Abstract
The benefits and risks of steroids for the treatment of IgA nephropathy remain uncertain. We systematically searched MEDLINE, EMBASE, and the Cochrane Library for randomized, controlled trials of corticosteroid therapy for IgA nephropathy published between 1966 and March 2011. We identified nine relevant trials that included 536 patients who had urinary protein excretion >1 g/d and normal renal function. Forty-six (8.6%) of these patients developed a kidney failure event, defined as doubling of the serum creatinine/halving of the GFR or ESRD. Overall, steroid therapy was associated with a lower risk for kidney failure (relative risk, 0.32 [95% confidence interval [CI], 0.15–0.67]; P=0.002) and a reduction in proteinuria (weighted mean difference, −0.46 g/d [95% CI, −0.63 to −0.29 g/d]), with no evidence of heterogeneity in these outcomes. Subgroup analysis suggested that the dose modifies the effect of steroids for renal protection (P for heterogeneity=0.030): Relatively high-dose and short-term therapy (prednisone >30 mg/d or high-dose pulse intravenous methylprednisolone with duration ≤1 year) produced significant renal protection, whereas low-dose, long-term steroid use did not. Steroid therapy was associated with a 55% higher risk for adverse events. The quality of included studies was low, however, limiting the generalizability of the results. In conclusion, steroids appear to provide renal protection in patients with IgA nephropathy but increase the risk for adverse events. Reliably defining the efficacy and safety of steroids in IgA nephropathy requires a high-quality trial with a large sample size.
IgA nephropathy is the most common primary glomerular disease worldwide, especially in young adults,1–3 and is among the most common causes of kidney failure. Many affected individuals develop chronic, slowly progressive renal injury. It is estimated that 1%–2% of all patients with IgA nephropathy will develop ESRD each year from the time of diagnosis.4 Previous studies have estimated that 13%–22% of people with IgA nephropathy will reach ESRD by 10 years, and up to 20%–30% will do so by 20 years. Impaired kidney function, sustained hypertension, persistent proteinuria (especially proteinuria over 1 g per day), and the nephrotic syndrome are markers of poor prognosis.5–8
No specific therapy is available for IgA nephropathy. BP lowering and renin-angiotensin system inhibition remain the cornerstone of management in people with IgA nephropathy,9 but better treatments are required.
The use of steroids in IgA nephropathy has been controversial. Breakthroughs in our understanding of the pathogenesis of IgA nephropathy have highlighted the immunologic basis of the condition. Identification of a specific autoantigen/autoantibody (characteristic of autoimmune diseases), the presence of immune-complex–mediated GN, and complement activation through lectin pathway10,11 have provided a clear potential rationale for immunosuppressive therapy in the management of progressive IgA nephropathy. Recently reported randomized, controlled trials (RCTs) have tested interventions intended to slow immune and inflammatory events implicated in progressive IgA nephropathy with corticosteroids. The results from the reported trials provided inconsistent results, and much uncertainty persists regarding the effectiveness of this therapy in progressive IgA nephropathy. Several reviews have evaluated the use of steroids in IgA nephropathy. However, these overviews were either a few years before without including the new trials12 or lacked good evaluation of hard endpoints13 or safety.14
In this systematic review, we sought to synthesize all available clinical trial data to better define the balance of risks and benefits associated with steroid therapy in IgA nephropathy.
Results
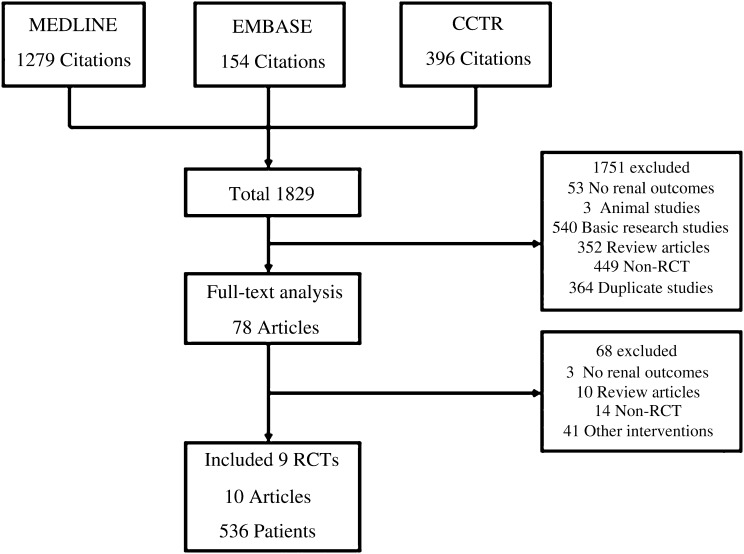
Trial Flow and Study Characteristics
The literature search yielded 1829 articles. Of these, 78 were reviewed in full-text form; 9 RCTs, reported in 10 publications,15–24 were identified as suitable for inclusion in this study. The reasons for exclusion are listed in Figure 1. The included trials provided information on a total of 536 patients who developed 46 kidney failure events. The characteristics of the included studies are summarized in Table 1. Among them, one study (34 patients) included patients mainly with proteinuria in the nephrotic range,15 six studies17–21,23,24 (435 patients) included patients with proteinuria 1–3.5 g/d, and two trials16,22 (67 patients) included those with proteinuria <1 g/d. Most studies17–19,22–24 (five studies with 356 patients) recruited patients with serum creatinine <1.5 mg/dl or estimated GFR >50 ml/min per 1.73 m2.
