Abstract
In patients with ESRD, the effects of online hemodiafiltration on all-cause mortality and cardiovascular events are unclear. In this prospective study, we randomly assigned 714 chronic hemodialysis patients to online postdilution hemodiafiltration (n=358) or to continue low-flux hemodialysis (n=356). The primary outcome measure was all-cause mortality. The main secondary endpoint was a composite of major cardiovascular events, including death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, therapeutic coronary intervention, therapeutic carotid intervention, vascular intervention, or amputation. After a mean 3.0 years of follow-up (range, 0.4–6.6 years), we did not detect a significant difference between treatment groups with regard to all-cause mortality (121 versus 127 deaths per 1000 person-years in the online hemodiafiltration and low-flux hemodialysis groups, respectively; hazard ratio, 0.95; 95% confidence interval, 0.75–1.20). The incidences of cardiovascular events were 127 and 116 per 1000 person-years, respectively (hazard ratio, 1.07; 95% confidence interval, 0.83–1.39). Receiving high-volume hemodiafiltration during the trial associated with lower all-cause mortality, a finding that persisted after adjusting for potential confounders and dialysis facility. In conclusion, this trial did not detect a beneficial effect of hemodiafiltration on all-cause mortality and cardiovascular events compared with low-flux hemodialysis. On-treatment analysis suggests the possibility of a survival benefit among patients who receive high-volume hemodiafiltration, although this subgroup finding requires confirmation.
Patients undergoing chronic intermittent hemodialysis have a high risk of cardiovascular morbidity and mortality. Of the potential risk factors involved, retention of uremic toxins in the middle molecular mass range (0.5–40 kD) might play an important role. During conventional hemodialysis with low-flux membranes, low molecular mass substances (<1.5 kD) are removed by diffusive transport, whereas larger uremic toxins accumulate in the body. Therefore, in the past, high-flux membranes have been developed, which enable the removal of larger molecules by convective transport. However, the amount of convective transport is uncertain and uncontrollable. Two large randomized controlled trials did not show a distinct clinical benefit of high-flux versus low-flux membranes.1,2
The magnitude of convective transport can be considerably increased by a technique that combines high-flux hemodialysis with the (ultra)filtration of large amounts of plasma water, so-called hemodiafiltration. Obviously, fluid balance can only be maintained if the amount of fluid withdrawn is substituted. In the last decades, the development of sophisticated water treatment systems and effective ultrafilters allowed the “online” production of sterile substitution fluid at acceptable costs. With modern online hemodiafiltration equipment, high convection and substitution volumes can be reached safely,3 resulting in markedly enhanced removal of middle molecular mass substances.4–7
Several nonrandomized observational studies suggested a survival benefit in patients who are treated with online hemodiafiltration.8–11 However, small randomized studies failed to show a difference between online hemodiafiltration and standard hemodialysis (as summarized by van der Weerd et al.12). On the basis of the above-mentioned theoretical considerations, the absence of conclusive clinical data, and the growing interest in convective techniques, the Convective Transport Study (CONTRAST) was initiated (ClinicalTrials.gov identifier: NCT00205556). This is a randomized controlled trial, comparing online hemodiafiltration and low-flux hemodialysis with respect to survival and various predefined secondary clinical endpoints.
Results
Patient Characteristics at Baseline
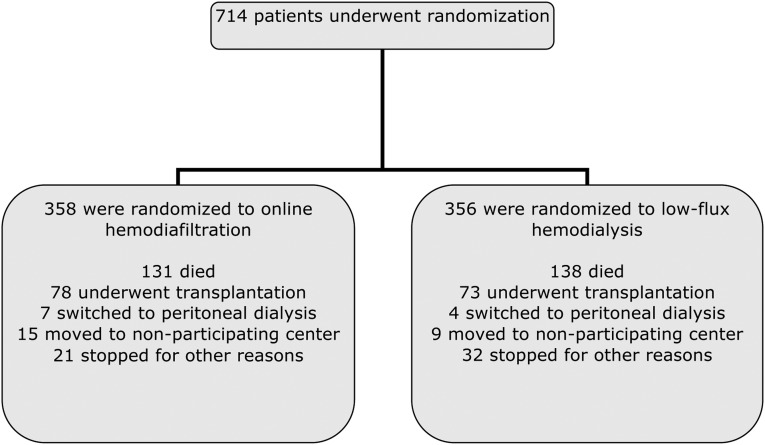
Between June 2004 and December 2009, 714 patients (597 in the Netherlands, 102 in Canada, and 15 in Norway) were enrolled and randomly assigned to start online hemodiafiltration (n=358) or to continue low-flux hemodialysis (n=356) (Figure 1). We achieved complete follow-up for all participants. The two groups were well balanced with respect to baseline characteristics (Table 1; additional information is provided in Supplemental Table 5). A small number of patients (6.1%) were treated with two sessions per week (26 in the hemodiafiltration group, and 18 in the hemodialysis group).
Figure 1.
Enrollment, randomization, and follow-up of study participants. For mortality and cardiovascular events, all patients were followed until the end of the study.
Table 1.
Characteristics of participants at baseline
| Variable | Online Hemodiafiltration (n=358) | Low-Flux Hemodialysis (n=356) |
|---|---|---|
| Age (yr) | 64.1±14.0 | 64.0±13.4 |
| Female sex | 144 (40) | 125 (35) |
| Race | ||
| Caucasian | 304 (85) | 296 (83) |
| Afro-Caribbean | 29 (8) | 29 (8) |
| Asian | 23 (6) | 22 (6) |
| other | 2 (1) | 9 (3) |
| Region | ||
| Netherlands | 300 (84) | 297 (83) |
| Canada | 51 (14) | 51 (14) |
| Norway | 7 (2) | 8 (2) |
| History of cardiovascular disease | 151 (42) | 162 (46) |
| Diabetes mellitus | 92 (26) | 78 (22) |
| Dialysis vintage (yr) | 2.8±2.9 | 3.0±2.8 |
| median (IQR) | 1.8 (1.0–3.7) | 2.1 (1.0–4.0) |
| Systolic BP (mmHg)a | 147±21 | 148±22 |
| Diastolic BP (mmHg)a | 75±12 | 76±12 |
| Vascular access | ||
| arteriovenous fistula | 279 (78) | 288 (81) |
| graft | 57 (16) | 43 (12) |
| central catheter | 22 (6) | 25 (7) |
| Treatments/wk | ||
| 3 | 332 (93) | 338 (95) |
| 2 | 26 (7) | 18 (5) |
| Duration of a dialysis session (min) | 226±26 | 227±22 |
| Blood flow (ml/min) | 302±39 | 299±41 |
| Dialysis single-pool Kt/Vurea | 1.41±0.24 | 1.38±0.19 |
| Residual kidney functionb | 186 (52) | 190 (53) |
| Estimated GFR | ||
| ml/min per 1.73m2c | 2.1±3.4 | 2.0±3.3 |
| median (IQR) | 0.32 (0–3.30) | 0.30 (0–3.35) |
| Hemoglobin (g/dl) | 11.9±1.3 | 11.8±1.2 |
| Phosphorus (mg/dl) | 5.12±1.58 | 5.05±1.46 |
| β-2-microglobulin (mg/L) | 30.7±14.3 | 32.3±13.6 |
| SGA classification | ||
| well nourished | 290 (81) | 297 (83) |
| mild to moderate malnutrition | 68 (19) | 58 (16) |
| severe malnutrition | 0 (0) | 1 (0) |
| BMI after dialysis (kg/m2) | 25.2±5.0 | 25.6±4.6 |
| Albumin (g/L) | 40.2±3.8 | 40.6±3.9 |
| C-reactive protein (mg/L) | 10.9 (20.2) | 10.2 (26.3) |
| median (IQR) | 4.1 (1.4–10.7) | 3.9 (1.3–10.2) |
| Creatinine (mg/dl), predialysis | 9.52±2.94 | 9.94±2.83 |
| Cholesterol (mg/dl) | 142±35 | 142±41 |
| Prescribed medication | ||
| β blocker | 184 (51) | 193 (55) |
| RAS inhibitor | 179 (50) | 170 (48) |
| platelet aggregation inhibitor | 111 (34) | 127 (36) |
| 1-OH and 1,25-OH vitamin D | 227 (63) | 254 (72) |
| statin | 198 (55) | 164 (46) |
| erythropoiesis stimulating agents | 314 (88) | 319 (90) |
Data are presented as n (%) or mean ± SD. SGA, subjective global assessment; RAS, renin-angiotensin system.
