Abstract
There are no FDA-approved pharmacotherapies for cannabis dependence. Cannabis is the most widely used illicit drug in the world, and patients seeking treatment for primary cannabis dependence represent 25% of all substance use admissions. We conducted a phase IIa proof-of-concept pilot study to examine the safety and efficacy of a calcium channel/GABA modulating drug, gabapentin, for the treatment of cannabis dependence. A 12-week, randomized, double-blind, placebo-controlled clinical trial was conducted in 50 unpaid treatment-seeking male and female outpatients, aged 18–65 years, diagnosed with current cannabis dependence. Subjects received either gabapentin (1200 mg/day) or matched placebo. Manual-guided, abstinence-oriented individual counseling was provided weekly to all participants. Cannabis use was measured by weekly urine toxicology and by self-report using the Timeline Followback Interview. Cannabis withdrawal symptoms were assessed using the Marijuana Withdrawal Checklist. Executive function was measured using subtests from the Delis–Kaplan Executive Function System. Relative to placebo, gabapentin significantly reduced cannabis use as measured both by urine toxicology (p=0.001) and by the Timeline Followback Interview (p=0.004), and significantly decreased withdrawal symptoms as measured by the Marijuana Withdrawal Checklist (p<0.001). Gabapentin was also associated with significantly greater improvement in overall performance on tests of executive function (p=0.029). This POC pilot study provides preliminary support for the safety and efficacy of gabapentin for treatment of cannabis dependence that merits further study, and provides an alternative conceptual framework for treatment of addiction aimed at restoring homeostasis in brain stress systems that are dysregulated in drug dependence and withdrawal.
Keywords: cannabis dependence, marijuana withdrawal, executive function, gabapentin, addiction treatment, randomized controlled trial
INTRODUCTION
Cannabis is the most widely used illicit drug in the world (UNODC, 2010). Globally, an estimated 147 million adults use illicit marijuana at least once annually, including 30 million Americans (SAMHSA, 2007; UNODC, 2010). Among those who used illicit marijuana in the past year, 10% (Koob et al, 2008) are estimated to meet the Diagnostic and Statistical Manual-Fourth Edition (DSM-IV; APA, 2000) criteria for cannabis dependence. Worldwide, patients seeking treatment for primary cannabis dependence represent 25% of all substance use admissions (UNODC, 2010). However, despite the prevalence of the disorder and the numbers of individuals seeking treatment for it, there are no FDA-approved medications for cannabis dependence.
Cannabis dependence is marked by compulsive use, inability to stop despite harmful consequences, and the emergence of a withdrawal syndrome upon cessation of use (APA, 2000). Withdrawal symptoms include disturbances in mood and sleep, and craving (Budney et al, 2003). Though seemingly inconsequential on their own, such symptoms have an additive effect, serving to undermine individuals' efforts to successfully abstain; and, instead, motivating them to continue to smoke marijuana in an effort to seek relief from these undesirable consequences of early abstinence (Budney et al, 2008; Copersino et al, 2006). The psychoactive constituent of cannabis, delta-9 tetrahydrocannabinol (THC) and its metabolites (11-hydroxy-THC and 11-nor-9-carboxyl-THC [THCCOOH]) have especially long half-lives, 25–57 hours and about 5 days, respectively (Caravati, 2003). As a result of this elimination profile, and because of the activation of brain stress circuitry caused by chronic heavy marijuana use and discontinuation (Koob and Le Moal, 2005, 2008; Koob and Volkow, 2010; Maldonado et al, 2011; Rodriguez de Fonseca et al, 1997), some withdrawal symptoms may persist for weeks or even months, as in the case of marijuana craving and sleep disturbances (Budney et al, 2003).
A pattern of continued heavy cannabis use and withdrawal also has been found to alter right prefrontal brain activity and impair executive functions, such as inhibition of impulses, cognitive flexibility, and complex information processing (Crean et al, 2011; Goldstein and Volkow, 2002). These changes in brain function may make it difficult for patients to effectively use the components of standard behavioral therapies, and psychosocial treatments for cannabis dependence have limited efficacy (Nordstrom and Levin, 2007). Cannabis-related impairment in executive function, eg, failure to inhibit impulses, also may contribute to the high dropout rates (≈60%) typically found in non-agonist clinical trials of primary cannabis dependence (Carpenter et al, 2009; Levin et al, 2004; McRae-Clark et al, 2009, 2010).
Clinical trials of drugs to treat cannabis dependence, such as buspirone (McRae-Clark et al, 2009), divalproex sodium (Levin et al, 2004), atomoxetine (McRae-Clark et al, 2010), nefazodone (Carpenter et al, 2009), bupropion (Carpenter et al, 2009), and oral THC (Levin et al, 2011), have shown no effect on cannabis use, relative to placebo. Given the prevalence of dependence, the risk of relapse posed by motivational symptoms of withdrawal, and the inability of dependent individuals to stop use despite harmful consequences, safe and effective pharmacotherapies for cannabis dependence have great clinical significance.