Figure 1.
Identification process for eligible studies. Results of a systematic literature search on corticosteroid therapy in IgA nephropathy. CCTR, Cochrane Central Register of Controlled Trials.
Table 1.
Characteristics of RCTs involved in the study
| Study | Patients | Kidney Functiona | Proteinuriaa | Sample Sizeb | Steroid Group | Control | Follow–up (mo) | Drop-in/Steroid Discontinuation | Events, n (%)c | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Duration of Steroid Therapy | ||||||||||
| Lai et al.,15 1986 (China) | IgA nephropathy with nephrotic syndrome | Scr: 1.36±0.59 mg/dl | 5.6±2.21 g/d | 34 (17/17) | Prednisone or prednisolone: initial dose, 40–60 mg/d, then tapered | 4 | No treatment | 38 | NA/0 | 0 | 0 |
| Julian and Barker,21 1993 (USA) | Ccr > 25 ml/min per 1.73 m2; proteinuria > 1 g/d or chronic lesions in renal specimen | Scr: 1.54±0.19 mg/dl | 3.35±0.6 g/d | 35 (17/18) | Prednisone: initial, 60 mg every other day for 3 mo, then tapered | 24 | No treatment | 6–24 | NA/2 | 1d (5.9) | 2d (11.1) |
| Shoji et al.,22 2000 (Japan) | Proteinuria < 1.5g/d; Scr < 1.5 mg/dl; mesangial cell proliferation or matrix accumulation involving >50% of glomeruli | Scr: 0.74±0.22 mg/dl | 0.75±0.31 mg/d | 19 (11/8) | Prednisolone: 0.8 mg/kg per day, then tapered; dipyridamole: 300 mg/dl | 12 | Dipyridamole, 300 mg/d | 12 | NA/0 | 0 | 0 |
| Katafuchi et al.,19 2003 (Japan) | Scr < 1.5 mg/dl; glomerular score: 4–7 | Scr: 0.91±0.22 mg/dl | UPC ratio: 1.63±1.53 | 90 (43/47) | Prednisolone: 20 mg/d, gradually tapered to 5 mg/d; dipyridamole: 150–300 mg/d | 24 | Dipyridamole, 150–300 mg/d | 65 | NA/1 | 3d (7.0) | 3d (6.4) |
| Pozzi et al.,24 2004 (Italy) | Scr < 1.5 mg/dl; proteinuria 1–3.5 g/d | Median Scr in steroid group: 1.10 mg/dl (IQR, 0.90–1.30); in control group: 0.98 mg/dl (IQR, 0.81–1.27) | Steroid group: median, 2.0 g/d (IQR, 1.6–2.4); control group: median, 1.8 mg/dl (IQR:1.4–2.4) | 86 (43/43) | Methylprednisolone: 1.0 g × 3 d; prednisone: 0.5 mg/kg every other day | 6 | Supportive therapy | 120 | 13/4 | 1e (2.3) | 13e (30.2) |
| Hogg et al.,18 2006 (USA) | UPC ratio > 1.0 or > 0.5 with renal pathologic lesions at risk; GFR > 50 ml/min per 1.73 m2 | Estimated GFR: 108.5±43.1 ml/min per 1.73 m2 | UPC ratio: 1.81±1.98 | 64 (33/31) | Prednisone: initial, 60 mg every other day for 3 mo, then tapered | 24 | Placebo | 24 | NA/NA | 2f (6.1) | 4f (12.9) |
| Koike et al.,16 2008 (Japan) | Mild inflammatory activities | Scr: 1.04±0.31 mg/dl | 0.93±0.63 g/d | 48 (24/24) | Prednisolone: 0.4 mg/kg per day, gradually tapered to 5–10 mg/d; | 24 | Dipyridamole or dilazep hydrochloride | 24 | NA/NA | NA | NA |
| dipyridamole or dilazep hydrochloride | |||||||||||
