Abstract
The clinical benefits of using “biocompatible” neutral pH solutions containing low levels of glucose degradation products for peritoneal dialysis compared with standard solutions are uncertain. In this multicenter, open-label, parallel-group, randomized controlled trial, we randomly assigned 185 incident adult peritoneal dialysis patients with residual renal function to use either biocompatible or conventional solution for 2 years. The primary outcome measure was slope of renal function decline. Secondary outcome measures comprised time to anuria, fluid volume status, peritonitis-free survival, technique survival, patient survival, and adverse events. We did not detect a statistically significant difference in the rate of decline of renal function between the two groups as measured by the slopes of GFR: −0.22 and −0.28 ml/min per 1.73 m2 per month (P=0.17) in the first year in the biocompatible and conventional groups, respectively, and, −0.09 and −0.10 ml/min per 1.73 m2 per month (P=0.9) in the second year. The biocompatible group exhibited significantly longer times to anuria (P=0.009) and to the first peritonitis episode (P=0.01). This group also had fewer patients develop peritonitis (30% versus 49%) and had lower rates of peritonitis (0.30 versus 0.49 episodes per year, P=0.01). In conclusion, this trial does not support a role for biocompatible fluid in slowing the rate of GFR decline, but it does suggest that biocompatible fluid may delay the onset of anuria and reduce the incidence of peritonitis compared with conventional fluid in peritoneal dialysis.
During the past 30 years, peritoneal dialysis (PD) has become an established form of renal replacement therapy for patients with ESRD.1 It is estimated that the number of patients currently receiving this therapy globally is approximately 200,000 and is increasing by >6% per annum.2
A key determinant of patient survival on PD is residual renal function.3,4 Numerous studies have shown that residual renal function declines more slowly in PD compared with hemodialysis5 and that this factor may account for the observed early survival advantage of PD in some studies.6,7 Recent evidence has also suggested that newer “biocompatible” PD fluids, which are neutral pH and low in potentially nephrotoxic glucose degradation products (GDPs), may be superior to conventional PD solutions for preserving residual renal function, thereby leading to improved clinical outcomes. A randomized crossover trial of conventional, acidic, lactate-buffered fluid with pH neutral, lactate-buffered, low GDP fluid in 86 prevalent PD patients demonstrated increases in renal urea and creatinine clearances over a 12-week period.8 Subsequently, a large, retrospective, observational cohort study in Korea9,10 demonstrated an association between the use of neutral pH, lactate-buffered, low GDP fluids and superior survival, although this finding was potentially limited by indication bias with residual confounding.11 Several small, short-term, randomized controlled trials have also reported that neutral pH, low GDP fluids were associated with either a beneficial12–14 or neutral15–19 effect on residual renal function. However, these studies were limited by insufficient statistical power, short-term follow-up, high drop-out rates, treatment-associated changes in fluid status and often poor methodologic quality, enrollment of prevalent PD patients, and single-center designs.
The aim of this multicenter, multicountry randomized controlled trial was to determine whether neutral pH, low GDP dialysate better preserves residual renal function in PD patients over a 2-year period compared with conventional dialysate.
Results
Patient Characteristics
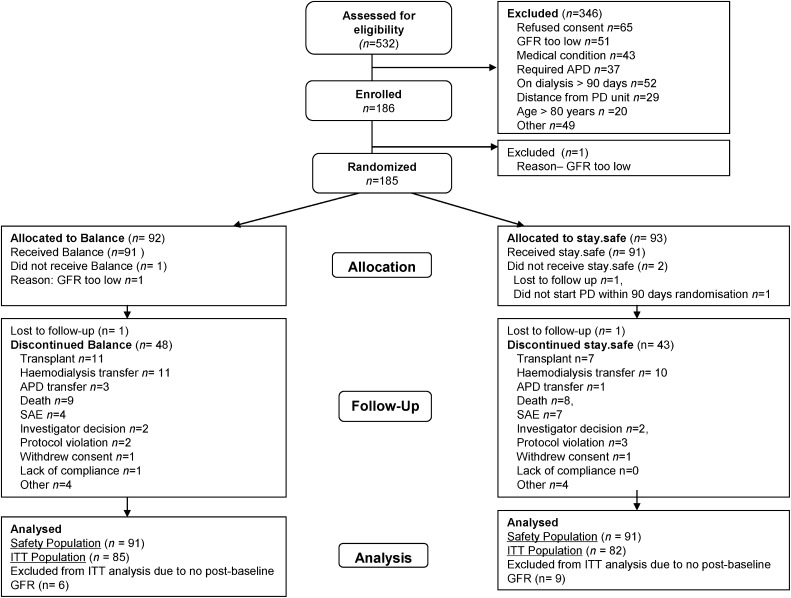
A total of 185 patients were randomized to receive either biocompatible (n=92) or control fluid (n=93) (Figure 1). The two groups were well matched for all baseline characteristics (Table 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram showing the number of patients recruited into the balANZ study, randomized, followed, and analyzed. APD, automated PD; SAE, serious adverse event; ITT, intention to treat.