Gabapentin (Neurontin) is an alkylated analog of gamma butyric acid (GABA) and is approved by the federal Food and Drug Administration (FDA) for the management of epileptic seizures and neuropathic pain. Gabapentin is believed to act by blocking a specific alpha-2d subunit of the voltage-gated calcium channel at selective presynaptic sites and, as a result, to indirectly modulate GABAergic mechanisms (Sills, 2006). Pre-clinical findings suggest that gabapentin normalizes the CRF-induced GABA activation in the amygdala (Roberto et al, 2008). That activation is associated with the development of dependence to alcohol and, by extrapolation, to cannabis as well, because cannabis withdrawal, like alcohol withdrawal, produces both an anxiogenic-like state and increased extrahypothalamic CRF release in the central nucleus of the amygdala in rodents (Roberto et al, 2008; Rodriguez de Fonseca et al, 1997). These GABA–CRF interactions and their role in the motivational aspects of cannabis relapse provide an excellent pre-clinical rationale for exploring the efficacy of gabapentin in cannabis dependence (Maldonado et al, 2011). Furthermore, in clinical studies of various disorders, gabapentin has been found to reduce craving and disturbances in sleep and mood (Ghaemi et al, 1998; Harden et al, 1999; Karam-Hage and Brower, 2000; Lo et al, 2010; Mason et al, 2009), which are among the most persistent symptoms of protracted cannabis withdrawal and a key reason patients resume smoking marijuana. Gabapentin also showed subtle cognitive-enhancing effects in the domains of attention, concentration, visual–motor functioning, inhibition, and set shifting in healthy volunteers (Salinsky et al, 2005). Thus, gabapentin, through its calcium-channel-GABAergic mechanism of action that has relevance for restoring homeostasis in brain stress (CRF) systems, may offer a novel treatment approach relative to the agonist, antagonist, or psychiatric drugs that have been explored to date for the treatment of cannabis dependence.
This proof-of-concept study assessed the efficacy of gabapentin vs placebo as a novel treatment for reducing marijuana use and withdrawal symptoms in a group of treatment-seeking unpaid outpatient volunteers with cannabis dependence. Additional resources acquired after study initiation enabled us to also examine the effect of gabapentin on executive function in the latter part of the sample. We hypothesized that gabapentin would significantly 1) decrease cannabis use; 2) reduce cannabis withdrawal symptoms, including those involving sleep, affect, and craving; and 3) improve cannabis-related physical, psychological, social, and functioning problems, as well as deficits in cognitive executive function, compared with placebo.
METHODS
Participants and Study Design
This was a 12-week, randomized, placebo-controlled, parallel groups phase IIa clinical trial. This single-site outpatient study was conducted in the Laboratory of Clinical Psychopharmacology at The Scripps Research Institute, La Jolla, CA and was carried out in accordance with the Declaration of Helsinki. The study was approved by the Scripps Institutional Review Board (Scripps-IRB), and written informed consent was obtained from all participants.
Unpaid volunteers with cannabis dependence were recruited primarily through newspaper and Internet advertisements between August 2006 and April 2008. The headline read, ‘Smoking too much pot? We want to help you stop.' To be eligible for the study, men or women (not pregnant or nursing) aged 18–65 years were required to meet DSM-IV criteria for current cannabis dependence; be seeking research-based outpatient treatment for cannabis dependence that involved daily medication; and have smoked marijuana at least once in the week prior to randomization. Exclusion criteria were treatment with medically prescribed marijuana; active suicidal ideation; meeting DSM-IV criteria for current abuse or dependence on substances other than cannabis or nicotine; significant medical or psychiatric disorders, including current depressive and anxiety disorders; treatment with an investigational drug during the previous month or ongoing treatment with medications that could affect study outcomes, eg, other psychoactive medications; and being mandated to treatment by a legal authority.
Randomization and Masking
Eligible subjects were randomly assigned to double-blind treatment with either gabapentin (1200 mg/day) or placebo, in a 1 : 1 ratio, on the basis of a computer-generated randomization code. Subjects, care providers, and those assessing outcomes were blinded to the identity of drug assignment. Gabapentin was purchased and over-encapsulated to match placebo capsules. The randomization code was kept by the study pharmacist, who provided subjects with a 1-week supply of medication in a blister card package at each weekly study visit. For the placebo group, each package contained two placebo capsules taken three times a day. For the active medication group, each package contained two capsules taken three times daily according to the following titration and dosing schedule: day 1: one 300 mg capsule in the evening; day 2: one 300 mg capsule in the morning and evening; day 3: one 300 mg capsule in the morning, at midday, and in the evening; day 4: one 300 mg capsule in the morning and at midday and two 300 mg capsules in the evening. Subjects maintained the 1200 mg/day dose until week 11. At that time, subjects were titrated off active medication by substituting one placebo capsule for one capsule of active medication per day, in the reverse order of the initial dose titration, with all subjects receiving only placebo by the end of week 12. Subjects returned their blister cards at each weekly study visit for drug accountability and compliance review. Correct drug assignment and ingestion of active drug were verified, retrospectively, by determining gabapentin concentration in plasma samples obtained at the week 2 study visit. Samples were frozen and shipped at the end of the study for analysis by gas chromatography/mass spectrometry in the laboratory of Mr Thomas Cooper (Nathan Kline Institute, Orangeburg, NY).
Visits and Assessments
Visits took place at screening (week −1), baseline (week 0), and weekly throughout the 12-week double-blind phase with a follow-up visit 1 week after the end of the double-blind treatment (week 13). Each study visit included assessments of cannabis use and withdrawal, and safety evaluations, as described below. Additionally, medical clearance by study physicians (MA, AB, MK, SR, and FS) included an electrocardiogram, pregnancy test and complete blood count with differential, urinalysis, blood chemistry, and physical exam. Concurrent with study medication, manual-guided, abstinence-oriented individual counseling was provided weekly throughout the trial by study clinicians (KB, RC, and SQ). Counseling components included motivation enhancement techniques to facilitate setting a quit date and cognitive behavioral techniques aimed at identifying and coping with relapse risk. Participants were also permitted to attend any self-help groups or psychosocial therapies they found beneficial; such attendance was documented at each study visit.