| Lv et al.,20 2009 (China) | Proteinuria 1–5 g/d; GFR > 30 ml/min per 1.73 m2 | Scr: 1.1±0.3 mg/dl | 2.26±0.85 g/d | 63 (33/30) | Prednisone: 0.8–1 mg/kg per day for 8 wk, then tapered; cilazapril | 6–8 | Cilazapril | 27.3 | 0/0 | 0 | 2d (6.7) |
| Manno et al.,17 2009 (Italy) | Proteinuria > 1 g/d; GFR > 50 ml/min per 1.73 m2; moderate renal lesions | Scr: 1.07±0.29 mg/dl | Steroid group: median, 1.7 g/d (IQR, 1.2–2.5); control group: median, 1.5 g/d (IQR, 1.4–2.3) | 97 (48/49) | Prednisone: initial, 1 mg/kg per day for 2 mo, then tapered; ramipril | 6 | Ramipril | 96 | 1/1 | 2e (4.2) | 13e (26.5) |
Drop in, patients who are randomized to standard/control arm but start taking/using the experimental treatment; Scr, serum creatinine; NA, not available; Ccr, creatine clearance; UPC, urine protein-to-creatinine; IQR, interquartile range.
Expressed as mean ± SD or median (IQR).
Expressed as total number of patients (number in steroid group/number in control group).
The event was defined as a composite of ESRD or either doubling in serum creatinine or halving of GFR.
ESRD.
Doubling in serum creatine.
Halving of GFR.
Quality of Trials
Reporting of key indicators of trial quality was suboptimal (Supplemental Table 1). Some studies in particular provided few details on the process of randomization, concealment of allocation, and use of intention-to-treat analysis. All of the trials were small (19–97 participants). Most trials (89%) used an open-label design, only three reported that they adequately concealed allocation, and five performed intention-to-treat analysis.
Effects on Renal Survival
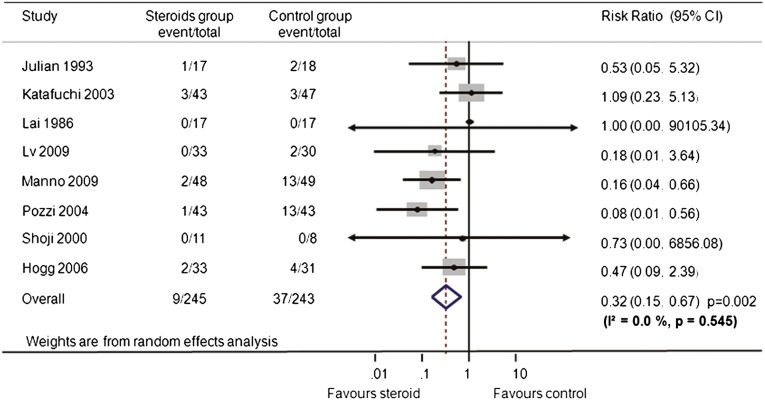
Overall, steroid therapy reduced the risk for ESRD or doubling of serum creatinine or halving of GFR by 68% (eight trials,17–24 488 patients; relative risk [RR], 0.32 [95% confidence interval (CI), 0.15–0.67]; P=0.002), with no evidence of heterogeneity (I2=0.0%; P=0.55) (Figure 2). When ESRD alone was considered, steroid therapy was also associated with a lower risk compared with that in the control group (RR, 0.40 [95% CI, 0.161–0.996], P=0.049; I2=0.0%, P for heterogeneity=0.765). The most data were provided by the studies by Pozzi24and Manno17 and colleagues (13.6% and 26.2% of the weight, respectively).
Figure 2.
Effect of steroids on composite renal endpoint (ESRD or doubling of serum creatinine or halving of GFR) in patients with IgA nephropathy. Boxes and horizontal lines represent relative risk and 95% CI, respectively, for each trial. Size of boxes is proportional to weight of that trial result. Diamonds represent the 95% CI for pooled estimates of effect and are centered on pooled relative risk. Dotted lines on the center of the diamonds represent pooled relative risk. Solid lines represent that the relative risk is 1.
