Abstract
The dopamine transporter (DAT) is the primary site of action for psychostimulant drugs such as cocaine, methylphenidate, and amphetamine. Our previous work demonstrated a reduced ability of cocaine to inhibit the DAT following high-dose cocaine self-administration (SA), corresponding to a reduced ability of cocaine to increase extracellular dopamine. However, this effect had only been demonstrated for cocaine. Thus, the current investigations sought to understand the extent to which cocaine SA (1.5 mg/kg/inf × 40 inf/day × 5 days) altered the ability of different dopamine uptake blockers and releasers to inhibit dopamine uptake, measured using fast-scan cyclic voltammetry in rat brain slices. We demonstrated that, similar to cocaine, the DAT blockers nomifensine and bupropion were less effective at inhibiting dopamine uptake following cocaine SA. The potencies of amphetamine-like dopamine releasers such as 3,4-methylenedioxymethamphetamine, methamphetamine, amphetamine, and phentermine, as well as a non-amphetamine releaser, 4-benzylpiperidine, were all unaffected. Finally, methylphenidate, which blocks dopamine uptake like cocaine while being structurally similar to amphetamine, shared characteristics of both, resembling an uptake blocker at low concentrations and a releaser at high concentrations. Combined, these experiments demonstrate that after high-dose cocaine SA, there is cross-tolerance of the DAT to other uptake blockers, but not releasers. The reduced ability of psychostimulants to inhibit dopamine uptake following cocaine SA appears to be contingent upon their functional interaction with the DAT as a pure blocker or releaser rather than their structural similarity to cocaine. Further, methylphenidate's interaction with the DAT is unique and concentration-dependent.
Keywords: dopamine, dopamine transporter, cocaine, self-administration, voltammetry, methylphenidate
INTRODUCTION
The primary mechanism for the acute euphorigenic and reinforcing actions of cocaine is its ability to increase extracellular dopamine (DA) levels via inhibition of DA uptake at the DA transporter (DAT), particularly at mesolimbic nerve terminals (Ritz et al, 1987, 1988; Kuhar et al, 1991; Peoples et al, 1998). In addition to the acute pharmacological effects of cocaine, chronic administration has been shown to modify presynaptic DA terminal function. For example, D2-type DA autoreceptor numbers and activity, tyrosine hydroxylase (TH) levels, DAT cell surface expression, and maximal rates of DA uptake (Vmax) are all changed by chronic or repeated cocaine exposure (Daws et al, 2002; Collins and Izenwasser, 2002; Self et al, 2004; Peraile et al, 2010). The direction and magnitude of these changes, DA uptake in particular, appear contingent upon time course, withdrawal period, and route of cocaine administration, among other factors (Ramamoorthy et al, 2010; Peraile et al, 2010; Mandt and Zahniser, 2010).
Given that basal extracellular DA levels as measured by microdialysis are influenced by release, diffusion and uptake by perisynaptic DATs at some distance from release sites, cocaine-induced alterations in the regulation of DA release and uptake will, in turn, have a long-term effect on basal DA levels (Chefer et al, 2003; Mateo et al, 2005; Ferris et al, 2011). Indeed, microdialysis studies have found decreases in basal extracellular DA levels and reductions in cocaine-induced DA release as a function of cocaine experience (Ferris et al, 2011; Mateo et al, 2005; Hurd et al, 1989). In addition to long-term alterations in DA release and uptake kinetics and corresponding DA tone, we have demonstrated a reduced ability of the DAT to be inhibited by cocaine, or cocaine tolerance, following high-dose cocaine self-administration (SA) (Ferris et al, 2011). Tolerance to cocaine effects at the DAT is induced by several schedules of SA, including discrete trial (Mateo et al, 2005) and fixed-ratio (Ferris et al, 2011) schedules, and persists for several weeks following cessation of cocaine intake (Ferris et al, 2011). Notably, the reduced sensitivity of the DAT to cocaine appears to be independent of changes in DAT number, or maximal uptake rate, Vmax. Indeed, robust decreases in cocaine sensitivity are present regardless of whether the Vmax for DA uptake (related to surface DAT number) increases, decreases, or remains unchanged following cocaine intake and cessation (Mateo et al, 2005; Ferris et al, 2011).
To date our knowledge of cocaine SA-induced DAT sensitivity changes is limited to cocaine itself; an understanding of the extent to which this effect generalizes to other compounds that bind to the DAT is lacking. Such understanding is particularly important for investigations into the mechanism(s) underlying reduced potency of cocaine at the DAT. Discovery of structural and/or functional characteristics of compounds that confer either sensitivity or insensitivity to changes in DAT function following cocaine SA will highlight domains of the DAT that may be particularly promising in the development of therapeutic targets for cocaine addiction. Therefore, the purpose of this research was to examine whether the reduced potency of cocaine following cocaine SA would extend, if at all, to other compounds based on their structural and/or functional similarity to cocaine. Therefore, we selected four DA uptake blockers (cocaine, nomifensine, bupropion, and methylphenidate (MPH)), four amphetamine-based DAT substrates/DA releasers (amphetamine, methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA), and phentermine), and one non-amphetamine-based releaser (4-benzylpiperidine (BPP)) for investigation of their ability to inhibit uptake of DA in naïve and cocaine-experienced rats. Taken together, these compounds possess varying degrees of either structural or functional similarity to cocaine and amphetamine. MPH is a compound of particular interest to this study, because it has structural similarity to DAT substrates, but is functionally more similar to uptake blockers (Sonders and Amara, 1997; Wayment et al, 1999; Dar et al, 2005).