Cannabis Use Measures
Primary efficacy endpoints for marijuana use were the number of grams of marijuana smoked per week, derived from the daily record of self-reported cannabis use obtained by the Timeline Followback Interview (Fals-Stewart et al, 2000), and THC metabolite concentrations derived from weekly urine toxicology.
Our subjects smoked marijuana in a variety of forms, eg, joints, blunts, bongs, and pipes, which can vary substantially in size. To standardize these amounts, we calculated grams of marijuana smoked per week, based on guidelines set forth by the California Department of Drug Programs (www.adp.ca.gov/marijuana): 1 ounce equals 28.5 grams; an average joint size is 1 gram, with approximately 28 joints in an ounce; an ‘Eighth' equals 3.5 grams; a ‘Dime Bag' has a $10 value and equals 3/4 grams; a ‘Zip' or ‘O' equals 1 ounce; a ‘Quad' or ‘Quarter' equals 7 grams. To support the Timeline Followback Interview in determining number of grams smoked per week, subjects were instructed to use Smoking Diaries to record how much marijuana was obtained at a time, how many days that amount lasted, and what amount, if any, was shared with others. Urine toxicology results were analyzed by Lab Corp using gas chromatography-mass spectrometry. Levels of THCCOOH, the primary metabolite of THC, were normalized to creatinine (CN) and reported as a ratio, CN-THCCOOH, to control for the variability in drug measurement attributable to urine dilution (Fraser and Worth, 1999).
Secondary measures included the number of days per week of marijuana use based on the Timeline Followback Interview and the weekly point prevalence of new marijuana use based on an increase of ⩾0.5 in CN-THCCOOH from the prior level. In chronic daily marijuana users, THCCOOH concentrations decrease rapidly at the start of abstinence, similar to occasional marijuana smokers, but then the rate of decrease slows, with an extended detection window of up to 30 days or more, making it difficult to differentiate between new marijuana use and residual cannabinoid excretion using absolute cutoff values. Alternatively, an increase in CN-THCCOOH ⩾0.5 from the preceding value has been found to be a valid and reliable indicator of new marijuana use (Huestis and Cone, 1998). Further confirmation of new marijuana use was provided by a more complex algorithm that considers the time interval between specimens as well as change from the prior specimen, with reference data selected for intervals ⩾96 hours and at the 95% limit of detection (Smith et al, 2009).
Cannabis Withdrawal Measures
Cannabis withdrawal symptoms were assessed by the Marijuana Withdrawal Checklist (Budney et al, 1999), in which subjects rate severity of each symptom as 0 (not at all), 1 (mild), 2 (moderate), or 3 (severe). The craving item from the checklist was examined as a measure of marijuana craving. Mood-associated symptoms were more specifically evaluated with the Beck Depression Inventory II (Beck et al, 1996). Multiple components of sleep disturbance were assessed using the Pittsburgh Sleep Quality Index, which was modified for weekly administration, with total scores >5 indicating sleep disturbance (Buysse et al, 1989). All marijuana use and withdrawal measures were obtained at intake, baseline, and weekly during the 12-week double-blind treatment phase, and at week 13 follow-up.
Cannabis Consequences Measures
Treatment effects on the negative consequences associated with marijuana dependence may support the clinical significance of changes found on primary measures of marijuana use. Thus, cannabis-related physical, psychological, social, and functioning problems were assessed using the Marijuana Problems Scale at baseline and at the end of treatment (week 12) (Stephens et al, 2000). The last 38 subjects enrolled were further assessed to evaluate any changes in executive function associated with gabapentin during the first 4 weeks of treatment. Executive functioning was assessed using three subtests from the Delis–Kaplan Executive Function System—trail making test, color–word interference test, and verbal fluency test—at baseline and week 4 (Delis et al, 2001).
Safety Assessments
Vital signs and the Systematic Assessment for Treatment Emergent Events-General Inquiry (SAFTEE-GI) were collected at every weekly study visit to assess any adverse effects of treatment (Levine and Schooler, 1986). Breath alcohol concentration and observed urine screens for drugs of abuse were collected at every visit. Specimens for blood chemistry and urinalysis were obtained at the end of the study. Subjects were evaluated on study outcome measures 1 week (week 13) after study completion to determine resolution of any adverse drug experiences and to assess for any rebound in drug use, craving, or symptoms of withdrawal.
Data Analysis
Baseline demographic and clinical characteristics were compared between groups using χ2 for categorical variables and t-tests for continuous variables. Mixed-effect modeling using PASW 17.0 software (IBM Corp., Armonk, NY) was applied to tests of primary hypotheses (Norusis, 2008; West, 2009), using the intention to treat (n=50, 25 per group) sample of participants, and assuming that missing observations were missing at random. Individual mixed-effect modelings were estimated for each marijuana use and withdrawal measure, with all models centered at week 12 and including the baseline value of the dependent variable as a covariate. Each model assumed unstructured covariance for random effects and related measures. The mixed-effects modeling technique accommodates a correlated design structure (allowing for within subject, ie, repeated measures), and unbalanced data sets, where cells may be missing data and data collected from individuals may not be collected at the same time points (Gueorguieva and Krystal, 2004; Laird and Ware, 1982; Xue et al, 2010). This technique allows the use of all available data without the need to replace missing values or make assumptions concerning post-study marijuana use in subjects who discontinue treatment early. For the purposes of this proof-of-concept study, we are interested in detecting whether treatment with gabapentin affects marijuana use and/or withdrawal symptoms relative to placebo. Therefore, results are reported as F values from type III tests of fixed effects (Levin et al, 2011). Additionally, treatment effects on secondary measures of new marijuana use based on CN-THCCOOH were assessed with generalized estimating equations and results reported as the Wald χ2 statistic (Huestis and Cone, 1998; Smith et al, 2009). Overall change on tests of executive function and Marijuana Problem Scale subtest scores was compared between groups using a t-test analysis of change scores. Baseline variables were evaluated as predictors of study completion using binary logistic regression. All tests were two-tailed with an alpha <0.05, which was considered statistically significant.
