Abstract
The circadian clock contributes to the control of BP, but the underlying mechanisms remain unclear. We analyzed circadian rhythms in kidneys of wild-type mice and mice lacking the circadian transcriptional activator clock gene. Mice deficient in clock exhibited dramatic changes in the circadian rhythm of renal sodium excretion. In parallel, these mice lost the normal circadian rhythm of plasma aldosterone levels. Analysis of renal circadian transcriptomes demonstrated changes in multiple mechanisms involved in maintaining sodium balance. Pathway analysis revealed the strongest effect on the enzymatic system involved in the formation of 20-HETE, a powerful regulator of renal sodium excretion, renal vascular tone, and BP. This correlated with a significant decrease in the renal and urinary content of 20-HETE in clock-deficient mice. In summary, this study demonstrates that the circadian clock modulates renal function and identifies the 20-HETE synthesis pathway as one of its principal renal targets. It also suggests that the circadian clock affects BP, at least in part, by exerting dynamic control over renal sodium handling.
Recent evidence indicates that the circadian clock is involved in BP control. In mice, suppression or decrease of the circadian clock activity via deletion of the circadian transcriptional activators Bmal1, Clock, or Npas2 leads to low BP, whereas its constitutive activation via deletion of the circadian repressors Cry1 and Cry2 results in salt-sensitive hypertension.1–4 Wang et al. have recently shown that mice simultaneously devoid of three proline- and acidic amino acid–rich basic leucine zipper circadian transcriptional factors Dbp, Hlf, and Tef exhibit a significant reduction in BP.5 Maintaining BP within the normal range strongly depends on the capacity of the kidney to precisely regulate sodium content in the extracellular space. Thus, dysregulation of molecular mechanisms involved in renal sodium handling could be partially responsible for the elevated or decreased BP observed in mice with genetically altered clocks. This hypothesis is supported by evidence in humans suggesting that alteration of circadian rhythms of urinary sodium excretion is the primary cause of disease in several forms of hyper- or hypotension. For instance, a decreased renal capacity to excrete sodium during the daytime correlates with nocturnal hypertension, whereas increased sodium excretion during the nighttime contributes to the maintenance of orthostatic hypotension.6,7 Of note, important changes in the amplitude or the circadian phase of urinary excretion of sodium can be provoked not only by a pathologic process but also by a misalignment between the endogenous circadian clock and the imposed rest-activity or feeding cycles, or by sleep disturbance. For instance, Kamperis et al. have shown that acute sleep deprivation in humans leads to excessive natriuresis and kaliuresis during the subjective night and attenuation of the nocturnal BP dip.8 Numerous studies have demonstrated an impairment of the sodium excretory rhythm and the development of hypertension in shift workers.9,10
The circadian clock can influence renal function via two types of circadian inputs: (1) entrainment of renal rhythms through the external circadian time cues, such as hormones, food, activity, and body temperature rhythms, and (2) the activity of the intrinsic renal circadian clock. For example, Doi et al. have shown that the circadian timing system controls sodium reabsorption in the distal nephron and the collecting duct via an effect on aldosterone production in the adrenal glands.3 On the other hand, Saifur Rohman et al. reported that the intrinsic renal clock directly regulates the activity of the Na+/H+ exchanger NHE3 in the proximal tubule,11 and Gumz et al. have shown that the circadian repressor period 1 (Per1) is capable of regulating the epithelial sodium channel expression in the collecting duct cells.12 We have recently demonstrated that the molecular clocks in the distal nephron and the collecting duct display robust circadian oscillations and that mice devoid of the clock gene exhibit a significant reduction in BP.2 However, the relationship between circadian clock activity and the rhythms of electrolyte excretion in urine has not been established. In addition, a systematic analysis of circadian mechanisms involved in maintaining electrolyte balance is still lacking.
