Abstract
Stressful and traumatic events can create aversive memories, which are a predisposing factor for anxiety disorders. The amygdala is critical for transforming such stressful events into anxiety, and the recently discovered neuropeptide S transmitter system represents a promising candidate apt to control these interactions. Here we test the hypothesis that neuropeptide S can regulate stress-induced hyperexcitability in the amygdala, and thereby can interact with stress-induced alterations of fear memory. Mice underwent acute immobilization stress (IS), and neuropeptide S and a receptor antagonist were locally injected into the lateral amygdala (LA) during stress exposure. Ten days later, anxiety-like behavior, fear acquisition, fear memory retrieval, and extinction were tested. Furthermore, patch-clamp recordings were performed in amygdala slices prepared ex vivo to identify synaptic substrates of stress-induced alterations in fear responsiveness. (1) IS increased anxiety-like behavior, and enhanced conditioned fear responses during extinction 10 days after stress, (2) neuropeptide S in the amygdala prevented, while an antagonist aggravated, these stress-induced changes of aversive behaviors, (3) excitatory synaptic activity in LA projection neurons was increased on fear conditioning and returned to pre-conditioning values on fear extinction, and (4) stress resulted in sustained high levels of excitatory synaptic activity during fear extinction, whereas neuropeptide S supported the return of synaptic activity during fear extinction to levels typical of non-stressed animals. Together these results suggest that the neuropeptide S system is capable of interfering with mechanisms in the amygdala that transform stressful events into anxiety and impaired fear extinction.
Keywords: restraint stress, fear extinction, anxiety disorder, neuromodulation, amygdala, neuropeptide S
INTRODUCTION
Intensively stressful and traumatic events often create aversive memories, which can be a predisposing factor of posttraumatic stress disorders (PTSDs) and other anxiety disorders (Heim and Nemeroff, 2009; Victor and Bernstein, 2009). These fear memories are readily retrieved by conditioned cues linked with the traumatic events, and weakening of this association might be helpful for removal of the aversive memories (see Ressler (2010) for review). In fact, an approach commonly used to treat certain forms of anxiety disorders (exposure therapy) is similar to that used to extinguish conditioned fear responses in experimental paradigms (Rothbaum and Davis, 2003; Monson et al, 2006). Fear extinction refers to a relatively simple form of fear behavior regulation, in which conditioned fear responses decrease when the relevant (conditioned, CS+) stimulus is represented repeatedly in the absence of a re-enforcing (unconditioned) stimulus (for reviews see: Maren and Quirk, 2004; Myers and Davis, 2007; Quirk and Mueller, 2008). It has indeed been proposed that PTSD combines aspects of severe stress responsiveness and either enhanced conditioned fear or an inability to extinguish fear memories (Corcoran and Quirk, 2007; Milad et al, 2008; Jovanovic et al, 2010; Ressler, 2010; Norrholm et al, 2011).
It is now widely assumed that the amygdala is critical for transforming stressful events into anxiety (Rainnie et al, 2004; Roozendaal et al, 2009). Data from neuroimaging studies show enhanced amygdala activation in patients with PTSD compared with control subjects, and the increased responses often extend beyond the trauma-relevant events (reviewed in Liberzon and Sripada (2008)). Neurobiologically, previous stress exposure facilitates fear learning, and it increases neuronal excitability and synaptic plasticity in the basolateral complex of the amygdala (BLA) (Vouimba et al, 2004; Rodriguez Manzanares et al, 2005; Kavushansky and Richter-Levin, 2006). Chronic immobility stress in addition results in an increase in spine density, a change in dendritic arborization and an overall hyperexcitability in neurons of the BLA, paralleled by a gradual increase in anxiety (Mitra et al, 2005; Rodriguez Manzanares et al, 2005; Roozendaal et al, 2009; Rosenkranz et al, 2010). A crucial element in the sequence of mechanisms leading to hyperexcitability in the amygdala upon chronic stress exposure is an attenuation of GABAergic inhibitory influence (Mitra et al, 2005; Rodriguez Manzanares et al, 2005; Roozendaal et al, 2009), although the types of GABAergic neurons and underlying mechanisms remain unknown.
The regulation of excitability of amygdaloid neurons, as for instance through GABAergic mechanisms, thus seems to be central to both stress- and extinction-related functions of the amygdala, and the search for endogenous systems apt to control these interactions is an important task. Within this theme it is interesting to note that neuropeptide (NPS), a neuropeptidergic transmitter acting via G protein-coupled receptors (NPS-Rs) to modulate anxiety and arousal (Xu et al, 2004; Jüngling et al, 2008; Pape et al, 2010), increases responsiveness of intercalated GABAergic neurons in the murine amygdala and thereby facilitates fear extinction learning and recall (Jüngling et al, 2008). Furthermore, a functional polymorphism in the NPS-R gene (rs324981 A/T) has been associated with overinterpretation of learned fear and panic disorders in humans and is associated with increased amygdala responsiveness to fear-relevant stimuli (Raczka et al, 2010; Dannlowski et al, 2011; Domschke et al, 2011; Donner et al, 2010). Of particular interest, here is that forced swim stress results in increased c-fos activity in NPS-synthesizing neurons in the brain stem (Liu et al., 2011) and increase extracellular levels of NPS in the BLA (Ebner et al, 2011), implying that the NPS system may be stimulated on stress exposure.
Therefore, the present study has been undertaken to test the hypothesis that NPS can interact with the stress-induced hyperexcitability in BLA synaptic networks, and thereby modulate the stress-induced impairment of fear extinction. The experimental strategy was (1) to combine an established restraint stress model (Mitra et al, 2005; Roozendaal et al, 2009) with auditory Pavlovian fear conditioning and extinction training in mice (Jüngling et al, 2008), (2) to locally apply NPS-R ant/agonists to the lateral amygdala (LA) before and after stress exposure, (3) to investigate the influence on the behavioral expression of fear during states of fear memory and extinction in vivo, and (4) to analyze excitatory synaptic activity in LA neurons in slices prepared ex vivo from the various groups of animals during different states of fear memory and extinction.
MATERIALS AND METHODS
Animals
Eight- to twelve-week-old C57BL/6J mice (Harlan, Germany) were kept under a 12/12 h light/dark cycle (lights on at 0700 hours) with food and water provided ad libitum. All experiments were carried out in accordance with the European Committees Council Directive (86/609/EEC) and were approved by the local authorities (LANUV NRW, AZ 87-51.042010).