MATERIALS AND METHODS
Subjects
Male, Sprague–Dawley rats (375–400 g; Harlan Laboratories) were used as subjects (N=70; naïve, n=41; cocaine SA, n=29), and multiple slices from cocaine SA animals were used to minimize the number of animals necessary for cocaine SA procedures (Nslices=88; naïve, n=41; cocaine SA, n=47). When multiple slices were used, the drug was counterbalanced so that both blockers and releasers were represented within the same animal. All animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
SA Procedures
The procedures for implantation of cannulae into the jugular vein and cocaine SA have been described previously (Mateo et al, 2005). Following surgery, animals were singly housed within chambers outfitted for both housing and SA procedures so that animals remained in chambers for the duration of SA procedures. Each animal was maintained on a reverse light cycle (0300 hours lights off; 1500 hours lights on), and all SA procedures occurred during the active/dark cycle (900–1500 hours). Each lever press resulted in the delivery of 1.5 mg/kg cocaine over 4 s. This dose was chosen because it is the most reinforcing dose, at the top of the dose–response curve in measures of reinforcing efficacy, and preferred over lower doses in choice studies (Richardson and Roberts, 1996). Concurrent with the start of each injection, the lever retracted and a stimulus light was activated for 20 s to signal a time-out period during which time responses produced no programmed consequence. The session was terminated after 40 injections or after 6 h, whichever occurred first. Typically under these conditions, animals acquired a stable pattern of cocaine SA and administered the full 40 injections allowed within 1–5 days. Once the animals reached the maximum number of injections allowed in a single session (ie, 40), they were required to self-administer 40 injections per day for five consecutive days before the voltammetry experiment.
Fast-Scan Cyclic Voltammetry
All voltammetry experiments were conducted in the morning (active/dark cycle) following final SA session (24 h from the start of the final session). Multiple coronal slices (400 μM) containing the nucleus accumbens (NAc) were prepared from each animal with a vibrating tissue slicer while immersed in oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl (126), KCl (2.5), NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), glucose (11), L-ascorbic acid (0.4) and pH was adjusted to 7.4. Once sliced, the tissue was transferred to the testing chambers containing bath aCSF (32°C), which flowed at 1 ml/min. After a 30-min equilibration period, a cylindrical carbon fiber microelectrode (100–200 μM length, 7 μM radius) and a bipolar stimulating electrode were placed into the core of the NAc. We selected the NAc core given dense innervations of DA nerve terminals and because it is a critical locus for the reinforcing and rewarding actions of cocaine. Further, our previous research has concentrated on plasticity of DATs in both the core and shell and demonstrated similar results. DA was evoked by a single, rectangular, electrical pulse (300 μA, 2 ms) and applied every 5 min. Extracellular DA was monitored at the carbon fiber electrode every 100 ms using fast-scan cyclic voltammetry (Kennedy et al, 1992) by applying a triangular waveform (−0.4 to +1.2 to −0.4V vs Ag/AgCl, 400 V/s). Once the extracellular DA response was stable (ie, did not exceed 10% variation in peak height for three successive stimulations), one of nine drugs (four blockers and five releasers) was applied cumulatively to the brain slice. The four DAT blockers were cocaine (0.3–30 μM), nomifensine (0.3–30 μM), bupropion (0.3–30 μM), and MPH (0.3–30 μM), whereas the five releasers were amphetamine (0.3–10 μM), methamphetamine (1–100 μM), MDMA (3–100 μM), phentermine (1–30 μM), and BPP (1–100 μM). The concentrations were chosen to equate inhibition constants in naïve animals whenever possible (John and Jones, 2007). Immediately following the completion of each concentration–response curve, recording electrodes were calibrated by recording their response (in electrical current; nA) to a known concentration of DA in aCSF (3 μM) using a flow-injection system. This value was then used to convert electrical current to DA concentration. To evaluate the effects of drugs, evoked levels of DA were modeled using Michaelis–Menten kinetics, as a balance between release and uptake (Wightman et al, 1988). Michaelis–Menten modeling provides parameters that describe the amount of DA released following stimulation (ie, the peak height of the signal), the maximal rate of DA uptake (Vmax), and inhibition of the ability of DA to bind to the DAT, or apparent uptake inhibition (apparent Km). For baseline modeling (pre-drug), we followed standard voltammetric modeling procedures by setting the apparent Km parameter to 160 nM for each animal based on well-established research on the affinity of DA for the DAT (Wu et al, 2001), whereas baseline Vmax values were allowed to vary as the baseline measure of rate of DA uptake. Indeed, while the affinity of DA for the DAT does not substantially vary from animal to animal, chronic cocaine SA has been shown to alter the Vmax by altering the number of DATs on the cell surface. Following drug application, apparent Km was then allowed to vary to account for changes in drug-induced DA uptake inhibition, while the respective Vmax value determined for that subject at baseline was held constant. The apparent Km parameter models the amount of DA uptake inhibition following a particular dose of drug rather than the explicit affinity of DA for the DAT (ie, Km) per se. All voltammetry data were collected and modeled using the Demon Voltammetry and Analysis Software (Yorgason et al, 2011). Baseline voltammetry data were compared across groups using one-way analysis of variance (ANOVA) using the Graphpad statistical software. Data obtained after administration of each drug was subjected to a two-way ANOVA, with experimental group and concentration of the drug as the factors. When significant main effects were obtained (p<0.05), differences between groups at each dose were tested using Bonferroni post-hoc tests.
