Abstract
Idiopathic nephrotic syndrome resistant to standard treatments remains a therapeutic dilemma in pediatric nephrology. To test whether the anti-CD20 monoclonal antibody rituximab may benefit these patients, we conducted an open-label, randomized, controlled trial in 31 children with idiopathic nephrotic syndrome unresponsive to the combination of calcineurin inhibitors and prednisone. All children continued prednisone and calcineurin inhibitors at the doses prescribed before enrollment, and one treatment group received two doses of rituximab (375 mg/m2 intravenously) as add-on therapy. The mean age was 8 years (range, 2–16 years). Rituximab did not reduce proteinuria at 3 months (change, −12% [95% confidence interval, −73% to 110%]; P=0.77 in analysis of covariance model adjusted for baseline proteinuria). Additional adjustment for previous remission and interaction terms (treatment by baseline proteinuria and treatment by previous remission) did not change the results. In conclusion, these data do not support the addition of rituximab to prednisone and calcineurin inhibitors in children with resistant idiopathic nephrotic syndrome.
Idiopathic nephrotic syndrome (INS) in children is a continuum of clinical disorders characterized by severe proteinuria, hypoalbuminemia, dyslipidemia, and hypercoagulability. INS includes pathologic variants that feature polymorphic podocyte injury as a unifying feature.1–3 Disease mechanisms are poorly understood, with the exception of the most severe cases, which are caused by molecular defects of one of the podocyte genes.4–7 For nongenetic forms of INS, responsiveness to prednisone alone or in combination with calcineurin inhibitors8,9 is the most important prognostic criterion to distinguish progressive from nonprogressive entities, given the known fibrogenic properties of persisting proteinuria.10,11
The chimeric monoclonal anti-CD20 antibody rituximab has recently emerged as a potential novel therapy for INS. Rituximab was first proposed for Hodgkin lymphoma and autoimmune diseases.12–17 Our group recently reported that rituximab successfully maintained short-term remission of proteinuria in children with INS responding to and dependent on both prednisone and calcineurin inhibitor, thereby allowing discontinuation of these standard medications.18
Anecdotal cases and few uncontrolled studies in small cohorts suggest that rituximab may induce disease remission in forms of INS unresponsive to prednisone and calcineurin inhibitors.19,20 The present multicenter randomized trial tested this hypothesis by comparing rituximab and the standard approach based on prednisone and calcineurin inhibitors in resistant forms of INS.
Results
Patient Characteristics
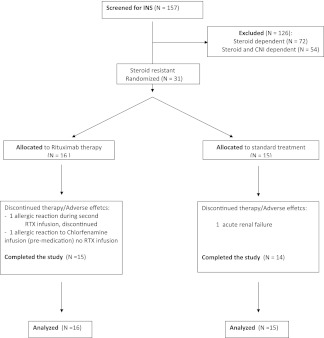
Between 2007 and 2010, we screened 157 children with INS and enrolled 31 eligible patients at four pediatric nephrology centers. Sixteen were randomly assigned to receive rituximab in double doses in addition to prednisone and calcineurin inhibitors and were compared with 15 patients randomly assigned to standard therapy (Figure 1). Baseline characteristics are summarized in Table 1. Children were on average 8 years of age and had a disease duration of 1.5 years. At randomization, all children were treated with prednisone (median dosage, 0.42 mg/kg per day [range, 0.1–1.9 mg/kg per day]), and most were receiving calcineurin inhibitors at the minimum oral amount necessary to achieve trough levels: 50–100 ng/ml for cyclosporine and 5–10 ng/ml for tacrolimus. In three cases, calcineurin inhibitors had been stopped 1 month before the start of the run-in period because of severe hypertension.
Figure 1.
Numbers of patients who were screened for the study, underwent randomization, and completed the study. CNI, calcineurin inhibitors; RTX, rituximab.
Table 1.