To address these questions, we studied functional and molecular aspects of urine excretory rhythms in wild-type mice and mice devoid of the clock gene. This model was selected because the Clock is essential for the rhythmicity of peripheral molecular oscillators but is not required for circadian behavior.13,14 The latter fact allows for minimizing the interference of confounding factors, such as changes in the circadian patterns of food and water intake or locomotor activity.2
Results
Circadian Rhythms of Urinary Sodium and Potassium Excretion in Wild-Type and clock-Knockout Mice
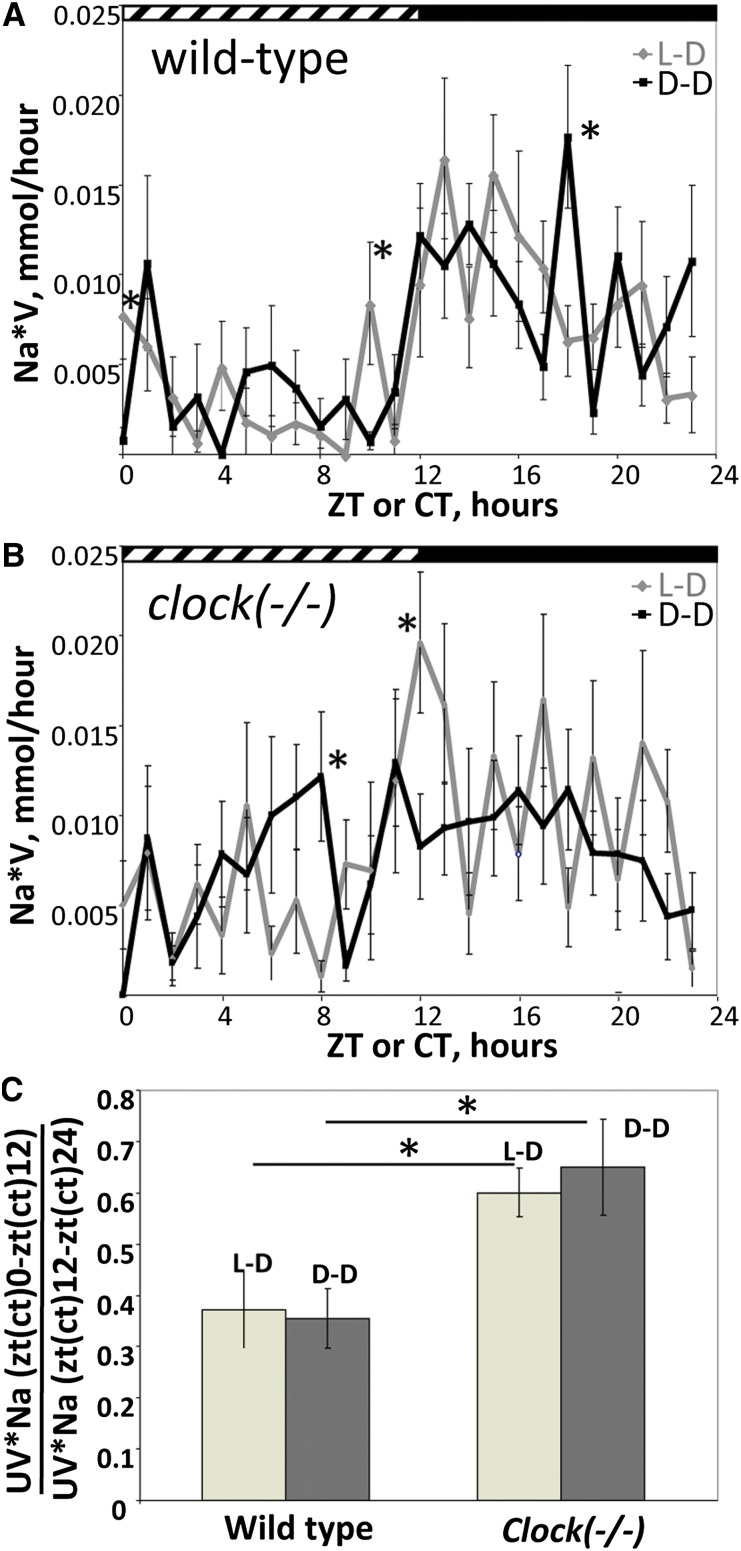
All mice were adapted to a 12-hour light and 12-hour dark cycle (LD) for 2 weeks. To exclude the influence of light on urinary rhythms, half the animals were placed in constant darkness (DD) 30 hours before urine collection. Urine was collected hourly from freely moving mice housed individually in the metabolic cages (see Concise Methods). As shown in Figure 1A, wild-type mice in LD conditions exhibited a well-marked circadian rhythm of sodium excretion with the maximal values in the first half of the activity phase (time is expressed in ZT, or Zeitgeber time units; ZT0 is the time of light on and ZT12 is the time of light off) and a trough during the inactive phase. This rhythm was maintained under DD conditions, with the exception of three time points (CT0, CT10, and CT18 [CT, circadian time, indicates the subjective circadian time independent of a Zeitgeber]), where the difference between the LD and DD conditions was statistically significant (Figure 1A).
Figure 1.
The circadian clock controls the temporal profile of urinary sodium excretion. (A) Temporal profile of renal sodium excretion rates in wild-type mice in LD or DD conditions. (B) Temporal profile of renal sodium excretion rates in clock(−/−) mice in LD or DD conditions. (C) Ratio between sodium excreted during the day (ZT0–ZT12) and the night (ZT12–ZT24) in LD conditions or during the subjective day (CT0–CT12) and subjective night (CT12–CT24) in DD conditions. Values are means ± SEM from 11 mice. Statistical significance was calculated using unpaired t test. UV*Na, urinary sodium excretion rate. *P<0.05.
