Abstract
Vasopressin controls transport in the renal collecting duct, in part, by regulating transcription. This complex process, which can involve translocation and/or modification of transcriptional regulators, is not completely understood. Here, we applied a method for large-scale profiling of nuclear proteins to quantify vasopressin-induced changes in the nuclear proteome of cortical collecting duct (mpkCCD) cells. Using stable isotope labeling and tandem mass spectrometry, we quantified 3987 nuclear proteins and identified significant changes in the abundance of 65, including previously established targets of vasopressin signaling in the collecting duct. Vasopressin-induced changes in the abundance of the transcription factors JunB, Elf3, Gatad2b, and Hmbox1; transcriptional co-regulators Ctnnb1 (β-catenin) and Crebbp; subunits of the Mediator complex; E3 ubiquitin ligase Nedd4; nuclear transport regulator RanGap1; and several proteins associated with tight junctions and adherens junctions. Bioinformatic analysis showed that many of the quantified transcription factors have putative binding sites in the 5′-flanking regions of genes coding for the channel proteins Aqp2, Aqp3, Scnn1b (ENaCβ), and Scnn1g (ENaCγ), which are known targets of vasopressin. Immunoblotting demonstrated that the increase in β-catenin in nuclear fractions was accompanied by an even larger increase in its phosphorylated form (pSer552). The findings provide a new online database resource for nuclear proteomics (http://helixweb.nih.gov/ESBL/Database/mNPD/) and generate new hypotheses regarding vasopressin-mediated transcriptional regulation in the collecting duct.
In the renal collecting duct, vasopressin mediates long-term regulation of NaCl and water transport by regulating the abundance of key transporter proteins such as aquaporin-2 (AQP2),1 aquaporin-3,2 and the β- and γ- subunits of the epithelial sodium channel (ENaCβ and ENaCγ, respectively).3 The measured increase in protein abundance is believed to be largely due to transcriptional regulation of the corresponding genes.4,5
Regulation of gene expression is mediated via differential DNA binding of transcription factors and co-regulators, as well as via chromatin modification. External stimuli, such as hormones like vasopressin, can influence activity and nuclear abundance of regulatory proteins through post-translational modifications, changes in transcription, and regulated translocation to and from the nucleus. So far, there is only limited information on transcription factors involved in the control of gene expression by vasopressin in renal collecting duct cells.6–10
We previously identified transcription factors potentially involved in regulation of transporter genes in collecting duct cells by transcriptomic profiling,11–13 and proteomic profiling of nuclear fractions.14 Uawithya et al.12 identified mRNAs coding for 824 transcription factors as well as numerous transcriptional co-regulators in rat inner medullary collecting duct. Nuclear proteomic profiling of inner medullary collecting duct cells identified 157 transcription factors and numerous other potential regulators of gene transcription such as transcriptional co-regulators, protein kinases, and protein phosphatases.14 However, these studies did not quantify changes in nuclear protein abundances in response to vasopressin. To this end, we used stable isotope labeling by amino acids in cell culture (SILAC)15 coupled to subcellular fractionation and state-of-the-art mass spectrometry (MS) to quantify vasopressin-induced changes in the abundances of 3987 proteins in the nuclei of cultured mpkCCD cells. Among these, 65 proteins were found to undergo changes.
Results
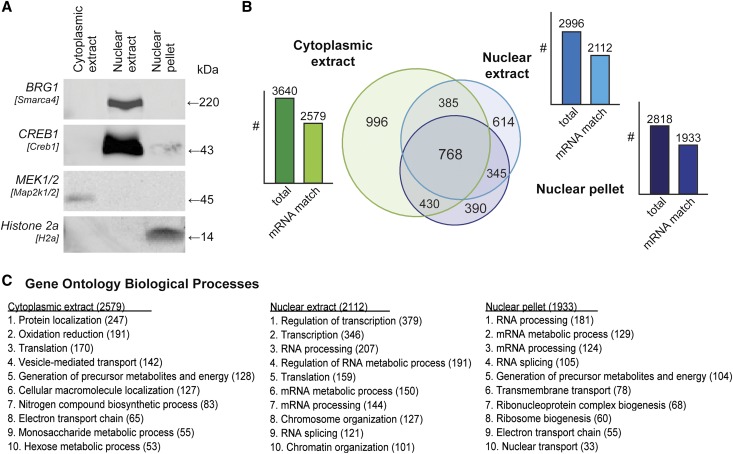
Cultured mpkCCD cells (recloned to maximize AQP2 response to 1-desamino-8-d-arginine vasopressin [dDAVP]11) underwent subcellular fractionation using a well characterized biochemical procedure that yielded three fractions16: cytoplasmic extract, nuclear extract, and nuclear pellet (Figure 1A). The nuclear markers Brg1 and Creb1 were enriched in the nuclear extract, whereas the chromatin marker Histone 2a was found only in the nuclear pellet. Map2k1/2 (MEK1/2) was found only in the cytoplasmic fraction. These results confirmed the success of the fractionation. The proteomes of these fractions were identified by liquid chromatography–tandem mass spectrometry (LC-MS/MS), using filters that restrict the false discovery rate to 1% at the peptide level. To obtain a list of the most confident protein level identifications, proteins were matched to the transcript list for these cells previously obtained using Affymetrix expression arrays.11 Of 6117 proteins identified in the three fractions, 64% had corresponding transcripts (Supplemental Tables 1 and 2). The distribution of this subset of proteins among the three fractions is shown in Figure 1B. Cellular Component Gene Ontology terms characterizing the three fractions were consistent with expectations (Figure 1C). Although the nuclear extract fraction was highly enriched in proteins identified as “nuclear proteins,” the nuclear pellet also contained a number of endoplasmic reticulum proteins, presumably indicative of the fact that the endoplasmic reticulum is continuous with the nuclear outer membrane. The proteins identified in the two nuclear fractions included 234 transcription factors/cofactors, 73 protein kinases, and 28 protein phosphatases (Supplemental Tables 3–5).