Baseline characteristics
| Characteristic | All Patients (n=31) | Control Group (n=15) | Rituximab Group (n=16) |
|---|---|---|---|
| Age (yr) | 7.9±4.1 | 7.3±3.7 | 8.5±4.4 |
| Body weight (kg) | 31±17 | 30±17 | 31±17 |
| Male, n (%) | 19 (61) | 9 (60) | 10 (62) |
| Disease duration (yr) | 1.5 (0.5, 11.8) | 1.4 (0.5, 8.1) | 2.5 (0.5, 11.8) |
| Drug resistance duration (yr) | 1.3 (0.5, 11.8) | 0.8 (0.5, 8.1) | 1.6 (0.5, 11.8) |
| Delayed resistance, n (%) | 15 (48) | 8 (53) | 7 (44) |
| Renal histology, n (%) | |||
| not performed | 4a (13) | 1 (7) | 3 (19) |
| FSGS | 19 (61) | 10 (67) | 9 (56) |
| minimal-change disease | 7 (23) | 3 (20) | 4 (25) |
| inadequate material | 1(3) | 1(3) | |
| CTX therapy, n (%) | 10 (32) | 5 (33) | 5 (31) |
| Prednisone dosage (mg/kg per day) | 0.42 (0.03, 1.9) | 0.35 (0.03, 1.1) | 0.51 (0.04, 1.9) |
| Tacrolimus (vs cyclosporine) | 16 (15) | 8 (7) | 8 (8) |
| FK506 dosage (mg/kg per day) | 0.1 (0.05, 0.17) | 0.1 (0.05, 0.14) | 0.13 (0.08, 0.17) |
| Cyclosporine dosage (mg/kg per day) | 3.6 (2.5, 4.5) | 3.6 (2.6, 4) | 3.6 (2.5, 4.5) |
| ARB or ACEI, n (%) | 25 (80) | 11 (73) | 14 (88) |
| Urinary protein (g/day per m2) | 2.7 (0.8, 8.8) | 2.5 (0.8, 8.8) | 2.8 (0.8, 6.6) |
| Serum albumin (g/dl) | 2.3±0.6 | 2.3±0.5 | 2.4±0.6 |
| Serum cholesterol (mg/dl) | 360±143 | 342±129 | 377±158 |
| Serum creatinine (mg/dl) | 0.54±0.36 | 0.55±0.41 | 0.53±0.35 |
| IgG (g/dl) | 322±220 | 324±200 | 320±243 |
Values are means ± SDs or medians (interquartile ranges) reported for quantitative variables (unit) and absolute (relative) frequencies for qualitative variables. CTX, previous use of cytotoxic agents (Endoxan, Leukeran); ARB, angiotensin-receptor blocker; ACEI, angiotensin-converting enzyme inhibitor.
Renal biopsy was not requested in patients who had presented at least one episode of drug responsiveness in the past.
All children had a history of resistance to both drugs that had lasted at least the 6 months before randomization (range, 6 months to 11.8 years; median, 1.3 years) (Table 1). Fifteen patients had developed resistance to treatments months or years after disease onset, during which time they had become dependent on steroids and calcineurin inhibitors. Thus, they were defined as “delayed resistant.” Development of unresponsiveness started at least 6 months before randomization. Children with delayed resistance were similarly represented in both treatment groups (Table 1).
At the time of enrollment, average prednisone and calcineurin inhibitor doses did not differ between groups. Additional therapies (angiotensin-receptor blocker and angiotensin-converting enzyme inhibitors) were used in the same proportions of patients. All clinical measures, including baseline proteinuria, kidney function, serum albumin, and cholesterol, were similar in the two groups.
Pharmacokinetics
Data on rituximab kinetics were measured in seven children at baseline and after the first infusion (Supplemental Table 1). Median serum levels were 0 µg/ml at the first infusion of rituximab, 313.4 µg/ml 15 minutes after infusion (range, 103.2–333 µg/ml), and 215.7 µg/mL 24 hours after infusion (range, 67.2–417.4 µg/ml). Similar levels were observed at the second infusion. The trend of serum levels of the patients analyzed was similar to that observed in previous studies in adults with clinical conditions requiring rituximab.21
Effectiveness
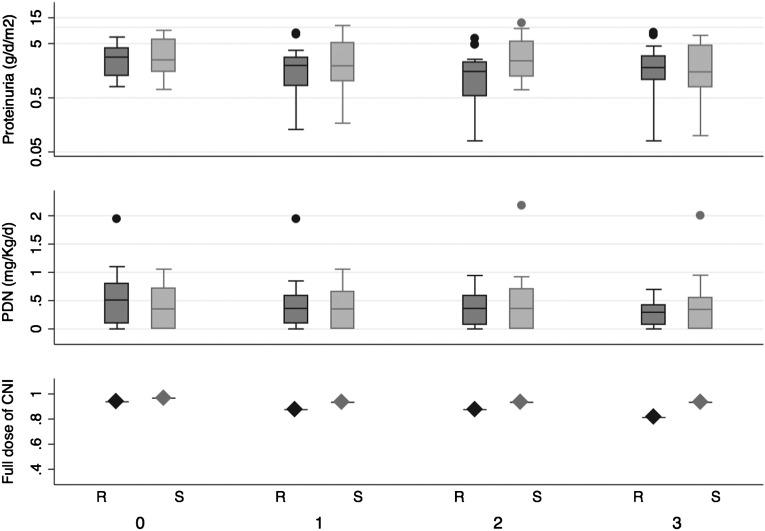
Figure 2 shows the distribution of proteinuria and prednisone dosage by group assignment over time (medians and interquartile ranges) and the proportion of patients receiving full doses of calcineurin inhibitors. Geometric mean values of proteinuria at baseline and study end were, respectively, 2.7 (95% confidence interval [CI], 1.7–4.2) g/d per m2 and 1.8 (95% CI, 0.9–3.4) g/d per m2 among controls and 2.4 (95% CI, 1.7–3.5) g/d per m2 and 1.4 (95% CI, 0.9–2.8) g/d per m2 among children assigned to rituximab. Six delayed-resistant patients (equally distributed by randomization group) achieved remission of proteinuria (Table 2). The doses of prednisone and calcium calcineurin inhibitor were reduced in these patients during the study.