Table 1.
Baseline characteristics of the balANZ trial participants
| Characteristic | Biocompatible (n=91) | Control (n=91) |
|---|---|---|
| Age (yr) | 59.3±14.20 | 57.9±14.72 |
| Female | 39 (42.9) | 43 (47.3) |
| Ethnicity | ||
| Caucasian | 77 (85) | 69 (76) |
| Aboriginal and Torres Strait Islander | 0 (0) | 2 (2) |
| Asian | 10 (11) | 13 (14) |
| Maori and Pacific Islander | 4 (4) | 7 (7) |
| Cardiovascular disease | 70 (76.1) | 71 (81.6) |
| Diabetic nephropathy | 30 (33.0) | 31 (34.1) |
| Medications | ||
| angiotensin converting enzyme inhibitor | 40 (44.0) | 41 (45.1) |
| angiotensin receptor blocker | 25 (27.5) | 29 (31.9) |
| β-blocker | 45 (49.5) | 51 (56.0) |
| statin | 67 (73.6) | 61 (67.0) |
| aspirin | 40 (44.0) | 46 (50.5) |
| nonsteroidal anti-inflammatory drug | 4 (4.4) | 2 (2.2) |
| anticoagulants | 16 (17.6) | 18 (19.8) |
| diuretics | 40 (44.0) | 46 (50.5) |
| exit-site mupirocin | 29 (31.9) | 36 (39.6) |
| exit-site gentamicin | 0 (0) | 0 (0) |
| Body mass index (kg/m2) | 27.7±5.02 | 28.4±6.16 |
| Systolic BP (mmHg) | ||
| supine | 139.8±21.4 | 138.9±21.8 |
| standing | 134.2±20.7 | 133.3±23.4 |
| Diastolic BP (mmHg) | ||
| supine | 76.6±11.3 | 78.1±11.0 |
| standing | 76.7±11.9 | 78.0±12.7 |
| Hemodialysis before PD | 13 (14.3) | 4 (4.4) |
| Initial PD modality | ||
| continuous ambulatory PD | 81 (89.0) | 82 (90.1) |
| automated PD | 10 (11.0) | 9 (9.9) |
| Prescribed dialysate volume (L/d) | 8 (2–10) | 8 (2–8.7) |
| Dialysate glucose exposure (g/d) | 122±35 | 124±36 |
| GFR (ml/min per 1.73 m2) | 7 (3–18) | 7 (3–18) |
| Urine volume (mL/d) | 1495 (379–3525) | 1365 (455–3359) |
| Weekly peritoneal urea clearance (L/wk per 1.73 m2) | 51.1±10.4 | 52.6±14.5 |
| Weekly peritoneal creatinine clearance (L/wk per 1.73 m2) | 38.3±9.0 | 36.3±11.9 |
| Peritoneal ultrafiltration (ml/d) | 700 (−700 to 3500) | 1090 (−400 to 2800) |
| Dialysate/plasma creatinine ratio at 4 hours (1 mo) | 0.67±0.10 | 0.62±0.10 |
| Normalized protein nitrogen appearance (g/kg/d) | 1.05±0.25 | 1.06±0.26 |
| Serum albumin (g/L) | 37.9±4.8 | 36.9±5.7 |
| Serum total calcium corrected (mmol/L) | 2.4±0.2 | 2.4±0.3 |
| Hemoglobin (g/L) | 115±17 | 115±17 |
Results are presented as number (%), mean ± SD, or median (range).