RESULTS
Subjects
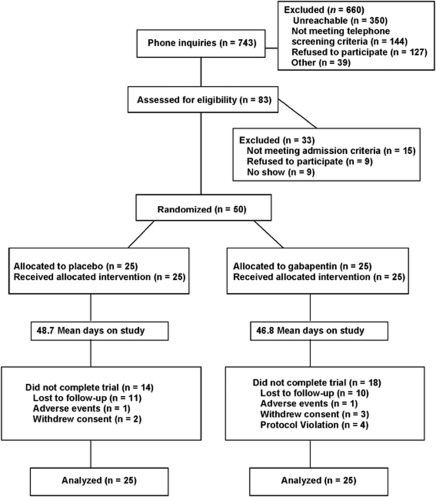
Recruitment for this study generated 743 phone inquiries (Figure 1). A face-to-face intake evaluation then was conducted with 83 individuals to yield the desired sample size of 50 randomized subjects. Primary reasons for non-randomization of screened subjects were the presence of excluded psychiatric disorders (n=12), medical disorders (n=4), or failure to return to clinic (n=9). Treatment groups did not differ on any baseline demographic nor clinical variable, as shown in Table 1.
Figure 1.
CONSORT flow diagram.
Table 1. Baseline (week −1) demographic and clinical characteristics.a.
| Characteristic | Total N=50 | Placebo N=25 | Gabapentin N=25 | p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 33.9 (9.7) | 34.4 (10.8) | 33.4 (8.5) | 0.72 |
| Male | 44 (88%) | 21 (84%) | 23 (92%) | 0.39 |
| White, non-Hispanic | 38 (76%) | 19 (76%) | 19 (76%) | 0.76 |
| Education, years | 14 (1.9) | 14.2 (1.9) | 13.6 (1.8) | 0.35 |
| College degree | 14 (28%) | 8 (32%) | 6 (24%) | 0.61 |
| Full-time employed | 31 (62%) | 13 (52%) | 18 (72%) | 0.36 |
| Married | 20 (40%) | 10 (40%) | 10 (40%) | 0.86 |
| Clinical assessments | ||||
| BDI-II (range 0–63) | 8.8 (5.1) | 9.6 (5.7) | 8.0 (4.5) | 0.29 |
| PSQI total score (range 0–21) | 5.9 (3.1) | 5.7 (3.1) | 6.0 (3.1) | 0.48 |
| MWC total score (range 0–84) | 14.2 (8.1) | 13.6 (8.3) | 14.8 (7.8) | 0.61 |
| MWC craving score (range 0–3) | 2.0 (0.8) | 1.9 (0.8) | 2.1 (0.7) | 0.44 |
| MPS total score (range 0–38) | 10.4 (5.9) | 10.5 (6.6) | 10.3 (5.2) | 0.88 |
| Body mass index | 27.4 (4.9) | 26.6 (4.5) | 28.1 (5.3) | 0.27 |
| Cannabis history | ||||
| MJ grams/weekb | 11.0 (18.5) | 9.0 (10.3) | 13.0 (24.1) | 0.46 |
| Urinary CN-THCCOOH | 684 (599) | 687 (371) | 682 (771) | 0.98 |
| Days of MJ abstinence prior to randomization | 0.9 (0.2) | 1.0 (20) | 0.9 (28) | 0.58 |
| Years daily MJ smoking | 11.6 (8.0) | 11.7 (8.5) | 11.5 (7.6) | 0.94 |
| Age at first MJ use | 14.5 (3.5) | 15.2 (2.6) | 13.8 (4.1) | 0.14 |
| Dollars spent/week on MJb | 86.5 (73.0) | 77.1 (48.0) | 96.0 (91.5) | 0.37 |
| Work days missed due to MJb | 2.6 (14.1) | 1.0 (3.0) | 4.1 (19.8) | 0.45 |
| Other substance history | ||||
| Cigarette smoker | 12 (24%) | 7 (28%) | 5 (20%) | 0.81 |
| Parental substance abuse | 20 (40%) | 9 (36%) | 11 (44%) | 0.39 |
Abbreviations: MJ, marijuana; BDI-II, Beck Depression Inventory-II; PSQI, Pittsburgh Sleep Quality Index; MWC, Marijuana Withdrawal Checklist; MPS, Marijuana Problems Scale; CN-THCCOOH, THCCOOH to creatinine ratio.
Values given are mean (SD) unless otherwise specified as number (%).
90 days prior to baseline.
Randomized subjects included 44 males (88%) and 6 females (12%), with a mean age of 33.9 (±9.7) years. Subjects typically began smoking marijuana at 14.5 (±3.5) years of age, had an average of 11.6 (±8.0) years of daily marijuana use, and were smoking an average of 11.0 (±18.5) grams per week of marijuana in the 90 days prior to randomization. On average, subjects met criteria for 6 (±1.0) of 7 possible DSM-IV criteria (of which, 3 are required to meet diagnostic criteria) for current cannabis dependence (Supplementary Table S1). Subjects endorsed an average of 9.7 (±4.6) withdrawal symptoms with a 1.5 (±1.7) (mild-to-moderate) level of severity for a total score of 14.2 (±8.1) on the Marijuana Withdrawal Checklist at their screening visit (see Supplementary Table S2).