Figure 1.
Nucleo-cytoplasmic separation and LC-MS/MS profiling. (A) Immunoblot of marker proteins for each subcellular compartment, labeled by common name with the gene symbol in brackets. (B) Venn diagram of common and unique proteins identified in cellular fractions. Bar graph represents the number of all proteins (left bar) and the number of proteins that have corresponding mRNA transcripts (right bar) in each cellular fraction. (C) Ten most frequent Biologic Processes terms (Gene Ontology) among identified proteins (count given in brackets, all false discovery rates<25%, all P<0.001).
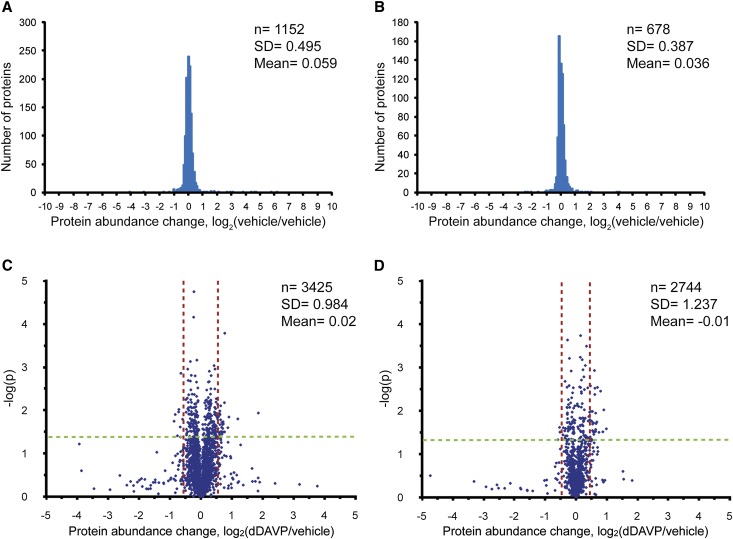
Next, we used SILAC coupled to LC-MS/MS to quantify changes in nuclear protein abundance in response to dDAVP. As a first step, we compared nominally identical samples of nuclear proteins from mpkCCD cells labeled with either light or heavy amino acids to determine the experimental variability of the method (Figure 2, A and B). The log2(vehicle/vehicle) was 0.059±0.495 (mean ± SD) for the nuclear extract and 0.036±0.387 for the nuclear pellet, indicating low intrinsic experimental variability. Subsequently, we used a threshold-for-change criterion based on these background SDs.
Figure 2.
SILAC quantification of nuclear fractions. Distribution of protein quantifications in (A) nuclear extract and (B) pellet in vehicle versus vehicle experiment. Distribution of protein quantifications in (C) nuclear extract and (D) pellet in 60-minute dDAVP (0.1 nM) versus vehicle experiments shown as “volcano plots.” Dashed vertical lines indicate 95% CI derived from vehicle versus vehicle experiment. Proteins of interest are those that exceed both the 95% CI requirement, as well as the P value requirement (see text).
We used SILAC to compare cells exposed for 60 minutes to the vasopressin analog dDAVP at a physiologic concentration (0.1 nM) versus vehicle-treated cells (Figure 2, C and D). Evaluating three biologic replicates, we quantified 3425 proteins in the nuclear extract and 2744 proteins in the nuclear pellet, accounting for a total of 3987 proteins in nuclear fractions. The combined database of 7514 proteins found in the nonquantitative and the quantitative study is available online (http://helixweb.nih.gov/ESBL/Database/mNPD/).
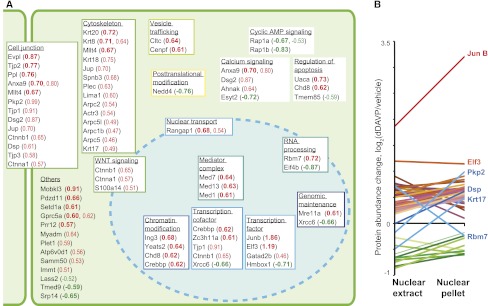
The full list of protein quantification results is given in Supplemental Table 6. To minimize false positive results, we required proteins to meet a dual statistical criterion (P<0.05 by t test and outside the 95% confidence interval [95% CI] defined by the vehicle versus vehicle experiment [vertical dashed lines in Figure 2, C and D]). This dual criterion was met by 65 proteins (Figure 3A; Supplemental Table 7). Several of these proteins are involved in aspects of transcriptional regulation. The transcription factors JunB and Elf3 stood out with the largest increases. Several chromatin modifiers, transcription factors, transcription cofactors, members of the mediator of RNA polymerase II complex (Mediator complex), and proteins involved in RNA processing were represented. In addition, the list included a large number of proteins usually associated with cytoplasmic functions, such as cytoskeletal and junctional proteins, consistent with prior observations (see the Discussion; Supplemental Table 8). The majority of these proteins with a significant change in either the nuclear extract or the nuclear pellet showed a corresponding change in the other fraction: 54 proteins were increased in both fractions (red in Figure 3B) and 9 proteins were decreased in both fractions (green in Figure 3B), whereas divergent changes were seen for only 4 proteins (blue in Figure 3B). Thus, the analysis of the two fractions provided nominally redundant information that provided internal confirmation of the findings.