In clock(−/−) mice, the difference between the amounts of sodium excreted during the light and dark phases (or subjective light and dark phases in DD conditions) was apparently reduced in both LD and DD conditions (Figure 1B). For the quantitative assessment of the difference, we calculated the ratio between the amounts of sodium excreted between ZT0 and ZT12 and between ZT12 and ZT24, or between CT0 and CT12 and between CT12 and CT24 in LD and DD conditions, respectively. As shown in Figure 1C, the ratio was significantly increased in clock(−/−) mice in both LD and DD conditions. Similar results were obtained for the rhythms of urinary potassium excretion (Supplemental Figure 1). The urinary excretion rates for sodium and potassium were higher in clock(−/−) mice; however, this difference reached statistical significance only for the sodium excretion rate in LD conditions (Table 1). In parallel, there was a tendency for increased food and water intake in clock-knockout mice in DD conditions; however, the statistical significance was reached only for water intake (Table 1).
Table 1.
Body weight, food and water intake, and sodium and potassium excretion rates in wild-type and clock-knockout mice
| Variable | Wild-Type Mice (n=11) | clock(−/−) Mice (n=11) | P Value |
|---|---|---|---|
| Body weight LD (g) | 25.82±0.19 | 26.14±0.26 | NS |
| Body weight DD (g) | 25.90±0.42 | 25.90±0.56 | NS |
| Food intake LD (g/g body weight) | 0.174±0.006 | 0.173±0.004 | NS |
| Food intake DD (g/g body weight) | 0.189±0.004 | 0.195±0.003 | NS |
| Water intake LD (ml/g body weight) | 0.193±0.009 | 0.209±0.009 | NS |
| Water intake DD (ml/g body weight) | 0.197±0.006 | 0.239±0.011 | <0.005 |
| UV*Na/g body weight LD (μmol/g) | 5.67±0.39 | 7.67±0.44 | <0.005 |
| UV*Na/g body weight DD (μmol/g) | 5.74±0.46 | 7.18±0.72 | NS |
| UV*K/g body weight LD (μmol/g) | 24.75±1.50 | 27.35±1.41 | NS |
| UV*K/g body weight DD (μmol/g) | 23.22±1.46 | 26.45±1.19 | NS |
Values are the mean ± SEM. UV*Na, urinary sodium excretion rate; UV*K, urinary potassium excretion rate.
Plasma Aldosterone Levels in Wild-Type and clock-Knockout Mice
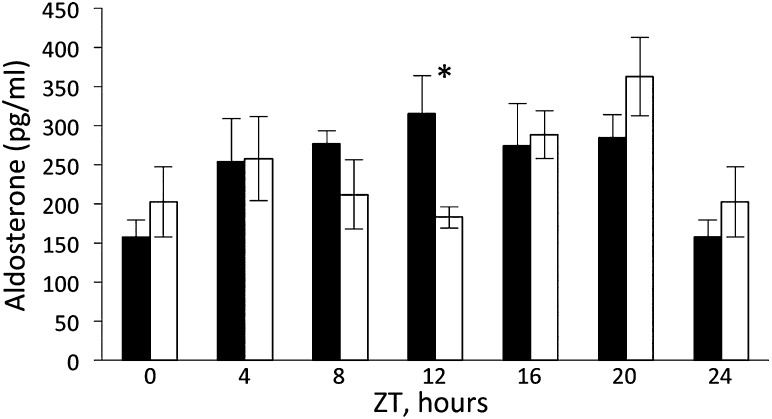
To check whether changes in the renin-angiotensin-aldosterone system may contribute to the impairment of sodium and potassium excretory rhythms in clock(−/−) mice, we analyzed plasma aldosterone levels in blood samples collected every 4 hours during a 24-hour period (six time points). As shown in Figure 2, aldosterone levels in wild-type mice vary between time points (P=0.003, one-way ANOVA) and follow a circadian temporal pattern with maximum at ZT12, the time of transition from the inactive to the active phase of the circadian cycle (fitted to the cosine function with P=0.007). Circadian fit was performed using a linear model with a pair of cosine and sine functions as the explanatory variable, with the frequency corresponding to 24-hour periodicity as described elsewhere.2 A similar circadian pattern of plasma aldosterone levels has been previously described in humans15,16 and rats.17 In clock(−/−) mice, although differences were still observed between time points (P=0.03, one-way ANOVA), the circadian pattern was disrupted (fitted to the cosine function with P=0.18) and a significant difference with plasma aldosterone levels in wild-type mice at ZT12 was observed. However, the 24-hour mean of plasma aldosterone levels did not differ between the wild-type and clock(−/−) mice (260.3±22.1 [mean ± SEM] pg/ml versus 250.8±27.4 pg/ml, respectively; P=0.7, two-way ANOVA). In addition, there was no difference in urinary Na+/K+ ratio, an indicator of plasma aldosterone activity; the exception was ZT23 in LD conditions, for which the difference was statistically significant (P<0.05) (Supplemental Figure 2).
Figure 2.
Temporal profiles of plasma aldosterone levels in wild-type (black bars) and clock(−/−) mice (white bars). Values are means ± SEM from five mice. Statistical significance was calculated using unpaired t test. *P<0.05.
