Abstract
The clinical application of interleukin 12 (IL-12) has been hindered by the toxicity associated with its systemic administration. To potentially overcome this problem, we developed a promoter designed to direct IL-12 expression within the tumor environment using an inducible composite promoter containing binding motifs for the nuclear factor of activated T cells (NFAT) linked to a minimal IL-2 promoter. In this study, the NFAT promoter was coupled to a single-chain human IL-12 gene and inserted into two γ-retroviral self-inactivating (SIN) vectors (SERS.NFAT.hIL12, and SERS.NFAT.hIL12.PA2) and one γ-retroviral vector (MSGV1.NFAT.hIL.12 PA2). Peripheral blood lymphocytes (PBLs) were double transduced with an antigen specific T cell receptor and the three NFAT.hIL12 vectors. Evaluation of inducible IL-12 expression, transduction efficiency, and vector production considerations, led to the choice of the MSGV1.NFAT.hIL12.PA2 vector for clinical application. MSGV1.NFAT.hIL12.PA2 PG13 retroviral vector producer cell clones were screened by transduction of tumor antigen specific PBLs. Based on expression studies in PBL, clone D3 was chosen to produce clinical-grade viral vector supernatant and was demonstrated to efficiently transduce young TIL. The vector-transduced young TIL with known tumor recognition demonstrated specific inducible IL-12 production following co-culture with HLA-matched tumor targets and had augmented effector function as demonstrated by increased IFN-γ secretion. These results support the clinical application of adoptive transfer of young TIL engineered with the NFAT.hIL12 vector as a new approach for cancer immunotherapy.
Keywords: NFAT responsive promoter, IL-12, Adoptive T cell therapy
Introduction
Interleukin 12 (IL-12) is an immuno-stimulatory cytokine, which enhances the cytotoxic activity of NK cells and CD8 T lymphocytes by stimulating interferon gamma (IFN-γ) production, and polarizes naïve CD4+ T cells to Th1 cells. Multiple animal studies have shown that the presence of IL-12 at the tumor site can augment the effector function in adaptive immunity 1. For example, Kerkar et al recently demonstrated that antigen-specific murine T cell (pmel-1 cell) expressing IL-12 dramatically augmented the tumor treatment efficacy 2. In human clinical trials, the therapeutic efficacy of the IL-12 administration was restricted by dose-limited toxicities associated with its systemic delivery 3. In an attempt to control the potential toxicity, we developed a γ-retroviral vector in which the expression of murine IL-12 was directed by a composite promoter containing binding motifs for the nuclear factor of activated T cells (NFAT) and a minimal IL-2 promoter, designated as MSGV1.NFAT.mIL12.PA2 4. We demonstrated that adoptive transfer of antigen specific murine lymphocytes (pmel-1 cells) genetically modified with this inducible vector produced IL-12 upon antigen-specific T cell activation and mediated the tumor treatment efficacy comparable to cells engineered with the constitutive IL-12 vector. Furthermore, while the toxicity was observed in this mouse melanoma treatment model using T cells engineered with the constitutive IL-12 expression vector, similar affects were not seen with the inducible vector 4.
In the present study, we sought to design an optimal gene transfer vector for the inducible expression of human single-chain IL-12 in patient-derived T cells. We compared IL-12 expression in two self-inactivating (SIN) γ-retroviral vectors; SERS.NFAT.hIL12, SERS.NFAT.hIL12.PA2, and the γ-retroviral vector, MSGV1.NFAT.hIL12.PA2. The type 2 SIN vectors used herein, eliminate the constitutive LTR promoter from the vector design5 and it has been suggested that SIN vectors may lessen potential genotoxicity associated with the preferential integration of γ-retroviral vectors into the promoter region of genes 6, 7. Here, we determined the specificity of IL-12 production upon encountering tumor targets (with concomitant low-background IL-12 production) for each vector design and evaluated the utility of these gene therapy reagents to be produced for human application.
Cell-based immunotherapy using autologous TIL combined with a lymphodepletion regimen was demonstrated to mediate tumor regression in metastatic melanoma patients 8. Others and we have recently reported that TIL isolation and expansion can be simplified and that these “young” TIL yield similar treatment efficacy 9,10. Herein we sought to test the potential therapeutic efficacy of NFAT.hIL12 vector-engineered young TIL for the treatment of patients with metastatic melanoma.
Materials and Methods
Construction of human single chain IL-12 vectors
Human single chain IL-12 (hscIL12) was designed by connecting the p40 and p35 subunits with G6S linker. The gene was synthesized by GeneArt (Life technologies, Grand island, NY) following codon optimization and cloned into NcoI/XhoI sites of the MSGV1 γ-retroviral vector to generate MSGV1.hIL12. The NFAT responsive promoter containing 6 repeats of NFAT binding motif linked to a minimal IL-2 promoter was from the SIN- (NFAT)6-GFP vector 11. PolyA signal sequence, PA2 (GGCCGCAATAAAATATCTTTATTTTCATTACATCTGTGTGTTGGTTTTTTGTG TGAG), was synthesized by Epoch Biolab (Sugar Land, TX). Vector MSGV1.NFAT.GFP.PA2 was constructed by ligating NFAT.GFP derived from SIN- (NFAT)6-GFP and PA2 into MSGV1 vector. IL-12 gene was inserted in place of GFP by using restriction sites NcoI and Not I to generate MSGV1.NFAT.hIL12.PA2.