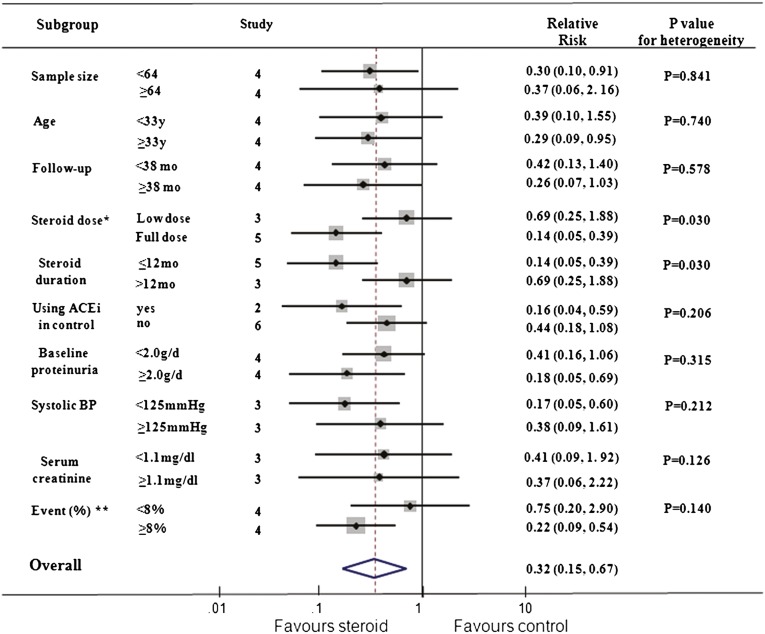
Subgroup analyses were performed for the composite renal endpoint (Figure 3). We noted a different magnitude of effect according to the steroid dose used in trials; the RR was 0.14 (95% CI, 0.05–0.39) for a higher-dose, short-term regimen (prednisone > 30 mg/d or methylprednisolone pulse therapy, ≤12 months) compared with 0.69 (95% CI, 0.25–1.88) for lower-dose, long-term therapy (P for heterogeneity=0.030). There was no apparent heterogeneity of effect between the trials with or without angiotensin-converting enzyme (ACE) inhibitors as a background therapy (P for heterogeneity=0.206). No clear evidence of heterogeneity was found in comparisons of summary results obtained from subsets of studies grouped by sample size, age, follow-up duration, the percentage of composite renal endpoint and renal function, proteinuria, and BP at baseline (P for heterogeneity>0.126 for all comparisons). However, the small sample size and few endpoints mean that the subgroup analyses have limited power to detect differences.
Figure 3.
Subgroup analysis for the effect of corticosteroid on composite renal endpoint (ESRD or doubling in serum creatinine or halving of GFR). *Full dose, prednisone > 30 mg/d or methylprednisolone pulse therapy; low dose, prednisone < 30 mg/d. **Percentage of patients progressing to composite renal endpoints in each trial. ACEi, ACE inhibitor. Boxes and horizontal lines represent relative risk and 95% CI, respectively, for each trial. Size of boxes is proportional to weight of that trial result. Diamonds represent the 95% CI for pooled estimates of effect and are centered on pooled relative risk. Dotted lines on the center of the diamonds represent pooled relative risk. Solid lines represent that the relative risk is 1.
A Begg funnel plot and Egger test disclosed no evidence of publication bias for the composite renal endpoint (Egger test P=0.93; Supplemental Figure 1).
Effects on Proteinuria
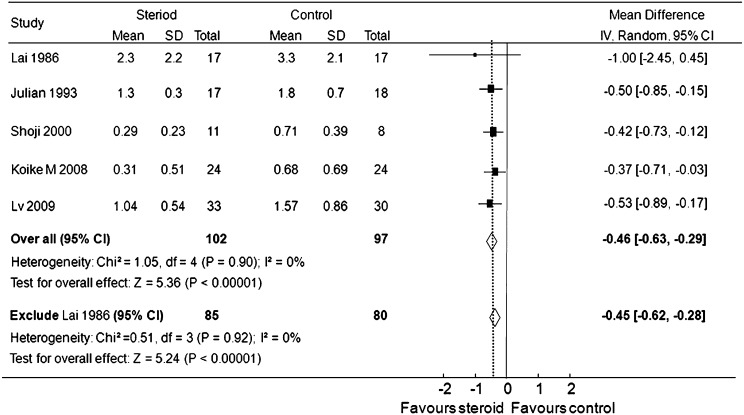
Proteinuria at the end of treatment or during follow-up was significantly lower in patients receiving steroid than in the control groups (five trials,15,16,20–22 199 patients; weighted mean difference, −0.46 g/d [95% CI, −0.63 to −0.29 g/d], P<0.001; I2=0%, P for heterogeneity=0.90).The trial of Lai et al. (1986)15 included some patients with nephrotic syndrome and minimal-change–type lesions on kidney biopsy, which may be more sensitive to steroid therapy. However, excluding this study did not influence the effect of steroids on proteinuria reduction (weighted mean difference, −0.45 g/d [95% CI, −0.62 to −0.28 g/d], P<0.01; I2=0%, P for heterogeneity=0.92) (Figure 4).
