida are generated specifically by presentation from Langerhans cells. Interestingly, generation of Th17 cells is blocked by signals from Langerin+ dermal DCs in favor of Th1 cells (Igyarto et al., 2011). In a vaginal candidiasis model, treating with halofuginone, which specifically blocks Th17 differentiation, resulted in a profound decrease in IL-17 production that correlated with an increase in fungal burden (Pietrella et al., 2011). Thus, similar to disseminated candidemia, mouse models of skin and mucosal candidiasis implicate IL-17 and IL-23 in immunity to Candida.
In addition to Th17 cells, there is an unexpected interplay between Th17 cells and regulatory T cells (Tregs), a subset of immunoregulatory T cells, suggesting both are required for effective host responses to Candida. Th17 and Treg cells both arise from signals from TGFβ (although this is still somewhat controversial, (Ghoreschi et al., 2010), with IL-6 and IL-1β providing the inflammatory switch that favors Th17 cells, and IL-2 driving Tregs and preventing Th17 differentiation (Laurence et al., 2007; Yang et al., 2011). Rag−/− mice, which lack both T and B cells, are extremely susceptible to OPC. However, transfer of naïve or Th17-polarized T effector cells alone is insufficient to mediate host defense to OPC; rather, co-transfer of regulatory T cells resulted in enhanced Th17 responses that were needed to prevent development of OPC. Mechanistically, this was shown to be due to a sequestration of IL-2 by Tregs via the high affinity IL-2 receptor complex. Consistently, depletion of Tregs in mice increases susceptibility to OPC (Pandiyan et al., 2011). This finding is in agreement with an earlier report that demonstrated a positive correlation between the levels of IL-17 produced by curdlan-stimulated BMDCs and the ratio of Treg to T effector cells (LeibundGut-Landmann et al., 2007). Interestingly, stimulation of BMDC with curdlan triggered conversion of Foxp3+IL-17- cells into IL-17-expressing Foxp3+ cells (Osorio et al., 2008), suggesting that dectin-1 enhances a Th17 response by converting Tregs. Thus Tregs play a previously unappreciated protective role in inflammatory responses during C. albicans infection.
Intriguingly, C. albicans appears to actively target the IL-17 pathway, presumably as an evasive strategy. Whereas heat-killed Candida stimulated PBMCs secrete IL-17, co-culture with live Candida strongly suppresses IL-17 production. This factor is apparently soluble (and thus far, unidentified), because live Candida exerted this suppressive effect even when separated from PMBC’s in a trans-well system. The mechanism appears to be via regulation of tryptophan metabolism. Specifically, suppression of IL-17 observed in co-culture was associated with reduced L-kynurenine (an indoleamine 2,3-dioxygenase [IDO]-dependent metabolite, representing one pathway of tryptophan metabolism) and increased 5-hydroxytryptophan (representing the alternate pathway). Consistently, ectopic application of 5-hydroxytryptophan inhibited IL-17 production (Cheng et al., 2010). Another recent report suggests that IL-17 might directly bind to Candida and induce nutrient starvation conditions in the organism (Zelante et al., 2012). Thus, there is still much to learn about the intricate interactions occurring between Candida and its host.