RESULTS
Escalation in the Rate of Cocaine Intake Across Sessions
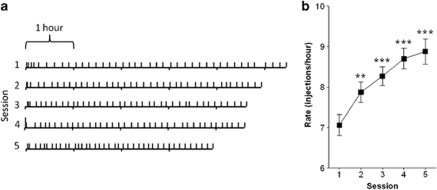
After animals achieved stable lever pressing behavior for cocaine, they were allowed to self-administer 40 injections per day for 5 days under an FR1 schedule of reinforcement. Figure 1a shows data from a representative animal depicting typical decreases in the inter-infusion interval over days, thus reducing the total session length. Figure 1b demonstrates a significant escalation of the rate of cocaine intake (F4, 76=24.75, p<0.0001).
Figure 1.
(a, Left panel) Event record of a representative animal self-administering cocaine intravenously. Each horizontal line represents a daily self-administration (SA) session. Each vertical line represents an injection of cocaine (1.5 mg/kg/inj). The top line shows acquisition of cocaine SA, with the animal demonstrating regular inter-injection intervals by the end of the 6 h session. The animal showed escalation of drug intake by self-administering 40 injections each day in successively shorter time periods. (b, Right panel) Significant escalation in the rate of intake shown in a group of 29 animals (**p<0.001, ***p<0.0001).
Cocaine SA Reduces Baseline-Stimulated Release and Slows Baseline Rate of DA Uptake
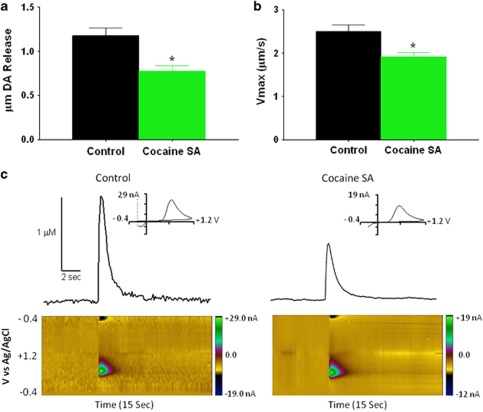
The baseline (pre-drug)-stimulated DA release and uptake parameters from each drug cohort were combined and analyzed to investigate cocaine SA-induced differences in baseline DA kinetics. As expected given previous work with this model (Ferris et al, 2011), baseline electrically stimulated DA release was significantly attenuated following cocaine SA (t86=3.76, p<0.001) as shown in Figure 2a and the representative traces in Figure 2c. In addition to blunted release, Figures 2b, c, and 4a (matched peak height) demonstrate that the maximal rate of DA uptake (Vmax) was significantly attenuated/slowed following cocaine SA (t86=4.08, p<0.0001).
Figure 2.
Cocaine self-administration (SA) significantly reduces electrically stimulated dopamine (DA) release (a) and slows the maximal rate of DA uptake (b; Vmax) in brain slices, as represented by the baseline means of all animals in the study acquired before drug application (*p<0.05). The representative trace in a naïve animal (c; left panel) exemplifies the higher peak height and faster uptake in these animals relative to the cocaine SA animal (c; right panel). The color plots represent the voltammetric currents (encoded in color in the z axis) plotted against the applied potential (y axis) and time (x axis). The background-subtracted cyclic voltammograms indicative of DA are shown as insets to the upper right of the concentration–time plots. These curves identify the detected analyte as DA. The colour reproduction of the figure is available at the Neuropsychopharmacology journal online.
Cocaine SA Reduces the Ability of DAT Blockers to Inhibit DA Uptake
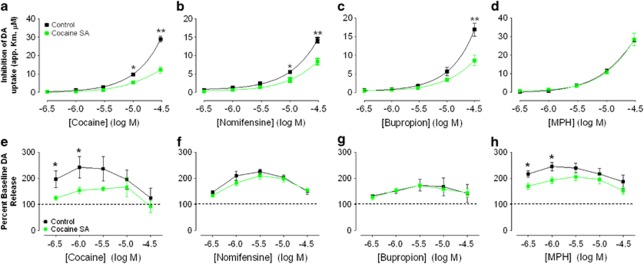
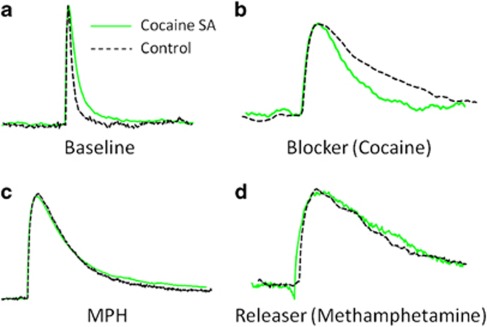
Multiple concentrations of cocaine, nomifensine, and bupropion were cumulatively added to brain slices of naïve animals and cocaine SA animals, while DA release and uptake kinetics were monitored. When cocaine was applied cumulatively to slices, a two-way mixed ANOVA using apparent Km (ie, DA uptake inhibition) as a dependent measure indicated a significant main effect of cocaine concentration (F4, 28=208, p<0.0001), a main effect of treatment (F1, 28=38.42, p<0.0001), and a Concentration × Treatment Interaction (F4, 28=36.14, p<0.0001). Indeed, while Figure 3a demonstrates that DA uptake inhibition increased in both groups, cocaine-induced uptake inhibition in cocaine SA animals was substantially reduced relative to naïve animals. Treatment comparisons of individual concentrations with Bonferroni correction demonstrated that both the 10 μM (p<0.05) and 30 μM (p<0.001) concentrations were significantly different. DA traces (matched for peak height) from individual animals shown in Figure 4 demonstrate a more rapid return to baseline in cocaine SA animals (Figure 4b).