The self-inactivating γ-retroviral vector SERS11MPSV.GFP 12 was used to construct the inducible human IL-12 vector. To create suitable digestion sites in SERS11MPSV.GFP, primers were designed to mutate the Sal I site at 2771bp and create a new Sal I restriction site at 1550bp upstream of MPSV promoter in the vector. The MPSV promoter in SERS11MPSV.GFP (Sal I/Nco I) was replaced by NFAT responsive promoter to generate SERS.NFAT.GFP. Vector SERS.NFAT.hIL12.PA2 was constructed by ligating NFAT (Sal I/Nco I), hIL12 (Nco I/Not I) and PA2 (Not I/ EcoR I) fragments into the SERS vector. hIL-12 was inserted into SERS.NFAT.GFP at the NcoI/NotI site to replace the GFP gene to get the SERS.NFAT.hIL12 vector. All vectors were confirmed by enzyme digestion and DNA sequencing.
To produce γ-retrovirus supernatant, 293GP cells, which stably express GAG and POL gene proteins. To begin, 9 μg of vector DNA and 4 μg of RD114 envelope plasmid DNA (provided by Francois-Loïc Cosset INSERM, Lyon, France) were mixed with lipofectamine 2000 (following the manufacture instructions, Life Technologies) in OptiMEM serum free medium (Life Technologies) and incubated at room temperature for 20 minutes. The mixture was applied to 5 × 106 293GP cells that had been plated the prior day on a 100-mm2 poly-lysine coated plate (Becton Dickinson). After 6 hours of incubation, the medium was replaced with DMEM (Life Technologies) with 10% FBS and the viral supernatants were harvested 48 hours later.
Cell culture media
PBMC were cultured in AIM V medium (Life technologies, Grand island, NY) supplemented with 5% human AB (blood type AB) serum (HS, Gemini Bioproducts, Woodland, CA), 300 IU IL-2 (Novartis, Basel, Switzerland), 100 U/ml penicillin and 100 μg/ml streptomycin (Life technologies). Complete medium (CM) consisted of RPMI-1640 medium (Life technologies), 10% human AB serum, 6000 IU IL-2, 100 U/ml penicillin and 100 μg/ml streptomycin, 55 μM 2-mercaptoethanol (Life technologies). 50/50 medium consisted of CM and AIM V medium mixture at ratio 1:1. R10 medium consisted of RPMI-1640 medium plus 10% fetal bovine serum (FBS) (Biofluids, Inc., Gaithersburg, MD), 100 U/ml penicillin and 100 μg/ml streptomycin. D10 consisted of DMEM medium (Life technologies) plus 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin.
PBL, cell lines, and young TIL transduction
PBL and TIL used in this study were taken from metastatic melanoma patients treated at the Surgery Branch, National Cancer Institute under IRB approved protocols. The cell lines used in experiments included two HLA-A2 + melanoma lines, mel526, mel624, and two HLA-A2- melanoma lines, mel888, mel938, were generated in the Surgery Branch from resected tumor lesions.
Transduction of stimulated human PBL has previously been described in details13. The “young” TIL were generated as previously described 10. Briefly, multiple independent cultures were initiated from the excised tumor specimens and plated in 24-well sterile tissue culture plates in complete medium (CM) containing 6000 IU/ml IL-2. Once TIL grew to be confluent (and all adherent cells are eliminated), the individual culture wells were pooled to yield a bulk TIL culture. The bulk TIL was expanded further by re-plating at 0.7-1.5×106 lymphocytes/ml in CM containing IL-2 until sufficient cells were obtained for therapy. The cells were examined by FACS to determine the percentages of CD3+ and CD8+ cells.
Transduction of TIL was initiated by stimulation using a rapid expansion protocol (REP)14 as follows. TIL were thawed and incubated in complete medium for 2 days. 2×106 TIL were then mixed with 2×108 allogeneic irradiate (4000 rad) peripheral blood mononuclear “feeder” cells and cultured in T175 flask (Nunc, Cole-Parmer, Vernon Hills, IL) with 150 ml 50/50 media containing 30 ng/ml of OKT3 (Ortho Biotech, Horsham, PA) and 3000 IU/ml of IL-2 (Novartis). Four or five days later, the cells were collected and counted for transduction. Transduction was performed using spin loading. RetroNectin (CH-296; Takara, Otsu, Japan)-coated non-tissue culture 6-well plates (Becton Dickinson) were loaded with viral vector supernatants and centrifuged at 2000g for 2 hours at 32°C. Vector supernatant was removed and cells were applied to the vector-coated plates at a concentration of 0.5×106/ml, using 2×106 per well, and centrifuged at 1000g for 10 minutes and incubated at 37°C and 5% CO2 for 18-24 hours. Next day, the transduced cells were transferred to fresh viral-vectors coated plates and the transduction repeated. After transduction, the cells were cultured in 50/50 media containing 5% human serum (HS) (Valley Biomedical, Inc., Winchester, VA) and 3000 IU/ml IL-2 (Novartis) until use.
Cytokine release assay
Cell cultures were tested for reactivity in cytokine release assays using a commercially available ELISA kit (human IL-12, IFN-γ, Endogen, Rockford, IL). For these assays, 1×105 responder cells (transduced PBLs or TILs) and 1×105 target cells (tumor lines) were incubated in a 0.2-ml culture volume in individual wells of 96-well plate overnight. Cytokine secretion was measured in culture supernatant diluted as to be in the linear range of the assay. Transduced TILs were treated with Phorbol 12-myristate 13-acetate (PMA) (10ng/ml) (Sigma, St. Louis, MO), Ionomycin (2.2 μM) (Sigma) overnight to activate the NFAT responsive promoter.