Figure 4.
Effect of steroids on proteinuria in patients with IgA nephropathy. Boxes and horizontal lines represent mean difference and 95% CI, respectively, for each trial. Size of boxes is proportional to weight of that trial result. Diamonds represent the 95% CI for pooled estimates of the effect and centered on pooled mean difference. Dotted line on the center of the diamond represents pooled mean difference. Solid line represents that the mean difference is 0. IV, inverse variance.
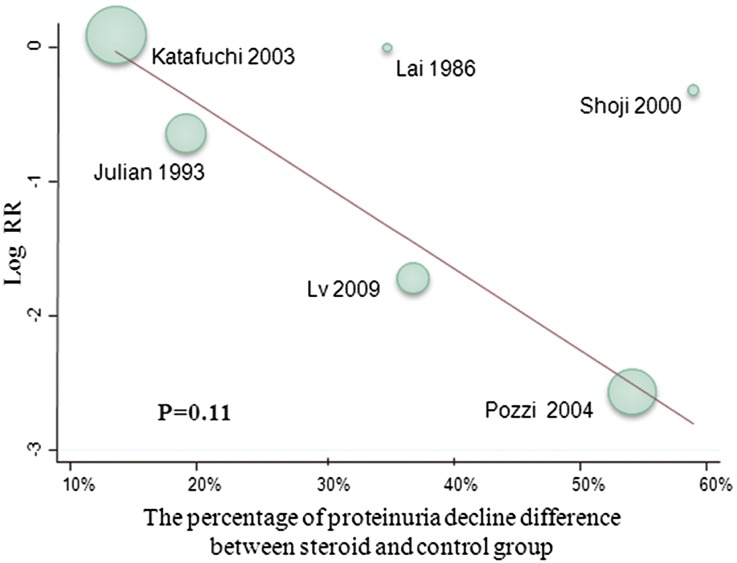
There was some indication that the reduction in risk for kidney failure with steroid therapy may be associated with the difference in proteinuria reduction between treatment groups (Figure 5), but this finding was not statistically significant (P=0.11).
Figure 5.
Meta-regression for the association of RR for composite renal endpoint and proteinuria reduction.
Adverse Events
Data on adverse outcomes potentially associated with treatment were collected from the trials but were inconsistently reported (Table 2). Several adverse events were reported (diabetes mellitus or impaired glucose intolerance, hypertension, gastrointestinal bleeding, cushingoid features, insomnia, headache, weight gain). No severe infections, fractures, or cardiovascular events were reported in these trials. Steroid therapy was associated with an increased risk for adverse events (RR, 1.55 [95% CI, 1.09–2.21]; P=0.02; Supplemental Figure 2), mainly because of an increased risk for cushingoid features. A trend toward increases in other risks (i.e., diabetes and weight gain) was observed but did not reach statistical significance (RR, 1.37 [95% CI, 0.899–2.088]).
Table 2.