Figure 3.
Cocaine self-administration (SA) significantly reduces the ability of multiple dopamine (DA) uptake blockers to inhibit DA uptake, with the exception of methylphenidate (MPH). The top row of graphs (a–d) demonstrates the effect of cocaine (a), nomifensine (b), bupropion (c), and MPH (d) on DA uptake, measured as apparent Km (y axis scaling varies across panels). Note that uptake inhibition is significantly reduced in animals with a history of cocaine SA (green squares) when compared with naïve animals (black squares) in panels a–c (**p<0.001; *p<0.05). When MPH is applied to the slice (d), however, the groups perfectly overlap, indicating no difference in the ability of MPH to inhibit DA uptake following cocaine SA. DA peak height for each concentration of cocaine (e), nomifensine (f), bupropion (g), and MPH (h), expressed as a percent of baseline peak height acquired before drug application. Blocker-induced increases in peak height (from baseline) are thought to largely represent increases in uptake inhibition when using brain slices. MPH, therefore, resembles cocaine in that normalized peak height (ie, uptake inhibition) at low concentrations is significantly higher in naïve animals (black squares) relative to cocaine SA animals (green squares), with no differences at higher concentrations. The colour reproduction of the figure is available at the Neuropsychopharmacology journal online.
Figure 4.
Representative dopamine (DA) traces (matched peak height) demonstrating no effect of cocaine self-administration (SA) on the ability of methylphenidate (MPH) (10 μM) to inhibit DA uptake, similar to the lack of effect demonstrated by releasers (methamphetamine (30 μM)), but not blockers (cocaine (10 μM)). Note that the descending limb of DA curve from animals with a history of cocaine SA (green traces) is slower and shifted to the right relative to naïve (black traces) at baseline (a). In addition, the descending limb of DA curve from animals with a history of cocaine SA is substantially reduced relative to naïve for cocaine (b), but not for MPH (c) and methamphetamine (d). The colour reproduction of the figure is available at the Neuropsychopharmacology journal online.
The effects of nomifensine and bupropion were almost identical to cocaine in that apparent Km data demonstrated a significant main effect of concentration (nomifensine, F4, 40=224, p<0.0001; bupropion, F4, 24=73.21, p<0.0001), a main effect of treatment (nomifensine, F1, 40=24.11, p<0.0001; bupropion, F1, 30=11.57, p<0.05), and a Concentration × Treatment Interaction (nomifensine, F4, 40=14.53, p<0.0001; bupropion, F4, 24=11.18, p<0.0001). Figure 3b and c demonstrate that DA uptake inhibition increased in both groups, but nomifensine- and bupropion-induced uptake inhibition in cocaine SA animals was substantially reduced relative to naïve animals. Treatment comparisons of individual concentrations with Bonferroni correction demonstrated that both the 10 μM (p<0.05) and 30 μM (p<0.001) concentrations were significantly different for nomifensine, whereas the 30 μM concentration (p<0.001) was significantly different for bupropion.
As there was no difference in apparent Km at the lowest cocaine concentrations of blockers tested, we investigated cocaine-induced changes in DA peak height. Because increases in DA peak height following application of blockers are thought to reflect DA uptake inhibition, any reduction in the ability of cocaine to increase peak height in animals with a history of cocaine SA would suggest a reduced ability to inhibit DA uptake. Indeed, low concentrations of DAT inhibitors often increase the peak height of signals before slowing the descending limb of the DA signal (Jones et al, 1995), explaining why effects on uptake inhibition manifest in the peak height before apparent Km. As expected, measures of DA peak height (expressed as percent baseline peak height) demonstrate that stimulated DA engendered an inverted U-shaped function across increasing doses of all blockers. Release data are expressed as a percent of baseline to account for any initial (pre-drug) differences in DA peak height that clearly influence the raw peak height of the dose response for each drug as shown with raw peak-height data in Supplementary Figure S1. Indeed, Figure 3e demonstrated a significantly smaller peak-height change in cocaine SA animals when the DA response following 300 nM (p<0.05) and 1 μM (p<0.05) cocaine was expressed as a percent baseline (pre-drug) response. Thus, cocaine SA animals demonstrated significantly reduced uptake inhibition across all concentrations, which was observed in at least one of two dependent variables (apparent Km in Figure 3a or peak height in Figure 3e). This reduction in peak height when expressed as a percent of baseline was not observed for nomifensine (Figure 3f) or for bupropion (Figure 3g), despite the reduced Km at higher doses for each drug. Bupropion did, however, demonstrate reduced DA release following cocaine SA that is attributable to baseline differences in DA release (Supplementary Figure S1; F1, 30=14.53, p<0.05).