Residual Renal Function Decline
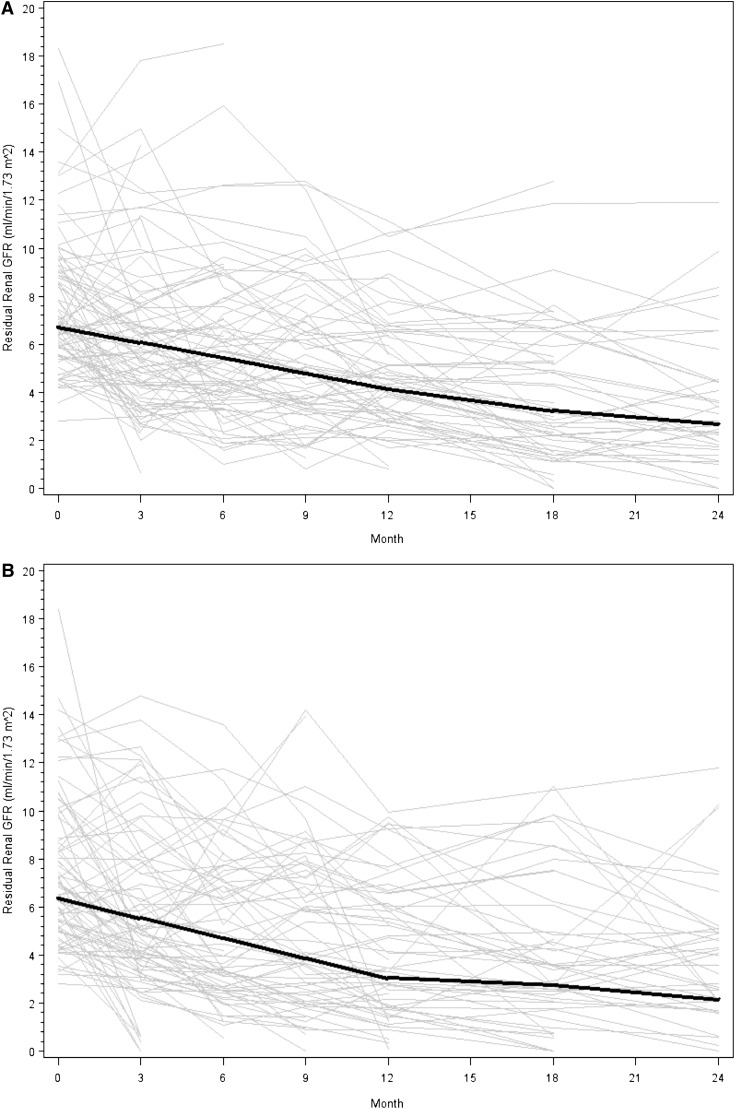
Participants in the biocompatible group experienced a nonsignificant slower decline of residual renal function than those in the control group, particularly in the first year. The mean slopes of GFR decline in the two groups were −0.22 and −0.28 ml/min per 1.73 m2 per month, respectively, in the first year (0.06 difference; 95% confidence interval [95% CI], −0.05 to 0.17; P=0.17) and −0.09 and −0.10 ml/min per 1.73 m2 per month in the second year (0.01 difference; 95% CI, −0.18 to 0.20; P=0.9). The differences in residual renal function decline between treatments across the two 12-month periods were not statistically significant (P=0.06; Figure 2). When the curvature of GFR decline was alternatively modeled by analyzing log-transformed data over the entire study period, the log scale trends for the biocompatible and control groups were −0.016 and −0.021, respectively (P=0.11). Similar results were found when the linear trend over 2 years was expanded to include a quadratic trend (0.005 versus 0.008; P=0.15). Use of angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs did not differ significantly between the two groups during the study (Table 2). Three patients in each group received radiographic contrast.
Figure 2.
Nonsignificant difference in residual renal function decline over 2 years in the biocompatible and control groups. (A) The biocompatible group. (B) Controls. Gray lines represent individual patient measurements, whereas solid lines represent predicted slopes in the first and second 12-month follow-up periods. The P value for the three-way interaction of year (first 12 months versus second 12 months) by GFR slope by dialysate was 0.06.
Table 2.