Comparison of Whole-Kidney Transcriptomes of Wild-Type and clock-Knockout Mice
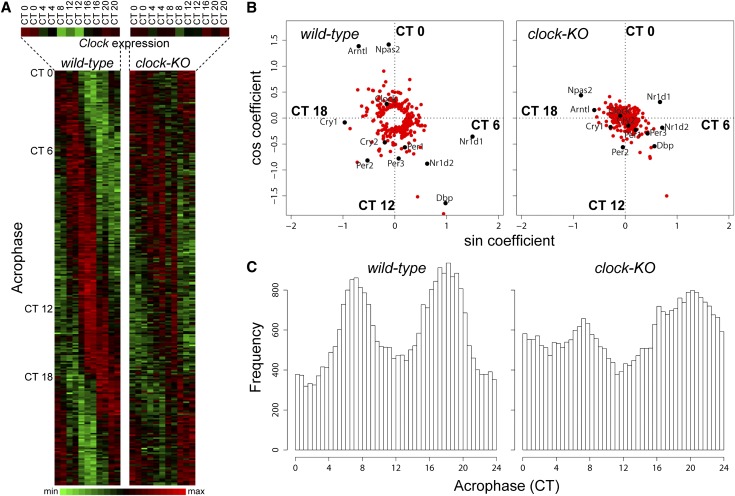
To identify molecular mechanisms underlying sodium and potassium excretory rhythms, we performed circadian profiling of renal transcriptomes in wild-type and clock(−/−) mice. The RNA was extracted from whole kidneys of mice adapted to LD conditions for 2 weeks and then placed in constant darkness (DD) for 30 hours before sacrifice. Mice were sacrificed every 4 hours over the course of circadian cycle. Hybridization data were analyzed with two distinct statistical protocols (Supplemental Methods). First, circadian oscillatory transcripts were identified independently in wild-type and clock(−/−) mice by fitting their temporal expression profiles to a cosine function with a period of 24 hours. A total of 277 and 174 transcripts in kidneys of wild-type and clock(−/−) mice met these criteria, respectively (Supplemental Tables 1 and 2, respectively; false discovery rate < 0.1). Of note, all principal elements of positive and negative limbs of the molecular clock (Bmal1 [Arntl], Clock, Npas2, Cry1, Cry2, Per1, Per2, Per3, and Rev-erb alpha [Nr1d1]) were identified as circadian transcripts in the kidneys of wild-type mice (Supplemental Table 1). For most of these genes, the circadian amplitude was significantly reduced in clock(−/−) mice (Figure 3, A and B). The whole-transcriptome distribution of acrophases was also significantly modified in clock(−/−) mice (Figure 3C).
Figure 3.
Deletion of the clock gene affects circadian patterns of gene expression in the kidney. (A) Phase ordering of 277 genes oscillating in wild-type mice. On the left are wild-type transcripts; on the right, clock(−/−) transcripts. The temporal expression of the clock gene is shown in the enlargement. Green and red represent minimal and maximal expression levels, respectively. The time of maximal transcript expression (acrophase) is indicated on the left. (B) Oscillating transcripts shown in A are plotted according to their amplitude and phase in wild-type and clock(−/−) mice. Principal elements of molecular clock are indicated in black. (C) Density distribution of acrophases for all 28,220 transcripts tested on microarrays in wild-type and clock(−/−) mice.
Analysis of circadian genes in wild-type mice revealed a large number of transcript-encoding proteins involved in various transcellular or paracellular transport functions: the amino acid transporters (Slc6a20a, Slc6a19, and Slc7a8), the monocarboxylate transporter 1 (Slc16a1), the Na+-dependent bile acid transporter (Slc10a2), the urate transporter (Slc2a9), the nucleoside transporter (slc29a3), claudins 1 and 10, the sodium/proton exchanger 3 (NHE3/Slc9a3), serine/threonine kinases Sgk1 and Sik1, nuclear receptors Thra and Ppara, modulators of distal sodium reabsorption Usp2 and Gilz (Tsc22d3), and enzymes involved in the synthesis/degradation of autocrine/paracrine regulators of sodium reabsorption and potassium secretion (thromboxane synthase [Tbxas1], dopamine decarboxylase [DDC], and catechol-O-methyltransferase [COMT1]) (Supplemental Table 1). Most of these transcripts did not meet the criteria for circadian oscillations in clock(−/−) mice, and the amplitudes of their diurnal variations were significantly reduced (with the exception of Gilz and Slc6a20a [Figure 3, A and B; Supplemental Table 2]).