Unique Effects of MPH on DA Uptake After Cocaine SA
Contrary to the robust effects of cocaine SA in attenuating the ability of other blockers to inhibit DA uptake, the ability of MPH to increase apparent Km for DA uptake was unaffected by cocaine SA. Figure 3d demonstrates no difference between naïve and cocaine SA animals at every concentration tested. In addition, DA traces (matched for peak height) from individual animals shown in Figure 4c demonstrate no difference in return to baseline in cocaine SA animals following MPH.
When examining peak-height changes, however, there was a main effect of cocaine SA on raw peak height (Supplementary Figure S1E; F1, 36=4.78, p<0.05), which was not solely attributed to baseline differences in release. Low concentrations of MPH were similar to cocaine in that MPH was less effective at increasing peak height in cocaine SA animals, as demonstrated when peak height is expressed as a percent of baseline response (Figure 3h). Indeed, there was a main effect of cocaine SA on peak height in response to MPH when expressed as a percent of baseline (F1, 36=6.46, p<0.05). Planned comparisons in Figure 3e demonstrated a significantly reduced peak height following 300 nM (p<0.05) and 1 μM (p<0.05) MPH in cocaine SA animals. As increases in DA peak height following application of blockers are thought to reflect DA uptake inhibition, the reduction in the ability of MPH to increase peak height in animals with a history of cocaine SA suggests a reduced ability to inhibit DA uptake. The cocaine SA-induced attenuation is similar to differences shown when low concentrations of cocaine are applied to the slice (Figure 3a). Therefore, cocaine SA history has a biphasic effect on MPH's ability to inhibit DA uptake, whereby inhibition is reduced at low concentrations (⩽1 μM) as indicated by the attenuation of peak height, while remaining intact at higher concentrations (⩾3 μM) as indicated by no change in either peak height or apparent Km.
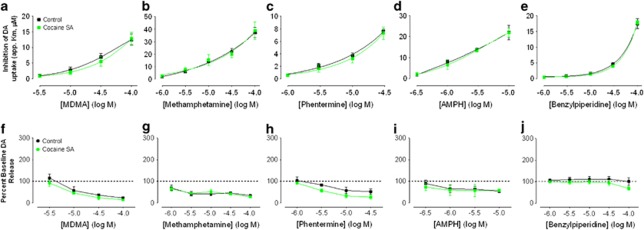
No Effect of Cocaine SA on the Ability of DAT Substrates to Inhibit DA Uptake
Contrary to the robust effects of cocaine SA on most of the blockers tested (except MPH), all five substrates/releasers failed to demonstrate any alteration in uptake inhibition as a result of cocaine SA history. This is the case when looking at both apparent Km measurements (Figure 5a–e, top row) and peak height when normalized to baseline release (Figure 5f–j) (all effects P>0.05). DA traces (matched for peak height) from individual animals shown in Figure 4d demonstrate no difference in return to baseline in cocaine SA animals following methamphetamine. The only effect present for two of the five releasers (phentermine; F1, 24=6.42, p<0.01; and BPP, F1, 20=17.14, p<0.01) is that the significantly lower raw DA peak-height level at baseline (pre-drug) remains significantly lower throughout the dose–response curves as demonstrated in Supplementary Figures S2A–S2E. These differences, however, can be attributed to the initial difference in baseline release demonstrated by no effect of cocaine SA after normalizing peak heights, and therefore are not an effect of the drug on the slice per se.
Figure 5.
Cocaine self-administration (SA) has no effect on the ability of multiple dopamine (DA) releasers to inhibit DA uptake. The top row of graphs (a–e) demonstrates the effect of 3,4-methylenedioxymethamphetamine (MDMA) (a), methamphetamine (b), phentermine (c), and amphetamine (d) and the non-amphetamine releaser 4-benzylpiperidine (BPP) (e) on DA uptake for both naïve (black squares) and cocaine SA animals (green squares), measured as apparent Km (y axis scaling varies across panels). Normalized stimulated DA release (bottom row; f–j), measured across all concentrations of drugs, indicated no effect of a history of cocaine SA on the decline in stimulated DA release to increasing doses of releasers. The colour reproduction of the figure is available at the Neuropsychopharmacology journal online.
DISCUSSION
The high-dose, fixed-ratio cocaine SA paradigm used in this study resulted in significant escalation in the rate of intake over sessions. Escalation during long-access SA of psychostimulants has been demonstrated with a number of drugs, including cocaine, MPH, and amphetamine, and is thought to be associated with the switch from abuse to addiction in humans (Ahmed and Koob, 1998, 1999; Marusich et al, 2010). The observed changes are reminiscent of changes in the patterns of SA when a lower dose of cocaine is substituted for a higher one, which would be consistent with reduced cocaine effects, or tolerance (Carelli and Deadwyler, 1996). A similar escalation in cocaine dosing also commonly occurs during bingeing in humans (Dackis and O'Brien, 2001), and humans self-report tolerance to the euphorigenic effects of cocaine following repeated use (Mendelson et al, 1998; Reed et al, 2009). Following escalation in the rate of cocaine intake, this study demonstrates reduced DA release and slower baseline rates of DA uptake. In addition, the ability of several DAT blockers to inhibit DA uptake was reduced following cocaine SA. On the other hand, experience with cocaine failed to attenuate the potency of either amphetamine- or non-amphetamine-based DAT substrates/DA releasers. Perhaps, the most intriguing finding was that, unlike all other blockers tested, the potency of MPH to alter apparent Km for DA uptake remained unchanged, demonstrating that MPH has a unique pharmacological profile that is distinct from prototypical DAT blockers.