Figure 3.
Proteins significantly changed in nuclear abundance. (A) Protein groupings were hand curated based on Gene Ontology terms, Swiss-Prot protein annotations, and Panther protein classes for each protein. Quantification results are given in parentheses as average log2(dDAVP/vehicle) for proteins meeting the dual statistical criterion (P<0.05 in t test and outside 95% CI derived from vehicle versus vehicle experiment). Values in bold refer to changes in the nuclear extract, and nonbold values refer to changes in the nuclear pellet. (B) Corresponding quantification results in nuclear extract and nuclear pellet for all proteins meeting the dual criterion in at least one of the nuclear fractions. For details, see Supplemental Tables 6 and 7.
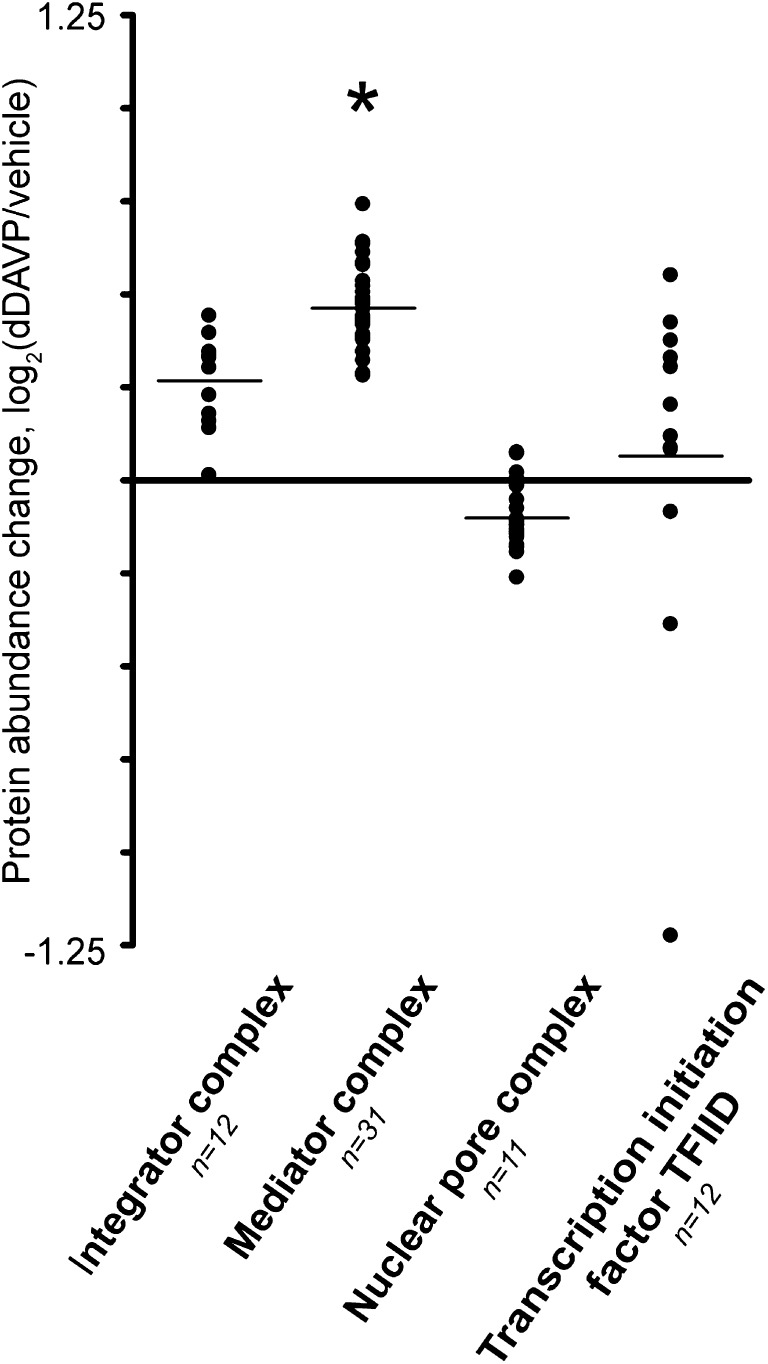
Three of 31 quantified subunits of the Mediator complex were significantly increased. Figure 4 shows the log2(dDAVP/vehicle) of all 31 quantified members of this complex compared with other complexes. The mean value for Mediator complex subunits was significantly greater than zero, in contrast to the other complexes. This suggests that the Mediator subunits may be co-regulated possibly via nuclear translocation of the intact complex.
Figure 4.
Protein complexes in the nuclear extract. Protein complexes were selected based on subunit count. *Quantification results significantly different from 0 (P<0.05). For details, see Supplemental Tables 6 and 7.