Cannabis Use
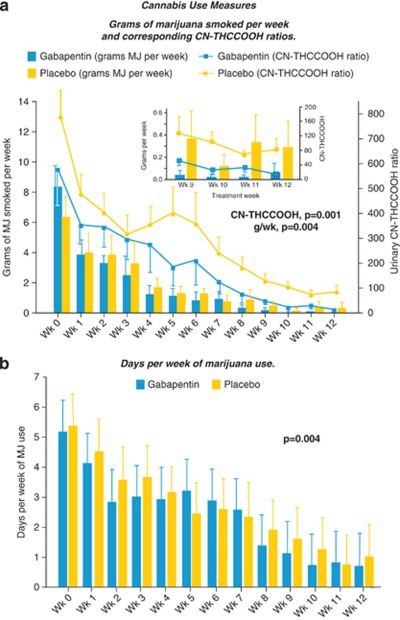
Gabapentin had a significant effect in decreasing marijuana use over the course of treatment, relative to placebo (Figure 2a). Those significant reductions were demonstrated using both the standardized self-report Timeline Followback Interview measure of grams of marijuana smoked per week (F=8.8, df=140, p=0.004), as well as through biochemical analysis of urinary CN-THCCOOH levels (F=12.2, df=216, p=0.001). There was no difference in slope between these two measures of marijuana use over the course of the study (Measure × Week: F=0.68, df=12, p=0.764) and the mean proportional change in both measures of use was nearly identical from study entry to study completion, with a mean difference of 0.002 (t=0.028, df=12, p=0.983). Gabapentin relative to placebo also significantly decreased the number of days of marijuana use per week (F=8.66, df=96, p=0.004; Figure 2b) and decreased the weekly point prevalence of new marijuana use (Wald χ2=8.2, p=0.004), applying the criteria of an increase ⩾50% in CN-THCCOOH from the earlier value (Huestis and Cone, 1998). Additionally, the more complex algorithm (Smith et al, 2009) that considers time between samples as well as change from the earlier specimen using normative data for intervals ⩾96 hours and at the 95% limit of detection likewise yielded a significant effect of gabapentin relative to placebo (Wald χ2=16.3, p<0.001).
Figure 2.
Effects of gabapentin vs placebo on cannabis use measures over the 12-week course of treatment (bars=SEM). (a) Grams of marijuana smoked per week and corresponding CN-THCCOOH ratios. Inset: Details for weeks 9 through 12. (b) Days per week of marijuana use.
Cannabis Withdrawal
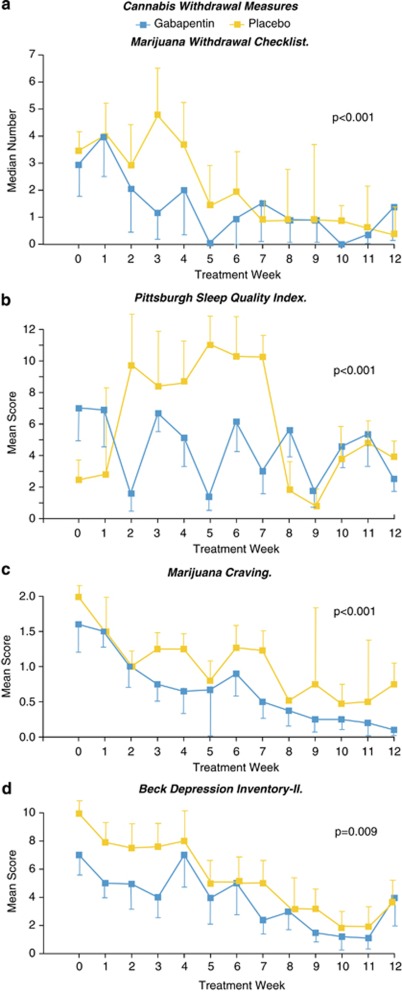
Consistent with the incremental reduction in cannabis use shown in Figure 2 and the long half-life of THC and its metabolites, acute withdrawal symptoms (as determined by items on the Marijuana Withdrawal Checklist) peaked at week 3 in the placebo group (Figure 3a). Those in the gabapentin group, however, experienced significant reductions in both the acute symptoms of withdrawal as well as in the more commonly persistent symptoms involving mood, craving, and sleep (Figure 3b–d). Over the course of treatment, gabapentin was associated with significantly greater improvement, compared with placebo, on the Marijuana Withdrawal Checklist (F=35.7, df=137, p<0.001), the craving item (F=15.2, df=245, p<0.001), the Beck Depression Inventory II (F=15.3, df=611, p=0.009), and the Pittsburgh Sleep Quality Index total score (F=17.0, df=150, p<0.001), as well as on the component scores for sleep duration (F=17.0, df=149.6, p<0.001), use of sleep medication (F=6.3, df=85.3, p=0.014), sleep efficiency (F=11.5, df=86.1, p<0.001), daytime dysfunction (F=6.5, df=208, p=0.012), and sleep disturbance (F=6.0, df=209, p<0.001).
Figure 3.