Local Application of Substances
The method was previously described (Jüngling et al, 2008); animals were transferred to individual housing 3 days before surgery, and were implanted with a 26-G stainless steel guide cannula bilaterally in the LA (stereotaxic coordinates: 1.7 mm posterior, 3.7 mm lateral from bregma, and 2.5 mm dorsoventral from brain surface) under deep pentobarbital anesthesia (50 mg/kg i.p.). Animals were allowed to recover from surgery for at least 7 days. Local drug infusion was performed under Forene inhalation anesthesia (isofluran, 1-chloro-2,2,2-trifluoroethyl-difluoromethylether; induction: 2.5%, maintenance: 1.5% in O2; flow rate 1 l/min). Using a 10 ml Hamilton microliter syringe, the following solutions were infused with a 33-G beveled needle injector that was 1 mm longer than the guide cannula: NPS-R antagonist SHA 68 (3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a] pyrazine-7-carboxylic acid 4-fluoro-benzylamide, 10 μM, in 10% Cremophor-PBS buffer; gift from R. Reinscheid, Department of Pharmacology, University of California, Irvine, USA), NPS (10 μM in 0.9 % saline solution; Ascent Scientific), and as vehicle controls, 10% Cremophor-PBS buffer, and 0.9% saline solution, respectively. Substances were bilaterally applied into LA (0.5 μl at 0.1 μl/min, each hemisphere) 20 min before, (a time window, which has been shown to be most effective during anxiety tests (Jüngling et al, 2008)), or immediately after (<1 min) immobilization stress (IS). Histological controls were performed through cresyl violet staining of 50-μm-thick coronal slices (Supplementary Figure S1).
Immobilization Stress
IS consisted of a single immobilization session of 2 h in a 50-ml Falcon tube perforated by 5 mm holes. The size of the tube restricted movements in all directions but did not interact with respiration. IS was performed between 0800 and 1200 hours, and the behavior was tested 1 day (IS-1D) or 10 days (IS-10D) after stress exposure and compared with non-stressed (NS) controls.
Elevated Plus Maze (EPM)
Mice were tested for general anxiety using the EPM, providing an indication of anxiety-like behavior that is sensitive to traumatic stressors (Pellow and File, 1986; Mitra et al, 2005; Roozendaal et al, 2009). The plus maze consisted of two open (30 × 5 cm2) and two wall-enclosed arms (30 × 5 × 25 cm3) connected by a central platform (5 × 5 cm2). Light intensity on the open arms was 120 lux. The apparatus was elevated 75 cm above the floor. Behavioral testing was started by placing a mouse in the central area facing a closed arm. Exploratory behavior was monitored by a video motility system (Video-Mot II, TSE, Bad Homburg, Germany) over a period of 10 min, quantified, and stored on videotape. Parameters for behavioral analyses were: percentage of time spent in the open/closed arms (related to total recording time) and entries into the open/closed arms.
Fear Conditioning and Extinction
Mice were fear conditioned using a Pavlovian fear-conditioning paradigms as described previously (Jüngling et al, 2008). On day 1, animals were adapted through two presentations of six CS− (2.5 kHz tone, 85 dB, stimulus duration 10 s, interstimulus interval 20 s; intertrial interval 6 h). On the next day, fear conditioning was performed through two sessions of three randomly presented CS+ (10 kHz tone, 85 dB, stimulus duration 10 s, randomized interstimulus interval 10–30 s; inter-session interval 6 h), each of which was co-terminated with an unconditioned stimulus (scrambled foot shock of 0.4 mA, duration 1 s). After 24 h (day 3), single animals were transferred to a new environment (retrieval context) and habituated over a period of 30 min, before being exposed to six retrieval sessions (R1–R6) for extinction training (inter-session interval 30 min), each consisting of a set of four CS− and (40 s later) a set of four CS+ (stimulus duration 10 s, interstimulus interval 20 s). After 24 h (day 4), recall of extinction (ER) was tested in the retrieval context by exposing the animal to one set of four CS− and 40 s later to a set of four CS+ (stimulus duration 10 s, interstimulus interval 20 s). Freezing time (complete immobilization except respiratory movements) was manually scored by an observer blind to the paradigm. For each session, cued fear was quantified by freezing time during CS (tone) presentation. Contextual fear was observed during the second fear training session and quantified by the freezing time in the fear-conditioning box during the first 2 min (before the CS delivery).
Electrophysiological Recordings
Slices were obtained from trained mice at two different stages of conditioned fear: 24 h after retrieval session R1 and 24 h after ER. Non-trained animals (NS, not subjected to behavioral experiments) were taken as controls. Mice were deeply anesthetized with Forene (2.5%) and killed by decapitation. A block of brain tissue containing the amygdala was rapidly removed and transferred into chilled oxygenated physiological saline containing (mM): KCl, 2.5; NaH2PO4 1.25; MgSO4, 10; CaCl2, 0.5; piperazine-N, N′-bis(ethanesulphonic acid), 20; glucose, 10; sucrose, 200 (pH 7.35 with NaOH). Coronal slices (300 μm thick) containing the LA were prepared on a vibratome (Leica VT1200S; Wetzlar; Germany), and were incubated in artificial cerebrospinal fluid (ACSF) of the following composition (in mM): NaCl 120, KCl 2.5, NaH2PO4 1.25, MgSO4 2, NaHCO3 22, CaCl2 2, glucose 20; pH 7.35 by gassing with carbogen. Slices were allowed to recover at 34 °C for 20 min and were then maintained for up to 8 h at room temperature. Single slices were placed in a submersion chamber at a perfusion rate of ∼2 ml/min (ACSF, 30 °C).
Whole-cell patch-clamp recordings were obtained using a patch-clamp amplifier (EPC-10, HEKA, Lambrecht/Pfalz, Germany) and sampled at 5 kHz. Patch pipettes were pulled from borosilicate glass (2.4–3.0 MΩ, GC150T-10; Clark Electromedical Instruments, Pangbourne, UK) and experiments were performed with a potassium-based internal solution containing the following (in mM): 88 K-gluconate, 20 K3-citrate, 10 NaCl, 10 HEPES, 3 BAPTA, 15 phoshocreatin, 0.5 CaCl2, 1 MgCl2, 3 MgATP, and 0.5 NaGTP, with pH adjusted to 7.25. Series resistance compensation of 30% was routinely used. Potential measurements were corrected for liquid junction potential of 10 mV. The series resistance (RS) was regularly monitored during the recordings. Neurons with resting membrane potential positive to −55 mV were rejected from analysis. Patch-clamp recordings and data analysis were performed under voltage- and current-clamp conditions using Pulse software (HEKA, Lambrecht/Pfalz, Germany). The data were analyzed off-line using Clampfit software (Molecular Devices, Sunnyvale, USA).
Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded under voltage-clamp conditions (holding potential: −70 mV) over a time-period of 5 min to determine amplitudes and frequencies of the recorded currents. The single sEPSCs have been detected by using the Clampfit ‘template event detection' function. For analysis, amplitudes and frequencies of the recorded sEPSCs were normalized to the average amplitude and frequency of non-trained animals. The recorded sEPSCs could be blocked by the addition of the selective non-NMDA receptor antagonist DNQX (10 μM; Tocris Bioscience, Bristol, UK), indicating that these currents were mediated by glutamate receptors.
The projection neurons (PNs) in LA were identified by their specific firing pattern in current-clamp recordings as described previously (Sosulina et al, 2006; Jüngling et al, 2008). Active and passive membrane properties of LA PNs were analyzed in the current-clamp mode. The resting membrane potential was obtained immediately after accessing the whole-cell configuration. To determine passive and active membrane properties, hyperpolarizing and depolarizing currents were injected (500 ms duration; from −40 pA to +80 pA; increment +10 pA). The input resistance (R) was calculated from the voltage-shift during a current-injection of −40 pA. The cell capacitance (C) was calculated by: C=τ/R, where the membrane time-constant τ was retrieved by a monoexponential fit of the hyperpolarizing voltage-deflection elicited by a −40 pA current-injection during current-clamp recordings. The action potential threshold was measured during injection of +60 pA depolarizing current. The instantaneous frequency was measured between the first consecutive action potentials elicited by depolarizing current injection.
Statistical Analysis
ANOVA was used to analyze the effects of stress and substance on general anxiety in the EPM and freezing behavior during fear acquisition. For extinction training (session R1–R6), ANOVA with session as repeated measures was performed to analyze freezing behavior. Post-hoc comparisons were done by Tukey-tests. Student's t-tests were performed for intra-group analysis of ER. For electrophysiological recordings, average of normalized sEPSCs frequencies and amplitudes were compared by non-parametric Mann–Whitney test. The data sets were tested for statistically significant outliers using the Grubb's test (significance level: p<0.05). All values are expressed as mean±SEM. Statistical significance for all experiments was p<0.05.
RESULTS
Stress-Aggravated Anxiety-like Behavior: Effects of NPS Application in the LA
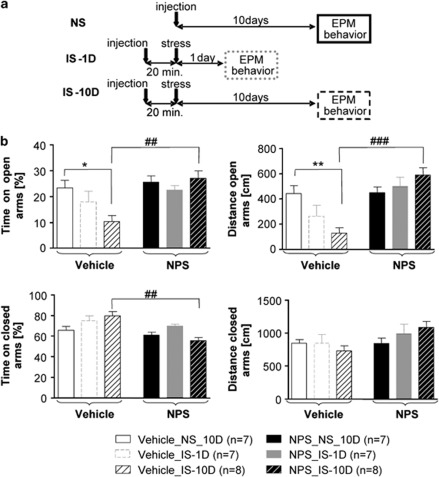
Mice were exposed to IS, either 1 day (group IS-1D) or 10 days (IS-10D) before the EPM test, and anxiety-like behavior was compared with a group without previous stress exposure (NS). In different behavioral groups, vehicle (NaCl 0.9 %) or NPS was bilaterally injected into the LA 20 min before IS in the stress-exposed groups or 10 days before EPM test in the NS controls. The paradigm is illustrated in Figure 1a, the results are illustrated in Figure 1b. Two-way ANOVA showed a significant stress X substance interaction for the time and distance in open arms (F(2,38)=4.004, p<0.05 and F(2,38)=6.914, p<0.01, respectively) and for time in closed arms (F(2,38)=4.807, p<0.05). In detail, in vehicle-injected mice, stress did not impair the time and the distance in closed arms but decreased the time and distance in open arms only when animals were evaluated 10 days after stress (post-hoc Tukey-test comparing NS and IS-10D group: p<0.05 and p<0.01, respectively). In NPS-injected mice, the time and the distance in open arms were not significantly different between control (NPS NS) and stressed animals. NPS application increased the time and the distance spent in open arms in IS-10D animals compared with vehicle (substance effect: p<0.01 and p<0.001, respectively). As NPS has been shown to induce anxiolytic-like effects (Xu et al, 2004; Jüngling et al, 2008; Fendt et al, 2010), which might influence the aversive component of IS, NPS injections were performed immediately after (<1 min) stress exposure in separate behavioral groups. Anxiety-like behavior, tested 10 days later showed a similar increase of distance and time in open arms in stressed NPS-injected animals (Supplementary Figure S2).
Figure 1.
Stress-aggravated anxiety-like behavior: effects of NPS injection in the LA before stress exposure. (a) Experimental design in the various groups of mice. NS: non-stressed animals, injection of NPS or vehicle bilaterally into the LA 10 days before EPM test. IS-1D: animals were exposed to IS (2 h) 1 day before EPM test, and NPS or vehicle was injected into the LA 20 min before IS. IS-10D: animals were exposed to IS (2 h) 10 days before EPM test, and NPS or vehicle was injected into the LA 20 min before IS. (b) Results of EPM tests in the various groups (symbols as indicated in a and below diagrams in b). Post-hoc Tukey HSD tests: stress effect: **p<0.01, *p<0.05. Substance effect: ###p<0.001, ##p<0.01.
Influence of Stress and NPS on Conditioned Fear and Fear Extinction
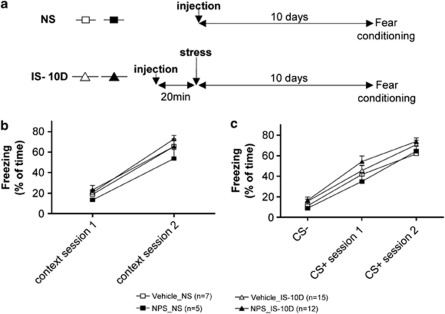
Fear acquisition was assessed as percentage of freezing to the context and conditioned stimuli during fear training. Freezing was compared between groups of mice exposed to IS 10 days before fear training (IS-10D) and non-IS-exposed controls (NS), and the effects of NPS and vehicle injection (20 min before IS in IS-10D and 10 days before fear conditioning in NS) were compared (Figure 2a). Contextual fear was determined as percentage of freezing on 2 min of exposure to the conditioning apparatus (contextual fear; Figure 2b) and on presentation of the CS (cued fear; Figure 2c) during the first and second fear training session. No significant effect of stress and/or substance could be detected by two-way ANOVA with repeated measures (Figure 2b). All groups showed a significant increase in contextual freezing during the second fear training session (session comparison: p<0.01 for all groups). Similarly for cued fear (Figure 2c), stress, and substance injection had no influence on freezing on CS+ presentation. In all groups, freezing evoked by CS+ exceeded that upon CS− presentation during session 1 (p<0.05 for NPS-NS and p<0.01 for the three other groups) and session 2 (p<0.0001 for all groups). To conclude freezing was not significantly different in mice that had been exposed to IS 10 days before training and NS controls, and LA application of NPS 20 min before IS had no effect on freezing.