It is not fully clear why DA release is blunted in cocaine SA animals. Repeated, prolonged increases in DA levels resulting from cocaine administration have been shown to desensitize presynaptic D2 receptors (Mateo et al, 2005; Beaulieu and Gainetdinov, 2011), and subsensitive autoreceptors would produce increases in stimulated DA release, not decreases as shown here. One possible explanation for blunted release could be decreased presynaptic DA synthesis in cocaine SA animals. Indeed, reductions in the rate-limiting enzyme TH and its phosphorylation have been demonstrated in the NAc following contingent and non-contingent administration of cocaine (Todtenkopf et al, 2000; Self et al, 2004). Nevertheless, the fact that there is no difference in amphetamine potency following cocaine SA suggests little or no difference in DA synthesis because amphetamine is particularly sensitivite to changes in DA synthesis (Sabol and Seiden, 1998). Another possibility is sequestration of DA-containing vesicles away from release sites following cocaine exposure (Farnsworth et al, 2009). A third possibility is cocaine-induced alterations in proteins that govern vesicular release of DA (Venton et al, 2006).
In addition to decreased stimulated release, previous work in our laboratory has demonstrated that regardless of whether the maximal rate of DA uptake is decreased, as in the current investigation, or increased following other schedules of cocaine reinforcement (Mateo et al, 2005), high-dose cocaine SA ultimately reduces the ability of cocaine to inhibit DA uptake (Mateo et al, 2005; Ferris et al, 2011). This dissociation of cocaine potency and maximal rate of DA uptake, which is linked to trafficking of DATs to the cell surface, suggests that there is an allosteric alteration in the DAT, rendering it less sensitive to cocaine, but fully capable of transporting DA. It was unclear, however, whether the reduced cocaine sensitivity extended to other drugs with functional similarity to cocaine (ie, DA uptake blockers).
Here we demonstrated that a history of cocaine SA is capable of reducing the ability of the uptake blockers nomifensine and bupropion, with structural dissimilarity to cocaine, to inhibit DA uptake. This is the first demonstration that pharmacodynamic tolerance to uptake inhibition extends beyond cocaine, and may underlie self-reports of psychological tolerance to multiple psychostimulants in cocaine-abusing humans (Mendelson et al, 1998; Reed et al, 2009). The putative conformational alterations in the DAT induced by cocaine SA could be due to changes in phosphorylation state, glycosylation, or lipid environment, which disrupt molecular interactions of uptake blockers (Foster et al, 2008; Chen et al, 2009). Our earlier work demonstrated that the exogenous substrate amphetamine, unlike cocaine, failed to demonstrate any change in potency following cocaine SA (Ferris et al, 2011). Further, amphetamine SA did not produce changes in the potency of cocaine or amphetamine (Ferris et al, 2011). It remained unclear, however, whether the lack of change in amphetamine effects was unique, or if it extended to other releasers/substrates.
Here we show that a history of cocaine SA fails to reduce the DAT-inhibiting ability of several amphetamine-based DAT substrates/DA releasers, including amphetamine, methamphetamine, phentermine, and MDMA, which display varying affinities for the DAT (John and Jones, 2007). Furthermore, cocaine SA does not affect the potency of the structurally distinct, non-amphetamine releaser BPP (Negus et al, 2009). Therefore, there appears to be a discrepancy between DA uptake blockers and releasers that act as substrates for the DAT in the ability of these compounds to interact with the DAT following a history of cocaine SA.
To test whether function or structure determined DAT sensitivity after cocaine SA, we selected MPH as a target molecule, given its functional similarity to cocaine and other DA uptake blockers on the one hand (Ukairo et al, 2005) and structural and binding similarities to amphetamine on the other. Cocaine SA failed to modulate MPH-induced DA uptake inhibition, similar to the lack of effect demonstrated for the DAT substrates/DA releasers. However, we noted that MPH effects on DA release/peak height (but not uptake) are similar to the uptake blockers. When examining release changes with different doses of MPH, it looks strikingly like cocaine, even demonstrating reduced potency in increasing peak height at low doses in SA animals. In fact, as increases in DA peak height induced by cocaine are thought to be driven by DA uptake inhibition during the release phase, the reduced ability of MPH to increase peak height in animals with a history of cocaine SA suggests a reduced ability to inhibit DA uptake. Low concentrations of cocaine and MPH increase the peak height of signals before slowing the descending limb of the DA. At higher concentrations, however, MPH retains normal potency similar to releasers when examining both the downward slope of the uptake curve (apparent Km) and the peak height when normalized to pre-drug release. Therefore, cocaine SA uniquely modulates the effects of MPH in the sense that MPH shares characteristics of both a DA blocker and DA releaser, depending on the concentration applied. This finding is consistent with binding studies demonstrating that MPH binds to both the cocaine/blocker and DA/substrate sites on the DAT, with higher affinity at the cocaine/blocker site (Wayment et al, 1999; Corera et al, 2001; Dar et al, 2005).