IL-12 Enzyme-Linked Immunosorbent Spot Assay (ELISPOT Assay)
96-well filtration plate (Millipore. Billerica, Massachusetts) was coated with 10μg/ml anti-h12 mAb in sterile PBS (MABTech, Cincinnati, OH) overnight at 4°C. The plate was then loaded with T cells double-transduced with the NFAT.IL12 and a TCR targeting MAGE-A3 antigen plus tumor line mel888 (HLA-A2-, MAGE-A3-) or mel624 (HLA-A2+, MAGE-A3+) at 1:1 ratio, incubating 37°C for 18 hours. Next day, the plate was washed with PBS for 5 times and 1μg/ml anti-hIL12mAb-Biotin (MABTech) was added. Two hours later, the plate was washed 5 times with PBS and diluted streptavidin (1:3000)(MabTech) was added. After 1-hour incubation, BCIP/NBT (MABTech) was added for 10 minutes or until distinct spots emerge. The dry plate was read and counted using immunoSpot Micro analyzer (C.T.L., Shaker Heights, OH)
Real-time PCR analysis of NFAT.hIL12 copy number
Real-time PCR was used to determine the vector copy number in engineered PBL. Total genomic DNA for each transduced cells was extracted using DNeasy Blood& Tissue kit (Qiagen, Hilden, Germany) following the manufacturer’s instruction. The concentration of DNA was determined from absorbance at 260nm using the NanoDrop ND-1000 spectrophotometer (Witec AG, Littau, Switzerland). Real-time PCR was performed using gene specific probe targeting single chain IL12 linker region G6S, which was absent in the endogenous IL-12 sequence. The IL-12 gene-specific primers and probe were listed: forward primer: 5′ GCTCCTGGTCCGAGTGG 3′, reverse primer: 5′ GTAGCCACGGGCAGGTT 3′, the probe sequence: CCTCCGCCCCCTCCGC. Conserved housekeeping gene β-actin (Taqman Gene Expression Assays; Applied Biosystems, Foster City, CA) was used as an internal reference. The experiment was performed in duplicate using Taqman 7900 real-time PCR machine (Applied Biosystem).
Generation of MSGV1.NFAT.hIL12.PA2 PG13 producer cell clones
PG13 packaging cell clones were generated using PG13 gibbon ape leukemia virus packaging cell line (ATCC, Manassas, VA), and human ecotropic package cell line, Phoenix Eco (Kindly provided by the National Gene Vector Biorepository (NGVB), Indiana university, Indianapolis, IN). All these cells were cultured in D10 medium at 37°C and 5% CO2. A PG13 retroviral packaging cell clone was generated as described previously 15, 16. Briefly, Phoenix Ecotropic cells were transfected with 9 μg of plasmid DNA (pMSGV1.NFAT.IL12.PA2) using Lipofectamine 2000 transfection reagent (Life technologies). After 48h, supernatant was harvested and used to transduce retroviral packaging cell line, PG13. Non-tissue culture treated 6-well plates were coated with 20 μg/ml RetroNectin (CH-296; Takara, Otsu, Japan). Retroviral vector supernatant (4 ml) was added to each well followed by centrifugation (2000 x g) at 32°C. After 2h, supernatant was removed and 5 × 105 PG13 cells were added to the well, centrifuged (1000 x g) for 10 min at 32°C. Two rounds of transduction were performed and then PG13 packaging clones were generated by limiting dilution cloning. The high titer clones were identified by RNA dot blot as described previously 15-17. Retroviral vector from the 6 highest titer clones was generated as described. Briefly, 175 cm2 tissue culture flasks were seeded at 4 × 104 cells/cm2, followed by a medium exchange (30 ml) on day 3. Supernatant was harvested 24h later, aliquoted and stored at −80°C until further use. Supernatant from each clone was used to transduce human PBL. Clone D3 was selected for the production of a master cell bank and subsequent good manufacturing practice (GMP) retroviral vector supernatant.
Generation of GMP retroviral vector supernatant
A total of 26 ×1700 cm2 expanded surface roller bottles were seeded on day 0 at a cell density of 4 × 104 cells/cm2 in 200 ml of DMEM medium. On day 3, the medium was changed and replaced with 120 ml DMEM medium. Medium containing the retroviral vector was harvested daily and the bottles were refed with fresh 120 ml of medium 16. Glucose levels were monitored daily using Roche’s Accu-check system (Roche, Basal, Switzerland). If glucose levels dropped below 2 g/L, the volume of the medium exchange was doubled to 240 ml/roller bottles for all subsequent harvests. All harvests were aliquoted and stored at −80°C until further use. All clinical products were subjected to an extensive biosafety-testing program in accordance with current regulatory guidelines 18-21.
Statistic Analysis
Two-way Anova was used to test the significant differences in enumeration assays. P<0.05 was considered significant.
Results and Discussion
Kerkar et al reported that the adoptive transfer of a small number (10,000) of murine IL-12 (mIL12) gene modified antigen-specific murine T cells (pmel-1 cells) mediated regression of B16 melanoma in the absence of administration of vaccine and IL-2, which were necessary in prior cell transfer therapies using pmel-1 cells 2. Interestingly, the therapeutic impact of IL-12 required the expression of IL-12 in tumor-antigen specific T cells and could not be mimicked with high doses of exogenous rIL-12 or by mixing cells separately transduced with pmel-TCR or IL-12 2. However, administration of doses greater than 500,000 transduced T cells constitutively expressing murine IL-12 resulted in severe toxicities. Most recently, we sought to limit IL-12 expression within the tumor environment using a composite NFAT responsive promoter. Pmel-1 cells engineered with NFAT.mIL12 vector dramatically augmented pmel-1 anti-tumor activity without observed toxicity 4.