Before hemodialysis.
Residual kidney function if diuresis >100 ml/24 h.
Mean of urea and creatinine clearance in 24-hour urine collection; mean plasma creatinine and urea concentrations during the collection period were estimated using the geometric mean of postdialysis and predialysis plasma samples.
Treatment Characteristics and Adherence
Treatment characteristics during the trial are shown in Table 2. During follow-up, spKt/Vurea increased in patients treated with hemodiafiltration (from 1.41 to 1.63; difference with hemodialysis, 0.18; P<0.001). Treatment time increased from 227 to 229 minutes in the hemodialysis group and remained stable in patients treated with hemodiafiltration (226 minutes; P=0.03 for the difference between the treatment modalities).
Table 2.
Mean characteristics of treatment during follow-up
| Characteristic | Online Hemodiafiltration | Low-Flux Hemodialysis | Mean Difference (SEM) | P Value for Difference |
|---|---|---|---|---|
| Duration of dialysis session (h) | 3.77 (0.01) | 3.81 (0.01) | −0.04 (0.02) | 0.02 |
| Blood flow (ml/min) | 332 (2.6) | 312 (2.3) | 20 (3.4) | 0.001 |
| Sessions/wk (n) | 2.99 (SD 0.6) | 2.99 (SD 0.7) | 0.002 (0.02) | 0.25 |
| Online hemodiafiltration | ||||
| number of sessions/wka | 2.71 (SD 0.35) | NA | ||
| convection volume (L)/treatment | 20.7 (SD 6.0) | NA | ||
| Single-pool Kt/Vurea | 1.63 (0.02) | 1.45 (0.02) | 0.18 (0.03) | <0.001 |
| β-2-microglobulin (mg/L) | 26.4 (0.37) | 35.4 (0.54) | −8.9 (0.7) | <0.001 |
| Albumin (g/L) | 39.4 (0.14) | 39.7 (0.15) | −0.30 (0.20) | 0.14 |
| CRP (mg/L) | 11.9 (1.4) | 11.4 (1.2) | 0.52 (1.9) | 0.77 |
| Hemoglobin (g/dl) | 11.8 (0.03) | 11.6 (0.03) | 0.11 (0.05) | 0.03 |
| Phosphorus (mg/dl) | 4.80 (0.06) | 4.95 (0.06) | 0.19 (0.06) | 0.02 |
| Cholesterol (mg/dl) | 143 (2) | 139 (2) | 2 (2) | 0.28 |
| Predialysis systolic pressure (mmHg) | 146 (1) | 145 (1) | 0.9 (0.5) | 0.49 |
| Interdialytic weight change, pre − post (kg) | 1.91 (0.05) | 1.85 (0.05) | 0.05 (0.07) | 0.51 |
Average levels during the trial and mean difference over time between treatment arms obtained through GEE analyses using the on trial measurements with adjustments for baseline measurements. Values are means (SEM) unless otherwise indicated. NA, not applicable; CRP, C-reactive protein.
Actual number of sessions per week delivered as online hemodiafiltration during the trial, the other sessions were delivered as high-flux hemodialysis.
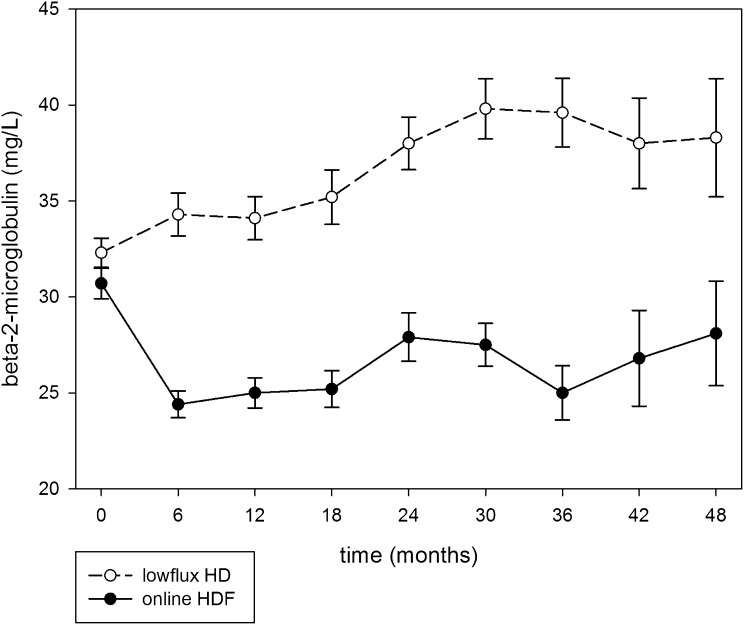
As shown in Figure 2, β2-microglobulin levels decreased in patients treated with hemodiafiltration (from 30.7 to 26.4 mg/L) and increased in hemodialysis patients (from 32.3 to 35.4 mg/L; difference between treatment arms, 8.9 mg/L; P<0.001).
Figure 2.
Predialysis β-2-microglobulin levels in patients treated with online hemodiafiltration and low-flux hemodialysis (mean ± SEM) using measurements of individuals at those time points. The difference between β-2-microglobulin levels for both treatments was significant (P<0.001).