Because the cosinor statistical treatment applies only to transcripts fitting to the cosine function, we also applied a second data analysis approach to identify genes differentially expressed between the clock(−/−) and wild-type kidneys, irrespective of their temporal expression patterns. As shown in Supplemental Table 3, 36 and 43 transcripts are up- or downregulated in kidneys of clock(−/−) mice, respectively (fold change > 50%; false discovery rate < 0.1). Of note, several of these transcripts (Cyp4a12a, Cyp4a12b, Cyp4a14, Cyp2c44, and Cyp2j13) encode enzymes involved in the conversion of arachidonic acid to different active metabolites. Three enzymes—Cyp4a12a, Cyp4a12b, and Cyp4a14—are required for the oxidation of arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE), a powerful endogenous regulator of renal sodium reabsorption and potassium secretion and of the renal vascular tone. Pathway enrichment analysis performed on the up- and downregulated transcripts confirmed a strong enrichment in transcripts involved in the arachidonic acid conversion pathways (10.8-fold enrichment; false discovery rate, 0.004 [Supplemental Table 4]).
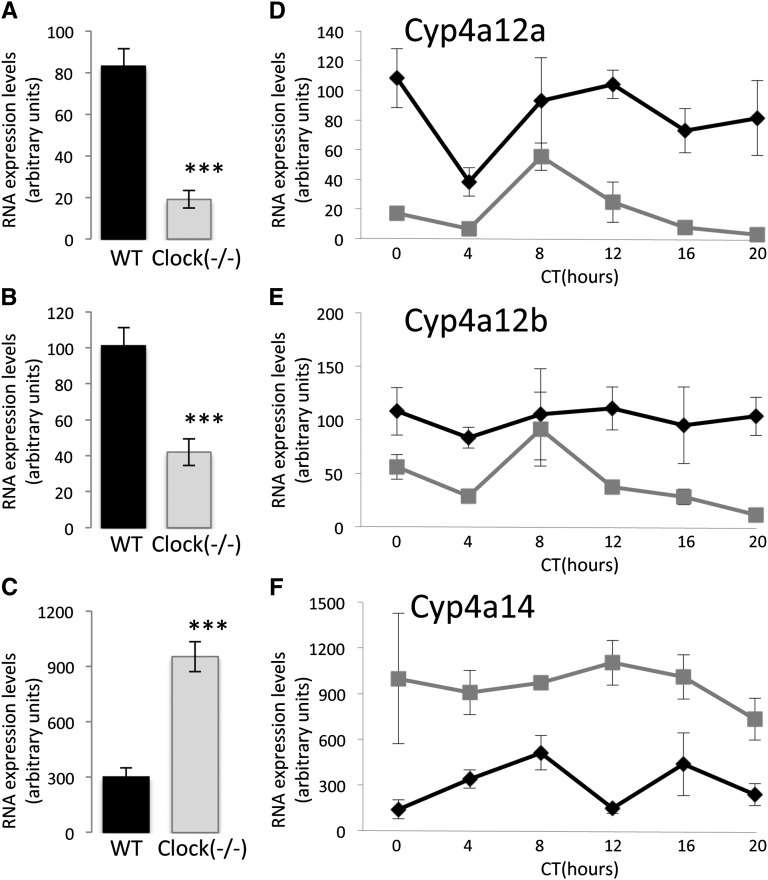
To validate the microarray results, real-time PCR was performed for the Cyp enzymes involved in the synthesis of 20-HETE on independent samples of RNA extracted from the kidneys of 30 wild-type and 30 clock(−/−) mice (five mice/time point). As shown in Figure 4, A–C, the 24-hour mean expression levels of Cyp4a12a and Cyp4a12b were significantly decreased in kidneys of clock(−/−) mice, whereas the expression level of Cyp4a14 was significantly increased, thereby confirming the results of the microarray analysis (Supplemental Figure 3, A–C, respectively, and Supplemental Table 3). However, the temporal patterns of Cyp4a12a, Cyp4a12b, and Cyp4a14 RNA expression significantly differed between the microarray analysis (Supplemental Figure 3, D–F, respectively) and the real-time PCR amplification (Figure 4, D–F, respectively). A possible explanation for this difference is that the members of the CYP4a subfamily share a high degree of nucleotide identity (e.g., 98% of nucleotide identity between Cyp4a12a and Cyp4a12b; see Supplemental Table 5). The high degree of sequence homology is a known source of errors in microarray analysis resulting from the cross-hybridization of related transcripts. In the LD condition, changes in the expression levels of Cyp4a12a, Cyp4a12b, and Cyp4a14 in clock(−/−) mice were similar to those observed in DD condition (Supplemental Figure 3, G–I 4).
Figure 4.
Expression levels of Cyp4a12a, Cyp4a12b, and Cyp4a14 transcripts in kidneys of wild-type and clock(−/−) mice. (A–C) Real-time PCR–based quantitation of 24-hour mean expression levels of Cyp4a12a, Cyp4a12b, and Cyp4a14, respectively. Values are means ± SEM from 30 mice. ***P<0.001, two-way ANOVA. (D–F) Real-time PCR–based temporal profiling of Cyp4a12a, Cyp4a12b, and Cyp4a14 RNA expression in kidneys of wild-type (black line) and clock(−/−) (gray line) mice, respectively. Values are means ± SEM from five mice.