Effects of gabapentin vs placebo on cannabis withdrawal variables over the 12-week course of treatment (bars=SEM). (a) Median number of Marijuana Withdrawal Checklist items endorsed on study. (b) Mean Pittsburgh Sleep Quality Index total scores. (c) Mean marijuana craving scores. (d) Beck Depression Inventory-II scores.
Cannabis Consequences
The gabapentin group, but not the placebo group, showed significant reductions from baseline to the end of treatment on the Marijuana Problems Scale total score (gabapentin change score=3.4, 95% CI 0.8–6.0, p=0.02). Gabapentin was associated with significantly greater improvement, compared with placebo, on subscales assessing marijuana-related psychological problems (t=2.5, df=12, p=0.028), and marijuana-related physical problems (t=2.3, df=10, p=0.046).
Executive function was measured at baseline in a subset of the last 38 subjects enrolled and compared with standardized normative data (see Supplementary Table S3). Although the IQ estimates of cannabis subjects were in the average range (105.2 (8.5)) based on the Reading subtest of the Wide Range Achievement Test-3 (Wilkinson and Robertson, 2006), and despite better than average performance on tests of simple attention and motor speed, cannabls subjects had significantly impaired performance on baseline measures of visual-motor functioning and cognitive flexibility on the trail making test, and in fluency, flexibility, and inhibition on the verbal fluency test, relative to normative data. The cannabis subjects were able to inhibit a prepotent response on a simple test of inhibition; however, as the task became more complex, their performance declined significantly more than did that of the normative sample.
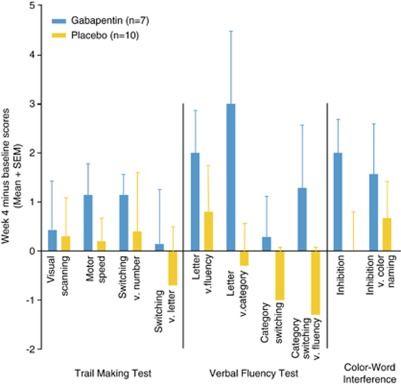
Baseline neurocognitive performance was compared with week 4 performance in subjects completing both assessments (n=17). Performance on each D-KEFS task improved numerically (but not significantly) from baseline to week 4 in the gabapentin group, whereas the placebo group's performance remained similar to baseline or even declined (Figure 4). Composite scores to reflect overall performance were created by summing the standard scores for each time point. Overall change was assessed by subtracting baseline from week 4 composite scores. Analysis of composite change scores showed overall improvement in performance across cognitive measures was significantly greater for gabapentin-treated subjects compared with those receiving placebo (t=−2.4, df=15, p=0.029).
Figure 4.
Change scores on Delis–Kaplan Executive Function System tests from baseline to week 4. Individual subtest scores were not significantly different between groups; however, mean improvement on composite scores was significantly better for gabapentin vs placebo (t=−2.4, df=15, p=0.029). Higher scores (>0) indicate greater improvement.
Safety, Tolerability, and Concomitant Therapy
Gabapentin was well tolerated and without significant side effects. There were no deaths and no serious drug-related adverse events. Two subjects discontinued the study because of adverse events: one placebo subject showed abnormal lab values and one gabapentin subject complained of headache. There were no differences between groups in type of adverse events (Supplementary Table S4). Both groups were also similar in the number (1.4(±1.6)) and severity (1.6(±0.7); 1=mild, 2=moderate) of adverse events reported. In addition, groups did not differ in the number of adverse events associated with the first 2 weeks of treatment, when rates of dropout were highest. Groups did not differ in body weight, vital signs, and on measures from urinalysis and blood chemistry testing that took place over the course of treatment. There was no evidence of drug substitution; no subjects tested positive for alcohol using weekly breathalyzer assessments and, of the total number of observed urine drug screens collected in our study (n=400), 14 (3%) were positive for other drugs of abuse, primarily prescription drugs. Nine subjects attended individual therapy or self-help groups during the course of the study. Of these, four had only one visit. Attendance was not associated with drug group or any outcome measure. Week 13 follow-up showed complete resolution of drug-related adverse events and no evidence of rebound in cannabis use, craving, or other symptoms of withdrawal.
Compliance and Treatment Retention
Mean rate of medication compliance, defined as number of pills taken divided by number prescribed, was 93.5% and was identical across treatment groups. Rate of study completion (36%) and average time on study (6.4 weeks) did not differ between treatment groups. Nine (18%) subjects were lost to follow-up immediately after randomization and did not return the following week (see Supplementary Figure S1 for rates of retention). Reasons for termination did not differ between groups.
A binary logistic regression evaluated the baseline variables in Table 1 and Supplementary Table S3 and identified a model of five variables that predicted study completion with 96.3% (p⩽0.001) accuracy (1 false positive): (1) years of daily marijuana use, (2) age at first marijuana use, (3) Marijuana Withdrawal Checklist score, and scores on the (4) Inhibition vs Color Naming and (5) Switch vs Letter Sequence tests of executive function.
DISCUSSION
This phase IIa proof-of-concept pilot study of gabapentin expands the limited amount of information about pharmacological treatment of cannabis dependence and withdrawal and represents an innovative approach to the treatment of cannabis dependence. Our study provides preliminary evidence that gabapentin (a) was associated with less marijuana use compared with placebo in a sample of unpaid treatment-seeking community-dwelling volunteers with cannabis dependence evaluated over a period of 12 weeks; (b) was found to attenuate withdrawal symptoms, including craving and disturbances in mood and sleep; and (c) was associated with greater overall improvement in marijuana-related problems and in tasks related to neurocognitive executive functioning, compared with placebo. Counseling alone (ie, placebo) resulted in less effective treatment of cannabis use and withdrawal, and no improvement in executive function or marijuana-related problems. The beneficial effects of gabapentin were obtained with an acceptable safety profile.