Figure 2.
Plots summarizing the distribution of proteinuria (g/d per m2 [log-scale]), prednisone (mg/kg per day), and the proportion of children receiving full-dose calcineurin inhibitors (cyclosporine or tacrolimus) over time in months from randomization (time zero). Dark gray bars represent patients assigned to rituximab-based strategy; light gray bars represent standard therapy. The line across the box plots (proteinuria and prednisone plots) is the median, the box hinges are the 25th and 75th percentiles, and the outliers are represented as dots lying beyond 1.5 times the interquartile range. CNI, calcineurin inhibitors; PDN, prednisone; R, rituximab; S, standard therapy.
Table 2.
Proteinuria, serum albumin, and serum creatinine values at beginning and end of study in patients with early and delayed resistance to drugs
| Early-Resistant Patients | Delayed-Resistant Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Rituximab Group (n=9) | Control Group (n=7) | Rituximab Group (n=7) | Control Group (n=8) | |||||
| T0 | T3 | T0 | T3 | T0 | T3 | T0 | T3 | |
| Proteinuria (g/day per m2) | 2.9 (1.2, 6.6) | 2.7 (1.6, 7.8) | 6 (1.5, 8.8) | 3.9 (1.2, 7.1) | 1.3 (0.8, 6.3) | 0.8 (0.1, 1.7) | 2.4 (0.8, 4.8) | 0.8 (0.1, 4.6) |
| Serum albumin (g/L) | 2.1±0.5 | 2.1±0.6 | 2.2±0.7 | 2.1±0.9 | 2.6±0.6 | 3.3±0.3 | 2.4±0.4 | 2.9±0.8 |
| Serum creatinine (mg/dl) | 0.6±0.2 | 0.7±0.3 | 0.7±0.4 | 0.7±0.4 | 0.5±0.4 | 0.6±0.4 | 0.5±0.3 | 0.5±0.3 |
| Remission (n) | 0 | 0 | 3 | 3 | ||||
Proteinuria values are medians (interquartile ranges). Albumin and creatinine values are means ± SDs.
Table 2 reports data on proteinuria, serum albumin levels, and creatinine levels by treatment group and history of previous responsiveness. Table 3 summarizes the results of regression analysis of log-transformed proteinuria. Rituximab did not reduce proteinuria after 3 months (model 1; analysis of covariance model adjusted for baseline proteinuria). The effect was larger but still nonsignificant in patients who had responded to standard therapy in the past and became resistant later (model 2; prespecified secondary analysis). No other factor (including histologic findings) affected the outcome or modified the effect of rituximab. CD20 counts were reduced to <1% at the first month in all rituximab-treated patients. After 3 months, CD20 count was still undetectable in all but one case. After 3 months, patients in the control group were offered alternative therapies, including plasmapheresis and protein A pheresis. Patients assigned to rituximab were monitored for an additional 3 months, and daily proteinuria did not change (geometric mean, 1.36 [95% CI, 0.65–2.87] g/d per m2). These patients were monitored for safety assessment for another 12 months.
Table 3.
Proteinuria at study end
| Variable | Group Mean (95% CI) | Percentage Reduction (95% CI) | P Value |
|---|---|---|---|
| Model 1 (R2 = 0.14) | |||
| all controls (n=15) | 0.36 (0.05–2.29) | −12 (−73 to 110) | 0.77 |
| all rituximab patients (n=16) | 0.32 (0.05–1.88) | ||
| Model 2 (R2 = 0.51) | |||
| Early resistance | |||
| control group (–n=7) | 1.49 (0.24–9.39) | −3 (−67 to 179) | 0.95 |
| rituximab group (no previous remission) (n=9) | 1.44 (0.21–9.99) | ||
| Delayed resistance | |||
| control group (previous remission (n=8) | 0.52 (0.08–3.27) | −48 (−79 to 93) | 0.40 |
| rituximab group (previous remission) (n=7) | 0.32 (0.07–1.51) |
Analysis of covariance model of log-proteinuria. Geometric means (95% confidence intervals) of daily proteinuria at study end and percentage change due to rituximab (exponentiated differences of log-values) without considering previous remission (model 1: intention-to-treat analysis) and by absence and presence of previous remission (model 2: secondary analysis; test for interaction, P=0.57). Both models include baseline proteinuria, rituximab group and prednisone dose. Last value was carried forward for children with missing data, as per intention-to-treat analysis. Both models failed to reject the null hypothesis of superiority of rituximab compared with standard therapy. CI, confidence interval.