In patients randomized to hemodiafiltration, 2.71 (91%) of 2.99 treatments per week were actually delivered as online hemodiafiltration during the trial. The remaining treatments were delivered as high-flux hemodialysis. The average convection volume, which includes weight loss, of all hemodiafiltration treatments given to participants during the entire trial was 20.7 L/treatment session, with a median of 19.8 L.
Primary Outcome: All-Cause Mortality
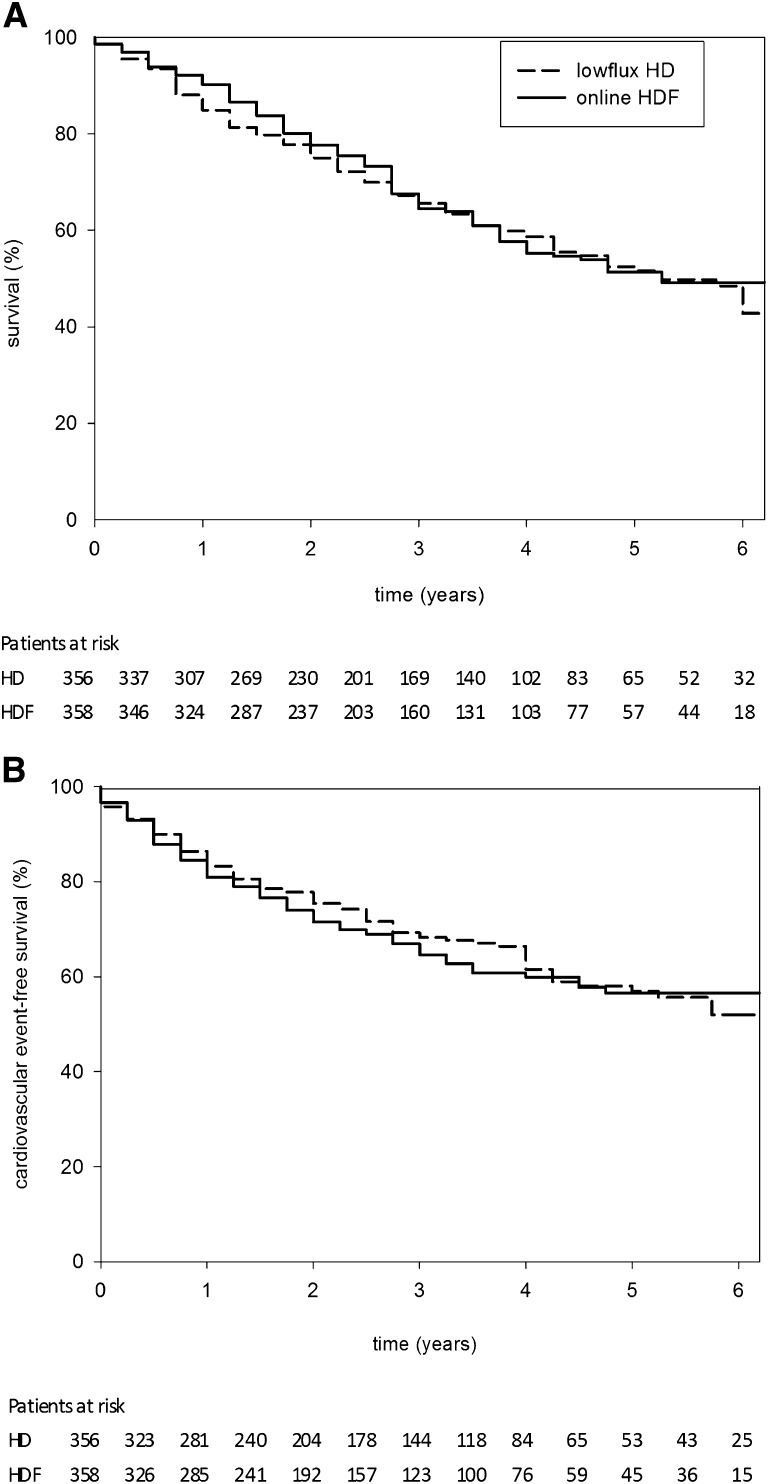
After a mean follow-up of 3.04 years (range, 0.4–6.6 years; median 2.9 years), the accumulating results met the criterion specified by the sequential analysis plan for termination of the study (Supplemental Figure 4). The incidence of all-cause mortality was not affected by treatment assignment (121 per 1000 person-years on hemodiafiltration versus 127 per 1000 person-years on low-flux hemodialysis; hazard ratio [HR], 0.95; 95% confidence interval [95% CI], 0.75–1.20) (Figure 3A). Both treatment groups had 1085 person-years of follow-up.
Figure 3.
The incidence of both all-cause mortality and cardiovascular events was not affected by treatment assignment. Survival curves for time to death from any cause (A) and for time to fatal or nonfatal cardiovascular event (B) based on life table analyses using 3-month time periods.
Secondary Outcomes
The incidence of fatal and nonfatal cardiovascular events was 127 per 1000 person-years in hemodiafiltration patients versus 116 per 100 person-years in patients treated with low-flux hemodialysis (HR, 1.07; 95% CI, 0.83–1.39) (Figure 3B). There were no significant differences between the two groups in the frequency and incidence of individual cardiovascular endpoints (Table 3). The incidence of renal transplants was similar in both treatment groups.
Table 3.
Primary and secondary outcomes
| Outcome | Online Hemodiafiltration | Low-Flux Hemodialysis | HR (95% CI)a | ||
|---|---|---|---|---|---|
| Number of Events | Person-Years of Follow-Up | Number of Events | Person-Years of Follow-Up | ||
| Primary outcome | |||||
| all-cause mortality | 131 | 1085 | 138 | 1085 | 0.95 (0.75–1.20) |
| Main secondary outcomes | |||||
| fatal and nonfatal cardiovascular events | 116 | 916 | 112 | 964 | 1.07 (0.83–1.39) |
| cardiovascular mortality | 37 | 1085 | 46 | 1085 | 0.80 (0.52–1.24) |
| nonfatal cardiovascular disease (first event) | 94 | 915 | 87 | 964 | 1.12 (0.83–1.49) |
| fatal and nonfatal CHD (MI, PTCA, CABG) | 38 | 1000 | 47 | 1022 | 0.81 (0.52–1.23) |
| fatal and nonfatal stroke | 19 | 1066 | 16 | 1062 | 1.17 (0.60–2.27) |
| amputation | 21 | 1058 | 16 | 1068 | 1.31 (0.68–2.51) |
| sudden death | 21 | 1085 | 21 | 1085 | 1.00 (0.55–1.83) |
| vascular intervention | 59 | 978 | 49 | 1010 | 1.23 (0.84–1.79) |
| transplantation | 78 | 800 | 73 | 798 | 1.06 (0.77–1.46) |
| hospital admissions due to infection | 130 | 800 | 110 | 798 | 1.21 (0.94–1.56) |
CHD, coronary heart disease; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass graft.