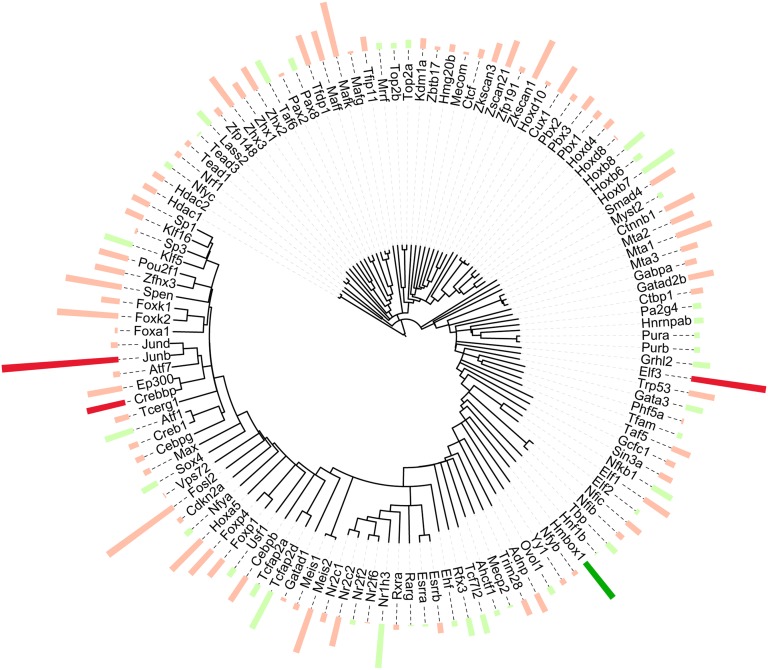
Because transcriptional regulation by vasopressin is a major focus of this study, we displayed the putative transcription factors and transcriptional co-regulators in a more useful format in Figure 5. Here, we performed protein sequence alignment for all proteins in the nuclear extract matching the Gene Ontology Molecular Function term “sequence-specific DNA binding transcription factor activity.” The circular dendrogram depicts the relatedness of their sequences, whereas the bars represent the quantification (red, increased; green, decreased). The increases in Elf3 and JunB and transcriptional coregulator CREB-binding protein (Crebbp) stood out, along with the decrease in the transcription factor homeobox-containing protein 1 (Hmbox1).
Figure 5.
Sequence-specific DNA binding transcription factors detected in the nuclear extract. A dendrogram showing proteins quantified in the nuclear extract that matched the Gene Ontology Molecular Function term “sequence-specific DNA binding transcription factor activity” aligned by amino acid sequence similarity (entire protein). The bars indicate quantification results, averages of |log2(dDAVP/vehicle)| >0 (red, increased; green, decreased). Bold colors implicate a significant difference meeting the dual criterion discussed above.
Many of the identified transcription factors matched transcription factor binding sites (TFBSs) in the 5′-flanking regions of downstream target proteins regulated by vasopressin in the kidney, namely Aqp2, Aqp3, Scnn1b (ENaCβ), and Scnn1g (ENaCγ) (Figure 6). Ninety-two transcription factors identified in this study matched 42 different TFBSs that are conserved among human, dog, rat, and mouse (within 1000 bp from transcriptional start site). Three of these transcription factors showed altered nuclear abundance: JunB (Aqp2), Elf3 (Aqp2, Aqp3, Scnn1b, Scnn1g), and Hmbox1 (Aqp2, Scnn1g). Twenty-two transcription factors matched to the five TFBSs that were common to all four genes, V$ETSF, V$SP1F, V$FKHD, V$RXRF, and V$CREB.
Figure 6.
Transcription factors putatively binding to cis-elements in the 5′-flanking regions of Aqp2, Aqp3, Scnn1b, and Scnn1g. Putative transcription factor binding elements (TFBSs) within 1000 bp upstream of the transcriptional start sites of (A) Aqp2, (B) Aqp3, (C) Scnn1b, and (D) Scnn1g analyzed using the Genomatix database and software suite. All shown TFBSs are conserved among human, dog, rat, and mouse. Transcription factors identified in this study that potentially bind to these TFBSs are indicated. Numbers represent center of motif in mouse. Transcription factors significantly changed above threshold are given in red (increase) or green (decrease). Genomatix TFBS family names are as follows: V$ABDB, abdominal B-type homeodomain transcription factors; V$AP1F, AP1 activating protein 1; V$AP1R, MAF and AP1 related factors; V$AP2F, activator protein 2; V$CAAT, CCAAT box binding proteins; V$CP2F, CP2-erythrocyte factor related to Drosophila Elf1; V$CREB, cAMP-responsive element-binding proteins; V$CTCF, CTCF and BORIS gene family transcriptional regulators; V$E2FF, E2F-myc activator/cell cycle regulator; V$EBOX, E-box binding factors; V$EGRF, EGR/nerve growth factor induced protein C and related factors; V$EREF, estrogen response elements; V$ETSF, human and murine ETS1 factors; V$FKHD, Fork head domain factors; V$GATA, GATA binding factors; V$HAML, human acute myelogenous leukemia factors; V$HESF, vertebrate homologs of enhancer of split complex; V$HNF1, hepatic NF 1; V$HOXF, paralog hox genes 1–8 from the four hox clusters A, B, C, D; V$IRFF, IFN regulatory factors; V$KLFS, Kruppel-like transcription factors; V$LEFF, LEF1/TCF; V$MAZF, Myc-associated zinc fingers; V$NFAT, NF of activated T cells; V$NFKB, NFκB/c-rel; V$NR2F, nuclear receptor subfamily 2 factors; V$OCT1, octamer binding protein; V$P53F, p53 tumor suppressor–negative regulator of the tumor suppressor Rb; V$PBXC, PBX1 - MEIS1 complexes; V$PTF1, pancreas transcription factor 1, heterotrimeric transcription factor; V$PURA, Pur-α binds both single-stranded and double-stranded DNA in a sequence-specific manner; V$RREB, Ras-responsive element-binding protein; V$RXRF, RXR heterodimer binding sites; V$SMAD, vertebrate SMAD family of transcription factors; V$SORY, SOx/sRY-sex/testis determining and related HMG box factors; V$SP1F, GC-Box factors SP1/GC; V$STAF, selenocysteine tRNA activating factor; V$STAT, signal transducer and activator of transcription; V$XBBF, X-box binding factors; V$YY1F, activator/repressor binding to transcription initiation site; V$ZBPF, zinc binding protein factors.