MPH is not a substrate for the DAT as are traditional releasers (Sonders and Amara, 1997); therefore, unlike releasers, MPH does not enter the cell and would not have the capability of interacting with the vesicular monoamine transporter and causing DA efflux through traditional substrate mechanisms. Even without being transported, it is possible that MPH could act at the substrate site(s) to change the orientation of the DAT to an inward-facing conformation, inhibiting uptake via reverse transport of DA as well as competition with DA for the substrate site(s). Such a change in orientation has been proposed with substrate binding to the DAT (Shan et al, 2011), ionic interactions with the DAT (Chen and Reith, 2004), and alternate access models for other symporters such as the serotonin transporter (Androutsellis-Theotokis and Rudnick, 2002).
Although it remains unclear why the DAT changes induced by cocaine SA do not affect the potency of DA releasers or MPH, one might speculate that reverse transport may mask any changes in uptake inhibition, because they are occurring at the same time. Reverse transport, however, would have to be greater in the cocaine SA group to offset reduced uptake inhibition, and the mechanism for greater reverse transport is unclear. Alternatively, the DAT alterations underlying releaser-evoked reverse transport may mitigate the cocaine SA-induced DAT changes, in effect resetting the transporters back to normal. A third possibility is that releasers and MPH may interact at sites on the DAT that are completely independent of the sites where cocaine and other blockers bind, with the two drug classes performing two separate types of uptake inhibition. The findings that MPH behaves as a releaser, but is not transported into the cytoplasm, restrict the possibilities of mechanisms involved in cocaine tolerance/releaser resistance to extracellular DAT interactions and rule out intracellular actions.
In summary, this study demonstrates that a history of high-dose cocaine SA reduces the ability of DAT blockers to inhibit uptake while leaving releaser potency unaltered. Further, MPH resembles a blocker in its effects on DA release at low concentrations, but a releaser on DA uptake and at high concentrations. This suggests differential effects of MPH contingent upon concentration, which has implications beyond cocaine SA. Indeed, this may underlie critical differential mechanisms of MPH when taken at the low doses used therapeutically and higher doses that are increasingly abused (Teter et al, 2006; Shaw et al, 2008).
The attenuated effects of cocaine documented in these experiments may help to explain the phenomenon of tolerance to the subjective effects of cocaine in human cocaine users (Mendelson et al, 1998; Reed et al, 2009). In addition, the fact that cocaine SA produces tolerance not only to cocaine, but also to other DAT blockers as well may provide some insight into why agonist pharmacotherapies based on high-affinity DAT blockers may be less promising than amphetamine or MPH in treating cocaine addiction (Grabowski et al, 2004; Negus et al, 2007; Rothman et al, 2008; Karila et al, 2011). Furthermore, due to pharmacodynamic tolerance at the DAT, cocaine and other blockers do not increase DA levels to levels reached in naïve animals (Mateo et al, 2005; Lack et al, 2008; Ferris et al, 2011). This brings up the question of what aspect(s) of cocaine taking is providing continued reinforcement during chronic cocaine use, if not DAT inhibition, but further studies will be required to address this issue. These results detailing the differential effects of cocaine SA on structurally dissimilar uptake blockers, releasers, and MPH may provide important information to further our understanding of acute and chronic effects of psychostimulants on DAT function.
Acknowledgments
The current research was supported by Grants R01DA021325 (SRJ), R01DA030161 (SRJ) P50DA006634 (SRJ and DCSR), R01DA014030 (DCSR), T31DA007246 (ESC), BIDA031533 (ESC), and T32DA007246 (MJF). We thank Joanne Konstantopoulos for her technical assistance and Dr Bruce Blough from Research Triangle Institute (RTI) for his expert advice.
The authors declare that, except for income received from primary employer, no financial support or compensation has been received from any individual or corporate entity for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Rudnick G. Accessibility and conformational coupling in serotonin transporter predicted internal domains. J Neurosci. 2002;22:8370–8378. doi: 10.1523/JNEUROSCI.22-19-08370.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dose-dependent transitions in nucleus accumbens cell firing and behavioral responding during cocaine self-administration sessions in rats. J Pharmacol Exp Therap. 1996;277:385–393. [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci. 2003;23:3076–3084. doi: 10.1523/JNEUROSCI.23-07-03076.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NH, Reith MEA. Interaction between dopamine and its transporter: role of intracellular sodium ions and membrane potential. J Neurochem. 2004;89:750–765. doi: 10.1111/j.1471-4159.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang MJ, Kim MN, Gereau RW, Leitges M, et al. Protein kinase C beta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Therap. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Corera AT, Do-Rego JC, Costentin J, Bonnet JJ. Differential sensitivity to NaCl for inhibitors and substrates that recognize mutually exclusive binding sites on the neuronal transporter of dopamine in rat striatal membranes. Neurosci Res. 2001;39:319–325. doi: 10.1016/s0168-0102(00)00230-3. [DOI] [PubMed] [Google Scholar]