Surrogate indices of fluid and nutritional status and selected medication usage in balANZ trial participants over a 2-year period
| Variable | 0 mo | 3 mo | 6 mo | 12 mo | 18 mo | 24 mo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Balance | Stay.safe | Balance | Stay.safe | Balance | Stay.safe | Balance | Stay.safe | Balance | Stay.safe | Balance | Stay.safe | |
| (n=91) | (n=91) | (n=85) | (n =80) | (n=76) | (n=75) | (n=62) | (n=66) | (n=53) | (n=59) | (n=42) | (n=48) | |
| Systolic BP (mmHg), supine | 139.8±21.4 | 138.9±21.8 | 134.9±20.0 | 134.8±21.6 | 133.6±19.7 | 131.0±22.5 | 131.7±20.8 | 130.8±22.3 | 132.4±18.7 | 132.9±22.3 | 127.0±16.3a | 137.8±24.2 |
| Diastolic BP (mmHg), supine | 76.6±11.3 | 78.1±11.0 | 76.6±12.7 | 76.6±13.5 | 75.1±13.2 | 74.5±14.4 | 75.2±13.3 | 74.3±10.5 | 74.2±12.2 | 75.2±12.7 | 72.3±12.4 | 75.4±13.8 |
| MAP (mmHg), supine | 96.7±12.4 | 97.4±12.5 | 95.1±13.1 | 95.1±14.1 | 93.6±13.4 | 92.4±15.3 | 93.1±13.7 | 92.2±13.0 | 92.7±12.4 | 93.5±13.9 | 89.6±11.8 | 95.3±15.4 |
| Actual weight (kg) | 76.3±16.5 | 76.8±18.9 | 77.8±17.3 | 76.8±18.1 | 79.3±17.9 | 78.3±18.6 | 81.9±18.3 | 79.4±19.1 | 84.1±18.7 | 80.8±19.2 | 83.2±19.8 | 82.1±19.0 |
| Physician-assessed dry weight (kg) | 76.2±16.6 | 75.6±18.2 | 77.7±17.3 | 77.0±17.8 | 79.4±18.0 | 78.1±18.6 | 81.9±18.3 | 79.9±18.8 | 84.4±18.7 | 80.8±19.5 | 83.5±20.1 | 82.1±19.0 |
| Hemoglobin (g/L) | 115±17 | 115±17 | 118±16 | 122±16 | 122±15 | 121±17 | 120±15 | 118±15 | 117±12 | 117±13 | 116±14 | 116±13 |
| Serum albumin (g/L) | 38±5 | 37±6 | 34±5 | 35±5 | 35±8 | 35±5 | 34±4 | 35±5 | 34±5 | 35±5 | 32±5a | 34±5 |
| Serum sodium (mmol/L) | 140±3 | 139±3 | 139±3 | 139±3 | 138±4 | 138±4 | 138±4 | 138±4 | 138±3 | 138±4 | 137±4 | 138±3 |
| Urine volume (ml/d) | 1556±691 | 1501±682 | 1228±674a | 991±685 | 1150±614a | 923±700 | 1081±656 | 731±573 | 957±822 | 754±743 | 814±624 | 699±639 |
| 24-h dialysate peritoneal ultrafiltration (ml/d) | ND | ND | 763±724a | 1105±625 | 811±588a | 1107±856 | 943±687 | 1196±748 | 1024±632 | 1002±873 | 1070±689 | 1009±762 |
| 24-h dialysate fill volume (ml/d) | 7198±1598 | 7137±1591 | 7506±1198 | 7758±1727 | 7676±1573 | 8228±2204 | 8036±1461 | 8602±1927 | 8503±1593 | 8623±1885 | 8569±1956 | 8928±2125 |
| Daily prescribed glucose exposure (g/d) | 121.5±35.3 | 123.6±36.3 | 142.1±43.5 | 133.9±36.9 | 143.7±40.5 | 140.5±43.5 | 150.0±41.0 | 150.9±48.5 | 158.4±49.2 | 150.2±50.5 | 161.7±54.1 | 157.4±52.3 |
| Urinary creatinine excretion (mmol/d) | 8.1±3.0 | 7.8±3.5 | 6.8±3.2 | 6.4±3.8 | 6.5±2.8 | 5.9±3.0 | 6.2±2.8 | 5.3±3.3 | 5.5±3.2 | 5.3±3.6 | 5.0±3.0 | 5.1±3.4 |
| Normalized protein nitrogen appearance (g/kg body wt per d) | ND | ND | ND | ND | 1.05±0.35 | 1.06±0.26 | 1.00±0.21 | 1.04±0.33 | 0.99±0.22 | 1.02±0.28 | 1.02±0.31 | 1.02±0.24 |
| ACE inhibitor | 40 (44) | 45 (45) | 34 (40) | 33 (41) | 30 (39) | 29 (39) | 23 (37) | 27 (41) | 19 (36) | 24 (41) | 14 (33) | 20 (42) |
| ARB | 25 (28) | 29 (32) | 24 (48) | 21 (26) | 21 (28) | 17 (23) | 19 (31) | 15 (23) | 15 (28) | 13 (22) | 12 (29) | 10 (21) |
| NSAID | 4 (4) | 2 (2) | 2 (2) | 1 (1) | 2 (3) | 1 (1) | 2 (3) | 1 (2) | 4 (8) | 1 (2) | 3 (7) | 0 (0) |
Results are presented as number (%), mean ± SD, or median (range). MAP, mean arterial pressure; ND, not done; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; NSAID, nonsteroidal anti-inflammatory drug.