Obtained through unadjusted Cox proportional hazards models.
Interactions of Treatment Interventions with Baseline Factors
Exploratory analyses for all-cause mortality and the combined fatal and nonfatal cardiovascular events were performed for various subgroups on the basis of age, sex, diabetes, albumin ≤40 g/L, residual kidney function, vascular access, and dialysis vintage.
The HRs for all-cause mortality during treatment with hemodiafiltration were 0.84 (95% CI, 0.60–1.18) in patients with residual kidney function and 1.06 (95% CI, 0.75–1.48) in patients without. Similarly, the HRs were not different between patients with a dialysis vintage above and below the median of 2.0 years (0.96 [95% CI, 0.68–1.35] and 0.91 [95% CI, 0.65–1.27], respectively). None of the P values of the interaction terms was significant (all >0.10).
Delivered Convection Volume and All-Cause Mortality
The variation in delivered convection volumes between participants enabled an exploration of the achieved delivered volume of convection during the trial and clinical outcome. In these post hoc analyses, separate models were run with extensive adjustments for factors that were related to the level of convection volume achieved per session, the risk of death, and center differences (Table 4). With respect to all-cause mortality, a significant inverse trend was observed (P=0.003), which remained after adjustment for potential confounders (Table 4). In the group of patients with the highest delivered convection volume (upper tertile >21.95 L) mortality was considerably lower than in patients randomized to low-flux hemodialysis (HR, 0.62; 95% CI, 0.41–0.83). This finding remained statistically significant after adjustment for determinants of convection volume and mortality (HR, 0.61; 95% CI, 0.38–0.98). Correction for body weight and facility did not materially alter these findings. A comparable tendency was observed for cardiovascular events (HR, 0.72; 95% CI, 0.44–1.19), which did not reach statistical significance (Table 4).
Table 4.
Risk of all-cause mortality and fatal and nonfatal cardiovascular events by achieved convection volume in liters per treatment
| Hemodialysis | Online Hemodiafiltration Convection Volume Tertiles | P for Trend | |||
|---|---|---|---|---|---|
| <18.17 L | 18.18–21.95 L | >21.95 L | |||
| Total mortality | |||||
| crude | 1.0 | 0.95 (0.66–1.38) | 0.83 (0.57–1.22) | 0.62 (0.41–0.93) | 0.010 |
| adjusteda | 1.0 | 0.79 (0.53–1.14) | 0.77 (0.51–1.14) | 0.65 (0.42–0.99) | 0.012 |
| adjustedb | 1.0 | 0.80 (0.52–1.24) | 0.84 (0.54–1.29) | 0.61 (0.38–0.98) | 0.015 |
| Fatal and nonfatal cardiovascular events | |||||
| crude | 1.0 | 1.37 (0.94–1.98) | 1.06 (0.72–1.56) | 0.76 (0.50–1.16) | 0.473 |
| adjusteda | 1.0 | 1.41 (0.92–2.11) | 0.93 (0.62–1.40) | 0.77 (0.48–1.21) | 0.369 |
| adjustedb | 1.0 | 1.35 (0.86–2.11) | 1.04 (0.66–1.62) | 0.72 (0.44–1.19) | 0.475 |
Results reported as HR and 95% confidence interval, from Cox proportional hazards models. Reference is treatment with low-flux hemodialysis.
Adjusted for determinants of mortality, i.e., age, sex, previous vascular disease, diabetes, previous transplantation, spKt/V, baseline eGFR, baseline albumin, baseline creatinine, baseline hematocrit, and use of α- and β-blockers, calcium antagonists, and angiotensin converting inhibitors at baseline (82 missing, 206 deaths, 182 cardiovascular events).
Adjusted for the above-mentioned determinates as well as for center differences (82 missing, 206 deaths, 182 cardiovascular events).
Discussion
In this study, chronic hemodialysis patients were randomized to treatment with online postdilution hemodiafiltration or to continuation of low-flux hemodialysis. After a mean follow-up of 3.04 years, treatment with hemodiafiltration did not result in lower all-cause mortality nor did it have a beneficial effect on the composite endpoint of fatal and nonfatal cardiovascular events.
This study is the first large-scale, randomized prospective trial designed to compare online hemodiafiltration with low-flux hemodialysis. Two earlier large randomized studies addressed the hypothesis that removal of larger uremic toxins would improve survival probability. Both the Hemodialysis (HEMO) study and the Membrane Permeability Outcome (MPO) study compared low-flux hemodialysis with high-flux hemodialysis.1,2 Neither study showed a difference in mortality risk between the treatment arms.
Convective transport, as quantified by a decrease in β-2-microglobulin levels, is higher during hemodiafiltration than during high-flux hemodialysis and is negligible during low-flux hemodialysis. In our study, the predialysis β-2-microglobulin levels in the hemodiafiltration group were consistently lower than those in hemodialysis patients. The difference in this study was more than twice the difference between the high-flux and low-flux arms achieved in the MPO study.2 Nonetheless, the main outcome of our study does not deviate from the results of both the HEMO and MPO studies. Therefore, it seems that the addition of convective transport does not improve survival in chronic dialysis patients, at least not when the average delivered convection volume over time is ≤20.7 L/treatment.
Secondary analyses in the HEMO and MPO studies suggested a survival benefit of high-flux hemodialysis in patients with a dialysis vintage >3.7 years,13 patients with diabetes, and patients with a serum albumin ≤40 g/L at baseline.2 In our study, neither dialysis vintage nor albumin levels or the presence of diabetes affected the direction and magnitude of the relation between hemodiafiltration and outcome. In a previous analysis of the CONTRAST study, we showed that the effect of online hemodiafiltration on β-2-microglobulin levels was especially present in patients without residual kidney function.7 However, neither residual kidney function, nor an analysis of other predefined subgroups, such as primary kidney disease, age, co-morbidity, and sex modified the effect of treatment in our study. Because the CONTRAST study was not powered to detect differences between low-flux hemodialysis and hemodiafiltration on outcome across subgroups, our “null” results in subgroup analyses do no exclude the presence of such a relation.
The lack of an overall beneficial effect of hemodiafiltration on survival might be explained by several factors. First, the positive effect of an increased removal of uremic toxins might be counteracted by the simultaneous loss of essential substances,14 and/or undesirable side effects of the treatment itself.15 Second, although the Kaplan–Meier curves did not even show a trend for a better outcome, the intervention period (mean 3.04 years) may have been too short for an effect on survival. Third, the positive effect of hemodiafiltration observed in observational studies might be due to the utilization of ultrapure dialysis fluids, which is mandatory for this treatment. The application of contaminated dialysis fluids during conventional hemodialysis might negatively influence patient health.16 In our study, ultrapure dialysis fluid was used in both treatment arms. Finally, because the actual convection volume per session (20.7 L/treatment) was below the arbitrary planned target of 24 L/treatment (6 L/h), and 9% of hemodiafiltration treatments were delivered as high-flux hemodialysis, the average amount of convective transport actually delivered to the participants during the study might have been too low to obtain an effect on outcome.