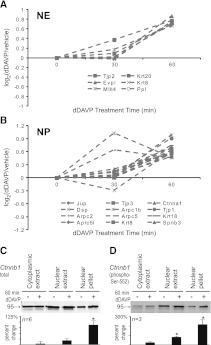
A particularly interesting finding from this study is the large number of junctional and cytoplasmic proteins that increased in the nucleus in response to dDAVP, presumably as a result of translocation. These included ZO-1 (Tjp1), ZO-2 (Tjp2), ZO-3 (Tjp3), α-catenin (Ctnna1), γ-catenin (Jup), and afadin (Mllt4), as well as three Arp2/3 complex proteins (Arpc1b, Arpc5, Arpc5l). We carried out separate MS experiments to address whether the changes in cytoskeletal and junctional proteins can be detected at an earlier time point (dDAVP versus vehicle, 30 minutes; Figure 7A). Indeed, 18 of the 20 proteins in this class successfully quantified at 30 minutes showed positive values (P<0.01, chi-squared versus random).
Figure 7.
Cytoskeletal and junctional proteins increased in nucleus. Values for cytoskeletal and junctional proteins in (A) nuclear extract fraction and (B) nuclear pellet fraction quantified at 30 minutes after dDAVP addition (0.1 nM) compared with values at 60 minutes of dDAVP addition. Eighteen of 20 of these proteins showed positive values at 30 min (P<0.01), chi-squared analysis versus random distribution of positive and negative (10/20). (C) Immunoblotting for total β-catenin in samples from three cellular fractions after 60 minutes of dDAVP (0.1 nM) or vehicle. (D) Immunoblotting for β-catenin phosphorylated at Ser-552. Results of densitometric quantification are shown in the bar graphs below the representative bands. *P<0.05 (t test). NE, nuclear extract; NP, nuclear pellet.
One junctional protein that is of particular interest in transcriptional regulation in the collecting duct is β-catenin, a multifunctional protein that has plays an important role in collecting duct development17 and in lithium toxicity.18 Prior studies showed that β-catenin phosphorylation at Ser-552 is regulated by vasopressin.19–21 However, none of these studies addressed changes in subcellular localization. Here, immunoblotting confirmed that β-catenin significantly increased in nuclear abundance in response to dDAVP (Figure 7B) and further showed parallel changes in β-catenin phosphorylated at Ser-552 (Figure 7C). The increase in this phosphorylated form is much greater than the change in total β-catenin protein in the nuclear fractions.
Discussion
Vasopressin regulates transport in the renal collecting duct in part through transcriptional regulation of transporter genes. This presumably occurs through changes in abundances of various regulatory proteins such as transcription factors and transcriptional co-regulators. Here, we used quantitative LC-MS/MS to identify nuclear proteins in cultured collecting duct cells (mpkCCD clone 1111) that undergo changes in abundance in response to the V2-receptor selective vasopressin analog dDAVP. We anticipated that changes in nuclear abundances of proteins would be largely due to translocation between cytoplasm and nucleus at the short time point chosen (60 minutes of dDAVP stimulation). However, changes in nuclear abundances can also occur by altered transcription or degradation, particularly for transcription factors, which often show rapid turnover.22 Preliminary studies demonstrated a high degree of technical precision in the SILAC-based quantification approach (Figure 2, A and B), allowing detection of small to moderate changes in nuclear abundance with high sensitivity. The sensitivity of the method is critical to its ability to view an entire landscape of changes that result from a physiologic stimulus, such as exposure to vasopressin. Indeed, several dozen proteins (of almost 4000 quantified) were found to increase or decrease.
Altogether, this study identified 7514 proteins from subcellular fractions of mpkCCD cells, representing the most comprehensive collection of proteomic data for cultured kidney collecting duct cells to date. These data have been placed online to provide a public resource ( http://helixweb.nih.gov/ESBL/Database/mNPD/). As a result of the subcellular fractionation procedure, we identified many nuclear proteins not detected in whole cell analyses, presumably because nuclear proteins contribute a relatively small proportion to the total protein mass of the cell.