Subjects were asked at study termination whether they believed they were treated with gabapentin or placebo. Responses indicate that neither group had >50% chance of correctly guessing the identity of their study drug. Similarly, careful adherence to the randomization protocol and maintenance of the double-blind conditions until all subjects had completed treatment indicates that the significant improvements associated with gabapentin are not likely to be a result of investigator bias. Manual-guided weekly counseling for all participants by experienced clinicians ensured that subjects received equal amounts of attention. Subjects were not paid and were self referred from the community, increasing the generalizability of study findings to clinical practice. Furthermore, all subjects met diagnostic criteria for cannabis dependence, indicating a degree of severity related to cannabis use for which an individual is likely to seek treatment. Our sample's continued access to marijuana while living in the community makes our results highly relevant to practitioners treating outpatients with cannabis dependence. State-of-the-art measures were employed for assessing study outcomes (Donovan et al, 2011), which reflect DSM-IV criteria for cannabis dependence and include assessment of cannabis use, withdrawal, and negative consequences. Our two, quite differently obtained (biochemical and self report), measures of cannabis use are consistent with hypothesized effects of treatment and highly concordant with each other, lending support to the validity of study results. Similarly, the reduction in overall withdrawal severity was concordant with independent assessments of sleep and mood disturbance in our sample, and our sleep data were consistent with that of a polysomnography study in heavy marijuana users undergoing abstinence (Bolla et al, 2010). The clinical relevance of the effects of gabapentin on cannabis use and withdrawal over the treatment course is supported by the improvement in executive function and marijuana-related problems in the gabapentin but not the placebo-treated group.
A limitation of this pilot study was the relatively small number of subjects in each group. The encouraging preliminary results we report here require replication in an adequately powered trial. Another limitation was a high rapid dropout rate, making outcome assessments unavailable for 18% of the sample. Our dropout rate was consistent with that of earlier non-agonist clinical trials of cannabis dependence, and with the large proportion of individuals who were lost following their original phone inquiry or who failed to return for the visit following their intake interview. Our analysis of baseline predictors of dropout found that individuals' impaired ability to inhibit impulses and process complex information were significant predictors for leaving treatment, as were the age at first marijuana use, years of daily marijuana use, and marijuana withdrawal severity. The risk for premature treatment termination posed by these cognitive factors and cannabis dependence severity underscores the importance of developing safe and effective pharmacological treatments for reducing marijuana use and withdrawal severity and for optimizing cognitive executive function. Our data suggest such pharmacological treatment may help individuals under treatment take better advantage of behavioral therapy aimed at supporting recovery, as gabapentin combined with abstinence-oriented counseling resulted in outcomes superior to those of counseling combined with placebo. The improvement in executive function found with gabapentin may represent a direct effect of the drug (Salinsky et al, 2005) and/or an indirect effect gained by decreasing marijuana withdrawal and use. Cognitive rehabilitation techniques directed at improving identified deficits that do not reverse with marijuana abstinence may further improve treatment retention and overall functioning in cannabis dependence (Sofuoglu et al, 2010).
Another potential limitation in this small proof-of-concept study was the use of a single, fixed dose of 1200 mg/day of gabapentin. This dose is in the midrange of approved dosing (900–1800 mg/day) for other indications (epilepsy and pain) and was chosen based on the significant reductions in craving and sleep disturbance, with no safety or dependence risks obtained in an earlier human laboratory study (Mason et al, 2009). Gabapentin 1200 mg/day was found to be safe and well tolerated, with high rates of medication compliance in the current study. Higher doses may result in a greater effect size. Gabapentin is not appreciably metabolized and does not interfere with the metabolism of commonly administered drugs (Neurontin package insert, 2007). It would not be expected to influence nor be influenced by marijuana use through hepatic-mediated mechanisms. Future trials may address the risks and benefits of higher doses. Of note, somnolence is a commonly reported adverse event in gabapentin pain and epilepsy trials (Neurontin package insert, 2007), but was not a common complaint among our cannabis-dependent subjects. Conversely, our subjects were experiencing sleep disturbance that significantly improved with gabapentin relative to placebo, as did cognition.
Current pharmacotherapies for addiction, in general, focus largely on agonist substitution or antagonist strategies that can be associated with problems of dependence and non-compliance, respectively. The significant effects of gabapentin compared with placebo on decreasing both cannabis use and withdrawal, with an acceptable safety profile and no evidence of dependence, suggest gabapentin may offer the most promising treatment for cannabis withdrawal and dependence studied to date. Our results support a novel pharmacotherapeutic strategy for the treatment of cannabis dependence that is aimed at treating the underlying neural dysregulation in stress systems associated with chronic heavy cannabis use and withdrawal. This alternative conceptual framework for the treatment of cannabis dependence also has implications for the treatment of drug addiction in general. The clinical relevance of the effects of gabapentin on cannabis use and withdrawal is supported by significantly greater improvement in executive function and in marijuana-related physical and psychological problems with gabapentin relative to placebo. The beneficial effects of gabapentin were obtained with an acceptable safety profile. Taken together, the preliminary results provided by this proof-of-concept study suggest that gabapentin may offer a valuable addition to the treatment of cannabis dependence and withdrawal that merits further study.
Acknowledgments
This project was funded by National Institute of Drug Abuse grants DA020766 and DA024194.