We thus explored the relation between delivered convection volume and mortality. Our post hoc analysis showed a significant inverse relation between delivered convection volume and mortality risk (P=0.010). Despite extensive adjustments for potential confounders, the HR for all-cause mortality was considerably lower in the group of patients treated with the highest delivered convection volumes (>21.95 L; HR, 0.62; 95% CI, 0.38–0.98), whereas a nonsignificant trend was observed for fatal and nonfatal cardiovascular events (HR, 0.72; 95% CI, 0.44–1.19). Although the finding of an association between convection dose and outcome is conceptually plausible and in agreement with observational data from the Dialysis Outcomes and Practices Pattern Study (DOPPS),8 this finding requires further investigation and confirmation. A survival benefit was observed in DOPPS patients with a substitution volume >15 L/session, which did not include net ultrafiltration (i.e., desired weight loss).8 Data from the Turkish Hemodiafiltration Study, a randomized trial comparing high-flux hemodialysis with online hemodiafiltration, show that the overall outcome was not affected by treatment allocation, whereas high convection volumes were associated with a significant survival benefit (48th Congress of the European Renal Association-European Dialysis Transplant Association, 2011, abstract LBCT2).
When our study was designed, a target convection volume of 24 L/treatment was merely based on the manufacturer’s guidelines and considered the maximum achievable dose in daily practice during one single treatment. Data linking convection volume to clinical outcome were lacking at that time. The finding that the target volume was not achieved in the majority of patients is a reflection of common practice and as such an important finding. In a previous analysis of the CONTRAST study, we showed that both the blood flow rate through the extracorporeal circuit and treatment time are potentially modifiable determinants of convection volume.17,18
Apart from survival, other endpoints might be of relevance. It was recently shown that the application of convective therapy was associated with better intradialytic hemodynamic stability.19 Other aspects, such as quality of life, erythropoietin resistance, vascular stiffness, left ventricular mass, nutritional status, and cost utility, are currently under investigation as predefined secondary endpoints of this study.20,21
The strengths of our study are its randomized design and the accurate and concise data collection. The external validity of our study is supported by the fact that the variables age, sex, and primary kidney disease of our patients are similar to those in all Dutch patients registered in the RENINE database (www.renine.nl). Because this trial was event driven, the number of events was sufficient for adequate and valid conclusions. In other words, a small sample size does not explain our null finding. A potential limitation may be the inclusion of prevalent patients (as being survivors), as illustrated by a mean dialysis vintage of 2.9 years. Whether the inclusion of incident patients would have resulted in a different outcome cannot be concluded from this study, although the interaction analysis did not show differences in results between patients with a low and a high dialysis vintage.
Recent guidelines advocate high-flux hemodialysis as first-line treatment.22 The fact that we did not find a difference in outcome between low-flux hemodialysis and hemodiafiltration suggests that comparison of high-flux hemodialysis with hemodiafiltration is even less likely to result in a difference in outcome.
Finally, because the relation between all-cause mortality and convection volume was found in an on-treatment analysis, this result should be interpreted with caution and needs confirmation.
In conclusion, our study showed that treatment with online hemodiafiltration did not result in a reduced mortality or less fatal and nonfatal cardiovascular events compared with treatment with low-flux hemodialysis. Subgroup analysis did not yield a benefit of hemodiafiltration over hemodialysis in patients with diabetes, cardiovascular disease, long dialysis vintage, low albumin, or lack of residual kidney function. On-treatment analysis suggests a survival advantage in patients receiving the highest convection volumes. These data must be confirmed by other studies in progress23,24 and/or by a meta-analysis of individual patient records from controlled clinical trials on this topic.
Concise Methods
Study Design
CONTRAST is a randomized controlled trial conducted in twenty-nine dialysis centers: the Netherlands (n=26), Canada (n=2), and Norway (n=1). This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by both a central medical ethics review board and local ethics committees. Written informed consent was obtained from all patients before enrollment. Detailed study methods were previously published.25
CONTRAST is an investigator-initiated trial that was designed, conducted, and analyzed independently of the financial contributors. Study data were collected and retained by the investigators and were not available for the financial contributors. The writing executive committee, whose membership did not include representatives of the financial contributors, has final responsibility for the interpretation of the data, the preparation of the manuscript, and the decision to submit for publication. The executive committee vouches for the validity and completeness of the reported data.
Patients
Patients with ESRD undergoing chronic intermittent hemodialysis for at least 2 months and aged ≥18 years were recruited from June 2004 through December 2009 and followed until December 31, 2010. Primary renal diagnoses were as follows: renal vascular disease (29%), diabetes mellitus (19%), primary glomerulopathy/GN (12%), interstitial nephropathy (9%), cystic kidney disease (7%), multisystem disease (4%), other (12%), or unknown (8%). Patients were eligible for inclusion if they were treated two or three times per week with low-flux hemodialysis. Exclusion criteria were as follows: treatment with hemo(dia)filtration or high-flux hemodialysis in the 6 months preceding randomization, a life expectancy <3 months due to nonrenal disease, participation in another clinical intervention trial evaluating cardiovascular outcomes, and severe nonadherence regarding frequency and/or duration of dialysis treatment.
Randomization and Blinding
All patients were randomized centrally by a computer-based randomization service (Julius Center University Medical Center, Utrecht, The Netherlands) into a 1:1 ratio for treatment with online hemodiafiltration or continuation of low-flux hemodialysis, stratified per participating center (permuted blocks). Because of the nature of the intervention, it was not possible to blind the patients, the local study nurses, or the investigators to the treatment assignment. The laboratory samples were measured in routine clinical care; hence, personnel were unaware of treatment assignment. The event adjudication committee was blinded to the treatment assignment.
Treatment and Procedures
Before randomization, all patients had to be stable with a minimum dialysis single-pool Kt/V for urea (spKt/Vurea) of 1.2. Treatment times were fixed during follow-up in both treatment arms, unless spKt/Vurea was <1.2.
Online hemodiafiltration was performed in the postdilution mode with a proposed target convection volume of 6 L/h, which was based on a targeted filtration rate between 25% and 33% of an extracorporeal blood flow rate between 300 and 400 ml/min (so-called filtration fraction). Blood flow rates could be increased in the hemodiafiltration arm to improve convection volumes. Because evidence relating convection volume and outcome was absent at the time of the start of the study, the proposed target was arbitrarily based on the level considered the maximal achievable convection volume in the postdilution mode.