Adverse events reported in the included RCTs
| Adverse Events | Studies Reporting (n) | Steroid Group (n/n) | Control Group (n/n) | RR (95% CI) | P Value |
|---|---|---|---|---|---|
| Total patients with adverse events | 8 | 60/245 | 34/243 | 1.55 (1.09–2.21) | 0.02 |
| Specific adverse events | |||||
| diabetes mellitus/impaired glucose intolerance | 3 | 4/108 | 1/110 | 2.55 (0.51–12.72) | 0.26 |
| hypertension | 3 | 12/93 | 15/90 | 0.78 (0.39–1.54) | 0.48 |
| insomnia, palpitations, increased perspiration, headache | 4 | 7/104 | 1/103 | 2.72 (0.60–12.44) | 0.20 |
| gastrointestinal bleeding | 1 | 1/17 | 0/17 | 3.00 (0.13–68.84) | 0.49 |
| cushingoid features | 3 | 9/82 | 0/84 | 7.18 (1.34–38.42) | 0.02 |
| weight increase | 1 | 22/33 | 13/31 | 1.56 (0.98–2.57) | 0.06 |
| cough | 2 | 2/81 | 3/79 | 0.75 (0.09–6.28) | 0.79 |
Discussion
The immunologic mechanisms now known to be involved in the development and progression of IgA nephropathy suggest a potential beneficial role for immunosuppressive therapies. This systemic review, including nine RCTs with more than 500 patients, provides evidence suggesting that a relatively short course of immunosuppression with steroids in IgA nephropathy may reduce the risk for kidney failure by two thirds compared with supportive therapy or ACE inhibitors alone. Consistent with this finding, steroid therapy also reduced proteinuria by 0.46 g/d (95% CI, −0.63 to −0.29 g/d). Of note, these results are mainly driven by two studies,17,24 one of which24 did not include optimal antiproteinuric and antihypertensive therapy. Conversely, steroid therapy increased the risk for adverse events, including diabetes, weight gain, and Cushing syndrome in most trials, whereas the effect on other, more serious adverse events remains uncertain.
This analysis has several important strengths. For example, we used rigorous methods, included more studies than did previous reviews, and focused on the critical clinical outcome of kidney failure. In addition, we attempted to tabulate adverse events. Overall, our analysis demonstrated the potential value of steroids as a treatment strategy for a short-term period (about 6 months). Because the therapy is inexpensive and appears to be mostly well tolerated, it offers great promise as a method of substantially reducing the burden of ESRD around the world. However, given the quality of the studies, and the shortcomings identified, this systematic review does not replace the need for a well conducted large clinical trial.
It remains premature to recommend the routine use of steroids in IgA nephropathy, for many reasons. The available studies are small and short-term and were generally conducted in a single center. Measures of study quality were suboptimal, and data on adverse effects were not collected consistently. Furthermore, individuals with significantly elevated creatinine levels or reduced estimated GFR were excluded, even though they could potentially derive the greatest benefit. It is also unknown whether immunosuppressive therapy adds benefit after supportive care has been optimized; a trial evaluating this issue, STOP-IgAN (Supportive Versus Immunosuppressive Therapy of Progressive IgA Nephropathy), is ongoing.25 As a result, the balance of benefits and risks remains uncertain, and it is not clear who should be treated with this therapy.
The results of this systematic review strongly support the need for a large, high-quality multicenter trial specifically powered to detect benefits for kidney failure in a broad population of people with high-risk IgA nephropathy and to collect reliable data on adverse outcomes. Such a trial is being planned by this study group across several countries (the TESTING [Therapeutic Evaluation of STeroids in IgA Nephropathy Global] study). This study will involve patients with high risk for kidney failure, defined as ESRD or 50% GFR decline. A target sample size of 1300 participants will provide excellent power to detect a 30% RR reduction (the upper limit of the 95% CI of the pooled results) with steroids over an average 5-year follow-up, and the involvement of additional centers and countries is welcome.
The results of this systematic review provide important additional information relevant to the design of future trials. First, our results suggest that the renal benefits varied according to the steroid dose used in the clinical trials. Treatment with high-dose regimens appeared effective, whereas low-dose regimens were possibly inferior. A high-dose regimen might therefore be preferred in future trials. Second, subgroup analysis revealed that renal protection achieved with steroids was not influenced by background therapy with or without ACE inhibitors, suggesting that the therapeutic effect of steroids may be independent of renin-angiotensin system inhibitors. Third, urine protein excretion was reduced, and this effect appeared to be possibly related to the kidney failure benefits according to meta-regression analysis; however, this analysis was underpowered in the regression model. This finding is consistent with the results of cohort studies suggesting that proteinuria remission will preserve kidney function in IgA nephropathy.7
In conclusion, the results of this systematic review examining published RCTs suggest that steroid therapy reduces proteinuria and may prevent ESRD in patients with IgA nephropathy who are proteinuric but have relatively preserved kidney function. The limitations of the current studies mean that a high-quality RCT with a large sample size is still needed to reliably define the efficacy and safety of steroids in IgA nephropathy. If these benefits are confirmed by such a trial, large numbers of people could be prevented from reaching ESRD, benefiting the affected individuals, their families, and their communities.