- Dackis CA, O'Brien CP. Cocaine dependence: a disease of the brain's reward centers. J Subst Abuse Treat. 2001;21:111–117. doi: 10.1016/s0740-5472(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Dar DE, Mayo C, Uhl GR. The interaction of methylphenidate and benztropine with the dopamine transporter is different than other substrates and ligands. Biochem Pharmac. 2005;70:461–469. doi: 10.1016/j.bcp.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, et al. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290:1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Farnsworth SJ, Volz TJ, Hanson GR, Fleckenstein AE. Cocaine alters vesicular dopamine sequestration and potassium-stimulated dopamine release: role of D2 receptor activaion. J Pharmacol Exp Ther. 2009;328:807–812. doi: 10.1124/jpet.108.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DCS, Jones SR. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biolog Psychiatry. 2011;69:201–207. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR, Vaughan RA. Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J Neurochem. 2008;105:1683–1699. doi: 10.1111/j.1471-4159.2008.05262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens—an in vivo Microdialysis Study. Brain Res. 1989;498:199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate–putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate–putamen and nucleus-accumbens. J Pharmacol Exp Therap. 1995;274:396–403. [PubMed] [Google Scholar]
- Karila L, Reynaud M, Aubin HJ, Rolland B, Guardia D, Cottencin O, et al. Pharmacological treatments for cocaine dependence: Is there something new. Curr Pharmaceut Des. 2011;17:1359–1368. doi: 10.2174/138161211796150873. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59:449–455. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Lack CM, Jones SR, Roberts DCS. Increased breakpoints on a progressive ratio schedule reinforced by IV cocaine are associated with reduced locomotor activation and reduced dopamine efflux in nucleus accumbens shell in rats. Psychopharmacology. 2008;195:517–525. doi: 10.1007/s00213-007-0919-4. [DOI] [PubMed] [Google Scholar]
- Mandt BH, Zahniser NR. Low and high cocaine locomotor responding male Sprague–Dawley rats differ in rapid cocaine-induced regulation of striatal dopamine transporter function. Neuropharmacology. 2010;58:605–612. doi: 10.1016/j.neuropharm.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2010;18:257–266. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology. 2005;30:1455–1463. doi: 10.1038/sj.npp.1300687. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology. 1998;18:263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine- versus food-maintained responding by monoamine releasers in Rhesus monkeys: benzylpiperazine, (+)phenmetrazine, and 4-benzylpiperidine. J Pharmacol Exp Therap. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate “agonist” medications for cocaine dependence: studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Therap. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Peoples LL, Gee F, Bibi R, West MO. Phasic firing time locked to cocaine self-infusion and locomotion: dissociable firing patterns of single nucleus accumbens neurons in the rat. J Neurosci. 1998;18:7588–7598. doi: 10.1523/JNEUROSCI.18-18-07588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraile I, Torres E, Mayado A, Izco M, Lopez-Jimenez A, Lopez-Moreno JA, et al. Dopamine transporter down-regulation following repeated cocaine: implications for 3,4-methylenedioxymethamphetamine-induced acute effects and long-term neurotoxicity in mice. Br J Pharmacol. 2010;159:201–211. doi: 10.1111/j.1476-5381.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Samuvel DJ, Balasubramaniam A, See RE, Jayanthi LD. Altered dopamine transporter function and phosphorylation following chronic cocaine self-administration and extinction in rats. Biochem Biophys Res Commun. 2010;391:1517–1521. doi: 10.1016/j.bbrc.2009.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Haney M, Evans SM, Vadhan NP, Rubin E, Foltin RW. Cardiovascular and subjective effects of repeated smoked cocaine administration in experienced cocaine users. Drug Alcohol Depend. 2009;102:102–107. doi: 10.1016/j.drugalcdep.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Progr Neuro-Psychopharmacol Biol Psychiatry. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dopamine/serotonin releasers as medications for stimulant addictions. Serotonin. 2008;172:385–406. doi: 10.1016/S0079-6123(08)00919-9. [DOI] [PubMed] [Google Scholar]
- Sabol KE, Seiden LS. Reserpine attenuates D-amphetamine and MDMA-induced transmitter release in vivo: a consideration of dose, core temperature and dopamine synthesis. Brain Res. 1998;806:69–78. doi: 10.1016/s0006-8993(98)00720-3. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan JF, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS One. 2011;6:e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SY, Shah L, Jolly AM, Wylie JL. Identifying heterogeneity among injection drug users: a cluster analysis approach. Am J Pub Health. 2008;98:1430–1437. doi: 10.2105/AJPH.2007.120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonders MS, Amara SG. Pharmacological agents as functional probes of the human dopamine transporter. Biophys J. 1997;72:TH357. [Google Scholar]
- Teter CJ, Mccabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, De Leon KR, Stellar JR. Repeated cocaine treatment alters tyrosine hydroxylase in the rat nucleus accumbens. Brain Res Bull. 2000;52:407–411. doi: 10.1016/s0361-9230(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Ukairo OT, Bondi CD, Newman AH, Kulkarni SS, Kozikowski AP, Pan S, et al. Recognition of benztropine by the dopamine transporter (DAT) differs from that of the classical dopamine uptake inhibitors cocaine, methylphenidate, and mazindol as a function of a DAT transmembrane 1 aspartic acid residue. J Pharmacol Exp Therap. 2005;314:575–583. doi: 10.1124/jpet.105.085829. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PEM, Wetsel WC, Gitler D, Greengard P, et al. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayment HK, Deutsch H, Schweri MM, Schenk JO. Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: potential as amphetamine antagonists. J Neurochem. 1999;72:1266–1274. doi: 10.1046/j.1471-4159.1999.0721266.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, et al. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith MEA, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.