Assessment of 20-HETE Levels
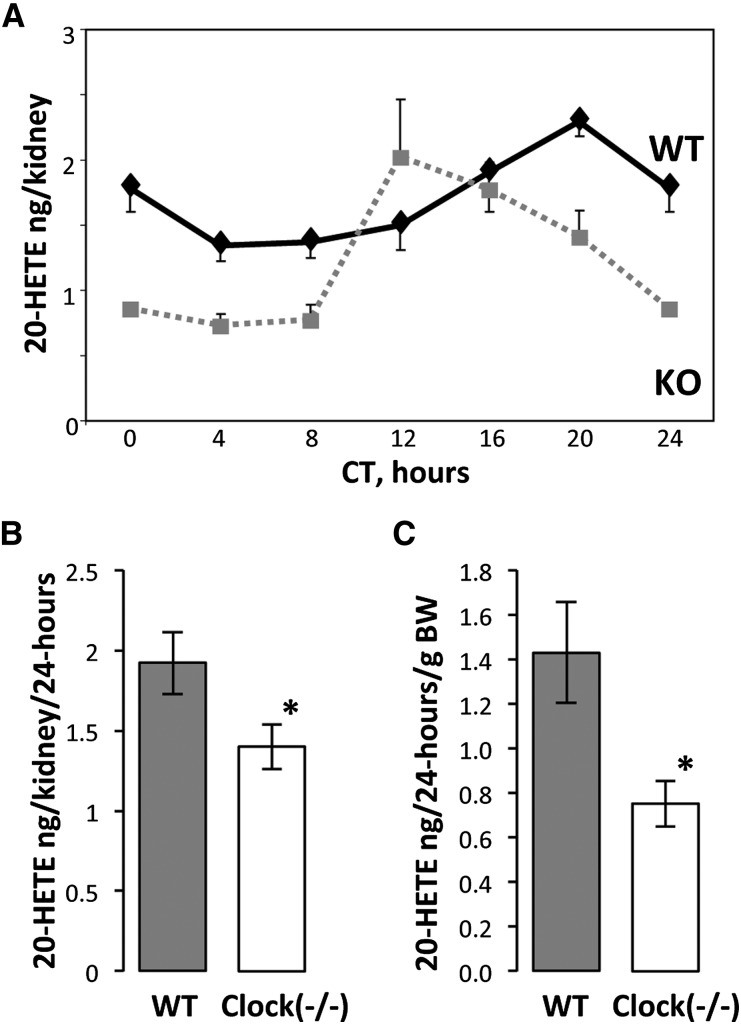
To determine whether renal content of 20-HETE is modified in clock(−/−) mice, we quantitatively analyzed this metabolite in renal microsomes and in urine. As shown in Figure 5A, in both wild-type and clock(−/−) mice, 20-HETE levels in microsomes change across time points (P=0.007 and P=0.004, respectively, one-way ANOVA) and follow a circadian-like temporal pattern (fitted to the cosine function with P<0.001 for wild-type and clock[−/−] mice). However, 20-HETE oscillations in clock(−/−) mice exhibit a significant shift in the acrophase (acrophase at CT20 in wild-type mice versus acrophase at CT12 in clock[−/−] mice) and a significant decrease in the 24-hour mean 20-HETE levels (P=0.02, two-way ANOVA; Figure 5B). A similar decrease was observed in 24-hour mean 20-HETE levels in urine of clock(−/−) mice (Figure 5C).
Figure 5.
The clock(−/−) mice exhibit a significant reduction in 20-HETE levels in kidney microsomes and in urine. (A) Temporal profiles of 20-HETE levels in kidney microsomes of wild-type (black lane) and clock(−/−) mice (gray lane). Values are means ± SEM from six mice. KO, knockout; WT, wild-type. (B) Twenty-four–hour mean 20-HETE levels in kidney microsomes. Values are means ± SEM from 35 mice. *P<0.05, two-way ANOVA. (C) Twenty-four–hour mean 20-HETE levels in the urine of wild-type and clock(−/−) mice. Values are means ± SEM from six mice. BW, body weight. *P<0.05, t test.
Discussion
Our results indicate that the circadian timing system controls the daily rhythms of sodium and potassium excretion by the kidney and suggest that dysfunction of these rhythms may have a significant influence on BP.
Analysis of clock(−/−) mice revealed several mechanisms by which the circadian timing system controls sodium and potassium excretion by the kidney. First, we demonstrated that the normal circadian rhythmicity of plasma aldosterone levels is lost in clock(−/−) mice. Because aldosterone is the principal hormone controlling sodium reabsorption in the distal nephron and the collecting duct, these changes are expected to directly influence the dynamic of sodium excretion by the kidney. Of note, 24-hour mean aldosterone levels were not different between the wild-type and clock(−/−) mice. These results are surprising because genetic ablation of the circadian transcriptional factors dbp, hlf, and tef results in a significant decrease in plasma aldosterone levels,5 whereas mice devoid of cry1 and cry2 display primary hyperaldosteronism.3 This finding indicates that the different elements of the circadian clock have a different effect on aldosterone synthesis or secretion.