Concise Methods
Data Sources and Search Strategy
We performed a systematic review of the published work according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for the conduct of meta-analyses of intervention studies.26 The search was limited to RCTs that assessed the effects of steroid therapy in IgA nephropathy and had a follow-up period of at least 6 months. No language restriction was used. Relevant studies were identified by searching MEDLINE via Ovid (from 1966 to March 2011), EMBASE (from 1988 to March 2011), and the Cochrane Library database (Cochrane Central Register of Controlled Trials), with relevant text words and Medical Subject Headings that covered GN, IgA, IgA nephropathy, IgA nephropathy, Berger disease, RCT, controlled clinical trial, and drug therapy. Reference lists from identified trials and review articles were manually scanned to identify any other relevant studies. The ClinicalTrials.gov website was also searched for randomized trials that were registered as completed but not yet published.
Study Selection
The literature search, data extraction, and quality assessment were undertaken independently by two authors (D. Xu and X. Ma) with a standardized approach, and differences were resolved by discussion with a third author (J. Lv).
Data Extraction and Quality Assessment
Published reports were obtained for each trial and standard information was extracted into a spreadsheet separately by two authors (D. Xu and X. Ma). The extracted data included baseline patient characteristics (age, gender, serum creatinine, GFR, proteinuria, BP), follow-up duration, mean proteinuria reduction, steroid doses and modalities of treatment, outcome events, and adverse events. Whenever data were not reported in the publications,16–18 the primary review investigators (J. Lv and D. Xu) contacted the study authors to seek the missing data.
Study quality was judged according to standard criteria (allocation concealment, blinding of patients, investigators and outcome assessors, completeness of follow-up, and use of intention-to-treat analysis).27
Outcomes
The primary outcome was kidney failure events, defined as a composite of ESRD or doubling in serum creatinine or halving of GFR. Secondary outcomes of interest were reduction in proteinuria and adverse outcomes.
Data Synthesis and Analysis
Dichotomous outcome data from individual trials were summarized using the pooled RR measure and its 95% CI. For continuous outcomes, the difference in means and their 95% CI at the end of follow-up were calculated for individual trials, and the weighted mean difference was used as a summary estimate. Pooled estimates across studies were obtained by means of random-effects models after log transformation. Studies were weighted according to an estimate of statistical size, defined as the inverse of the variance of the log RR.28 We used a random-effects model in this study because it is generally considered to be more conservative.29 When zero events were observed in one or both treatment groups of a study, the reciprocal of the size of the opposite treatment group was added to each cell of the 2×2 table as a continuity correction factor. Heterogeneity of treatment effects between studies was investigated statistically through chi-squared and I2 statistics.30 Potential publication bias was assessed with the Egger test and represented graphically with Begg funnel plots of the natural log of the RR versus its SEM.31 We explored potential heterogeneity in estimates of treatment effect by comparing summary results obtained from subsets of studies grouped by the median value of included studies (e.g., sample size, age, follow-up duration, BP at baseline) or according to condition (e.g., whether ACE inhibitors were used as background therapy). Meta-regression was used to assess the association of proteinuria reduction between the intervention and control groups and the RR for the composite renal endpoint. All the reported P values are two-sided, and P<0.05 was considered to represent statistically significant differences. All analyses were calculated using Stata software, version 11.0, and Review Manager 5.0.
Disclosure
None.
Supplementary Material
Acknowledgments
This study is supported by the Peking University Clinical Research Program (PUCRP201102) and the National Natural Science Foundation for Innovative Research Groups of China (Grant 81021004).
TESTING Study Group—Steering Committee: Daniel Cattran, Tak Mao Chan, John Feehally, Jürgen Floege, Richard J. Glassock, Carmel Hawley, Vivek Jha, David W. Johnson, Adeera Levin, Zhihong Liu, Vlado Perkovic (Deputy Chair), Giuseppe Remuzzi, Haiyan Wang (Chair), David C. Wheeler, Mark Woodward, Yangfeng Wu, and Hong Zhang.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011111112/-/DCSupplemental.