P<0.05 versus stay.safe.
Time to Anuria
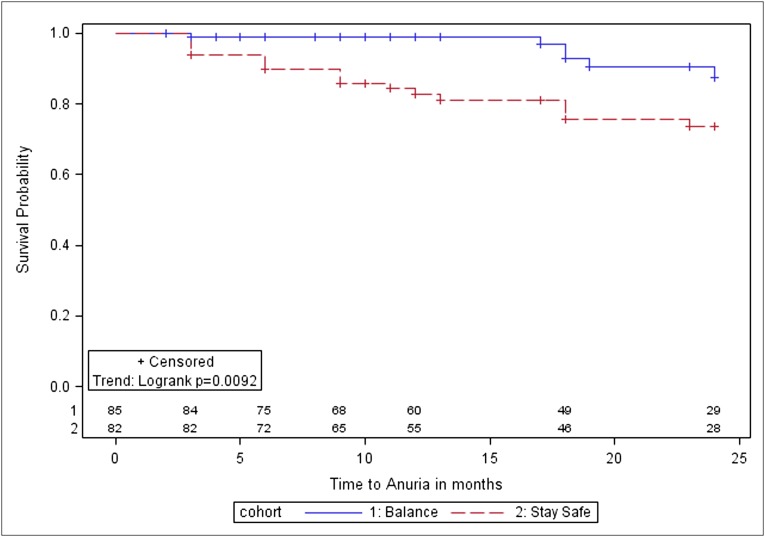
Six patients (7%) in the biocompatible group became anuric compared with 18 (20%) in the control group. Time to anuria was significantly longer in the biocompatible group (P=0.01; Figure 3). After adjustment for diabetic nephropathy, baseline GFR, and dialysis modality (automated versus continuous ambulatory), biocompatible fluid use was associated with a lower hazard of anuria (adjusted hazard ratio [HR], 0.36; 95% CI, 0.13–0.96).
Figure 3.
Kaplan–Meier survival analysis for time to anuria in balANZ trial participants over the 2-year study period. The differences between the biocompatible and control groups were statistically significant (P=0.009). Numbers at risk are indicated above the abscissa.
Indices of Fluid Status
Systolic and diastolic BP, mean arterial pressure, actual weight, serum sodium, serum albumin, and hemoglobin were comparable (and not statistically significantly different) at all time points between the two groups, except for significantly lower BPs and serum albumin levels in the biocompatible group at 24 months (Table 2). Peritoneal ultrafiltration and urine volumes were comparable between the two groups, except at 3 and 6 months when patients in the biocompatible group had significantly lower peritoneal ultrafiltration and higher urine volumes (Table 2). No significant differences were observed between the two groups with respect to prescribed fill volumes, dialysate glucose exposure, or nutritional indices (physician-assessed dry weight, serum albumin, creatinine excretion, or normalized protein nitrogen appearance). Icodextrin use was comparable between the biocompatible and control groups throughout the study: baseline (0 versus 1, respectively), 3 months (1 versus 1), 6 months (3 versus 2), 12 months (3 versus 4), 18 months (5 versus 5), and 24 months (8 versus 8).