Adverse Events
Acute Adverse Events
One patient developed a severe reaction with bronchospasm and hypotension during the second rituximab infusion; treatment was discontinued and the patient spontaneously recovered. Another child had a severe acute allergic reaction to the bolus of chlorpheniramine maleate during the premedication therapy. The child had extreme dizziness, skin rash, and bronchospasm, and the protocol was stopped (without any infusion of rituximab). Other minor side effects were more frequent and consisted of abdominal pain (four cases), skin rash (three cases), and mild dyspnea (two cases). Resolution was rapidly and completely achieved in all cases by reducing the infusion rate.
Delayed Adverse Events
After 18-month follow-up, no important side effects were observed.
Discussion
The potential use of anti-CD20 antibodies (rituximab) in children with INS has recently emerged from uncontrolled observations19,20,22–24 and a randomized, controlled trial in children with INS dependent on both steroids and calcineurin inhibitors.18 Although the pathogenesis of INS is not fully understood, recent observations on potential mechanisms suggest that rituximab may be a possible therapeutic option for patients with INS who are unresponsive to calcineurin inhibitors and steroids and therefore have no other therapeutic options.25,26
First, rituximab interacts with regulatory elements of the cytoskeleton27 and may, in this way, directly modify podocyte structure. Second, rituximab affects regulatory elements of B cells positive for CD20 that are implicated in innate immunity and affects Th17 cells.28–31 Finally, preliminary observations suggest that rituximab reduces expression of soluble urokinase-type plasminogen activator receptor by monocytes (Ghiggeri, personal observation). This is of particular importance because this receptor has recently been identified as a circulating factor that plays a direct pathogenetic role in FSGS by interacting with β3 integrin.32,33 However, evidence from randomized, controlled trials is necessary to promote its use in clinical practice.
To our knowledge, this is the first randomized, controlled trial in children with INS unresponsive to standard combination therapy with calcineurin inhibitors and prednisone. The study indicates that two intravenous doses of rituximab do not change 3-month proteinuria by the hypothesized amount (70% reduction) compared with the standard approach. The trend of serum levels of rituximab in patients analyzed was not influenced by nephrotic syndrome per se, and levels were similar to those observed in previous studies in adults with autoimmune disorders.21 Although the treatment appears to be safe in the short term, acute and potentially harmful allergic reactions may occur. Although midterm side effects did not occur, long-term follow-up safety data are warranted. Our study population included patients with delayed resistant forms of the disease, reflecting the heterogeneity of the population of children with INS. Although in this latter group the point estimate of the treatment effect was clinically more relevant (reduction of proteinuria with amelioration of serum albumin), results were similarly nonsignificant. This aspect may reflect important pathogenetic differences between children who are resistant ab initio and those with delayed resistance. Overall, our study indicates that rituximab should not be considered an alternative therapy in children unresponsive to steroids and calcineurin inhibitors, especially in those unresponsive ab initio.
Few data in the literature are available on the use of rituximab in patients with persistent resistance to the classic combination of prednisone and calcineurin inhibitors. A short series reported by Fernandez-Fresnedo et al.23 showed no effect in six of eight patients and a partial response in the remaining two patients after four or six treatments. Gulati et al.20 reported complete remission in a variable number of patients, including those with delayed-resistant INS. Enrollment of delayed-resistant cases may partly explain these positive results, which we could not confirm. Thus, a randomized, controlled trial should be done to assess this topic.
A possible explanation of the negative results of our study is related to the current limited knowledge and understanding of the disease. Although molecular analysis of NPHS2 and WT1 allowed the identification and exclusion of the most severe forms of known genetic disorders, other genetically mediated forms of INS may have been included in our cohort of stable unresponsive children.6,34 In this respect, it is possible that delayed-resistant forms of INS (half of the sample of the present trial) may benefit from antibody-based therapies, including rituximab.