Figure 2.
Effect of stress exposure and prior NPS injection into the LA on fear acquisition. (a) Experimental design. NS: non-stressed animals, injection of NPS (NPS_NS) or vehicle (Vehicle_NS) bilaterally into the LA 10 days before fear conditioning. IS-10 D: exposure to IS 10 days before fear conditioning, with injection of NPS (NPS_IS-10D) or vehicle (Vehicle_IS-10D) 20 min before IS. (b) Freezing to the context during a period of 2 min before the first CS+ presentation for session 1 and 2. (c) Freezing to the CS. The data represent the mean of freezing for 6-block CS- and 3-block CS+.
Furthermore, fear memory retrieval upon CS+ presentation during the first retrieval session (R1), was similar for all behavioral groups, and the stress X substance interaction was not significant (two-way ANOVA: F(2,35)=0.194, n.s.; Figure 3a). Freezing during R1 was significantly higher for CS+ than CS− in all groups, showing that all mice learned to specifically discriminate the CS+ (t-test: p<0.0001 for all groups).
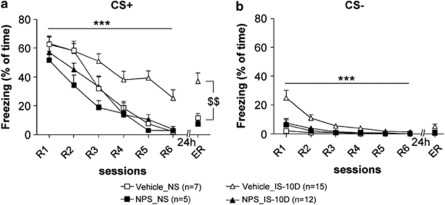
Figure 3.
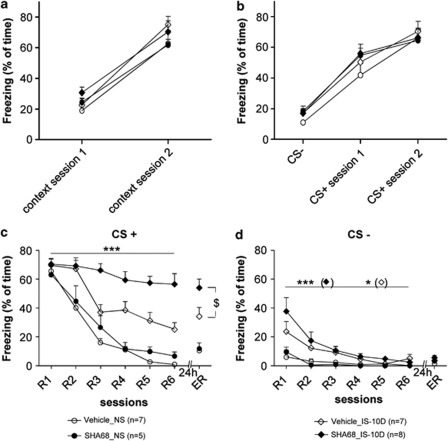
Stress-induced alterations in conditioned fear responses: compensatory effect of prior application of NPS. NS: non-stressed animals, injection of NPS (NPS_NS) or vehicle (Vehicle_NS) bilaterally into the LA 10 days before fear conditioning. IS-10 D: exposure to IS 10 days before fear conditioning, with injection of NPS (NPS_IS-10D) or vehicle (Vehicle_IS-10D) 20 min before IS. CS+ (a) and CS− (b) evoked freezing during fear memory retrieval (R1 session), extinction training (R1–R6) and extinction recall (ER). Stress effect (two-way ANOVA with repeated measures in vehicle-injected animals): ***p<0.001. t-test for ER (intra-group analysis for stressed and non-stressed vehicle-injected animals): $$p<0.01.
Next, fear extinction learning was assessed by a three-way ANOVA with a within-subjects factor (six recall sessions; R1–R6) and two between-subjects factors (drug and stress; Figure 3a). Interestingly, the three-way ANOVA indicates that stress, drug, and rate of extinction interact (three-way ANOVA: for CS+ : F(5,175)=2.987, p<0.01). More specifically, stress exposure resulted in maintained high levels of CS+ freezing during extinction training in vehicle-injected animals (comparison IS-10D and NS: two-way-ANOVA: F(5,100)=4.475, p<0.001). In contrast, upon injection of NPS 20 min before stress exposure, freezing evoked by CS+ declined in stressed mice (NPS_IS-10D) similar to that in NS (vehicle) controls (F(5,75)=0.889, n.s.). NPS injection had a significant effect on fear extinction in mice exposed to IS (comparison of stressed groups, two-way ANOVA; F(5,152)=2.495, p<0.05). Interestingly, the three-way ANOVA also revealed a significant stress X substance interaction during freezing upon CS− exposure (F(5,175)=3.248, p<0.001; Figure 3b). Post-hoc analysis showed that the freezing time during CS− of stressed vehicle-injected mice was higher than in the three other groups for session R1 (post-hoc test: p<0.0001 for all comparisons).
During ER session 24 h after extinction training, CS+ freezing in stressed vehicle-injected mice maintained at significantly higher levels as compared with NS controls (t-test: p<0.01; Figure 3a). In contrast, previous stress did not affect recall of fear extinction after NPS injection into the LA (NPS_IS-10D vs NPS_NS; Figure 3a), and freezing in stressed vehicle-injected mice was higher than in stressed NPS-injected mice (p<0.001). No differences were found between groups for freezing evoked by CS− during extinction recall.
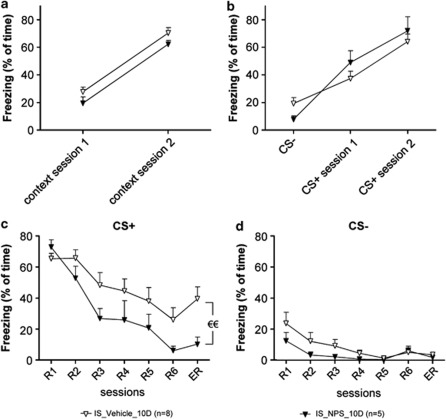
Overall these results suggest that previous stress results in maintained conditioned fear responses during fear extinction, and that NPS in the LA during stress exposure can prevent these stress-induced effects. NPS induced similar effects when injected into the LA 20 min before (as described above) or immediately (<1 min) after IS (Figure 4). Specifically, in animals injected with NPS immediately after stress, NPS was not found to modify fear acquisition (Figure 4a and b). Contextual fear (Figure 4a) in NPS-injected mice was not different from vehicle group in the first or second training session (two-way ANOVA in stressed animals: F(1,11)=1.157, n.s.). Similarly, NPS did not alter freezing to the CS-, or to the CS+ (Figure 4b) of session 1 and session 2 (two-way ANOVA: F(2,42)=1.364, n.s.). During fear extinction (Figure 4c), NPS did not significantly affect extinction learning (two-way ANOVA from R1 to R6 sessions and substance effect: F(5,55)=1.631, n.s.). However, animals injected with NPS immediately after stress exposure (IS-10D) showed a significant decrease in freeding during ER (t=2.802 for E session, p<0.01), while an effect on CS− freezing was not observed (Figure 4d).