In addition to IL-17A, other Th17-derived cytokines such as IL-17F and IL-22 may also participate in anti-Candida immunity. In contrast to IL-17A−/− mice, IL-17F knockouts are resistant to systemic candidiasis (Saijo et al., 2010), although the role of IL-17F in experimental mucosal candidiasis has not yet been evaluated. As noted in Table 1, dominant negative mutations in IL-17F in humans are linked to CMC, although the mutations affect IL-17A as well as IL-17F signaling (Puel, 2011). Surprisingly, IL-22−/− mice inoculated orally with C. albicans had a significantly lower fungal burden than IL-17RA−/− or IL-23−/− mice, despite the observation that IL-22 mRNA is strongly induced in WT mice following oral Candida infection (Conti et al., 2009). Similarly, in dermal candidiasis there was no major role for IL-22 (Kagami et al., 2010). However, De Luca et al. reported that IL-22−/− mice are susceptible to both systemic and gastric candidiasis, due in part to impaired production of antimicrobial peptides (AMPs) such as S100A8, S100A9, RegIIIβ and RegIIIγ. In addition, blockade of IFNγ in IL-22−/− mice resulted in fungal dissemination, suggesting that IL-22 together with IFNγ may provide a first line of defense in preventing dissemination from the gastric mucosa (De Luca et al., 2010b). In a recent study where human keratinocytes were infected in vitro with C. albicans, IL-22 in combination with TNFα led to decreased cell death and epidermal damage caused by infection (Eyerich et al., 2011). However, IL-22 is impaired in several human CMC syndromes (Table 1), and thus its role in human candidiasis cannot be ruled out.
Most of the studies in experimental candidiasis have described a protective role for IL-17 in host responses to C. albicans. However, a detrimental role of this cytokine has also been reported, specifically in a model of gastric candidiasis. IL-23p19−/− and IL-12/IL-23p40−/− mice inoculated intragastrically with C. albicans showed 100% survival over a 2 week period, whereas only 25% of IL-12p35−/− mice survived. Furthermore, expression of IL-12p70 and IFNγ in stomach correlated with protection in IL-23−/− mice, whereas levels of IL-23p19 and IL-17 were linked to severity of disease (Zelante et al., 2007). Candida does colonize the gut, but the clinical relevance of the gastric candidiasis disease model is unclear. Although some cases of gastric candidiasis have been reported, they are extremely rare due to the low pH and inhospitable conditions of the stomach (Filler, 2012). Regardless, these studies indicate that lessons of immunity in the gut mucosa cannot necessarily be applied to other sites.
Experiments of nature: IL-17 and candidiasis in humans
The identification of Th17 cells and CLRs as elements of anti-fungal immunity facilitated characterization of human genetic deficiencies underlying development of CMC, either in isolation (CMC disease) or in the context of an immune disorder (Table 1). The most direct link of IL-17 to the etiology of CMC disease comes from a recent report describing rare human pedigrees with mutations in the IL-17 signaling axis. One individual had an autosomal recessive mutation in IL-17RA, and cells from this patient did not express the receptor and hence were refractory to IL-17 signaling. Another cohort exhibited an autosomal dominant mutation in IL-17F that prevents signaling through IL-17A, IL-17F and the IL-17A/F heterodimer (Puel, 2011). In separate studies, gain of function mutations in signal transducer and activator of transcription (STAT) 1 were identified as causes of CMC disease, and were associated with reduced Th17 frequency (Liu et al., 2011; van de Veerdonk et al., 2011b). It is not fully clear why this STAT1 mutation causes CMC and reduced Th17 cell numbers, but STAT1-activating cytokines such as IFNα/β, IFNγ and IL-27 are all inhibitors of Th17 differentiation, and therefore Th17 cells appear to be abnormally restrained in these patients.
In scenarios where CMC is present in conjunction with other infections and/or inflammatory defects, identifying a single cause for recurrent candidiasis poses a challenge. Strikingly, in many cases defects in some component of Th17 differentiation or the pattern recognition pathways that promote it have been linked to disease. For example, Hyper IgE (Job’s) Syndrome (HIES) patients suffer from recurrent Staphylococcus aureus and C. albicans infections, and exhibit a Th17 deficiency due to dominant negative mutations in STAT3 (Ma et al., 2008; Milner et al., 2008; Minegishi et al., 2007). STAT3 is critical for Th17 differentiation in mice (Chen et al., 2006; Yang et al., 2007), as it lies is downstream of signaling by IL-23, IL-21 and IL-6 (all involved in induction and maintenance of Th17 cells) as well as IL-22 (produced by Th17 cells). Although a failure to differentiate Th17 cells is the most obvious cause of their susceptibility to Candida, these patients also have impaired antifungal activity of saliva (Conti et al., 2011), and their keratinocytes show defective responses to Th17 cytokines (Minegishi et al., 2009).