Transcription Factors Regulated by Vasopressin
Several of the transcription factors identified here matched binding sites in the 5′-flanking region of vasopressin-regulated target genes including Aqp2, Aqp3, Scnn1b (ENaCβ), and Scnn1g (ENaCγ) (Figure 6). However, of the large number of transcription factors quantified, only four changed in nuclear abundance. Specifically, JunB, Elf3, and Gatad2b increased, whereas Hmbox1 decreased. Yasui et al. showed c-jun binding to the V$AP1F site in Aqp2 after treatment with vasopressin,6 compatible with the idea that either nuclear translocation or rapid transcriptional regulation of JunB may contribute to the transcriptional regulation of Aqp2 by vasopressin. The transcription factor Elf3 has conserved binding sites (V$ETSF) in all four vasopressin-regulated target genes examined in this study (Figure 6). Furthermore, Elf3 mRNA levels have been shown to correlate with Aqp2 expression in mpkCCD clones, and vasopressin was directly shown to increase AQP2 transactivation by Elf3 in promotor-reporter studies.11 The activity of Elf3 can be positively modified by Crebbp,23 a transcriptional coregulator that also increased in our study. In contrast, Hmbox1 was decreased in nuclear abundance with vasopressin. This observed decrease might lead to increased transcriptional activity of target genes, because Hmbox1 acts chiefly as a repressor in other systems.24 To our knowledge, Hmbox1 has not been previously associated with vasopressin signaling.
Post-Translational Modifiers Regulated by Vasopressin
Post-translational modifications are an important mode of protein regulation, and we hypothesized that enzymes that catalyze such modifications would be found to translocate in response to vasopressin. However, among this category of proteins, only Nedd4, an E3 ubiquitin ligase, increased in nuclear abundance. Nedd4 has been implicated in vasopressin-mediated control of AQP2 protein abundance25 and a closely related protein, Nedd4-2, plays a key role in regulation of ENaC abundance.26 In addition, nuclear proteins such as RNA polymerase II subunits can undergo in vivo ubiquitinylation by Nedd4.27 One may hypothesize that Nedd4 is a nuclear effector of vasopressin-mediated changes in protein ubiquitinylation and degradation.
Known Components of Vasopressin Signaling in Collecting Duct Cells Altered by Vasopressin in This Study
Rap1a and Rap1b (decreased in nuclear abundance) are Ras-like GTPases regulated in part by cAMP-sensitive Epac proteins that are involved in vasopressin-related intracellular calcium mobilization.28 Our study showed that vasopressin leads to diminished nuclear abundance of these signaling molecules. Rap1 isoforms lie upstream of the Raf-MEK-ERK signaling pathway, which is downregulated in response to vasopressin in renal collecting duct.29
Junctional and Cytoskeletal Proteins
Among the proteins increased in the nucleus, we detected a large number of junctional and cytoskeletal proteins (Figure 3A) that play roles in determination of epithelial cell polarity and regulated trafficking to the apical plasma membrane.30–33 Although superficially surprising, nuclear transport of junctional proteins is well established in the literature, particularly with regard to β-catenin and the tight junction proteins ZO-1, 2, and 3, which can function as transcriptional co-regulators.34,35 This study identified increases in all four of these proteins in the nucleus in response to vasopressin, and extends the finding to a number of other cytoskeletal and junctional proteins (Figure 3A). These presumed translocation events therefore provide an alternative mechanism (to activation of protein kinase cascades) whereby extracellular signals may result in changes in transcription. Additional MS experiments focusing on cytoskeletal and junctional proteins confirmed the general finding and showed that increases in nuclear abundance could be observed after only 30 minutes of vasopressin exposure for many of these proteins (Figure 7, A and B).
Mediator Complex
The Mediator complex is a key element in transcriptional regulation, forming a bridge between transcriptional activators bound to cis-regulatory elements and RNA polymerase II.36 Interestingly, three subunits of the Mediator complex were significantly increased in the nuclear extract (Figure 3A), and when plotted together (Figure 4) the nuclear abundances of all 31 mediator complex proteins seemed to cluster in a range consistent with nuclear increases. These results are what would be expected if the complex assembles in the cytoplasm and translocates as a unit in response to vasopressin, although separate regulation of the each subunit cannot be ruled out.
β-Catenin and Vasopressin Signaling
An interesting transcriptional coregulator that increased in the nuclear fractions is β-catenin. There is an emerging recognition of a role for β-catenin in vasopressin signaling in the renal collecting duct.17–21 β-catenin is a multifunctional protein acting in at least three ways: (1) as a transcriptional coregulator, (2) as a component of the Wnt signaling network, and (3) as a component of adherens junctions. We also identified numerous proteins that putatively interact with β-catenin in collecting duct nuclei such as Cdh, Vcl, Scrib, Crebbp, Ep300, Tcf7l2, and Tax1bp3.37 Furthermore, we demonstrated vasopressin stimulated β-catenin phosphorylation at the activating Ser-552 site in both nuclear fractions, a phosphorylation event that is thought to be mediated by protein kinase A.38 The finding that both total and phosphorylated β-catenin increase in response to vasopressin points to the possibility that β-catenin may play a role in mediating vasopressin-dependent regulation of gene transcription.