Figure 4.
NPS injection into the LA immediately after stress exposure: effects on conditioned fear responses. IS_Vehicle_10D, IS_NPS-10D: injection of vehicle and NPS <1 min after IS. (a, b) Fear acquisition. Freezing to the context during 2 min before the first CS+ presentation for session 1 and 2 (a), and freezing to the CS− (b). Data represent the mean of freezing for 6-block CS- and 3-block CS+. (c, d) Conditioned fear responses. CS+ (c) and CS− (d) evoked freezing during fear memory retrieval (R1), extinction training (R1–R6) and extinction recall (ER). t-test for ER session: €€p<0.01.
Effect of an NPS Receptor Antagonist on Stress-Modulated Conditional Fear
In a next series of experiments, the NPS receptor antagonist SHA68 (Okamura et al, 2008) was injected bilaterally into the LA 20 min before IS, and conditioned fear was tested 10 days after stress exposure, using the same paradigms as before. Cremophor-injected (vehicle) and NS animals were used as controls. Data are illustrated in Figure 5. Similar to NPS, SHA68 did not affect CS-evoked freezing during fear acquisition (Figure 5a and b) and recall (R1; Figure 5c), regardless of IS exposure. During fear extinction, the three-way ANOVA revealed no interaction between factors across the extinction recall sessions for CS+ and CS− (F(5,110)=1.707, n.s.; F(5,110)=0.767, n.s., respectively), whereas stress (F(5,115)=8.604, p<0.001; F(5,110)=6.714, p=0.001; for CS+ and CS−, respectively) and substance (F(5,115)=4.070, p=0.01; for CS+) showed a significant main effect (Figure 5c and d). SHA68 did not affect CS-evoked freezing in non-stressed animals (extinction training: two-way ANOVA: F(5,50)=0.903, n.s.; ER: t-test, t=0.365, n.s.). IS resulted in maintained high levels of CS+ freezing in both control (vehicle injected) and SHA68-injected animals during extinction training as compared with NS controls (two-way ANOVA: F(5,60)=2.916, p<0.05 and F(5,55)=6.501, p<0.0001 for vehicle- and SHA68-injected animals respectively). Interestingly, conditioned freezing in SHA68-injected stressed animals exceeded that in vehicle-injected stressed controls during fear extinction training (two-way ANOVA: F(5,65)=4.501, p<0.001) and extinction recall (t-test for ER session: t=2.287, p<0.05). With respect to CS−, stress resulted in increased freezing in SHA68 and cremophor-injected animals (two-way ANOVA: F(5,55)=4.562, p<0.001 and F(5,55)=2.237, p<0.05, respectively).
Figure 5.
Stress exposure and injection of an NPS receptor antagonist into the LA: effects on conditioned fear. NS: non-stressed animals, injection of SHA68 (SHA68_NS), or vehicle (cremophore, Vehicle_NS) bilaterally into the LA 10 days before fear conditioning. IS-10 D: exposure to IS 10 days before fear conditioning, with injection of SHA68 (SHA68_IS-10D) or vehicle (cremophore, Vehicle_IS-10D) 20 min before IS. (a, b) Fear acquisition. Freezing to the context during a period of 2 min before the first CS+ presentation for session 1 and 2 (a), and freezing to the CS− (b). The data represent the mean of freezing for 6-block CS- and 3-block CS+. (c, d) Conditioned fear responses. (c) CS+-evoked and (d) freezing evoked by CS− during fear memory retrieval (R1 session), extinction training (R1–R6), and extinction recall (ER). Substance effect (two-way ANOVA with repeated measures for extinction training (R1–R6) in stressed animals): ***p<0.001; substance effect for ER session, t-test: $p<0.05. Stress effect in SHA68-(***p<0.001) and vehicle-injected (*p<0.05) animals.
Excitatory Synaptic Activity in the LA: Influence of Conditioned Fear, Stress, and NPS
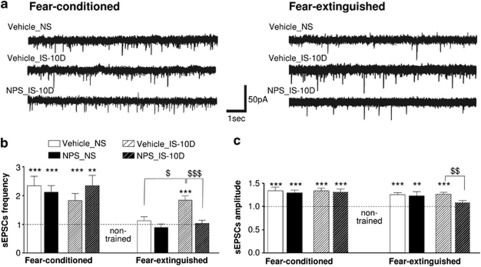
In a next experimental step, slices were prepared from the amygdala of the behavioral groups, and sEPSCs were recorded from putative LA PNs ex vivo (Figure 6a). The sEPSCs were recorded at a holding potential of −70 mV using a low chloride (K+-gluconate) internal pipette solution. Under these experimental conditions, the reversal potential for GABAA receptor-mediated currents is close to the holding potential. Furthermore, the recorded sEPSCs were sensitive to application of 10 μM DNQX (a non-NMDA receptor antagonist), indicating that the recorded sEPSCs are mediated by AMPA receptors. The two groups of non-trained animals received vehicle or NPS injection into the LA 10 days before ex vivo recordings. Mean amplitudes and frequencies of sEPSCs were −19.2±0.8 pA and 1.2±0.2 Hz (vehicle group; n=21 cells from two animals) and −20.3±0.8 pA and 1.3±0.2 Hz (NPS group; n=18/2), respectively. The sEPSCs of the two groups were not significantly different and pooled for all further comparisons. All following acquired data were normalized to these values in non-trained mice.
Figure 6.
Spontaneous excitatory postsynaptic currents in LA projection neurons ex vivo. Recordings obtained in slices from the different behavioral groups as indicated (abbreviations as in Figure 1), prepared 24 h after retrieval of fear memory (R1; fear-conditioned group) and 24 h after recall of extinction (ER; fear-extinguished group). (a) Representative original traces of sEPSCs in the various groups. (b) Average sEPSCs frequency. (c) Average sEPSCs amplitude. Values were normalized to those obtained in non-trained controls (dashed line in diagrams). Data are presented as means with standard error of the mean (±SEM). Note the overall increase in sEPSC frequency and amplitude in conditioned as compared with non-trained animals, the return to baseline of sEPSCs frequency upon fear extinction in non-stressed but not in stressed animals, and the compensatory effect of NPS in stressed animals. Numbers of recorded cells and numbers of animals are as follows (cells/animals). Non-trained control group: 40/4. Fear-conditioned group: vehicle-injected/non-stressed (Vehicle NS): 20/2; NPS-injected non-stressed (NPS NS): 30/3; vehicle-injected/stressed (Vehicle IS-10D): 25/3; NPS-injected/stressed (NPS IS-10D): 26/3. Fear-extinguished group: Vehicle NS: 33/4; NPS NS: 17/2; Vehicle IS-10D: 55/6; NPS IS-10D: 27/3. Comparison to non-trained controls: ***p<0.001, **p<0.01; group comparisons: $$$p<0.001, $$p<0.01, $p<0.05.