The list of renal genes involved in resistant forms of INS is now growing (up to 12 already characterized), and it is currently difficult to perform a systematic analysis. When only two genes are considered (NPHS2 and WT1), the estimated prevalence of genetic forms of INS in patients without a familial history of the disease indicates that as many as 15%–20% of cases carry a causative mutation.35 The prevalence of other genetic mutations resulting in INS unresponsive to available drugs (including rituximab) is unknown. Although we searched for the preceding two major genes involved in INS before enrollment, we cannot exclude the possibility that some patients may carry genetic disorders that could clinically affect outcomes. In this view, genes involved in dominant INS (INF2 and ACTN4) were not tested because patients with a family history (indicating a dominant trait) were not included in the study. Among recessive variants, only some of the participants were screened for CD2AP and TRCP6, which are responsible for rare cases with recessive inheritance. However, the incidence of CD2AP and TRCP6 in our database of 400 cases of INS is less than 2%.36,37 The development of next-generation techniques for molecular analysis will enable a more comprehensive analysis of all genes potentially involved in INS and characterization of patient populations in whom new therapies may be successful.
Our study has limitations. First, we powered the study to detect a large effect (70% reduction of proteinuria at 3 months); false-negative results cannot be excluded, especially if a true effect exists and is smaller. We chose such a large difference because of both feasibility and biologic considerations. For example, to show that a smaller reduction of 50% (e.g., 2.5–1.25 g/d per m2 instead of 0.75 g/d per m2) was statistically significant at a two-sided P value of 0.05, a study three times as large (n=90) and with the same power as ours would be necessary. More important, we believe that the balance between clinically relevant effects and costs should be considered when new interventions are tested. Smaller effects are of limited clinical relevance and do not justify the biologic costs or economic implications of rituximab use. On the other hand, the point estimate of the effect among delayed-resistant cases (secondary analysis) is close to (but smaller than) the hypothesized effect of rituximab (52% reduction). The width of the CIs might reflect the heterogeneity of children with resistant forms of INS included in this study, partly because of different genetic background or sensitivity to rituximab.27 In fact, recent data suggest a potentially variable sensitivity to rituximab in relation to the sphingomyelinase content of the podocyte membrane. Rituximab may affect transmembrane signaling pathway regulation by binding acid-sphingomyelinase epitopes.27 Overall, a larger study in late nonresponders that also include a sensitivity essay for rituximab may be justified.
Second, our study follow-up was relatively short. The 3-month term was chosen on the basis of recent observations of the effect of rituximab in patients with INS who have the same characteristics as our cohort.20 Those findings indicated that in patients in whom rituximab therapy was successful, remission of proteinuria was achievable at 3 months. Moreover, as a result of severe clinical conditions and therapy side effects, patients in the control group were offered alternative therapies, including plasmapheresis and protein A pheresis, because we considered further delays in treatment to be unethical. Thus, possibilities for prolonging the study protocol were limited. Patients assigned to rituximab were monitored for an additional 15 months to exclude delayed effects.
Third, we chose two infusions on the basis of available literature data and positive results in patients with INS who are dependent on drugs.18 We cannot exclude the possibility that higher rituximab doses may be necessary to reduce proteinuria in resistant forms of INS. Moreover, it is important to emphasize that rituximab toxicity, including lung fibrosis38 and progressive multifocal leukoencephalopathy,39 could be a function of the number of infusions and cumulative dose. A program of virus monitoring in urine and blood cells of our patient cohort (e.g., JC virus, the etiologic agent of progressive multifocal leukoencephalopathy) for the next few years is in place.
In conclusion, our study suggests that rituximab is not effective in forms of INS resistant to steroids and calcineurin inhibitors. This seems to be especially true in children with INS who never responded to standard drugs. Further studies may be necessary in children with delayed-resistant forms of INS. An improvement in the basic molecular work-up for genetic disorders causing INS is necessary to better characterize the target population of new therapies for INS.
Concise Methods
Design Overview
Eligible participants entered a 1-month run-in period during which proteinuria was monitored and adherence assessed. During the run-in period, instructions on urine collection and dipstick readings were carefully reviewed. After run-in, children were centrally allocated to continue standard therapy alone or to have rituximab added, independent of the current level of proteinuria. Allocation was concealed by contacting the holder of the allocation schedule at central administration at the University of Calgary, Calgary, Alberta, Canada (P.R.). Random sequences were generated using R software (version 2.13.1). Assignments followed permuted block randomization with blocks of variable size. Clinical investigators, study nurses enrolling patients, and the statistician were not blinded to group assignment. However, study staff responsible for facilitating follow-up data measurements by contacting patient families by phone were kept blinded. An independent data safety monitoring board reviewed safety data when half the participants had been enrolled and at study end. The protocol and consent documents were approved by the ethics committees at each participating center (EudraCT 2007-007796-16). All patients provided written informed consent.