Role of Large-Scale Analysis in Physiology
This study further supports the conclusion that large-scale proteomic analysis is a powerful means of identifying new hypotheses about physiologic regulation. Large-scale studies like this one have a negative aspect that needs to be recognized, but can be dealt with; namely, they depend on a large number of statistical tests with false positive rates (P values) that can potentially produce false positive conclusions about individual proteins. This problem is mitigated here by several factors that put the conclusions in a much stronger position statistically than would otherwise be the case for large-scale analysis. First, values for each protein are determined by quantifying multiple peptides, meaning that several independent observations go together to give a value for one protein. Second, in the systems biology paradigm, the focus is not on individual proteins, but rather on groups of proteins. One example is the Mediator complex (Figure 4), which shows an increase in nuclear abundance for all members of the complex in response to vasopressin. Therefore, although the probability of a false positive for any of them might be considered to be 0.05, the probability for the whole group approaches (0.05)n, where n is the number in the group. Third, in general, proteins that were changed in the nuclear pellet were also changed in the nuclear extract as shown in Figure 3B. Fourth, to limit false positives, we have included dual statistical criteria such as statistical significance at a 0.05 level and a fractional change defined by the 95% CI from the preliminary vehicle versus vehicle study. Additional studies will be needed to identify the mechanistic basis of the changes observed and to discover the signaling mechanisms that convert a change in peritubular vasopressin concentration to a change in the nuclear proteome.
Concise Methods
Cell Culture and Sample Preparation
All studies were done in an AQP2-expressing mpkCCD clonal cell line (clone 11)11 grown on membrane supports. We carried out three series of MS experiments, namely, a nonquantitative profiling study and two series of experiments using the SILAC method for quantification. An LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) system was used for all studies. In each study, samples were subjected to nuclear and cytoplasmic separation using a commercial kit (NE-PER Nuclear and Cytoplasmic Extraction Reagent Kit; Pierce, Rockfort, IL).16 This is a widely used method that consists of two basic steps: (1) separation of cytoplasm from nucleus using gentle lysis of cell membranes followed by low-speed centrifugation to harvest the nuclei, and (2) extraction of soluble components of nucleus (nuclear extract) from genomic DNA and nuclear RNA along with their bound proteins (nuclear pellet). According to the manufacturer, there is typically <10% contamination between nuclear and cytoplasmic fractions using the standard approach. To enhance the purity of the separation of cytoplasm and nucleus for the quantitative studies, the steps extracting the cytoplasm were carried out twice before proceeding with the nuclear sample. The total protein ratio for these fractions was 8:1:3 for cytoplasmic extract, nuclear extract, and nuclear pellet, respectively.
The two series of studies (each done in three biological replicates) quantifying the response to the vasopressin analog dDAVP (0.1 nM) were done using SILAC14 culture media containing the amino acids arginine and lysine labeled with stable isotopes (heavy: 13C6 15N4 arginine, 13C6 lysine; light: 12C6 14N4 arginine, 12C6 lysine) for 16 days (three passages). This labeling period is sufficient to achieve >98% saturation of labeling.20 At the beginning of an experiment (Supplemental Figure 1), labeled cells were grown in the presence of dDAVP (0.1 nM) for 4–5 days to assure that all vasopressin-dependent proteins are expressed.13 In the first quantitative series, the dDAVP was then withdrawn for 24 hours before re-exposure to vasopressin or vehicle for 60 minutes. In the second series observations were made after 30 minutes of exposure to dDAVP. For these experiments, cells (60–90 µg) were pooled in a 1:1 ratio (dDAVP/vehicle) and subjected to nuclear and cytoplasmic separation, as described above, before mass spectrometry.
MS
Samples were reduced with dithiothreitol (10 mM), and alkylated with iodoacetamide (40 mM) before one-dimensional SDS-PAGE (4%–15% gradient). The gels were cut to yield multiple pieces followed by in-gel trypsin digestion. Tryptic peptides were analyzed on an LTQ Orbitrap Velos mass spectrometer system. MS spectra were searched using the SEQUEST, Inspect, and OMSSA algorithms as described14 using the most recent mouse RefSeq database. Data were filtered using the target-decoy approach39 to obtain <1% false discovery rate at a peptide level. Quantification of light and heavy peptides was performed using QUIL software.40 Uniqueness or multiplicity of putative peptide assignment was evaluated using ProMatch software.14 Protein quantifications were median-normalized in each of experimental pair. Dual statistical criteria were applied: t test (paired, P<0.05) and outside the 95% CI defined by vehicle versus vehicle experiments.
Bioinformatics
Clustering of transcription factors was done using the Muscle algorithm41 and results were displayed as a dendrogram using the Interactive Tree of Life tool.42 Conserved TFBSs in the 5′-flanking regions of specific genes were found using the online Genomatrix software suite and TFBS database (http://www.genomatix.de/).
Immunoblotting
Immunoblotting was carried out as described.29
Disclosures
None.
Supplementary Material
Acknowledgments
We thank R. Lance Miller and Christina van Itallie for helpful discussion regarding the study, Jennifer Huling for creating the online database, Fahad Saeed for help with the sequence alignment, and Guanghui Wang for help with MS.
L.K.S. was supported by the German Academic Exchange Service (Bonn, Germany) and the Biomedical Sciences Exchange Program (Hannover, Germany). Mass spectrometry was performed in the National Heart, Lung, and Blood Institute Proteomics Core Facility (Director Marjan Gucek). This work was funded by the Division of Intramural Research of the National Heart, Lung, and Blood Institute (Project ZO1-HL001285 to M.A.K.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011070738/-/DCSupplemental.