In this study, we evaluated whether principles learned from these murine vector studies could be applied to human cells. Using the same NFAT composite promoter, but now linked to human single-chain IL-12, (NFAT.hIL12) we assembled three expression vectors (Figure 1A); a γ-retroviral (MSGV1.NFAT.hIL12.PA2) and two self-inactivating (SIN) γ-retroviral vectors (SERS.NFAT.hIL12, SERS.NFAT.hIL12.PA2). In SIN vector SERS.NFAT.hIL12, the IL-12 expression cassette is in the same orientation as the viral LTR promoter, but the LTR promoter is deleted following target cell transduction due to the SIN design and thus the expression of IL12 was driven by the NFAT-responsive promoter. In order to further minimize the possibility of transient IL-12 expression due to leaky promoter activity from the 5′ LTR, the NFAT.hIL12 expression cassette was inserted into two vectors in the reverse direction with respect to the LTR (MSGV1.NFAT.hIL12.PA2 and SERS.NFAT.hIL12.PA2). These vectors also required addition of a polyA signal (PA2) in order to facilitate mRNA processing (Fig 1A). To best compare these vectors all viral supernatants were produced by transient vector production in 293GP cells using the envelop from the RD114 virus that reproducibility yields high-quality vector preparations for transduction of human cells. The three vectors were evaluated by transducing human PBLs made antigen-reactive by co-transduction with a TCR expressing vector targeting the MAGE-A3 cancer testis antigen.
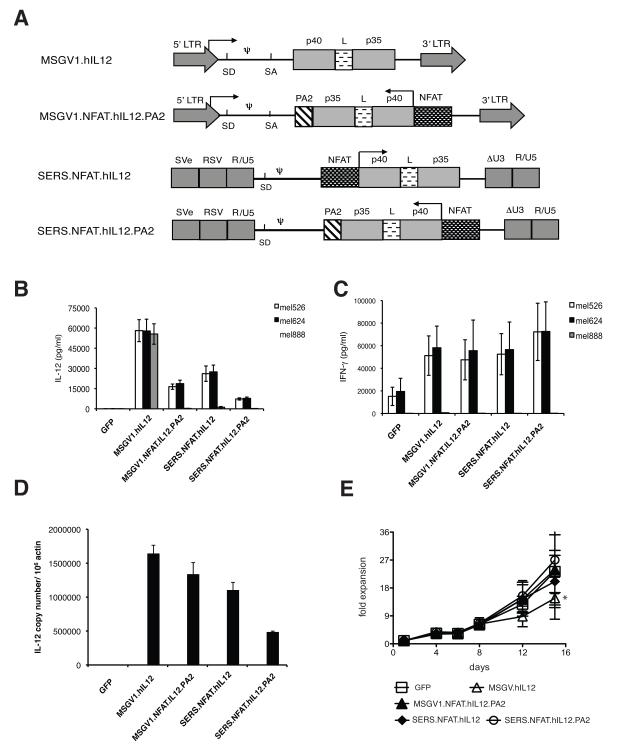
Figure 1. Evaluation of NFAT.hIL12 in different γ-retroviral vectors.
(A) Diagram of γ-retroviral vectors containing NFAT.hIL12 expression cassette (LTR, long terminal repeat; SD, splice donor; SA, splice acceptor; PA2, polyadenylation signal; NFAT, composite NFAT-responsive promoter element; SVe, SV40 virus enhancer; RSV, Rous sarcoma virus promoter; ΔU3, enhancer and promoter region deleted LTR). Arrows indicate the direction of transcription. Human PBLs were stimulated and transduced a TCR recognized tumor antigen MAGE-A3, then sequentially transduced with IL-12 constitutive expression vector MSGV1.hIL12 or NFAT-IL12 vectors: MSGV1.NFTA.hIL12.PA2, SERS.NFAT.hIL12, SERS.NFAT.hIL12.PA2. The transduced cells were then co-cultured with HLA-A2+/ MAGE A3+ positive tumor lines (mel526 and mel624) or HLA-A2-/ MAGE A3+ tumor line (mel888). (B) The IL-12 level in the supernatant was measured by ELISA. (C) Effector cytokine IFN-γ in the co-culture supernatant was measured by ELISA assay. (D) DNA isolated from the transduced cells was used in real-time PCR assay using specific probe targeting single chain IL-12. The vector copy number was calculated base on the amplification of standard curve using plasmid DNA and internal β-actin control. (E) The number of viable cells in the different cultures was enumerated at each time point by trypan blue staining. Cells transduced by MSGV1.hIL12 vector grew less well compared with the cells transduced by NFAT.IL12 or GFP (* P<0.05 on day15). Results presented are the mean values of data from three different donors. Values represented mean + SD.