Setting and Participants
Participants in this study had to be 16 years of age or younger and have an estimated creatinine clearance >60 ml/min per 1.73 m2. They had to have a history of INS unresponsive to the combination of prednisone and calcineurin inhibitors for at least 6 months. INS was defined by the presence of nephrotic-range proteinuria (>40 mg/m2 per hour or >1 g/d per m2) or a protein-to-creatinine ratio (>4 mg/mg in a single urine specimen) associated with low serum albumin (<2.5 g/dl) and high cholesterol (>220 mg/dl); in case of lower proteinuria values (5–40 mg/m2 per hour), the association with hypoalbuminemia and dyslipidemia was considered sufficient for the definition of INS. Children could be enrolled once drug unresponsiveness had persisted for at least 45 days with full-dose steroids (60 mg/m2), three pulses of methylprednisolone (10 mg/kg) given every other day, and 100 days of combination therapy with full doses of prednisone (2 mg/kg) and calcineurin inhibitors (cyclosporine, 5 mg/kg; tacrolimus, 0.1 mg/kg). A 1-month run-in period followed this 5-month clinical history, for an overall period of drug resistance of at least 6 months. Overall, the interval of drug resistance was 2 years (median) and ranged from 6 months to 11.8 years (Table 1). Fifteen patients were classified as delayed-resistant because they had developed drug resistance after a phase of responsiveness to and dependence on both steroids and calcineurin inhibitors. These patients were randomly assigned after 6-month unresponsiveness was documented.
Negative results on genetic testing for NPHS2 and WT1 were required in all cases. Exclusion criteria were infantile onset (<1 year); previous episodes of macrohematuria; hepatitis B virus, hepatitis C virus, or HIV infection; positivity for any marker of autoimmunity (antineutrophil antibody, nuclear DNA, antineutrophil cytoplasmic antibody); and low C3 levels. Renal biopsy had to be performed in children who never responded to steroids and calcineurin inhibitors (Table 1).
Common therapies for nephrotic syndrome, such as diuretics, anticoagulant therapy, angiotensin-converting enzyme inhibitors, or angiotensin-receptor blockers, were used at the discretion of the investigators but were kept constant during the study (Table 1).
Intervention Strategy and Standard Therapy
The intervention strategy was based on rituximab (Mabthera), 375 mg/m2, given intravenously twice (at randomization and after 2 weeks). The medication was diluted in normal saline (1 mg/ml) and administered at increasing speeds (0.5–1.5 ml/min) over approximately 6 hours, in the absence of side effects. The infusion was preceded by chlorpheniramine maleate, 2.5–5 mg intravenously; methylprednisolone, 2 mg/kg in normal saline intravenously; and paracetamol, 8 mg/kg orally. Children in both groups had to continue prednisone (median, 0.42 mg/kg per day) and calcineurin inhibitors (cyclosporine, 3.6 mg/kg per day, or tacrolimus, 0.1 mg/kg per day; Table 1) at the same dosage as before enrollment for a month. Starting at 30 days, the prednisone dose had to be tapered off by 0.3 mg/kg per week if proteinuria was <1 g/d per m2. After 2 weeks from the prednisone withdrawal, the calcineurin inhibitor dose had to be decreased by 50% and treatment stopped after 2 additional weeks.
Outcomes and Follow-up
Children were seen by the nephrologist responsible for the study at the beginning and at the end of the study (3 months), and as many times as necessary. Study coordinators maintained ongoing contact with the children and the families to monitor clinical status and report potential adverse events. The family pediatrician contacted the primary nephrologist for data reporting and clinical update. At baseline and after 3 months, proteinuria was determined at a central laboratory. Kidney function, plasma proteins, cell blood counts, and cholesterol were obtained monthly. The primary efficacy measure was the percentage change in daily proteinuria at 3 months by treatment group (study end). Patients treated with rituximab were further monitored for 15 months to ascertain medium-term safety of the drug. Patients in the control group were offered alternative therapies after the completion of the trial.
Pharmacokinetics Study
Serum samples were collected from patients assigned to rituximab immediately before and 15 minutes and 24 hours after each infusion. Serum samples from all time points were kept frozen at −20°C until analysis, which was carried out within 3 months of sampling. Serum concentrations were evaluated by a very sensitive ELISA.40 For comparison, kinetics data were obtained in adults with different clinical conditions who required rituximab infusion (22 with lymphoproliferative disorders, 22 with follicular non-Hodgkin lymphoma, and 14 with autoimmune diseases).
Statistical Analyses
We estimated that with 30 participants (the anticipated recruitment rate), we could detect a reduction in proteinuria by at least 70% at 3 months between treatment groups as statistically significant at a two-sided P value of 0.05, with a power of 90%. We assumed a log-normal distribution of the response, with a coefficient of variation of 1.5 on the original scale (mean ± SD, 3±4.5 g/d per m2), and a geometric mean ratio of 0.3 as the superiority margin. The estimation accounted for a 5% risk for withdrawals.41 The primary outcome was analyzed according to the intention-to-treat principle. Three-month log-transformed proteinuria was modeled using an analysis of covariance model with log-transformed baseline proteinuria as a covariate and treatment as the main effect. Prespecified secondary analyses tested the modification effect of previous sensitivity to standard therapy (partial or full remission). Missing values at 3 months in patients who did not complete the study were replaced by carrying forward their last available value (intention-to-treat analysis). Analyses were performed with Stata software, version 11.2 (Stata Corp., College Station, TX), and R 2.13.0 (http://www.R-project.org).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors acknowledge the external support of Rossella Rossi, Nunzia Miglietti, and Marina Vivarelli. The corresponding author certifies that all persons who contributed significantly to the work have been here acknowledged.