In fear-conditioned animals, recordings in LA slices prepared 24 h after R1 revealed a significant increase in normalized frequencies and amplitudes of sEPSCs in all behavioral groups as compared with non-trained controls (Figure 6b and c). No significant differences were detected within or between stressed and NS groups. On fear extinction in NS animals, sEPSC frequencies returned to levels indistinguishable from those in non-trained animals in both, vehicle- and NPS-injected groups (Figure 6b). The normalized mean amplitudes remained increased as compared with non-trained animals (Figure 6c). By contrast, sEPSC frequencies remained at an elevated level in stressed, vehicle-injected animals (Figure 6b, p<0.0001). This sustained increase in sEPSC frequencies and amplitudes was not detected in stressed animals that had received NPS injection into the LA. Both, frequencies and amplitudes, were reduced to levels of NS mice, indistinguishable from those of non-trained control (Figure 6b and c). The observed increase of sEPSC frequencies in stressed, vehicle-injected animals was significantly higher than in stressed, NPS-injected, and NS, vehicle-injected animals (p<0.001 and p<0.05, respectively; Figure 6b). The normalized amplitudes of sEPSCs in stressed, NPS-injected animals were not significantly different from non-trained controls, but significantly reduced compared with stressed, vehicle-injected animals (p<0.01; Figure 6c). These differences in sEPSCs properties between behavioral groups were corroborated by cumulative frequency and amplitude distributions (Supplementary Figure S3). Furthermore, analyses of intrinsic properties of LA PNs revealed no alterations apt to explain the differences in sEPSC properties (Supplementary Table S1).
DISCUSSION
The data of the present study indicate that (1) acute IS in mice results in an increase in anxiety-like behavior, and an enhancement of conditioned fear responses during extinction 10 days after stress exposure, (2) NPS application into the LA around stress exposure can prevent, whereas an NPS-R antagonist aggravates, the stress-induced changes in anxiety and conditioned fear responses, (3) excitatory synaptic activity in LA PNs is increased upon fear conditioning and returns to pre-conditioning values on fear extinction, and (4) IS results in a sustainment of increased excitatory synaptic activity in LA PNs during fear extinction, whereas NPS in the LA around stress exposure supports a return of synaptic activity upon fear extinction to levels typical of NS animals. Together these results suggest that the NPS transmitter system is capable of interfering with mechanisms in the amygdala that transform stressful events into anxiety and impaired extinction.
Prevention of Stress-Induced Alteration in Fear Responsiveness through NPS Action in the LA
Acute IS increased anxiety-like behavior tested in the EPM 10 days but not 1 day after stress exposure in mice in the present study. These delayed and long-term effects of stress exposure are in line with previous studies performed in rats (Mitra et al, 2005; Roozendaal et al, 2009). Different from previous studies reporting on a facilitation of auditory and contextual fear on acute exposure to a single restraint stress session (Cordero et al, 2003; Rodriguez Manzanares et al, 2005), acquisition and retrieval of conditioned fear was similar in the stressed and NS mice in the present study. Differences in species, fear training parameters and temporal delay between stress exposure and fear training might contribute to these differences between studies. Similar to previous findings (Izquierdo et al, 2006; Miracle et al, 2006; Akirav and Maroun, 2007; Baratta et al, 2007; Garcia et al, 2008; Muigg et al, 2008; Yamamoto et al, 2008; Baran et al, 2009; Gourley et al, 2009), acute IS resulted in elevated fear responses to the CS+ during early and late phases of extinction in the stressed compared with the NS mice. During acquisition and retrieval, fear responses were similar in the various groups, irrespective of previous stress exposure and application of NPS in the LA. These results largely rule out the possibility that enhanced fear responses during extinction reflect higher levels of re-experiencing fear. They are also in line with previous findings of a lack of NPS effects in the amygdala on acquisition and retrieval of fear in mice (Jüngling et al, 2008).
Local injection of NPS into the LA around stress exposure prevented the long-term increase in anxiety and enhancement of conditioned responses during fear extinction. Various groups have shown acute anxiolytic-like effects of NPS, as for instance in the EPM, defensive burying, or social interaction test (Jüngling et al, 2008; Leonard et al, 2008; Rizzi et al, 2008; Vitale et al, 2008; Fendt et al, 2010). Moreover, NPS was found to block acute stress-induced changes in physiological parameters, such as hyperthermia, oxidative stress damage and release of serotonin and noradrenaline in the frontal cortex (Xu et al, 2004; Castro et al, 2009; Raiteri et al, 2009). In addition to the acute effects, NPS has been shown to induce long-lasting effects, such as a reduction in conditioned aversive responses in contextual paradigms (Meis et al, 2008), a rapid decrease of cued conditioned fear during extinction and a facilitation of extinction recall (Jüngling et al, 2008), and an enhancement of memory during the consolidation phase irrespective of the emotional content (Okamura et al, 2011). In the present study, pre- or post-stress application of NPS was effectively interacting with the stress-induced changes in anxiety and fear extinction tested 10 days after stress exposure, whereas NPS in NS animals had no lasting effect if tested on the same parameters. These findings indicate a lack of acute anxiolytic-like influence of NPS during stress exposure. Application of the NPS-R antagonist SHA 68 (Okamura et al, 2008) evoked effects on stress-modulated fear responses opposite to that of NPS, suggesting that ambient NPS and thus the endogenous NPS system contribute to stress-coping (Xu et al, 2004; Pape et al, 2010; Okamura et al, 2011). Along the same line, NPS and SHA68 modified freezing also in response to the non-conditioned stimulus (CS−), suggesting that the NPS system may limit stress-induced generalization of fear responses. It is interesting to note that the NPS-induced enhancement of memory consolidation observed in a previous study was of a transient nature, with peak effects 2–4 days post memory training, and that the NPS effect interacted with the noradrenergic transmitter system in the brain (Okamura et al, 2011). In view of the central role for noradrenaline in regulating stress effects on memory consolidation, particularly also in the amygdala (for review see Roozendaal et al (2009)), NPS might thus act as a salience or arousal mechanism in concert with other memory systems to temporarily regulate the valence of the relevant signals during stressful encounters and thereby contribute to balance behavioral responses to the stressor.