Synthetic high-flux dialyzers were used for hemodiafiltration (ultrafiltration coefficient [KUF] >20 ml/mmHg per h; FX80: 27%, FX100: 11%, and Optiflux F200NR: 8% [Fresenius Medical Care, Bad Homburg, Germany]; Polyflux 170H: 25% and Polyflux 210H: 27% [Gambro AB, Stockholm, Sweden]; or other dialyzers: 2%). Hemodialysis patients were treated with synthetic low-flux dialyzers (KUF<20 ml/mmHg per h; F6HPS: 5%, F8HPS: 45%, and Optiflux 18NR: 9% [Fresenius]; Polyflux 14L: 2% and Polyflux 17L: 30% [Gambro], or other: 9%). More detailed information on dialyzer characteristics is provided in Supplemental Table 6. When hemodiafiltration could temporarily not be performed due to technical reasons, the patients concerned were treated with high-flux membranes. Both hemodiafiltration and hemodialysis were performed with ultrapure dialysis fluids, defined as <0.1 colony forming units per milliliter and <0.03 endotoxin units per milliliter. In a subset of the participating centers, 99% of the samples were within reference quality levels.26 Routine patient care was performed according to national and international quality of care guidelines.
At baseline, information on demography, history of cardiovascular disease, diabetes mellitus, medication, type of vascular access, and duration of dialysis (dialysis vintage) was assessed. At each quarterly visit, information on clinical events, dialysis treatment, medication, and laboratory values was recorded. History of cardiovascular disease was defined as a confirmative answer on any of the questions regarding a previous acute myocardial infarction, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, angina pectoris, stroke, transient ischemic attack, intermittent claudication, amputation, percutaneous transluminal angioplasty, peripheral bypass surgery, and renal percutaneous transluminal coronary angioplasty. In addition, blood flow rate, intradialytic weight loss, treatment time, infusion volume, and predialysis BP were assessed. In hemodiafiltration patients, infusion volumes (liters per treatment) were reported as the mean value of three consecutive treatment sessions preceding the quarterly visit. Convection volumes (liters per treatment) were calculated as the sum of the intradialytic weight loss and the substitution volume per session. For calculation of the convection volume in the on-treatment analysis, at every quarterly visit, the actual number of hemodiafiltration treatments in the past 3 months was assessed. Patients could miss hemodiafiltration treatments (and then be treated with high-flux hemodialysis) due to technical problems with the water installation or vacation schedules. The mean delivered convection volume during the trial was estimated with the following formula: mean delivered convection volume = (hemodiafiltration treatments/total number of treatments) × mean convection volume of the three treatments preceding the quarterly visit.
Every 3 months, blood samples were drawn before dialysis (the day was chosen according to local schemes), and when appropriate, after the session. Serum β-2-microglobulin (molecular mass of 11.8 kD) was assessed at baseline and every 6 months thereafter. All samples were analyzed in the local laboratories of the participating hospitals by standard laboratory techniques. Interdialytic urinary samples were collected during each quarterly visit in patients with a urinary production ≥100 ml/d. In these patients, residual kidney function was expressed as the estimated GFR (eGFR), calculated by the mean of 24-hour urinary creatinine and urea clearances and adjusted for body surface area (milliliters per minute per 1.73 m2). The plasma concentrations used for this calculation were the mean of the values before and after dialysis. The eGFR was considered zero in patients with a urinary production <100 ml/d. Dialysis adequacy was expressed as spKt/Vurea, calculated with the second-generation Daugirdas formula.27
Outcomes
The primary study outcome was all-cause mortality. Deaths were reported within 24 hours to the data management center by fax or email. The main secondary endpoint was a composite of fatal and nonfatal cardiovascular events. Cardiovascular events were defined as death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, therapeutic coronary procedure (coronary artery bypass graft, percutaneous transluminal coronary angioplasty, and/or stenting), therapeutic carotid procedure (endarterectomy and/or stenting), and vascular intervention (revascularization, percutaneous transluminal angioplasty, and/or stenting), or amputation. Congestive heart failure was not considered as a cardiovascular event and hence was not formally evaluated by the event adjudication committee because the distinction with fluid overload is often difficult to make in patients with ESRD. Hospitalizations, duration of the hospitalizations, and main diagnosis (including infections) were recorded during the study period.
After participants stopped randomized treatment due to renal transplant (n=151), switch to peritoneal dialysis (n=11), move to another (non-CONTRAST) hospital (n=24), or other reasons (n=53), they continued to be followed for the primary and main secondary outcome, being death and (non)fatal cardiovascular events. These patients were not followed for the occurrence of noncardiovascular events such as hospitalization due to infection.
Interim Analyses and Data Monitoring
An independent endpoint adjudication committee, whose members were not aware of the treatment assignments, reviewed source documentation for all primary outcome events (deaths), as well as nonfatal cardiovascular events and infections.
A separate, independent data and safety monitoring board, evaluated the results of sequential interim analyses. Every 2 months, sequential unblinded analyses were performed on the cumulative database. On the basis of this design, the board decided whether the data provided enough evidence of either efficacy or futility with respect to the primary outcome and sufficient support to formulate recommendations for the executive committee to continue the trial. Guidelines for early discontinuation were previously published.25 In December 2010, the board recommended to stop the trial because enough evidence was provided for futility (i.e., no clear efficacy of hemodiafiltration over hemodialysis).
Statistical Analyses
The CONTRAST study was designed to have 80% statistical power to detect a relative risk reduction of ≥20% for online hemodiafiltration compared with low-flux hemodialysis for the primary outcome, with a two-sided α level of 5%. A 3-year incidence of death from any cause of 44% was expected in hemodialysis patients and a relative risk reduction of ≥20% by hemodiafiltration was assumed. The primary outcome was time to death, and a relative risk reduction was translated to a HR of 0.75, which is equal to the ratio of the logarithm of the expected cumulative survival proportion under hemodiafiltration (1 – 0.352 = 0.648) and the logarithm of the expected cumulative survival proportion under hemodialysis (1 – 0.44 = 0.56). For a sequential design, no fixed sample size estimate can be provided. Approximately 772 patients needed to be enrolled and followed for about 3 years. To reach a decision, approximately 250 endpoints were expected in these patients. A double sequential triangular test as proposed by Whitehead28 was used to monitor accumulating data and to test the primary hypothesis.