Mutations in the PRR pathway have also been identified that cause CMC, reinforcing results from knockout mouse studies. Some CMC patients show deficiencies in CARD9 or dectin-1. Notably, the dectin-1 mutation appears to be a polymorphism found in a population-wide search in individuals from both Europe and Africa and is associated with increased Candida colonization in immunosuppressed hematopoietic stem cell transplant recipients (Ferwerda et al., 2009; Glocker et al., 2009; Plantinga et al., 2009).
Another cohort of patients with CMC have autoimmune polyendocrinopathy syndrome (APS)-1, characterized by genetic defects in central tolerance due to mutations the transcription factor AIRE (Browne and Holland, 2010). The cause of CMC in APS-1 was enigmatic, until it was discovered in 2010 that these patients produce neutralizing autoantibodies against Th17 cytokines, namely IL-17A, IL-17F and IL-22 (Kisand et al., 2010; Puel et al.). These observations, together with the fact that the majority of C. albicans-specific T cells in human peripheral blood exhibit a classical Th17 phenotype (Acosta-Rodriguez et al., 2007), strongly support a crucial role for Th17 cells in immunity to C. albicans.
Mechanisms of IL-17-mediated immunity
Pioneering studies of IL-17 in a Klebsiella pneumoniae infection model demonstrated that IL-17 is a major inducer of granulopoiesis and neutrophil chemotaxis (Ye et al., 2001). A plethora of studies in other infection systems, many conducted long before the recognition of Th17 cells, showed that IL-17 mobilizes neutrophils. IL-17 activates neutrophils indirectly, primarily by upregulating expression of G-CSF and CXC chemokines in mucosal epithelial cells as well as the local stroma (Khader et al., 2009) (Figure 2C). Although IL-17RA is highly expressed in neutrophils, there is no convincing data showing that IL-17 acts directly on these cells, since neutrophils lack the co-receptor IL-17RC (Pelletier et al., 2010). With respect to candidiasis, IL-17 appears to mediate many of its protective effects via mobilization of neutrophils to peripheral inflammatory sites, although there is reason to postulate that other antimicrobial mechanisms are also important. In systemic candidemia, IL-17R-deficient mice exhibited decreased absolute neutrophil counts (ANC) in peripheral blood, which correlated with impaired neutrophil recruitment and myeloperoxidase (MPO) activity in kidney (Huang et al., 2004). In OPC, both IL-23p19 −/− and IL-17R−/− mice exhibit decreased neutrophil numbers the oral mucosa compared to resistant mouse strains. Moreover, microarray analysis of tongue tissue showed induction of prototypical IL-17 target genes that serve to expand or recruit neutrophils, such as CXCL1 (KC, Groα), CXCL2 (MIP2), CXCL5 (LIX) and CSF3 (G-CSF) (Conti et al., 2009). In addition, C. albicans can stimulate oral epithelial cells (OECs) to secrete CXCL8 (IL-8), another potent neutrophil chemoattractant (Dongari-Bagtzoglou and Kashleva, 2003; Dongari-Bagtzoglou et al., 2005). Similarly, in a murine model of vulvovaginal candidiasis (VVC) 75% of the total vaginal infiltrate at 48 hours post-infection were found to be neutrophils, coinciding with elevated IL-17 and IL-23 in vaginal fluid (Pietrella et al., 2011). In addition, it has been demonstrated that β-glucans can induce human neutrophil chemotaxis and that neutrophil extracellular traps can capture and kill both C. albicans yeast and hyphae (Urban et al., 2006). Therefore, IL-17-dependent recruitment of neutrophils to the site of infection is likely an important element in host responses to C. albicans.