Mechanisms Underlying NPS-Mediated Regulation of Stress Effects on Conditioned Fear
Spontaneous EPSCs occurred at increased frequency and amplitude in putative LA PNs in slices prepared ex vivo after fear conditioning, as compared with non-trained controls, irrespective of the presence or absence of restraint stress 10 days before conditioning. The EPSCs were recorded at −70 mV and were blocked by DNQX, suggesting mediation predominantly by AMPA-type glutamate receptors with little if any contribution by NMDA and GABAA receptors. Intrinsic membrane properties, in particular membrane resting potential and input resistance, were not significantly altered upon fear conditioning. Since both frequency and amplitude of spontaneous EPSCs were increased upon conditioning, mediating mechanisms may involve both pre- and postsynaptic sites. In fact, there is ample evidence indicating that fear conditioning induces long-term changes in synaptic efficacy at cortical and thalamic inputs to the LA, with both pre- and postsynaptic mechanisms contributing (recent review see Pape and Pare (2010)). One line of findings demonstrated that postsynaptic AMPA receptor trafficking in the LA is essential for auditory conditioned fear, in that conditioning was found to drive GluR1 receptor subunits into LA synapses, and blockade of AMPA receptor incorporation blocked both long-term synaptic potentiation of thalamic inputs to the LA in vitro and retention of conditioned fear in vivo (Rumpel et al, 2005). Furthermore, it is generally assumed that re-organization of actin and associated stabilization of spines provides a mechanism of structural plasticity during memory stabilization (for review see Yuste and Bonhoeffer (2001)). Fear conditioning indeed alters the expression of cytoskeletal proteins including neurofilament and a-actinin (Ressler et al, 2002), and actin dynamics regulate AMPA receptor trafficking and spinogenesis after contextual fear conditioning in the hippocampus (Fischer et al, 2004). This is important here for two major reasons. First, removal of surface AMPA receptors through endocytosis has been suggested a critical mechanism of depotentiation at LA synapses underlying fear extinction (Kim et al, 2007; Clem and Huganir, 2010). In the present study, the increase and decrease in sEPSC frequency on fear conditioning and extinction in LA neurons may thus reflect the respective changes in expression of surface AMPA receptors. Second, a single 2-h episode of IS led to a delayed increase in spine density in PNs of the BLA 10 days after stress exposure, and this was accompanied by an increase in anxiety-like behavior (Mitra et al, 2005). A very similar stress exposure protocol in the present study resulted in a stress-induced enhancement of conditioned fear responses during extinction, and concomitant sustainment of sEPSC activity at pre-extinction levels. This sEPSC activity may then reflect the increased spine density and surface expression of synaptic AMPA receptors, contributing to the enhanced fear responses during extinction. Future studies are certainly needed to clarify this issue, for instance through interfering with regulated AMPA receptor exo- or endocytosis during stress-impaired fear extinction.
How can application of NPS around stress exposure prevent the stress-induced sustainment of EPSC activity in LA neurons and enhancement of fear responses during extinction? One possible route is via GABAergic mechanisms in the amygdala (Harris and Westbrook, 1998; Akirav et al, 2006; Heldt and Ressler, 2007; Makkar et al, 2010). First, intercalated GABAergic neurons are critically involved in fear extinction recall (Likhtik et al, 2008), mediated to an important part via influences from the infralimbic prefrontal cortex (Quirk and Mueller, 2008). Second, NPS decreases conditioned fear responses during extinction (Jüngling et al, 2008). Third, a stress-induced transient decrease in GABAergic influence is considered a critical element in triggering the cascade of events leading to hyperexcitability and spinogenesis in the basolateral amygdaloid complex (Rodriguez Manzanares et al, 2005; Roozendaal et al, 2009). Importantly, pretreatment of stressed animals with midazolam, a positive modulator of GABAA sites, prevented the stress-induced increases in contextual fear in vivo and hyperexcitability in the BLA in vitro (Rodriguez Manzanares et al, 2005), although the exact cellular sites of GABAergic action were not identified. NPS acts through stimulation of GABAergic intercalated cells of the medial paracapsular cluster in the amygdala (Jüngling et al, 2008), which may provide a mechanism capable of interacting with the stress-induced GABAergic disinhibition. The findings that NPS targets connections of LA PNs to intercalated GABAergic neurons (Jüngling et al, 2008), that NPS had no effects on conditioned fear tested 10 days after application in NS animals (present study), are in line with the conclusion of a rather specific influence of NPS on stress-impaired extinction.
In conclusion, the present results provide evidence indicating that the NPS system can prevent stress-induced increases in anxiety and impairment of fear extinction, most likely through an influence on the excitation–inhibition balance in the amygdala. The NPS system might thus participate to a potential endogenous mechanism that controls the transformation of stressful events into anxiety and thereby might be relevant as a new pharmacological way for stress disorders such as PTSD. In view of the relationship between anxiety-like traits and individual differences in behavioral and neurobiological vulnerability to stress (Luksys et al, 2009, Salehi et al., 2010; as reviewed by Sandi and Richter-Levin, 2009; Mahan and Ressler, 2011), future studies are needed to identify the possible contribution of the NPS system and genetic variations of the NPS receptor (as quoted in the Introduction) to individual differences in vulnerability to develop anxiety disorders when exposed to stressful or traumatic events (see, eg, Segman and Shalev (2003)).