Several analyses were performed. First, all primary analyses were conducted according to the intention-to-treat principle. Data for patients were censored at their date of death, date of last visit (for those still alive at the end of the follow-up at December 31, 2010), or date when last known to be alive (for those with unknown vital status). The primary endpoint was analyzed cumulatively by sequential analysis28 using the PEST program (version 4.4).29 Point and interval estimates were adjusted for cumulative testing. Effects of treatment on secondary study endpoints were estimated with the use of unadjusted Cox proportional hazards models, involving the time to the first relevant endpoint in any individual patient. Second, homogeneity of treatment effects across subgroups was tested by adding interaction terms to the relevant Cox models. Third, to study differences in continuous and dichotomous variables between the two treatment arms during the follow-up period, we applied linear mixed models (generalized estimating equation [GEE]). The main assumption of the GEE approach is that measurements are assumed to be dependent within participants and independent between participants. The correlation matrix representing the within-patient dependencies was estimated using an autoregressive relationship.30 For these analyses, the interest was in the mean difference over time in risk factor levels between treatment arms. GEE analyses were performed using the on-trial measurements adjusted for the level of the variable of interest at baseline. Finally, based on the existing literature, we explored whether the treatment effect depended on the average amount of convection volume that was delivered to the hemodiafiltration patients during the trial. For this purpose, the actual (on-treatment) delivered convection volume was divided in tertiles, which were introduced in a Cox proportional hazards model as dummy variables. Patients who were treated by low-flux hemodialysis served as the reference group (no convection volume). In this post hoc analysis, elaborate adjustments were made for factors that have previously been related to convection volume (age, sex, albumin, and hematocrit),18 and for potential determinants of mortality. Furthermore, to account for potential differences between study facilities, center adjustments were made.
All P values were two sided, and P values <0.05 were considered significant. No adjustment for multiple statistical testing was made.31 Analyses were performed with SPSS 17 software (SPSS Inc, Chicago, IL).
Disclosures
M.P.C.G. reports research funded by Fresenius, Gambro, and Baxter. M.A.v.d.D. reports research funded by Amgen. R.L. reports research funded by Amgen Canada. M.J.N. reports research funded by Baxter and Fresenius as well as honoraria received from Fresenius and Baxter for lectures. P.M.t.W. reports research funded by Abbott, Baxter, Gambro, Fresenius, and Roche as well as honoraria received from Amgen, Roche, Genzyme, and Fresenius for lectures. P.J.B. reports research funded by Fresenius, Gambro, Roche, Amgen, and Novartis, as well as consultant fees and honoraria for lectures from Fresenius, Gambro, Solvay, Medtronic, and Novartis.
Supplementary Material
Acknowledgments
This study was supported by a grant from the Dutch Kidney Foundation (Nierstichting Nederland Grant C02.2019), and unrestricted grants from Fresenius Medical Care, Netherlands, and Gambro Lundia AB, Sweden. Additional support was received from the Dr. E.E. Twiss Fund, Roche Netherlands, the International Society of Nephrology/Baxter Extramural Grant Program, and the Netherlands Organization for Health Research and Development (ZONMw Grant 170882802).
The following committee members and investigators participated in the CONTRAST study. Executive Committee. University Medical Center Utrecht: P.J. Blankestijn (co-chair); VU University Medical Center, Amsterdam: M.P.C. Grooteman, M.J. Nubé, P.M. ter Wee (co- chair); Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht: M.L. Bots; and Maasstad Hospital, Rotterdam: M.A. van den Dorpel. Research Physicians. E.L. Penne, N.C. van der Weerd, A.H.A. Mazairac, and C.H. den Hoedt. Endpoints Committee. Medical Center Alkmaar, Alkmaar: A.E.R. Arnold, W. Bronsveld, F. Stam; University Medical Center Utrecht: W.H. Boer, P.A. Doevendans, L.J. Kappelle, F.L.J. Visseren; VU University Medical Center, Amsterdam: A.J. Kooter, Y.M. Smulders, M.C. Visser, G. Veen; and College voor Zorgverzekeringen, Diemen: G. Ligtenberg. Data and Safety Monitoring Board. Julius Center for Health Sciences and Primary Care, Utrecht: I. van der Tweel; Leiden University Medical Center, Leiden: T.J. Rabelink; and Maastricht University Medical Center, Maastricht: C.D.A. Stehouwer. Investigators. Canada: Georges-L. Dumont Regional Hospital, Moncton: M. Dorval; CHUM St. Luc Hospital, Montréal: R. Lévesque. The Netherlands: Academic Medical Center, Amsterdam: M.G. Koopman; Catharina Hospital, Eindhoven: C.J.A.M. Konings; Dialysis Clinic Noord, Beilen: W.P. Haanstra; Dianet Dialysis Centers, Utrecht: M. Kooistra and B. van Jaarsveld; Fransiscus Hospital, Roosendaal: T. Noordzij; Gelderse Vallei Hospital, Ede: G.W. Feith; Groene Hart Hospital, Gouda: H.G. Peltenburg; Haga Hospital, The Hague: M. van Buren; Isala Clinics, Zwolle: J.J.G. Offerman; Jeroen Bosch Hospital, Hertogenbosch: E.K. Hoogeveen; Maasland Hospital, Sittard: F. de Heer; Maasstad Hospital, Rotterdam: P.J. van de Ven; Martini Hospital, Groningen: T.K. Kremer Hovinga; Medical Center Alkmaar: W.A. Bax; Onze Lieve Vrouwe Gasthuis, Amsterdam: J.O. Groeneveld; Oosterschelde Hospital, Goes: A.T.J. Lavrijssen; Rijnland Hospital, Leiderdorp: A.M. Schrander-Van der Meer; Rijnstate Hospital, Arnhem: L.J.M. Reichert; Slingeland Hospital, Doetinchem: J. Huussen; St. Elisabeth Hospital, Tilburg: P.L. Rensma; St. Fransiscus Gasthuis, Rotterdam: Y. Schrama; University Medical Center St. Radboud, Nijmegen: H.W. van Hamersvelt; University Medical Center Utrecht, Utrecht: W.H. Boer; VieCuri Medical Center, Venlo: W.H. van Kuijk; VU University Medical Center, Amsterdam: M.G. Vervloet; Zeeuws-Vlaanderen Hospital, Terneuzen: I.M.P.M.J. Wauters. Norway: Haukeland University Hospital, Bergen: I. Sekse.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “On-Line Hemodiafiltration: Not a Self-Fulfilling Prophecy,” on pages 974–975.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2011121140/-/DCSupplemental.