Based on co-culture results with MAGE-A3 TCR and NFAT.hIL12 double-transduced cells; all three NFAT vectors produced IL-12 only when the MAGE-A3 TCR expressing cells encounter HLA-A2 matched, MAGE-A3 positive tumor cell targets: mel526 and mel624 (Fig.1B). Coordinately, the cells expressing IL-12 produced more IFN-γ during specific antigen stimulation (Fig. 1C). We determined the copy numbers of different vector insertions by real-time PCR using gene specific probe targeting single-chain IL-12. The results indicated that the copy numbers of the inserted vector genes were similar for MSGV.hIL12, MSGV1.NFAT.hIL12.PA2 and SERS.NFAT.hIL12, while the SERS.NFAT.hIL12.PA2 transduced cells had a copy number about half that of other populations (Fig.1D). As transduced cell vector copy number is correlated with the vector titer, we estimate that the MSGV1.NFAT.hIL12.PA2 and SERS.NFAT.hIL12 vectors had similar titers while the titer of the SERS.NFAT.hIL12.PA2 was less than one half of these vectors. All of the NFAT.hIL12 transduced cells proliferated slightly better than the cells constitutively expressing IL-12 (MSGV2.hIL12) (P<0.05 day 15), similar to the cells transduced by the GFP vector (Fig.1E).
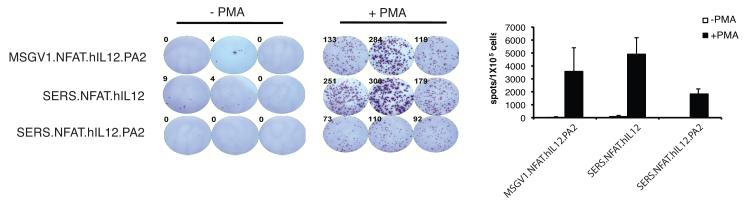
The estimation of vector titer by DNA PCR-based vector copy number determination does not necessarily equate with transduction efficiency. To quantify the number of IL-12 producing cells in a bulk transduced cell population, an IL-12 ELISPOT assay was developed to further evaluate the NFAT.hIL12 vectors (Fig. 2). We observed that the NFAT.hIL12 vector-engineered cells only produced IL-12 upon activation through PMA/Ionomycin stimulation (Fig. 2). Similar to the PCR results, there was no difference in the number of IL-12 secreting cells observed when comparing the MSGV1.NFAT.hIL12.PA2 and SERS.NFAT.hIL12 vectors but, the SERS.NFAT.hIL12.PA2 vector produced about half as many IL-12 secreting cells in the same assay (Fig. 2).
Figure 2. IL-12 secreting cells monitored by ELISPOT assay.
Human PBLs were stimulated by OKT3 (50ng/ml) and sequentially transduced with a TCR recognized MAGE-A3 and three NFAT.hIL12 γ-retroviral vectors separately. The transduced cells were with PMA (10 ng/ml)/Ionomycin (2.2 μM) and after 16 hours, the IL-12 specific spots were imaged and counted by immunoSpot Micro analyzer (left). The numbers of spots were determined (right) from three wells and values presented represented the mean + SD. M, MAGE-A3 TCR.
The design and utility of SIN vectors has greatly improved since their original description and the current type 2 designs used in this report have minimal residual promoter activity5, which is essential for an inducible gene system such as reported here. In comparison to LTR-driven γ-retroviral vectors, it has been suggested that SIN vectors may also lessen potential geontoxicity7. While, it is technically more difficult to scale-up SIN-vector production for clinical application as these vectors are produced by transient DNA transfection of packaging cell lines, such efforts are now being pursued by investigators developing treatments for X-SCID22. Based on the IL-12 induction levels, cell expansion in vitro, and vector transduction efficiency, we found no difference between vectors MSGV1.NFAT.hIL12.PA2 and SERS.NFAT.hIL12. Therefore, based mainly on vector production considerations, we chose vector MSGV1.NFAT.hIL12.PA2 for clinical application. Interestingly, this reverse-orientation vector design generally produces low-titer vectors due to the converging transcripts coming from the LTR and the reverse orientation internal promoter. We did not observe this phenomenon likely due to the lack of the activity of the composite NFAT promoter during vector production in the packaging cell line.
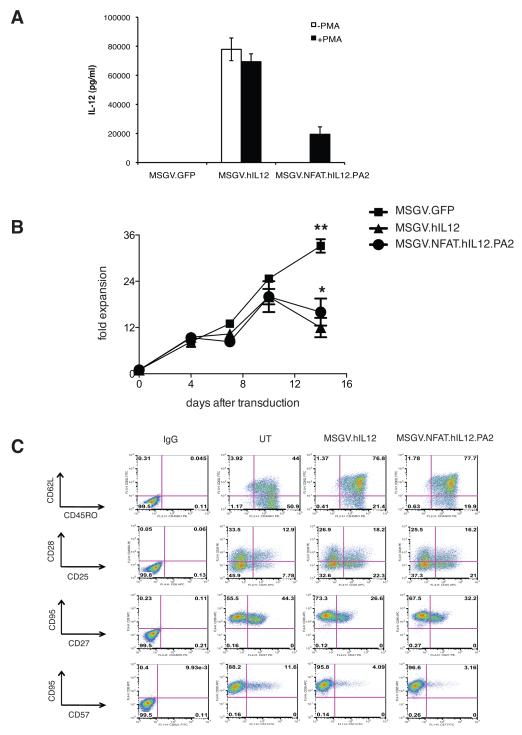
Since the NFAT-responsive promoter drove gene expression in antigen specific fashion in TCR co-transduced PBL, we sought to test the NFAT.hIL12 vector for reactivity in tumor infiltrating lymphocytes (TIL). If successful, we would envision that TIL, would traffic to and be activated at tumor sites, leading to the accumulation of IL-12 in the tumor microenvironment. To verify transduction, TIL were activated by PMA/ Ionomycin after transduction. Similar to the result with PBLs, the MSGV1.NFAT.hIL12.PA2 transduced TIL secreted IL-12 only when cells were activated, while the cells transduced by MSGV1.hIL12 expressed high levels of IL-12 with or without PMA/Ionomycin treatment (Fig. 3A). The IL-12 modified TIL could expand for 7-10 days but then viable cell numbers dropped rapidly. MSGV1.NFAT.IL12.PA2 vector transduced cells proliferated slightly better than cells constitutively expressing IL-12 at day 14, P<0.05 (Fig. 3B). The REP procedure results in a transient induction of the NFAT composite promoter and this is associated synthesis of IFN-γ, which may account for the reduced cell expansion compared to the GFP vector transduced TIL (data not shown). Analysis of cell surface markers suggest that cells expressing IL-12 displayed increased CD62L and CD25 expression and a decrease in CD27 and CD57 expression but transduction did not substantially affect the other surface makers analyzed; CD45RO CD28, and CD95 (Fig. 3C).