Second, cosinor analysis of renal transcriptomes revealed many circadian transcripts that encode proteins involved in tubular reabsorption and secretion of various substrates, including sodium and potassium. In clock(−/−) mice, circadian rhythmicity of most of these transcripts was lost. One of these transcripts encodes the sodium-proton exchanger NHE3, a transporter that is directly regulated by the circadian clock.11 Of note, NHE3 is the major transporter mediating sodium reabsorption in the proximal tubule, and mice devoid of the NHE3 gene exhibit mild hypotension.18 Another interesting transcript is Sgk1, a serine-threonine kinase that regulates a variety of sodium transporters all along the renal tubule. Bozek et al. have recently proposed that this kinase is also directly regulated by the circadian clock.19 These two examples clearly demonstrate that the impairment of renal sodium handling in clock(−/−) mice might be directly related to the dysfunction in the intrinsic renal clock.
Third, this study reveals a major role of the circadian timing system in the regulation of the expression of renal cytochrome p450 enzymes involved in the formation of 20-HETE, a powerful mediator of BP control by the kidney.20–22 In parallel, we demonstrate that the renal content of 20-HETE exhibits a clear circadian pattern and that this pattern is significantly modified in kidneys of clock(−/−) mice. 20-HETE has a potent prohypertensive effect by acting as a vasoconstrictor of preglomerular arterioles, but it can also inhibit several important sodium transporters in the proximal tubule and the thick ascending limb (Na+-K+-adenosine triphosphatase, NHE3, NKCC2 Na+-K+-Cl− cotransporter 2),23–26 thereby promoting sodium excretion and lowering BP. 20-HETE is mainly produced by a Cyp4a subfamily of enzymes located in both microvessels and tubular cells. Disruption of the Cyp4a14 gene in mice causes gender-specific hypertension in males, which results from the increased plasma androgen levels and upregulation of androgen-sensitive Cyp4a12 a and b isoforms, the predominant 20-HETE synthases in the male mouse kidney.27 Holla et al. proposed that a Cyp4a12-mediated increase in renal 20-HETE levels is responsible for hypertension in Cyp4a14-knockout mice.28 This mechanism seems to mirror our findings in clock(−/−) mice. Indeed, the increase in Cyp4a14 expression levels and the decrease in Cyp4a12a and Cyp4a12b expression levels in clock(−/−) mice correlates with the lower renal content of 20-HETE and decreased BP.
We propose that the changes in the renal content of 20-HETE could be one of the possible causes in the dysfunction of the renal excretory rhythms and BP control in clock(−/−) mice. Our findings that 20-HETE levels in the kidney exhibit circadian rhythms may have clinical importance. Indeed, drugs targeting the 20-HETE axis present an interesting therapeutic potential for vascular and salt-sensitive hypertension, acute kidney injury, and renal cancer.22,29,30 Some of these compounds are being tested in preclinical studies.22 The circadian rhythmicity of 20-HETE levels indicates that the safety and efficiency of these drugs could vary depending on the time they are administered.
Concise Methods
Animals
A colony of clock-deficient mice was established from breeding pairs of clock(+/−) heterozygous mice originally generated by Debruyne et al.13 The clock(+/−) mice were backcrossed to C57BL/6J mice for more than nine generations. All experiments with animals were performed in accordance with the Swiss guidelines for animal care, which conform to the National Institutes of Health animal care guidelines. Animals were fed with a standard mouse diet (#3800) from KLIBA (Kaiseraugst, Switzerland). This diet contains 0.23% sodium (www.kliba-nafag.ch).
Urine Collection
Mice were housed in individual metabolic cages (Tecniplast, Italy). Urine was collected after a 3-day adaptation period. Hourly urine collection was simultaneously obtained from six wild-type and six knockout mice with the help of a 12-channel peristaltic pump (IPC, Ismatec, Switzerland) connected to a fraction collector (FC204, Gilson, Switzerland). Both the peristaltic pump and the fraction collector were automatically switched on every hour for 2 minutes. This protocol rendered unnecessary the experimenter’s presence during the 24-hour collection period. Urine was collected under mineral oil to avoid evaporation. Urine content of sodium and potassium was determined by flame photometry.
Analysis of Plasma Aldosterone Levels
Plasma aldosterone was measured by a conventional radioimmunoassay (DPC). All blood samples were collected retro-orbitally and stored on ice.
Analysis of 20-HETE Levels
20-HETE levels were measured using Detroit R&D kits. Microsomes were prepared according to the manufacturer’s protocol (Detroit R&D).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank David Weaver (University of Massachusetts Medical School) for the generous gift of the clock-deficient mice and Otto Hagenbuchle, Keith Harshman, Alexandra Paillusson, and Mélanie Dupasquier from Lausanne Genomic Technologies Facility for the microarray profiling studies.