Time to First Peritonitis Episode
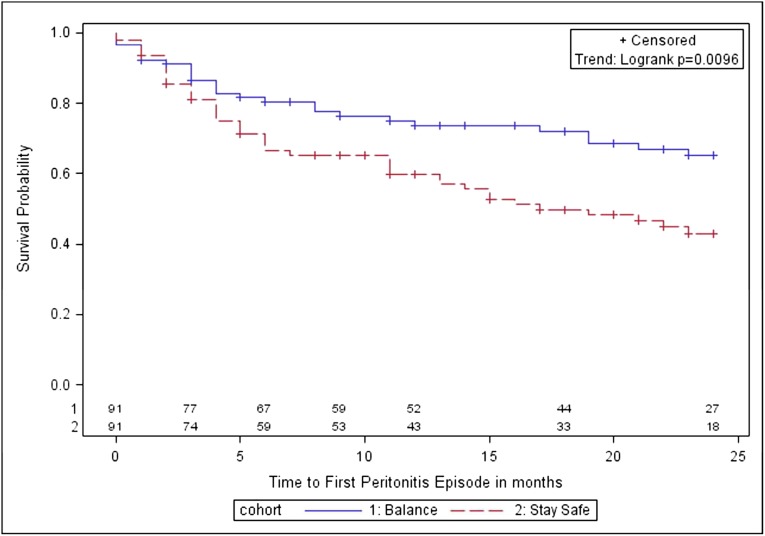
The number of patients experiencing peritonitis was 27 (30%; 95% CI, 20%–40%) in the biocompatible group and 45 (49%; 95% CI, 39%–59%) in the control group (P=0.006). Compared with controls, the biocompatible group had a significantly longer time to first peritonitis episode (P=0.01; Figure 4) and a significantly lower overall peritonitis rate (0.30 versus 0.49 episodes per patient-year; P=0.01). Using negative binomial regression, the incidence rate ratio for peritonitis in the biocompatible group was 0.64 (95% CI, 0.42–0.98), after adjustment for age, sex, body mass index, diabetes, cardiovascular disease, baseline renal function, and peritoneal transport status.
Figure 4.
Kaplan–Meier survival analysis for time to first peritonitis episode in balANZ trial participants over the 2-year study period. The difference between the biocompatible and control groups was statistically significant (P=0.01). Numbers at risk are indicated above the abscissa.
Technique Survival
Sixteen patients (18%) in the biocompatible group transferred to hemodialysis due to peritonitis (n=5), catheter tunnel infection (n=2), catheter malfunction (n=1), hernia (n=1), dialysate leaks (n=2), inadequate solute clearance (n=2), social reasons (n=2), and other causes (n=1). Sixteen patients (18%) in the control group transferred to hemodialysis due to peritonitis (n=5), hernia (n=3), dialysate leaks (n=2), inadequate solute clearance (n=2), and social reasons (n=4). Kaplan–Meier analysis showed that technique survival was not significantly different between the two groups (P=0.85). This finding was not altered by including death as a technique failure (P=0.79).
Patient Survival
Nine patients (10%) in the biocompatible group died of cardiovascular (n=6), infectious (n=1), and other causes (n=2). Eight patients (9%) in the control group died of cardiovascular (n=5), infectious (n=1), and other causes (n=2). Kaplan–Meier analysis showed that patient survival was not significantly different between the two groups (P=0.20).
Adverse Events
Adverse events are shown in Table 3 and were not significantly different between the two groups, except for more frequent occurrence of peritonitis and non-PD–related infections in the control group. Non-PD–related infections included pneumonia (2 episodes in 2 biocompatible patients versus 13 episodes in 9 controls), urosepsis (2 episodes in 1 biocompatible patient versus 5 episodes in 4 controls), viral infection (0 episodes in biocompatible patients versus 3 episodes in 3 controls), septicemia (1 episode in 1 biocompatible patient versus 3 episodes in 3 controls), cellulitis (1 episode in 1 biocompatible patient versus 1 episode in 1 control), and endocarditis (1 episode in 1 biocompatible patient versus 0 episodes in controls).
Table 3.
Adverse events in the balANZ trial
| Event | Biocompatible | Control |
|---|---|---|
| (n=91) | (n=91) | |
| SAEs (n) | 162 | 209 |
| Patients with at least 1 SAE | 67 | 74 |
| Requiring permanent discontinuation of dialysis (i.e., withdrawn due to SAE) | 12 | 13 |
| Dialysate-related SAE | — | — |
| Death | 9 | 8 |
| cardiovascular death | 6 | 5 |
| infectious death | 1 | 1 |
| other | 2 | 2 |
| Infection | ||
| peritonitis | 27 | 45 |
| exit-site infection | 4 | 6 |
| tunnel infection | 1 | 2 |
| non-PD–related infection | 4 | 20 |
| Inadequate dialysis | 1 | 1 |
| Ultrafiltration failure | — | — |
| Hernia | 10 | 11 |
| Peritoneal leak | 1 | 3 |
| Gastrointestinal disorder | 9 | 4 |
| Constipation | 5 | 2 |
| Catheter blockage | 5 | 4 |
| Malposition | 1 | 2 |
| Hepatic disorder | 0 | 1 |
| Fluid overload | 1 | 3 |
| Newly diagnosed cancer | 4 | 3 |
| Hospitalization | ||
| cardiac | 12 | 18 |
| vascular | 13 | 9 |
| other | 24 | 23 |
The serious adverse events (SAEs) row indicates the total number of events, whereas all other rows denote the number of patients experiencing an event.