The Institute Giannina Gaslini provided financial and logistic support to the trial. This work was also supported by the Italian Ministry of Health Ricerca Corrente, the Renal Child Foundation, Fondazione Mara Wilma e Bianca Querci (project “Ruolo dello stress reticolare nella progressione del danno renale e tumurale”), Fondazione La Nuova Speranza (“Progetto integrato per la definizione dei meccanismi implicati nella glomerulo sclerosi focale”).
DSMB members included Antonella Trivelli, Giovanni Candiano, Giorgio Piaggio, and Gianluca Caridi.
The present study was investigator initiated and driven. All members of the study steering committee are listed as authors of the present report, had access to the study data, and vouch for the accuracy and completeness of the data reported.
Footnotes
A.M. and P.R. contributed equally to the work.
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Rituximab in Steroid-Resistant Nephrotic Syndrome in Children: A (False) Glimmer of Hope?,” on pages 975–978.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011080775/-/DCSupplemental.
References
- 1.Cohen AH, Border WA, Glassock RJ: Nehprotic syndrome with glomerular mesangial IgM deposits. Lab Invest 38: 610–619, 1978 [PubMed] [Google Scholar]
- 2.Korbet SM: Primary focal segmental glomerulosclerosis. J Am Soc Nephrol 9: 1333–1340, 1998 [DOI] [PubMed] [Google Scholar]
- 3.McAdams AJ, Valentini RP, Welch TR: The nonspecificity of focal segmental glomerulosclerosis. The defining characteristics of primary focal glomerulosclerosis, mesangial proliferation, and minimal change. Medicine (Baltimore) 76: 42–52, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Tryggvason K, Wartiovaara J: Molecular basis of glomerular permselectivity. Curr Opin Nephrol Hypertens 10: 543–549, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Caridi G, Bertelli R, Di Duca M, Dagnino M, Emma F, Onetti Muda A, Scolari F, Miglietti N, Mazzucco G, Murer L, Carrea A, Massella L, Rizzoni G, Perfumo F, Ghiggeri GM: Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol 14: 1278–1286, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Aucella F, Bisceglia L, De Bonis P, Gigante M, Caridi G, Barbano G, Mattioli G, Perfumo F, Gesualdo L, Ghiggeri GM: WT1 mutations in nephrotic syndrome revisited. High prevalence in young girls, associations and renal phenotypes. Pediatr Nephrol 21: 1393–1398, 2006 [DOI] [PubMed] [Google Scholar]
- 8.ISKDC : Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int 20: 765–771, 1981 [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli C, Rizzoni G, Edefonti A, Altieri P, Rivolta E, Rinaldi S, Ghio L, Lusvarghi E, Gusmano R, Locatelli F, et al. : A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int 43: 1377–1384, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Ghiggeri GM, Catarsi P, Scolari F, Caridi G, Bertelli R, Carrea A, Sanna-Cherchi S, Emma F, Allegri L, Cancarini G, Rizzoni GF, Perfumo F: Cyclosporine in patients with steroid-resistant nephrotic syndrome: An open-label, nonrandomized, retrospective study. Clin Ther 26: 1411–1418, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Catarsi P, Ravazzolo R, Emma F, Fruci D, Finos L, Frau A, Morreale G, Carrea A, Ghiggeri GM: Angiotensin-converting enzyme (ACE) haplotypes and cyclosporine A (CsA) response: A model of the complex relationship between ACE quantitative trait locus and pathological phenotypes. Hum Mol Genet 14: 2357–2367, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Jayne D: Role of rituximab therapy in glomerulonephritis. J Am Soc Nephrol 21: 14–17, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T: Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350: 2572–2581, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Smith KG, Jones RB, Burns SM, Jayne DR: Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: Remission, relapse, and re-treatment. Arthritis Rheum 54: 2970–2982, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Stasi R, Stipa E, Del Poeta G, Amadori S, Newland AC, Provan D: Long-term observation of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis treated with rituximab. Rheumatology (Oxford) 45: 1432–1436, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Chiurchiu C, Abbate M, Perna A, Cravedi P, Bontempelli M, Remuzzi G: Rituximab for idiopathic membranous nephropathy: Who can benefit? Clin J Am Soc Nephrol 1: 738–748, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC: Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117–125, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, Benetti E, Scozzola F, Pasini A, Dallera N, Sica F, Belingheri M, Scolari F, Ghiggeri GM: Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial [published online ahead of print February 16, 2012]. Clin J Am Soc Nephrol doi:10.2215/CJN.07990811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas B, Niaudet P, Nivet H, Raynaud N, Taque S, Ronco P, Bouissou F: Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: A multicentric series of 22 cases. Pediatr Nephrol 23: 1269–1279, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, Srivastava RN, Moudgil A, Bagga A: Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: Multicentric report. Clin J Am Soc Nephrol 5: 2207–2212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regazzi MB, Iacona I, Avanzini MA, Arcaini L, Merlini G, Perfetti V, Zaja F, Montagna M, Morra E, Lazzarino M: Pharmacokinetic behavior of rituximab: A study of different schedules of administration for heterogeneous clinical settings. Ther Drug Monit 27: 785–792, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Benz K, Dötsch J, Rascher W, Stachel D: Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 19: 794–797, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Fresnedo G, Segarra A, González E, Alexandru S, Delgado R, Ramos N, Egido J, Praga M, Trabajo de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) : Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 4: 1317–1323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prytuła A, Iijima K, Kamei K, Geary D, Gottlich E, Majeed A, Taylor M, Marks SD, Tuchman S, Camilla R, Ognjanovic M, Filler G, Smith G, Tullus K: Rituximab in refractory nephrotic syndrome. Pediatr Nephrol 25: 461–468, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Orman SV, Schechter GP, Whang-Peng J, Guccion J, Chan C, Schulof RS, Shalhoub RJ: Nephrotic syndrome associated with a clonal T-cell leukemia of large granular lymphocytes with cytotoxic function. Arch Intern Med 146: 1827–1829, 1986 [PubMed] [Google Scholar]
- 26.Vincenti F, Ghiggeri GM: New insights into the pathogenesis and the therapy of recurrent focal glomerulosclerosis. Am J Transplant 5: 1179–1185, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW, 3rd: Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3: 85ra46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ: B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J Immunol 177: 4481–4487, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Bertelli R, Trivelli A, Magnasco A, Cioni M, Bodria M, Carrea A, Montobbio G, Barbano G, Ghiggeri GM: Failure of regulation results in an amplified oxidation burst by neutrophils in children with primary nephrotic syndrome. Clin Exp Immunol 161:151–158, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertelli R, Bodria M, Nobile M, Alloisio S, Barbieri R, Montobbio G, Patrone P, Ghiggeri GM: Regulation of innate immunity by the nucleotide pathway in children with idiopathic nephrotic syndrome. Clin Exp Immunol 166: 55–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, Timmermans K, Di Padova F, Koenders MI, Gutierrez-Roelens I, Durez P, Netea MG, van der Meer JW, van den Berg WB, Joosten LA: The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum 63: 1507–1516, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Caridi G, Trivelli A, Sanna-Cherchi S, Perfumo F, Ghiggeri GM: Familial forms of nephrotic syndrome. Pediatr Nephrol 25: 241–252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caridi G, Perfumo F, Ghiggeri GM: NPHS2 (Podocin) mutations in nephrotic syndrome. Clinical spectrum and fine mechanisms. Pediatr Res 57: 54R–61R, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Gigante M, Pontrelli P, Montemurno E, Roca L, Aucella F, Penza R, Caridi G, Ranieri E, Ghiggeri GM, Gesualdo L: CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS). Nephrol Dial Transplant 24: 1858–1864, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Gigante M, Caridi G, Montemurno E, Soccio M, d’Apolito M, Cerullo G, Aucella F, Schirinzi A, Emma F, Massella L, Messina G, De Palo T, Ranieri E, Ghiggeri GM, Gesualdo L: TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol 6: 1626–1634, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Chaumais MC, Garnier A, Chalard F, Peuchmaur M, Dauger S, Jacqz-Agrain E, Deschênes G: Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol 24: 1753–1755, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Bord E, Tompkins T, Miller J, Tan CS, Kinkel RP, Stein MC, Viscidi RP, Ngo LH, Koralnik IJ: Asymptomatic reactivation of JC virus in patients treated with natalizumab. N Engl J Med 361: 1067–1074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iacona I, Lazzarino M, Avanzini MA, Rupolo M, Arcaini L, Astori C, Lunghi F, Orlandi E, Morra E, Zagonel V, Regazzi MB: Rituximab (IDEC-C2B8): validation of a sensitive enzyme-linked immunoassay applied to a clinical pharmacokinetic study. Ther Drug Monit 22: 295–301, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Wolfe R, Carlin JB: Sample-size calculation for a log-transformed outcome measure. Control Clin Trials 20: 547–554, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.