Figure 3. Evaluation of MSGV1.NFAT.hIL12.PA2 vector in TIL.
(A) TIL were stimulated by irradiated allogeneic feeder and high dose (6000 IU/ml) IL2 and transduced with MSGV1.hIL12 or MSGV1.NFAT.hIL12.PA2. The transduced cells were stimulated by PMA/Ionomycin (+PMA) and IL-12 production in the supernatant was measured by ELISA two days later. (B) The number of viable cells in the different cultures was enumerated at each time point by trypan blue staining. The fold expansion of the MSGV1.hIL12 transduced TIL was less than that of cells transduced by GFP (**, P<0.01) or by NFAT.hIL12 (*, P<0.05) on day 14 post-transduction. (C) The transduced cells were staining with various surface makers, CD62L, CD45RO, CD95, CD28, Cd27, CD25, CD57 and analyzed by flow cytometry. Figure A, B are the average of data from three different donors, and values presented represented the mean + SD. Figure C is representative data from one of three TIL lines.
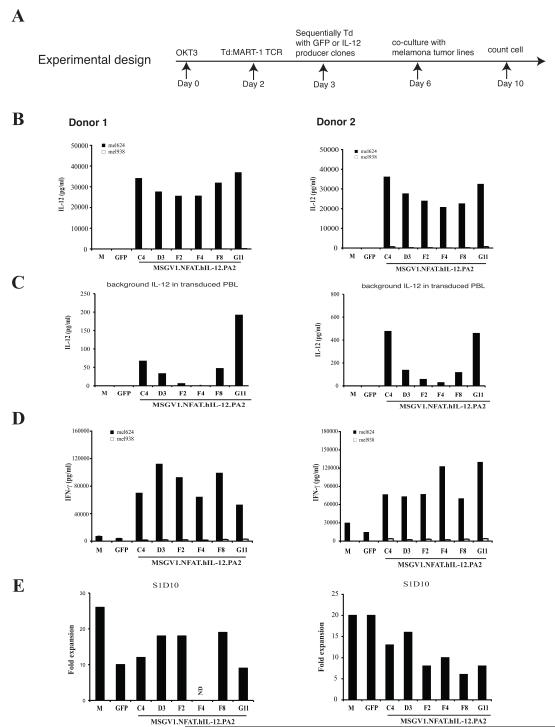
For clinical application, PG13 packaging clones stably expressing MSGV1.NFAT.hIL12.PA2 were developed. Six clones (C4, D3, F2, F4, F8, G11) were selected for evaluation based on high vector RNA copy number with low IL-12 background levels (data not shown). Human PBLs were sequentially transduced by the clones with a T cell receptor recognizing the MART-1 antigen followed by the NFAT.hIL12 vector (Fig. 4A). The transduced cells were then incubated with an HLA-A2+ and MART-1 positive tumor line (mel624) to stimulate T cells through specific antigen recognition. The results with two donor lymphocytes indicated that 100,000 IL-12 transduced cells could produce greater than 20 ng/ml of IL-12 upon activation (Fig. 4B) with minimal background of IL-12 secretion (< 500 pg/ml) (Fig. 4C). As in Figure 1, the transduced PBL displayed enhanced activity as demonstrated by 10 to 20-fold higher levels of IFN-γ production in co-culture compared with the cells only expressing MART-1 TCR (M) or cells transduced with the control GFP viral vector (GFP) (Fig. 4D). To determine the effect of vector transduction on cell growth, viable cells were enumerated at day10 and cell expansion was compared (Fig. 4E). In these two NFAT.hIL12-transduced PBL cultures, we did not observe a significant difference in cell expansion compared to GFP transduced cells. Vector-containing supernatant from clones D3 and F4 was further evaluated in young TIL, with clone D3 yielding slightly more IL-12 synthesis post-stimulation in one of two cultures (data not shown). Clone D3 were chosen for clinical production based on the ability of transduced cells to produce high amounts of cytokine (IL-12 and IFN-γ), lower IL-12 background, and adequate cell proliferation in vitro.
Figure 4. Screening NFAT.hIL12 viral producing clones in human PBL.