References
- 1.D’Amico G: The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med 64: 709–727, 1987 [PubMed] [Google Scholar]
- 2.Julian BA, Waldo FB, Rifai A, Mestecky J: IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med 84: 129–132, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Donadio JV, Grande JP: IgA nephropathy. N Engl J Med 347: 738–748, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Nachman PH, Jennette JC, Falk RJ: IgA nephropathy. In: Brenner and Rector's the Kidney, 8th Ed, edited by Brenner BM, Philadelphia, Saunders, 2007, pp. 1024–1032 [Google Scholar]
- 5.Lv J, Zhang H, Zhou Y, Li G, Zou W, Wang H: Natural history of immunoglobulin A nephropathy and predictive factors of prognosis: A long-term follow up of 204 cases in China. Nephrology (Carlton) 13: 242–246, 2008 [DOI] [PubMed] [Google Scholar]
- 6.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid S, Cawthon PM, Craig JC, Samuels JA, Molony DA, Strippoli GF: Non-immunosuppressive treatment for IgA nephropathy. Cochrane Database Syst Rev (3): CD003962, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glassock RJ: Analyzing antibody activity in IgA nephropathy. J Clin Invest 119: 1450–1452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuels JA, Strippoli GF, Craig JC, Schena FP, Molony DA: Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev (4): CD003965, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Zhang X, Zhang W, He Q, Tao X, Chen J: Efficacy and safety of glucocorticoids therapy for IgA nephropathy: A meta-analysis of randomized controlled trials. Am J Nephrol 30: 315–322, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Zhou YH, Tang LG, Guo SL, Jin ZC, Wu MJ, Zang JJ, Xu JF, Wu CF, Qin YY, Cai Q, Gao QB, Zhang SS, Yu DH, He J: Steroids in the treatment of IgA nephropathy to the improvement of renal survival: A systematic review and meta-analysis. PLoS ONE 6: e18788, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai KN, Lai FM, Ho CP, Chan KW: Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: A long-term controlled trial. Clin Nephrol 26: 174–180, 1986 [PubMed] [Google Scholar]
- 16.Koike M, Takei T, Uchida K, Honda K, Moriyama T, Horita S, Ogawa T, Yoshida T, Tsuchiya K, Nitta K: Clinical assessment of low-dose steroid therapy for patients with IgA nephropathy: A prospective study in a single center. Clin Exp Nephrol 12: 250–255, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Hogg RJ, Lee J, Nardelli N, Julian BA, Cattran D, Waldo B, Wyatt R, Jennette JC, Sibley R, Hyland K, Fitzgibbons L, Hirschman G, Donadio JV, Jr, Holub BJ, Southwest Pediatric Nephrology Study Group : Clinical trial to evaluate omega-3 fatty acids and alternate day prednisone in patients with IgA nephropathy: Report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 1: 467–474, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Katafuchi R, Ikeda K, Mizumasa T, Tanaka H, Ando T, Yanase T, Masutani K, Kubo M, Fujimi S: Controlled, prospective trial of steroid treatment in IgA nephropathy: A limitation of low-dose prednisolone therapy. Am J Kidney Dis 41: 972–983, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, Wang H: Combination therapy of prednisone and ACE inhibitor versus ACE-inhibitor therapy alone in patients with IgA nephropathy: A randomized controlled trial. Am J Kidney Dis 53: 26–32, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Julian BA, Barker C: Alternate-day prednisone therapy in IgA nephropathy. Preliminary analysis of a prospective, randomized, controlled trial. Contrib Nephrol 104: 198–206, 1993 [PubMed] [Google Scholar]
- 22.Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, Imai E, Hori M, Tsubakihara Y: Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. Am J Kidney Dis 35: 194–201, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, Locatelli F: Corticosteroids in IgA nephropathy: A randomised controlled trial. Lancet 353: 883–887, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, Ponticelli C, Locatelli F: Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Eitner F, Ackermann D, Hilgers RD, Floege J: Supportive Versus Immunosuppressive Therapy of Progressive IgA nephropathy (STOP) IgAN trial: Rationale and study protocol. J Nephrol 21: 284–289, 2008 [PubMed] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151: 264–269, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Green S: Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.2, The Cochrane Collaboration, 2009. Available at http://www.cochrane-handbook.org. Accessed March 10, 2011 [Google Scholar]
- 28.Woodward M: Epidemiology: Study Design and Data Analysis, 2nd Ed, Boca Raton, FL, Chapman and Hall/CRC, 2005 [Google Scholar]
- 29.Thompson SG, Pocock SJ: Can meta-analyses be trusted? Lancet 338: 1127–1130, 1991 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenbroucke JP: Bias in meta-analysis detected by a simple, graphical test. Experts' views are still needed. BMJ 316: 469–470,1998 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.