References
- 1.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA: Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci USA 91: 8984–8988, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA: Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol 269: F663–F672, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA: Vasopressin-mediated regulation of ENaC abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG: Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852–1863, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stokes JB, Sigmund RD: Regulation of rENaC mRNA by dietary NaCl and steroids: Organ, tissue, and steroid heterogeneity. Am J Physiol 274: C1699–C1707, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Yasui M, Zelenin SM, Celsi G, Aperia A: Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol 272: F443–F450, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Furuno M, Uchida S, Marumo F, Sasaki S: Repressive regulation of the aquaporin-2 gene. Am J Physiol 271: F854–F860, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Hozawa S, Holtzman EJ, Ausiello DA: cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol 270: C1695–C1702, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, Bens M, Doucet A, Wincker P, Artiguenave F, Horisberger JD, Vandewalle A, Rossier BC, Firsov D: Transcriptome of a mouse kidney cortical collecting duct cell line: Effects of aldosterone and vasopressin. Proc Natl Acad Sci USA 98: 2712–2716, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasler U, Leroy V, Jeon US, Bouley R, Dimitrov M, Kim JA, Brown D, Kwon HM, Martin PY, Féraille E: NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem 283: 28095–28105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA: Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA: Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khositseth S, Pisitkun T, Slentz DH, Wang G, Hoffert JD, Knepper MA, Yu MJ: Quantitative protein and mRNA profiling shows selective post-transcriptional control of protein expression by vasopressin in kidney cells. Mol Cell Proteomics 10: M110.004036, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tchapyjnikov D, Li Y, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA: Proteomic profiling of nuclei from native renal inner medullary collecting duct cells using LC-MS/MS. Physiol Genomics 40: 167–183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M: Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1: 376–386, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Smirnova IV, Bittel DC, Ravindra R, Jiang H, Andrews GK: Zinc and cadmium can promote rapid nuclear translocation of metal response element-binding transcription factor-1. J Biol Chem 275: 9377–9384, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Marose TD, Merkel CE, McMahon AP, Carroll TJ: Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol 314: 112–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen J, Hoffert JD, Knepper MA, Agre P, Nielsen S, Fenton RA: Proteomic analysis of lithium-induced nephrogenic diabetes insipidus: Mechanisms for aquaporin 2 down-regulation and cellular proliferation. Proc Natl Acad Sci USA 105: 3634–3639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, Knepper MA: Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci USA 107: 15653–15658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA: Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107:3882–3887, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal AD, Hoffert JD, Pisitkun T, Hwang S, Chou CL, Boja ES, Wang G, Knepper MA: Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. J Am Soc Nephrol 21: 303–315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers S, Wells R, Rechsteiner M: Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis. Science 234: 364–368, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Fang R, Cho JY, Libermann TA, Oettgen P: Positive and negative modulation of the transcriptional activity of the ETS factor ESE-1 through interaction with p300, CREB-binding protein, and Ku 70/86. J Biol Chem 279: 25241–25250, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Saiyin H, Zeng X, Xi J, Liu X, Li X, Yu L: Isolation and functional analysis of human HMBOX1, a homeobox containing protein with transcriptional repressor activity. Cytogenet Genome Res 114: 131–136, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Lee YJ, Lee JE, Choi HJ, Lim JS, Jung HJ, Baek MC, Frøkiær J, Nielsen S, Kwon TH: E3 ubiquitin-protein ligases in rat kidney collecting duct: Response to vasopressin stimulation and withdrawal. Am J Physiol Renal Physiol 301: F883–F896, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D: Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16: 6325–6336, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anindya R, Aygün O, Svejstrup JQ: Damage-induced ubiquitylation of human RNA polymerase II by the ubiquitin ligase Nedd4, but not Cockayne syndrome proteins or BRCA1. Mol Cell 28: 386–397, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Yip KP: Epac-mediated Ca(2+) mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol 291: F882–F890, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA: Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295: F1030–F1043, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edemir B, Pavenstädt H, Schlatter E, Weide T: Mechanisms of cell polarity and aquaporin sorting in the nephron. Pflugers Arch 461: 607–621, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Noda Y, Horikawa S, Kanda E, Yamashita M, Meng H, Eto K, Li Y, Kuwahara M, Hirai K, Pack C, Kinjo M, Okabe S, Sasaki S: Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J Cell Biol 182: 587–601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou CL, Christensen BM, Frische S, Vorum H, Desai RA, Hoffert JD, de Lanerolle P, Nielsen S, Knepper MA: Non-muscle myosin II and myosin light chain kinase are downstream targets for vasopressin signaling in the renal collecting duct. J Biol Chem 279: 49026–49035, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Brown D: The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284: F893–F901, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Bauer H, Zweimueller-Mayer J, Steinbacher P, Lametschwandtner A, Bauer HC: The dual role of zonula occludens (ZO) proteins. J Biomed Biotechnol 2010: 402593, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapin HC, Caplan MJ: The cell biology of polycystic kidney disease. J Cell Biol 191: 701–710, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik S, Roeder RG: The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet 11: 761–772, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhurinsky J, Shtutman M, Ben-Ze’ev A: Plakoglobin and beta-catenin: Protein interactions, regulation and biological roles. J Cell Sci 113: 3127–3139, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO: Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem 281: 9971–9976, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Elias JE, Gygi SP: Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods 4: 207–214, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Wang G, Wu WW, Pisitkun T, Hoffert JD, Knepper MA, Shen RF: Automated quantification tool for high-throughput proteomics using stable isotope labeling and LC-MSn. Anal Chem 78: 5752–5761, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Edgar RC: MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Letunic I, Bork P: Interactive Tree Of Life v2: Online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 39: W475– W478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.