This work was supported by Swiss National Science Foundation Research Grant 31003A-132496 (to D.F.)
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011080842/-/DCSupplemental.
References
- 1.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA: Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA 104: 3450–3455, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, Bonny O, Firsov D: Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA 106: 16523–16528, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H: Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16: 67–74, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Rudic RD, Fulton DJ: Pressed for time: The circadian clock and hypertension. J Appl Physiol 107: 1328–1338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Maillard M, Schibler U, Burnier M, Gachon F: Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF. Am J Physiol Regul Integr Comp Physiol 299: R1013–R1019, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Pechère-Bertschi A, Nussberger J, Biollaz J, Fahti M, Grouzmann E, Morgan T, Brunner HR, Burnier M: Circadian variations of renal sodium handling in patients with orthostatic hypotension. Kidney Int 54: 1276–1282, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Burnier M, Coltamai L, Maillard M, Bochud M: Renal sodium handling and nighttime blood pressure. Semin Nephrol 27: 565–571, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC: Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol 299: F404–F411, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Nogawa K: Shift work is a risk factor for increased blood pressure in Japanese men: A 14-year historical cohort study. Hypertension 52: 581–586, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Conroy RT, Elliott AL, Mills JN: Circadian excretory rhythms in night workers. Br J Ind Med 27: 356–363, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saifur Rohman M, Emoto N, Nonaka H, Okura R, Nishimura M, Yagita K, van der Horst GT, Matsuo M, Okamura H, Yokoyama M: Circadian clock genes directly regulate expression of the Na(+)/H(+) exchanger NHE3 in the kidney. Kidney Int 67: 1410–1419, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS: The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM: A clock shock: Mouse CLOCK is not required for circadian oscillator function. Neuron 50: 465–477, 2006 [DOI] [PubMed] [Google Scholar]
- 14.DeBruyne JP, Weaver DR, Reppert SM: Peripheral circadian oscillators require CLOCK. Curr Biol 17: R538–R539, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Rittig S, Matthiesen TB, Pedersen EB, Djurhuus JC: Circadian variation of angiotensin II and aldosterone in nocturnal enuresis: Relationship to arterial blood pressure and urine output. J Urol 176: 774–780, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hurwitz S, Cohen RJ, Williams GH: Diurnal variation of aldosterone and plasma renin activity: Timing relation to melatonin and cortisol and consistency after prolonged bed rest. J Appl Physiol 96: 1406–1414, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hilfenhaus M: Circadian rhythm of the renin-angiotensin-aldosterone system in the rat. Arch Toxicol 36: 305–316, 1976 [DOI] [PubMed] [Google Scholar]
- 18.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE: Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Bozek K, Relógio A, Kielbasa SM, Heine M, Dame C, Kramer A, Herzel H: Regulation of clock-controlled genes in mammals. PLoS ONE 4: e4882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao CM, Breyer MD: Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int 71: 1105–1115, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Capdevila JH: Regulation of ion transport and blood pressure by cytochrome p450 monooxygenases. Curr Opin Nephrol Hypertens 16: 465–470, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Williams JM, Murphy S, Burke M, Roman RJ: 20-hydroxyeicosatetraeonic acid: A new target for the treatment of hypertension. J Cardiovasc Pharmacol 56: 336–344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ominato M, Satoh T, Katz AI: Regulation of Na-K-ATPase activity in the proximal tubule: Role of the protein kinase C pathway and of eicosanoids. J Membr Biol 152: 235–243, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Yu M, Lopez B, Dos Santos EA, Falck JR, Roman RJ: Effects of 20-HETE on Na+ transport and Na+ -K+ -ATPase activity in the thick ascending loop of Henle. Am J Physiol Regul Integr Comp Physiol 292: R2400–R2405, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Quigley R, Chakravarty S, Zhao X, Imig JD, Capdevila JH: Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiol 113: 23–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amlal H, Legoff C, Vernimmen C, Paillard M, Bichara M: Na(+)-K+(NH4+)-2Cl- cotransport in medullary thick ascending limb: Control by PKA, PKC, and 20-HETE. Am J Physiol 271: C455–C463, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH: Mouse Cyp4a isoforms: Enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J 403: 109–118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH: Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA 98: 5211–5216, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoff U, Lukitsch I, Chaykovska L, Ladwig M, Arnold C, Manthati VL, Fuller TF, Schneider W, Gollasch M, Muller DN, Flemming B, Seeliger E, Luft FC, Falck JR, Dragun D, Schunck WH: Inhibition of 20-HETE synthesis and action protects the kidney from ischemia/reperfusion injury. Kidney Int 79: 57–65, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panigrahy D, Kaipainen A, Greene ER, Huang S: Cytochrome P450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev 29: 723–735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.