Discussion
Use of neutral pH, lactate-buffered, low GDP (Balance) solution in PD patients was not associated with a significantly slower decline of residual renal function in the first 12 months (P=0.17) or with a significant difference between the slope differences across the two 12-month periods (P=0.06), but was associated with a significantly delayed onset of anuria compared with conventional (stay.safe) solution (P=0.01). Biocompatible solution was also associated with superior peritonitis-free survival and significantly lower peritonitis rates. These results have important implications for the practice of nephrology and demonstrate that newer, biocompatible solutions confer clinically important benefits to PD patients.
Previous studies yielded conflicting results regarding the effect of biocompatible PD solutions on residual renal and peritoneal membrane function, leading to cautious guideline suggestions by the International Society of Peritoneal Dialysis.20,21 However, these studies have had significant limitations. In the EuroBalance trial,8 Balance solution was associated with significant increases in renal urea and creatinine clearances over 12 weeks in 86 prevalent PD patients compared with conventional solution. However, this increase in renal function was accompanied by increases in urine volume and reciprocal decreases in peritoneal ultrafiltration, raising the possibility that renal function was improved by augmented plasma volume. Moreover, the use of a crossover study design to evaluate renal function was problematic, given the nonlinear decline of residual renal function over time.22,23 Similar difficulties applied to a randomized crossover trial of bicarbonate-buffered dialysate versus conventional dialysate in 55 prevalent PD patients over 12 weeks.14 More recently, the BalNet study group12 reported that Balance solution was associated with a nonsignificant slower decline in renal function in 48 incident PD patients in four Korean centers compared with 43 patients receiving stay.safe solution. This difference became statistically significant only after multivariable adjustment for age, sex, comorbidity, and GFR at 1 month. However, peritoneal ultrafiltration fell significantly in the intervention group, despite similar dialysate glucose loads, leading to the possibility of volume-driven renal functional improvement. The DIUREST study group similarly reported a significant attenuation of residual renal function decline in 43 prevalent patients randomly allocated to Gambrosol trio over 18 months compared with 26 patients receiving one of a range of three different conventional PD solutions.13 However, this study was limited by lack of information on peritoneal ultrafiltration volume, by an approximately two-fold higher prescription of angiotensin converting enzyme inhibitors in the intervention group, and by high drop-out rates, particularly in the control group, leading to possible informative censoring.
In contrast, a number of randomized controlled trials have not shown any beneficial effect of biocompatible fluids on residual renal function.15–19 Common features of these trials were that they were underpowered, did not consider nonlinear decline of renal function in their analytic models, and generally enrolled prevalent patients with lower baseline levels of renal function. As with other studies,22,24 we observed that renal function decline over time was nonlinear, was most marked in the first year of PD, and tended to be slower with biocompatible fluid use in this first year (although not statistically significant). A possible renoprotective benefit of biocompatible (Balance) solution was reinforced by the finding of a significantly delayed onset of anuria (an alternative outcome measure of residual renal function decline) in this group.
One potential mechanism underpinning a possible benefit of biocompatible, low GDP fluid on residual renal function is reduced systemic absorption of reactive carbonyls (GDPs) from the peritoneal cavity.25 This could lead to a reduction in the systemic load of advanced glycation end products, which have been shown to exert direct proinflammatory, proapoptotic, and pro-oxidative nephrotoxicity.26 Because biocompatible solutions are quite variable with respect to their GDP content, such heterogeneity might explain potentially conflicting trial results. For example, the absence of beneficial effect in the randomized trial by Fan et al.15 could be explained by the fact that 91% of patients in the intervention group received Physioneal, which is reported to contain levels of 3-dexoyglucosone (70–161 μmol/L depending on dialysate glucose concentration27) that are intermediate between those of the intervention group (42–60 μmol/L) and the control group (173–324 μmol/L) in this study.28
An alternative explanation for a possible renoprotective benefit of biocompatible fluid is reduced peritonitis risk. Numerous studies previously demonstrated that peritonitis and/or its subsequent treatment with nephrotoxic antibiotics are major risk factors for residual renal function decline.24,29,30 In this investigation, biocompatible (Balance) solution was associated with an important reduction in peritonitis risk. Similar findings have been reported in 3-year31 and 5-year32 nonrandomized studies of biocompatible fluid. In contrast, previous small, single-center randomized controlled trials8,12–19 have not shown significant differences in peritonitis rates, possibly due to insufficient statistical power, short follow-up durations, low peritonitis rates, Neyman bias, and drop-outs due to anuria. This investigation also showed that biocompatible fluid use was associated with a significant reduction in non-PD–related infections. The mechanisms of this observation are uncertain but could potentially relate to reductions in residual renal function decline to the point of anuria and/or the systemic load of advanced glycation end products.