Despite its potent effects on neutrophils, is not clear whether mobilization of neutrophils is the primary underlying mechanism by which IL-17 mediates antifungal effects. Although patients undergoing chemotherapy associated with neutropenia are highly susceptible to various forms of disseminated candidiasis, individuals with isolated neutropenia or neutrophil defects such as chronic granulomatous disease (CGD) are not particularly prone to Candida infections (Del Favero, 2000; Grigull et al., 2006; Huppler et al., 2012; Maertens et al., 2001). In this regard, IL-17 induces antimicrobial peptide (AMP) expression in a variety of settings, and many of the IL-17-induced AMPs have direct antifungal activity towards Candida (Gorr, 2009). In skin, IL-17 signals cooperatively with IL-22 to induce AMPs such as S100A7 and β-defensin 2 (BD2) in keratinocytes and epithelial cells (Liang et al., 2006). In humans with HIES, this effect is significantly impaired (Minegishi et al., 2009), perhaps explaining the susceptibility to dermal candidiasis in these patients. In VVC, production of BD2 by vaginal epithelial cells is inhibited following ablation of Th17 responses (Pietrella et al., 2011). Moreover, in murine OPC, AMPs such as S100A8, S100A9 and β-defensin 3 (murine homologue of BD2) are induced in tongue in WT mice but impaired in IL-17R−/− animals (Conti et al., 2009). Saliva is another important defense mechanism to limit oral candidiasis; notably, saliva from HIES patients has increased C. albicans colonization and significantly decreased levels of BD2 and histatins (salivary AMPs specific to primates) compared to healthy donors. Furthermore, this study also showed IL-17 could directly induced histatin expression in human salivary gland acinar cells, identifying a previously unrecognized target organ for this cytokine (Conti et al., 2011). Thus, IL-17 mediates antifungal host defense by orchestrating the recruitment of neutrophils to the site of infection and also by upregulating expression of AMPs at mucosal surfaces (Figure 2C).
Although there is data implicating IL-17 and/or Th17 cells in immunity to Candida in various settings, its relative importance in different anatomic sites is not fully elucidated. As noted, studies in both humans and mice overwhelmingly support a role for IL-17 in oral candidiasis, based on susceptibility in HIV+ patients, IL-17R-deficient and IL-17F-deficient humans and IL-17R−/− and IL-23−/− knockout mice (Filler, 2012; Gaffen et al., 2011). Dermal candidiasis also involves IL-17, based on mouse studies (Hirota et al., 2011; Igyarto et al., 2011; Kagami et al., 2010) as well as human CMC syndromes (Table 1). In disseminated candidemia, IL-17A and IL-17RA−/− mice are susceptible in experimental model systems (Huang et al., 2004), but the data for a non-redundant role for IL-17 in humans is less compelling. For example, HIV+ individuals and other Th17-deficient cohorts (e.g., HIES or APS1 patients) do not routinely experience systemic candidiasis. Instead, systemic disease is associated with only with combined lymphopenia and neutropenia, and CARD9 is the only molecule to date genetically linked to systemic candidiasis in humans (Glocker and Grimbacher, 2010; Glocker et al., 2009). Finally, the role of IL-17 in vaginal candidiasis is controversal. Although HIV/AIDS patients do not experience high rates of VVC, HIES patients and Dectin-1-deficient humans do (Table 1, reviewed in (Freeman and Holland, 2008) (Yano et al., 2012)). Some mouse studies implicate IL-17 in VVC (Pietrella et al., 2011), but others have failed to find a strong connection (Yano et al., 2012). Thus, IL-17 plays tissue-specific roles in immunity to C. albicans.