(A). The experimental design. PBLs were stimulated with soluble OKT3 at day 0. On day 2, the cells were suspended in fresh medium with IL-2 and transduced with MART-1 TCR. Next day, the MART-1 transduced cells were divided into 7-groups, and transduced with vector supernatant from six MSGV1.NFAT.hIL-12.PA2 vector producer clones: C4, D3, F2, F4, F8, G11 or GFP respectively. Three days after the 2nd transduction (day 6), the transduced cells were co-cultured with HLA-A2+/MART-1+ tumor line: mel624 and HLA-A2-/MART-1+ tumor line: mel938. The levels of IL-12 (B & C) and IFN-γ (D) in the culture were measured by ELISA (shown are the mean values of duplicate determinations). (E). The number of viable cells in different cultures was enumerated on day 10 by trypan blue staining. The data was repeated using PBLs from two donors. M, MART-1 TCR transduced.
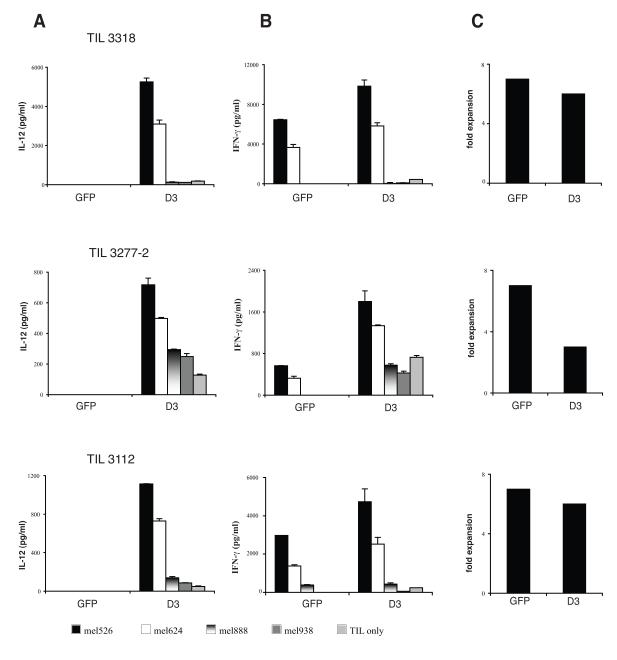
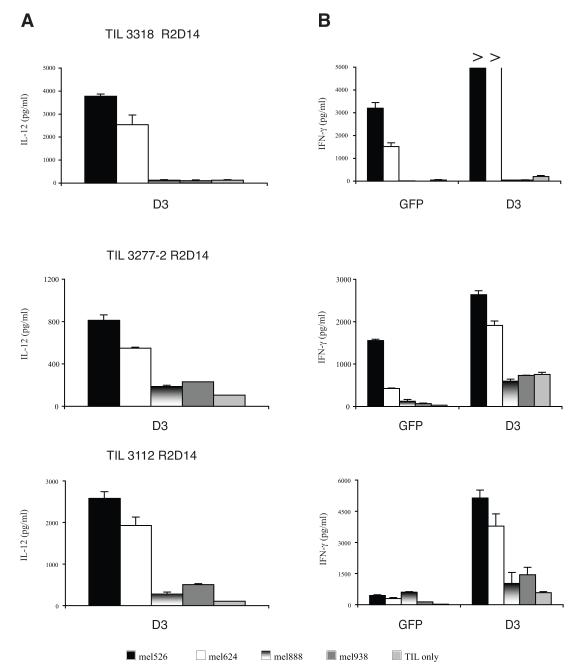
One potential clinical application for the NFAT.hIL12.PA2 vector would be a trial in which TIL were transduced with the NFAT.hIL12.PA2 vector and administered to metastatic melanoma patients. In our previous clinical trial reports, TIL were extensively screened for the recognition of tumor antigen as measured by IFN-γ production when the cells were co-cultured with tumor lines 23. In more recent clinical applications, others and we have developed a rapid “young” TIL protocol that eliminates the screening step9,10. To test the NFAT.hIL12 vector in non-selected young TIL, we stimulated these cultures by a rapid expansion protocol (REP) and transduced them with vector supernatant from clone D3 (Figure 5). Three young TIL cultures (3318, 3277-2, and 3112) were chosen for transduction because they had been retrospectively determined to recognize antigens presented by melanoma lines mel526 and/or mel624. As expected, IL-12 was induced when the cells were activated through antigen recognition (Fig. 5A). Enhanced effector cell function was demonstrated by the production of higher amounts of the effector cytokine, IFN-γ (Fig 5B). The cells transduced with clone D3 proliferated as well as the control cells transduced with the GFP vector in 2 of 3 young TIL cultures (Fig. 5C). To mimic the production of large number of cells for clinical application, we performed a second rapid expansion (REP) of NFAT.hIL12.PA2 engineered TIL on day 10. Fourteen-days after the second REP, the activity of the expanded TIL were again measured by co-culture with tumor targets. The results indicated that the expanded cells retained their property of IL-12 induction and enhancement of IFN-γ production when encountering the specific tumor antigen targets (mel526 and mel624) (Fig. 6A & 6B).
Figure 5. Young TIL transduced with NFAT.IL12 vector clone D3 produce IL-12 production upon antigen recognition.
The CD8 enriched TILs were subjected to REP (day 0) and transduced with clone D3 and GFP vector on day 4. Two days later, the transduced cells were co-cultured with antigen-positive and HLA-A2+ tumor lines: mel526, mel624, and HLA-A2-tumor lines: mel888 and mel938. The level of IL-12 (A) and IFN-γ B in the cultures was measured by ELISA (shown are the mean values of triplicate determinations, +/− standard deviation). (C) The fold expansion of transduced cells was calculated by counting viable cell number on day 14.