References
- 1.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R, Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R, Membrane Permeability Outcome (MPO) Study Group : Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ledebo I, Blankestijn PJ: Haemodiafiltration-optimal efficiency and safety. NDT Plus 3: 8–16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CL, Yang CW, Chiang CC, Chang CT, Huang CC: Long-term on-line hemodiafiltration reduces predialysis beta-2-microglobulin levels in chronic hemodialysis patients. Blood Purif 19: 301–307, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Lornoy W, Becaus I, Billiouw JM, Sierens L, Van Malderen P, D’Haenens P: On-line haemodiafiltration. Remarkable removal of beta2-microglobulin. Long-term clinical observations. Nephrol Dial Transplant 15[Suppl 1]: 49–54, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Pedrini LA, De Cristofaro V, Comelli M, Casino FG, Prencipe M, Baroni A, Campolo G, Manzoni C, Colì L, Ruggiero P, Acquistapace I, Auriemma L: Long-term effects of high-efficiency on-line haemodiafiltration on uraemic toxicity. A multicentre prospective randomized study [published online ahead of print. Nephrol Dial Transplant 26: 2617–2624, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Penne EL, van der Weerd NC, Blankestijn PJ, van den Dorpel MA, Grooteman MP, Nubé MJ, Ter Wee PM, Lévesque R, Bots ML, CONTRAST investigators : Role of residual kidney function and convective volume on change in beta2-microglobulin levels in hemodiafiltration patients. Clin J Am Soc Nephrol 5: 80–86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canaud B, Bragg-Gresham JL, Marshall MR, Desmeules S, Gillespie BW, Depner T, Klassen P, Port FK: Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int 69: 2087–2093, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Jirka T, Cesare S, Di Benedetto A, Perera Chang M, Ponce P, Richards N, Tetta C, Vaslaky L: Mortality risk for patients receiving hemodiafiltration versus hemodialysis. Kidney Int 70: 1524–1525, author reply 1524–1525, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, Rindi P, Donati G, Antonelli A, Panicucci E, Tripepi G, Tetta C, Palla R, RISCAVID Study Group : Chronic inflammation and mortality in haemodialysis: Effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant 23: 2337–2343, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Vilar E, Fry AC, Wellsted D, Tattersall JE, Greenwood RN, Farrington K: Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: A comparative analysis. Clin J Am Soc Nephrol 4: 1944–1953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Weerd NC, Penne EL, van den Dorpel MA, Grooteman MP, Nube MJ, Bots ML, ter Wee PM, Blankestijn PJ: Haemodiafiltration: Promise for the future? Nephrol Dial Transplant 23: 438–443, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Cheung AK, Levin NW, Greene T, Agodoa L, Bailey J, Beck G, Clark W, Levey AS, Leypoldt JK, Ornt DB, Rocco MV, Schulman G, Schwab S, Teehan B, Eknoyan G: Effects of high-flux hemodialysis on clinical outcomes:Results of the HEMO study. J Am Soc Nephrol 14: 3251–3263, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Morena M, Cristol JP, Bosc JY, Tetta C, Forret G, Leger CL, Delcourt C, Papoz L, Descomps B, Canaud B: Convective and diffusive losses of vitamin C during haemodiafiltration session: A contributive factor to oxidative stress in haemodialysis patients. Nephrol Dial Transplant 17: 422–427, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Gritters-van den Oever M, Grooteman MP, Bartels PC, Blankestijn PJ, Bots ML, van den Dorpel MA, Schoorl M, Schoorl M, Ter Wee PM, Nubé MJ: Post-dilution haemodiafiltration and low-flux haemodialysis have dissimilar effects on platelets: A side study of CONTRAST. Nephrol Dial Transplant 24: 3461–3468, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Hoenich NA, Levin R, Ronco C: How do changes in water quality and dialysate composition affect clinical outcomes? Blood Purif 27: 11–15, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Penne EL, van Berkel T, van der Weerd NC, Grooteman MP, Blankestijn PJ: Optimizing haemodiafiltration: Tools, strategy and remaining questions. Nephrol Dial Transplant 24: 3579–3581, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Penne EL, van der Weerd NC, Bots ML, van den Dorpel MA, Grooteman MP, Lévesque R, Nubé MJ, Ter Wee PM, Blankestijn PJ, CONTRAST investigators : Patient- and treatment-related determinants of convective volume in post-dilution haemodiafiltration in clinical practice. Nephrol Dial Transplant 24: 3493–3499, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Locatelli F, Altieri P, Andrulli S, Bolasco P, Sau G, Pedrini LA, Basile C, David S, Feriani M, Montagna G, Di Iorio BR, Memoli B, Cravero R, Battaglia G, Zoccali C: Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol 21: 1798–1807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffl H: Prospective randomized cross-over long-term comparison of online haemodiafiltration and ultrapure high-flux haemodialysis. Eur J Med Res 12: 26–33, 2007 [PubMed] [Google Scholar]
- 21.Tiranathanagul K, Praditpornsilpa K, Katavetin P, Srisawat N, Townamchai N, Susantitaphong P, Tungsanga K, Eiam-Ong S: On-line hemodiafiltration in Southeast Asia: A three-year prospective study of a single center. Ther Apher Dial 13: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Tattersall JE, Martin-Malo A, Pedrini L, Basci A, Canaud B, Fouque D, Haage P, Konner K, Kooman J, Pizzarelli F, Tordoir JH, Vennegoor M, Wanner C, ter Wee PM, Vanholder R: EBPG guideline on dialysis strategies. Nephrol Dial Transplant 22[Suppl 2]: ii5–ii21, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Canaud B, Morena M, Leray-Moragues H, Chalabi L, Cristol JP: Overview of clinical studies in hemodiafiltration: What do we need now? Hemodial Int 10[Suppl 1]: S5–S12, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Foraster A, Soler J, Galceran JM, Martinez-Castelao A, Online Hemodiafiltration Study Group from the Catalonian Society of Nephrology : Design and patient characteristics of ESHOL study, a Catalonian prospective randomized study. J Nephrol 24: 196–202, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Penne EL, Blankestijn PJ, Bots ML, van den Dorpel MA, Grooteman MP, Nubé MJ, van der Tweel I, Ter Wee PM, the CONTRAST study group : Effect of increased convective clearance by on-line hemodiafiltration on all cause and cardiovascular mortality in chronic hemodialysis patients - the Dutch CONvective TRAnsport STudy (CONTRAST): Rationale and design of a randomised controlled trial [ISRCTN38365125]. Curr Control Trials Cardiovasc Med 6: 8, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penne EL, Visser L, van den Dorpel MA, van der Weerd NC, Mazairac AH, van Jaarsveld BC, Koopman MG, Vos P, Feith GW, Kremer Hovinga TK, van Hamersvelt HW, Wauters IM, Bots ML, Nubé MJ, Ter Wee PM, Blankestijn PJ, Grooteman MP: Microbiological quality and quality control of purified water and ultrapure dialysis fluids for online hemodiafiltration in routine clinical practice. Kidney Int 76: 665–672, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Whitehead J: The Design and Analysis of Sequential Clinical Trials, John Wiley & Sons Ltd, Chichester, UK, 1997 [Google Scholar]
- 29.MPS Research Unit: PEST 4: Operating Manual, University of Reading, Reading, UK, 2000 [Google Scholar]
- 30.Twisk J, de Vente W: Attrition in longitudinal studies. How to deal with missing data. J Clin Epidemiol 55: 329–337, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Schulz KF, Grimes DA: Multiplicity in randomised trials I: Endpoints and treatments. Lancet 365: 1591–1595, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.