Concluding remarks and future perspectives
The discovery of Th17 cells as a subset distinct from Th1 and Th2 cells has been of paramount importance in understanding antifungal host defense mechanisms. It is now clear that IL-17-mediated recruitment of neutrophils and induction of AMPs at peripheral inflammatory sites are essential for host defense against Candida albicans, and probably other extracellular fungal organisms as well. However, the cellular source(s) of IL-17 production and the niche(s) it occupies during C. albicans infections in different anatomical sites remain an open and exciting research topic. Based on the protective roles of IL-23 and IL-17 in most experimental and human C. albicans infections, it has been generally assumed that “classical” CD4+Th17 cells are responsible for IL-17 production and subsequent fungal clearance. This view is supported by the high sensitivity of CD4-depleted HIV/AIDS patients and Th17-deficient HIES patients to oral thrush. However, in recent years a number of innate sources of IL-17 have been reported, including NKT, γδ T, macrophage and innate lymphoid cells (ILC) (Cua and Tato, 2010). In many settings, these rather than Th17 cells have been shown to play crucial roles in mucosal immunity. For example, in dermal candidiasis the γδ T cell subsets are critical, at least in acute infection models (Hirota et al., 2011; Kagami et al., 2010). Nonetheless, it is clear that immunocompetent humans mount strong CD4+ Th17 responses to Candida (Acosta-Rodriguez et al., 2007), and to date the relative contributions of innate versus adaptive IL-17 responses in C. albicans infections have not been well elucidated. This is likely to be an important area of inquiry in the future, as the integration of innate and adaptive sources of IL-17 becomes better understood.
Biologic drugs that target inflammatory cytokines have revolutionized treatment of autoimmune diseases such as rheumatoid arthritis and psoriasis. However, an inevitable risk with these therapies is infection. Drugs targeting TNFα do not predispose to Candida infections (Strangfeld and Listing, 2006), consistent with observations that TNFα−/− mice are resistant to candidiasis (Farah et al., 2006). Antibodies targeting IL-17A and IL-17RA are now in clinical trials to treat various autoimmune conditions (Genovese et al., 2010; Hueber et al., 2010), and as noted above, APS-1 patients with naturally-occurring neutralizing antibodies against IL-17 routinely develop CMC (Table 1) (Kisand et al., 2010; Puel et al., 2010). Although available data have not yet identified CMC as a major adverse event associated with clinical application of anti-IL-17 therapies, this is nonetheless a potential clinical issue that should be monitored.
Acknowledgments
SLG was supported by NIH grants AR054389 and DE022550. NHS was supported by AR054389-S1 and T32 grant CA082084. SLG has received a research grant from Amgen and travel reimbursements and honoraria from Amgen and Novartis. We thank HR Conti and AR Huppler for critical reading.
Abbreviations
- APS-1
autoimmune polyendocrine syndrome-1
- AMP
antimicrobial peptide
- AR
autosomal recessive
- ASC
apoptosis associated speck-like protein
- BMDC
bone marrow dendritic cells
- OPC
oropharygeal candidiasis
- CMC
chronic mucocutaneous candidiaisis
- HIES
Hyper-IgE Syndrome
- LOF
loss of function
- NLR
NOD-like receptor
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- VVC
vulvo-vaginal candidiasis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ghilardi N, Xie MH, De Sauvage FJ, Gurney AL. Interleukin 23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin 17. J Biol Chem. 2002;3:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- Ashman RB, Papadimitriou JM, Fulurija A, Drysdale KE, Farah CS, Naidoo O, Gotjamanos T. Role of complement C5 and T lymphocytes in pathogenesis of disseminated and mucosal candidiasis in susceptible DBA/2 mice. Microb Pathog. 2003;34:103–113. doi: 10.1016/s0882-4010(02)00211-5. [DOI] [PubMed] [Google Scholar]
- Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- Bi L, Gojestani S, Wu W, Hsu YM, Zhu J, Ariizumi K, Lin X. CARD9 mediates dectin-2-induced IkappaBalpha kinase ubiquitination leading to activation of NF-kappaB in response to stimulation by the hyphal form of Candida albicans. J Biol Chem. 2010;285:25969–25977. doi: 10.1074/jbc.M110.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD. How fungi have shaped our understanding of mammalian immunology. Cell Host Microbe. 2010;7:9–11. doi: 10.1016/j.chom.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Browne SK, Holland SM. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol. 2010;10:534–541. doi: 10.1097/ACI.0b013e3283402b41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, Kullberg BJ, Torensma R, Williams DL, Figdor CG. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008;283:20590–20599. doi: 10.1074/jbc.M709334200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E, Mencacci A, Del Sero G, d’Ostiani CF, Mosci P, Bacci A, Montagnoli C, Kopf M, Romani L. IFN-gamma is required for IL-12 responsiveness in mice with Candida albicans infection. J Immunol. 1998;161:3543–3550. [PubMed] [Google Scholar]
- Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle KH, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, van de Veerdonk F, Smeekens S, Joosten LA, van der Meer JW, Kullberg BJ, Netea MG. Candida albicans Dampens Host Defense by Downregulating IL-17 Production. J Immunol. 2010;185:2450–2457. doi: 10.4049/jimmunol.1000756. [DOI] [PubMed] [Google Scholar]
- Cheng SC, van de Veerdonk FL, Lenardon M, Stoffels M, Plantinga T, Smeekens S, Rizzetto L, Mukaremera L, Preechasuth K, Cavalieri D, et al. The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. Journal of leukocyte biology. 2011;90:357–366. doi: 10.1189/jlb.1210702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal immunology. 2011;4:448–455. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010;12:518–527. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010a;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal immunology. 2010b;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- Del Favero A. Management of fungal infections in neutropenic patients: more doubts than certainties? Int J Antimicrob Agents. 2000;16:135–137. doi: 10.1016/s0924-8579(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Kashleva H. Candida albicans triggers interleukin-8 secretion by oral epithelial cells. Microb Pathog. 2003;34:169–177. doi: 10.1016/s0882-4010(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Dongari-Bagtzoglou A, Villar CC, Kashleva H. Candida albicans-infected oral epithelial cells augment the anti-fungal activity of human neutrophils in vitro. Med Mycol. 2005;43:545–549. doi: 10.1080/13693780500064557. [DOI] [PubMed] [Google Scholar]
- Esteban A, Popp MW, Vyas VK, Strijbis K, Ploegh HL, Fink GR. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc Natl Acad Sci U S A. 2011;108:14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, Schaller M, Behrendt H, Ring J, Schmidt-Weber CB, et al. IL-22 and TNF-alpha represent a key cytokine combination for epidermal integrity during infection with Candida albicans. European journal of immunology. 2011;41:1894–1901. doi: 10.1002/eji.201041197. [DOI] [PubMed] [Google Scholar]
- Farah CS, Gotjamanos T, Seymour GJ, Ashman RB. Cytokines in the oral mucosa of mice infected with Candida albicans. Oral Microbiol Immunol. 2002;17:375–378. doi: 10.1034/j.1399-302x.2002.170607.x. [DOI] [PubMed] [Google Scholar]
- Farah CS, Hu Y, Riminton S, Ashman RB. Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene-targeting. Oral Microbiol Immunol. 2006;21:252–255. doi: 10.1111/j.1399-302X.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–1767. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler SG. Insights from human studies into the host defense against candidiasis. Cytokine. 2012;58:129–132. doi: 10.1016/j.cyto.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AF, Holland SM. The hyper-IgE syndromes. Immunology and allergy clinics of North America. 2008;28:277–291. viii. doi: 10.1016/j.iac.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen S, Hernandez-Santos N, Peterson A. IL-17 Signaling in host defense against Candida albicans. Immunologic Res. 2011;50:181–187. doi: 10.1007/s12026-011-8226-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese M, Van den Bosch F, Roberson S, Bojin S, Biagini I, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis. Arth Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Ouyang W. Targeting the development and effector functions of Th17 cells. Semin Immunol. 2007;19:383–393. doi: 10.1016/j.smim.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker E, Grimbacher B. Chronic mucocutaneous candidiasis and congenital susceptibility to Candida. Curr Opin Allergy Clin Immunol. 2010;10:542–550. doi: 10.1097/ACI.0b013e32833fd74f. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, Salzer U, Pfeifer D, Veelken H, Warnatz K, Tahami F, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–1735. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr SU. Antimicrobial peptides of the oral cavity. Periodontol 2000. 2009;51:152–180. doi: 10.1111/j.1600-0757.2009.00310.x. [DOI] [PubMed] [Google Scholar]
- Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigull L, Beilken A, Schmid H, Kirschner P, Sykora KW, Linderkamp C, Donnerstag F, Goudeva L, Heuft HG, Welte K. Secondary prophylaxis of invasive fungal infections with combination antifungal therapy and G-CSF-mobilized granulocyte transfusions in three children with hematological malignancies. Support Care Cancer. 2006;14:783–786. doi: 10.1007/s00520-005-0910-8. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell host & microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A, Shen F, Conti H, Patel N, Childs E, Peterson A, Hernandez-Santos N, Kolls J, Kane L, Ouyang W, et al. IL-17RC is required for immune signaling via an extended SEFIR domain in the cytoplasmic tail. J Immunol. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Hube B. Fungal adaptation to the host environment. Current opinion in microbiology. 2009;12:347–349. doi: 10.1016/j.mib.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- Huppler A, Bishu S, Gaffen SL. Mucocutaneous candidiasis: Implications for targeted immunotherapy. Arth Res Ther. 2012 doi: 10.1186/ar3893. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. Journal of immunology. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–411. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. The Journal of Experimental Medicine. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Normal Host Defense during Systemic Candidiasis in Mannose Receptor-Deficient Mice. Infection and Immunity. 2003:71. doi: 10.1128/IAI.71.1.437-445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. The Journal of Experimental Medicine. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. Th1-Th17 Cells Mediate Protective Adaptive Immunity against Staphylococcus aureus and Candida albicans Infection in Mice. PLoS Pathogens. 2009:5. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, Toubiana J, Itan Y, Audry M, Nitschke P, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. The Journal of Experimental Medicine. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens J, Vrebos M, Boogaerts M. Assessing risk factors for systemic fungal infections. Eur J Cancer Care (Engl) 2001;10:56–62. doi: 10.1046/j.1365-2354.2001.00241.x. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Seminars in immunology. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired TH17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, Yamada M, Kawamura N, Ariga T, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- Netea MG. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. The Journal of Clinical Investigation. 2006:116. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008a;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–3718. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- Netea MG, van de Veerdonk F, Verschueren I, van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol. 2008b;52:118–123. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002;185:1483–1489. doi: 10.1086/340511. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Osorio F, LeibundGut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, Reis e Sousa C. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38:3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d’Enfert C, Vecchiarelli A. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PloS one. 2011;6:e22770. doi: 10.1371/journal.pone.0022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, Donnelly JP, Brown GD, Kullberg BJ, Blijlevens NM, et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–732. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- Puel A. Chronic Mucocutaneous Candidiasis in Humans with Inborn Errors of Interleukin-17 Immunity. Science. 2011 doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. The Journal of Experimental Medicine. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A. Dectin-1 diversifies Aspergillus fumigatus– specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. The Journal of Experimental Medicine. 2011 doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–2051. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L. Immunity to fungal infections. Nature reviews Immunology. 2011;11:275–288. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol. 2011;23:467–472. doi: 10.1093/intimm/dxr046. [DOI] [PubMed] [Google Scholar]
- Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, et al. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck TG, Moors MA, Buckley HR, Blank KJ. Protection against murine disseminated candidiasis mediated by a Candida albicans-specific T-cell line. Infect Immun. 1993;61:3540–3543. doi: 10.1128/iai.61.8.3540-3543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Strangfeld A, Listing J. Infection and musculoskeletal conditions: Bacterial and opportunistic infections during anti-TNF therapy. Best practice & research. 2006;20:1181–1195. doi: 10.1016/j.berh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, et al. Syk Kinase-Coupled C-type Lectin Receptors Engage Protein Kinase C-delta to Elicit Card9 Adaptor-Mediated Innate Immunity. Immunity. 2012 doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara K, Tokieda S, Nagaoka K, Inaba K. Efficient capture of Candida albicans and zymosan by SIGNR1 augments TLR2-dependent TNF-alpha production. Int Immunol. 2012;24:89–96. doi: 10.1093/intimm/dxr103. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalka J, Ganesan S, Azodi E, Patel K, Majmudar P, Hall BA, Fitzgerald KA, Hise AG. A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog. 2011;7:e1002379. doi: 10.1371/journal.ppat.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cellular microbiology. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Joosten LA, Shaw PJ, Smeekens SP, Malireddi RK, van der Meer JW, Kullberg BJ, Netea MG, Kanneganti TD. The inflammasome drives protective Th1 and Th17 cellular responses in disseminated candidiasis. European Journal of Immunology. 2011a;41:2260–2268. doi: 10.1002/eji.201041226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Kullberg BJ, Verschueren IC, Hendriks T, van der Meer JW, Joosten LA, Netea MG. Differential effects of IL-17 pathway in disseminated candidiasis and zymosan-induced multiple organ failure. Shock. 2010;34:407–411. doi: 10.1097/SHK.0b013e3181d67041. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, et al. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG, Jansen TJ, Jacobs L, Verschueren I, van der Meer JW, Kullberg BJ. Redundant role of TLR9 for anti-Candida host defense. Immunobiology. 2008;213:613–620. doi: 10.1016/j.imbio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, Arts P, Rosentul DC, Carmichael AJ, Smits-van der Graaf CA, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011b;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- van der Graaf CA, Netea MG, Franke B, Girardin SE, van der Meer JW, Kullberg BJ. Nucleotide oligomerization domain 2 (Nod2) is not involved in the pattern recognition of Candida albicans. Clin Vaccine Immunol. 2006;13:423–425. doi: 10.1128/CVI.13.3.423-425.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautier S, Sousa Mda G, Brown GD. C-type lectins, fungi and Th17 responses. Cytokine Growth Factor Rev. 2010;21:405–412. doi: 10.1016/j.cytogfr.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamon E, Gozalbo D, Roig P, O’Connor JE, Fradelizi D, Gil ML. Toll-like receptor-2 is essential in murine defenses against Candida albicans infections. Microbes Infect. 2004;6:1–7. doi: 10.1016/j.micinf.2003.09.020. [DOI] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, et al. The Macrophage-Inducible C-Type Lectin, Mincle, Is an Essential Component of the Innate Immune Response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- Werninghaus K, Babiak A, Gross O, Holscher C, Dietrich H, Agger EM, Mages J, Mocsai A, Schoenen H, Finger K, et al. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRgamma-Syk-Card9-dependent innate immune activation. J Exp Med. 2009;206:89–97. doi: 10.1084/jem.20081445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willment JA, Brown GD. C-type lectin receptors in antifungal immunity. Trends in microbiology. 2008;16:27–32. doi: 10.1016/j.tim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. The Journal of biological chemistry. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano J, Noverr MC, Fidel PL., Jr Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine. 2012;58:118–128. doi: 10.1016/j.cyto.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Wilhelmus KR. Toll-like receptors involved in the pathogenesis of experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2010;51:2094–2100. doi: 10.1167/iovs.09-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, De Luca A, Arroyo J, Blanco N, Servillo G, Sanglard D, Reichard U, Palmer GE, Latge JP, et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat Commun. 2012;3:683. doi: 10.1038/ncomms1685. [DOI] [PubMed] [Google Scholar]
- Zenaro E, Donini M, Dusi S. Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, Mannose Receptor, and DC-SIGN. J Leukoc Biol. 2009;86:1393–1401. doi: 10.1189/jlb.0409242. [DOI] [PubMed] [Google Scholar]