Figure 6. NFAT.hIL12 transduced young TIL retain the ability to produce IL-12 and IFN-γ after REP.
The transduced CD8 enriched TILs were re-stimulated following the rapid expansion protocol on day 13 (REP2, R2). The activity of the expanded cells was measure two-week later by co-culture with antigen-positive and HLA-A2+ tumor lines: mel526, mel624, and HLA-A2- tumor lines: mel888 and mel938. The level of IL-12 (A) and IFN-γ (B) in the culture was measured by ELISA (shown are the mean values of triplicate determinations, +/− standard deviation).
The initial clinical study using recombinant IL-12 reported several toxicities including two patients death, which hindered the clinical application of this cytokine 3, 24. In an effort to limit its systemic toxicity we sought to deliver the cytokine only at the tumor site. We recently reported that the NFAT responsive promoter drove murine IL-12 gene expression in mouse lymphocytes when the cells were activated by specific antigen recognition following TCR engagement 4. This novel inducible vector design was successfully evaluated in the murine B16 melanoma treatment model and demonstrated robust tumor treatment with minimal toxicity 4.
For potential clinical application of this inducible vector, we compared the IL-12 production from three different NFAT.hIL12 γ-retroviral vectors by transducing human lymphocytes. The MSGV1.NFAT.hIL12.PA2 vector was chosen for clinical application, and vector producer clone D3 demonstrated to efficiently transduce PBL and young TIL. The transduced cells induced IL-12 production only when the cells were activated by tumor recognition or PMA/Ionomycin stimulation. Transduced TIL retained their ability to specifically produce IL-12 even after expansion to a large cell numbers. Using this novel vector design, we have proposed a clinical trial where NFAT-hIL12 transduced young TIL are administered to melanoma patients without concomitant high-dose IL-2 or vaccine therapy.
Acknowledgement
We thank the FACS laboratory and the TIL laboratory in the Surgery Branch, National Cancer Institute, for providing technical support and maintenance of tumor cells from patients. This work is supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
Competing interests statements
The authors declare that they have no competing financial interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 2.Kerkar SP, Muranski P, Kaiser A, et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 2010;70:6725–6734. doi: 10.1158/0008-5472.CAN-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Kerkar SP, Yu Z, et al. Improving Adoptive T Cell Therapy by Targeting and Controlling IL-12 Expression to the Tumor Environment. Mol Ther. 2011 doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu W, Russ JL, Eiden MV. Evaluation of Residual Promoter Activity in gamma-Retroviral Self-inactivating (SIN) Vectors. Mol Ther. 2012;20:84–90. doi: 10.1038/mt.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozturk F, Park PJ, Tellez J, et al. Expression levels of the PiT2 receptor explain, in part, the gestational age-dependent alterations in transduction efficiency following in Utero retroviral-mediated gene transfer. J Gene Med. 2012 doi: 10.1002/jgm.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modlich U, Bohne J, Schmidt M, et al. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545–2553. doi: 10.1182/blood-2005-08-024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 10.Dudley ME, Gross CA, Langhan MM, et al. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooijberg E, Bakker AQ, Ruizendaal JJ, et al. NFAT-controlled expression of GFP permits visualization and isolation of antigen-stimulated primary human T cells. Blood. 2000;96:459–466. [PubMed] [Google Scholar]
- 12.Schambach A, Galla M, Maetzig T, et al. Improving transcriptional termination of self-inactivating gamma-retroviral and lentiviral vectors. Mol Ther. 2007;15:1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- 13.Frankel TL, Burns WR, Peng PD, et al. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J Immunol. 184:5988–5998. doi: 10.4049/jimmunol.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 15.Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman SA, Goff SL, Xu H, et al. Rapid production of clinical-grade gammaretroviral vectors in expanded surface roller bottles using a "modified" step-filtration process for clearance of packaging cells. Hum Gene Ther. 2011;22:107–115. doi: 10.1089/hum.2010.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onodera M, Yachie A, Nelson DM, et al. A simple and reliable method for screening retroviral producer clones without selectable markers. Hum Gene Ther. 1997;8:1189–1194. doi: 10.1089/hum.1997.8.10-1189. [DOI] [PubMed] [Google Scholar]
- 18.F. a. D. A. U S Department of Health and Human Service; Center for Drug Evaluation and Research; Center for Biologic Evaluation and Reseach, editor. Guidance for industry: INDs-Approaches to Complying with cGMP during Phase I (Draft) 2006. [Google Scholar]
- 19.F. a. D. A. U S Department of Health and Human Servicez; Center for Drug Evaluation and Research; Center for Biologic Evaluation and Reseach, editor. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy. 1998. [Google Scholar]
- 20.F. a. D. A. U S Department of Health and Human Service; Center for Drug Evaluation and Research; Center for Biologic Evaluation and Reseach, editor. Referenece Point to Consider in the Production and Testing of New Drug and Biologicals Produced by Recombinant DNA Technology. 1985. [Google Scholar]
- 21.F. a. D. A. U S Department of Health and Human Service; Center for Drug Evaluation and Research; Center for Biologic Evaluation and Reseach, editor. Points to Consider in the Use of Cell Lines to Produce Biologicals. 1993. [Google Scholar]
- 22.Herzog RW. Gene therapy for SCID-X1: round 2. Mol Ther. 2010;18:1891. doi: 10.1038/mt.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudley ME, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34:524–531. doi: 10.1053/j.seminoncol.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]