Acknowledgments
Support for this research was provided by the German Research Foundation (DFG; SFB-TR58, TPA02, A03 to H.-C.P., T.S) the IZKF (Interdisciplinary Centre for Clinical Research, Münster; PaHC3/003/10 to H.-C.P. and K.J.), and a Max Planck Research Award (to H.-C.P). F.C. gratefully acknowledges Alexander von Humboldt foundation for a personal post-doctoral stipend. We thank R. Reinscheid for drug gift, S. Kiesling, P. Berenbrock, and E. Boening for excellent technical support.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:1–11. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Raizel H, Maroun M. Enhancement of conditioned fear extinction by infusion of the GABA(A) agonist muscimol into the rat prefrontal cortex and amygdala. Eur J Neurosci. 2006;3:758–764. doi: 10.1111/j.1460-9568.2006.04603.x. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, et al. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;4:1495–1503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AA, Moretti M, Casagrande TS, Martinello C, Petronilho F, Steckert AV, et al. Neuropeptide S produces hyperlocomotion and prevents oxidative stress damage in the mouse brain: a comparative study with amphetamine and diazepam. Pharmacol Biochem Behav. 2009;4:636–642. doi: 10.1016/j.pbb.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;6007:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;3:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003;4:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Franke F, Stuhrmann A, Hohoff C, Zwanzger P, et al. Neuropeptide-S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology. 2011;9:1879–1885. doi: 10.1038/npp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K, Reif A, Weber H, Richter J, Hohoff C, Ohrmann P, et al. Neuropeptide S receptor gene—converging evidence for a role in panic disorder. Mol Psychiatry. 2011;9:938–948. doi: 10.1038/mp.2010.81. [DOI] [PubMed] [Google Scholar]
- Donner J, Haapakoski R, Ezer S, Melén E, Pirkola S, Gratacòs M, et al. Assessment of the neuropeptide S system in anxiety disorders. Biol Psychiatry. 2010;68:474–483. doi: 10.1016/j.biopsych.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Ebner K, Rjabokon A, Pape HC, Singewald N. Increased in vivo release of neuropeptide S in the amygdala of freely moving rats after local depolarisation and emotional stress. Amino Acids. 2011;4:991–996. doi: 10.1007/s00726-011-1058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Imobersteg S, Bürki H, McAllister KH, Sailer AW. Intra-amygdala injections of neuropeptide S block fear-potentiated startle. Neurosci Lett. 2010;3:154–157. doi: 10.1016/j.neulet.2010.03.028. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci. 2004;8:1962–1966. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem. 2008;4:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;3:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 1998;1:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of posttraumatic stress disorder. CNS Spectr. 2009;1 (Suppl 1:13–24. [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;12:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;21:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Phifer JE, Weiss T, Davis M, et al. Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD. Psychoneuroendocrinology. 2010;6:846–857. doi: 10.1016/j.psyneuen.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüngling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, et al. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;2:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. J Neurosci Res. 2006;7:1580–1587. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, et al. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci USA. 2007;52:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, et al. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;4:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog.Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zeng J, Zhou A, Theodorsson E, Fahrenkrug J, Reinscheid RK. Molecular fingerprint of neuropeptide S-producing neurons in the mouse brain. J Comp Neurol. 2011;519:1847–1866. doi: 10.1002/cne.22603. [DOI] [PubMed] [Google Scholar]
- Luksys G, Gerstner W, Sandi C. Stress, genotype and norepinephrine in the prediction of mouse behavior using reinforcement learning. Nat Neurosci. 2009;12:1180–1186. doi: 10.1038/nn.2374. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2011;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;8:1625–1652. doi: 10.1038/npp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;11:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T. Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. PLoS ONE. 2008;7:e2695. doi: 10.1371/journal.pone.0002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;7:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;3:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;26:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson CM, Schnurr PP, Resick PA, Friedman MJ, Young-Xu Y, Stevens SP. Cognitive processing therapy for veterans with military-related posttraumatic stress disorder. J Consult Clin Psychol. 2006;5:898–907. doi: 10.1037/0022-006X.74.5.898. [DOI] [PubMed] [Google Scholar]
- Muigg P, Hetzenauer A, Hauer G, Hauschild M, Gaburro S, Frank E, et al. Impaired extinction of learned fear in rats selectively bred for high anxiety—evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur J Neurosci. 2008;11:2299–2309. doi: 10.1111/j.1460-9568.2008.06511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;2:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;6:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Garau C, Duangdao DM, Clark SD, Jüngling K, Pape HC, et al. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology. 2011;4:744–752. doi: 10.1038/npp.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J Pharmacol Exp Ther. 2008;3:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Jüngling K, Seidenbecher T, Lesting J, Reinscheid RK. Neuropeptide S: a transmitter system in the brain regulating fear and anxiety. Neuropharmacology. 2010;1:29–34. doi: 10.1016/j.neuropharm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;2:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;3:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;1:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczka KA, Gartmann N, Mechias ML, Reif A, Büchel C, Deckert J, et al. 2010A neuropeptide S receptor variant associated with overinterpretation of fear reactions: a potential neurogenetic basis for catastrophizing Mol Psychiatry 1110451067–1074. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;14:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiteri L, Luccini E, Romei C, Salvadori S, Calò G. Neuropeptide S selectively inhibits the release of 5-HT and noradrenaline from mouse frontal cortex nerve endings. Br J Pharmacol. 2009;157:474–481. doi: 10.1111/j.1476-5381.2009.00163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry. 2010;12:1117–1119. doi: 10.1016/j.biopsych.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;18:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, et al. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol. 2008;2:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Manzanares PA, Isoardi NA, Carrer HF, Molina VA. Previous stress facilitates fear memory, attenuates GABAergic inhibition, and increases synaptic plasticity in the rat basolateral amygdala. J Neurosci. 2005;38:8725–8734. doi: 10.1523/JNEUROSCI.2260-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;6:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;12:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann NY Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;5718:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 2010;17:522–530. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- Sandi C, Richter-Levin G. From high anxiety trait to depression: a neurocognitive hypothesis. Trends Neurosci. 2009;32:312–320. doi: 10.1016/j.tins.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Segman RH, Shalev AY. Genetics of posttraumatic stress disorder. CNS Spectr. 2003;8:693–698. doi: 10.1017/s1092852900008889. [DOI] [PubMed] [Google Scholar]
- Sosulina L, Meis S, Seifert G, Steinhauser C, Pape HC. Classification of projection neurons and interneurons in the rat lateral amygdala based upon cluster analysis. Mol Cell Neurosci. 2006;33:57–67. doi: 10.1016/j.mcn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Victor AM, Bernstein GA. Anxiety disorders and posttraumatic stress disorder update. Psychiatr Clin North Am. 2009;1:57–69. doi: 10.1016/j.psc.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Vitale G, Filaferro M, Ruggieri V, Pennella S, Frigeri C, Rizzi A, et al. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides. 2008;12:2286–2291. doi: 10.1016/j.peptides.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, Diamond D, Richter-Levin G. Effects of inescapable stress on LTP in the amygdala versus the dentate gyrus of freely behaving rats. Eur J Neurosci. 2004;7:1887–1894. doi: 10.1111/j.1460-9568.2004.03294.x. [DOI] [PubMed] [Google Scholar]
- Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;4:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;9:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.