Dr Mason's work is funded by the National Institutes of Health. Over the past 3 years, she has received compensation as a member of the scientific advisory board for Addex Pharmaceuticals and the Lohocla Research Corporation, and as a consultant for Johnson and Johnson Pharmaceutical Research and Development, LLC; Lilly USA, LLC; and Merck Serono. A patent has been filed based in part on this work. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- American Psychiatric Association 2000Diagnostic and Statistical Manual of Mental Disorders4th edn, Text Revision.American Psychiatric Press: Washington, DC [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lesage SR, Gamaldo CE, Neubauer DN, Wang NY, Funderburk FR, et al. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med. 2010;11:882–889. doi: 10.1016/j.sleep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Caravati EM.2003Marijuana and other cannabinoidsIn: Dart RC (ed).Medical Toxicology3rd edn.Lippincott Williams & Wilkins: Philadelphia, PA [Google Scholar]
- Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double-blind comparison of nefazodone, bupropion-SR, and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18:53–64. doi: 10.1080/10550490802408936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E. Manual for the Delis-Kaplan Executive Function System (D-KEFS) Psychological Corporation: San Antonio, TX; 2001. [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, et al. 2011Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials Addiction(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Fraser AD, Worth D. Urinary excretion profiles of 11-nor-9-carboxy-delta9 tetrahydrocannabinol: a delta9-THCCOOH to creatinine ratio study. J Anal Toxicol. 1999;23:531–534. doi: 10.1093/jat/23.6.531. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Katzow JJ, Desai SP, Goodwin FK. Gabapentin treatment of mood disorders: a preliminary study. J Clin Psy. 1998;59:426–429. doi: 10.4088/jcp.v59n0805. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated- measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Harden CL, Lazar LM, Pick LH, Nikolov B, Goldstein MA, Carson D, et al. A beneficial effect on mood in partial epilepsy patients treated with gabapentin. Epilepsia. 1999;40:1129–1134. doi: 10.1111/j.1528-1157.1999.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J Anal Toxicol. 1998;22:445–454. doi: 10.1093/jat/22.6.445. [DOI] [PubMed] [Google Scholar]
- Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psy. 2000;157:151. doi: 10.1176/ajp.157.1.151. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kandel DB, Volkow ND.2008Psychiatric pathophysiology: addictionIn: Tasman A, Kay J, Lieberman J, First MB, Maj M (eds). Psychiatry3rd edn. John Wiley & Sons: West Sussex, England [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side' of drug addiction. Nature Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, et al. Pharmacotherapy for marijuana dependence: a double-blind, placebo-controlled pilot study of divalproex sodium. Am J Addict. 2004;13:21–32. doi: 10.1080/10550490490265280. [DOI] [PubMed] [Google Scholar]
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacol Bull. 1986;22:343–381. [PubMed] [Google Scholar]
- Lo HS, Yang CM, Lo HG, Lee CY, Ting H, Tzang BS. Treatment effects of gabapentin for primary insomnia. Clin Neuropharmacol. 2010;33:84–90. doi: 10.1097/WNF.0b013e3181cda242. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Berrendero F, Ozaita A, Robledo P. Neurochemical basis of cannabis addiction. Neuroscience. 2011;181:1–17. doi: 10.1016/j.neuroscience.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, Wahlquist AE, Simpson SA, et al. A placebo-controlled trial of buspirone for the treatment of marijuana dependence. Drug Alcohol Depend. 2009;105:132–138. doi: 10.1016/j.drugalcdep.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Killeen TK, Carpenter MJ, White KG, Brady KT. A placebo-controlled trial of atomoxetine in marijuana-dependent individuals with attention deficit hyperactivity disorder. Am J Addict. 2010;19:481–489. doi: 10.1111/j.1521-0391.2010.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurontin (gabapentin) [package insert LAB-0106-9.0]. Pfizer, New York. Revised January 2007.
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am J Addict. 2007;16:331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- Norusis MJ. SPSS Statistics 17.0 Advanced Statistical Procedures Companion. Prentice Hall Inc.: Upper Saddle River, NJ; 2008. [Google Scholar]
- Roberto M, Gilpin NW, O'Dell LE, Cruz MT, Morse AC, Siggins GR, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Storzbach D, Spencer DC, Oken BS, Landry T, Dodrill CB. Effects of topiramate and gabapentin on cognitive abilities in healthy volunteers. Neurology. 2005;64:792–798. doi: 10.1212/01.WNL.0000152877.08088.87. [DOI] [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine- normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sugarman DE, Carroll KM. Cognitive function as an emerging treatment target for marijuana addiction. Exp Clin Psychopharmacol. 2010;18:109–119. doi: 10.1037/a0019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended vs brief treatments for marijuana use. J Consult Clin Psychol. 2000;68:898–908. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2006 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-32, DHHS Publication No. SMA 07-4293) SAMHSA: Rockville, MD; 2007. [Google Scholar]
- United Nations Office on Drugs and Crime . World Drug Report 2010. United Nations: New York, NY; 2010. [Google Scholar]
- West BT. Analyzing longitudinal data with the linear mixed models procedure in SPSS. Eval Health Prof. 2009;32:207–228. doi: 10.1177/0163278709338554. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test-4 Professional Manual. Psychological Assessment Resources, Inc: Lutz, FL; 2006. [Google Scholar]
- Xue X, Gange SJ, Zhong Y, Burk RD, Minkoff H, Massad LS, et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev. 2010;19:159–169. doi: 10.1158/1055-9965.EPI-09-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.