Finally, in light of the observation that patients in the biocompatible group had significantly lower peritoneal ultrafiltration volumes and higher urine volumes than controls at 3 and 6 months, it is possible that some of the observed differences in GFR decline and onset of anuria between the two groups may have been volume driven. Nevertheless, the absence of significant differences in BP, weight, serum sodium, serum albumin, or hemoglobin during the study argues against significant differences in hydration between the two groups.
The strengths of this study include its large sample size, enrollment of incident patients, 2-year follow-up period, low drop-out rate, analytic consideration of nonlinear residual renal function decline, and involvement of participants from a range of centers and countries with varying approaches to PD. Stratification for center also minimized “center effects” on outcomes between the two groups. These strengths must be weighed against the study’s limitations, the principal one of which was recruitment to 55% of the prespecified recruitment target of 336 patients. The major barriers to recruitment were limited center participation due to clinician bias for or against biocompatible fluid, contractual commitments of centers to other dialysis fluid suppliers, and insufficient clinical or research staff to carry out extra procedures required for the trial. For those centers participating in the trial, the major barriers to recruitment were difficulty meeting inclusion criteria (particularly GFR ≥5 ml/min per 1.73 m2 and dialysis duration <90 days at time of randomization). Although this suboptimal recruitment reduced the power of the study, event rates were higher than anticipated and the study had sufficient observed statistical power to detect significant differences between the biocompatible and control groups. Moreover, the balANZ study is the largest randomized controlled clinical trial to date of biocompatible versus conventional PD fluids. The open-label design of the study potentially introduced observer bias, although this was countered by the use of clearly defined, objective outcome measures. The possibility of co-intervention bias due to the open-label nature of the study could not be excluded. We also did not measure serum phosphate or baseline peritoneal ultrafiltration. Furthermore, the low proportion of automated PD patients in this study may limit the generalizability of the study findings to the automated PD setting.
In conclusion, administration of a neutral pH, lactate-buffered, low GDP fluid (Balance) to incident PD patients was associated with a numerically slower, nonsignificant decline of GFR compared with conventional, standard, lactate-buffered solution (most apparent in the first year, P=0.17), which was not statistically significantly different between years (P=0.06). However, biocompatible (Balance) fluid use was associated with statistically significant and clinically important benefits with respect to delayed onset of anuria and reduced risk of peritonitis. These results are likely to have important implications for existing international clinical practice guidelines.
Concise Methods
Study Oversight
The study design and methodology were previously described.28 This study was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12606000044527) and the study protocol was approved by ethics committees at all participating centers. All patients provided written informed consent before trial participation.
Participants
The balANZ trial included adult patients (aged ≥18 years) with ESRD who were receiving PD, had commenced dialysis for the first time within the preceding 90 days, and had both a residual measured GFR ≥5 ml/min per 1.73 m2 and a measured urine volume ≥400 ml/d at enrollment. Exclusion criteria included an anticipated life expectancy <12 months, pregnancy, breastfeeding, history of malignancy within the last 5 years (other than a successfully and completely treated cutaneous squamous cell or basal cell carcinoma or cervical intraepithelium-like neoplasia), acute infection at the time of enrollment, contraindications to PD therapy, any mental or physical condition that interfered with the patient’s ability to comply with the study protocol, known or suspected allergy to the trial product or related products, or participation in any other clinical trial in which an intervention was designed to moderate rate of change of residual renal function. Biocompatible solution was not available for cycler therapy until December 6, 2006, such that patients receiving automated PD were only included in the study after this time.
Study Treatment
Patients were randomized 1:1 to receive either neutral pH, lactate-buffered, low GDP Balance solutions in Biofine plasticizer-free solution bags (Fresenius Medical Care, Bad Homburg, Germany) or conventional, standard, lactate-buffered PD solutions (stay.safe) in Biofine plasticizer-free solution bags. To ensure adequate concealment of allocation, such that clinicians and participants were unaware of upcoming treatment assignments, randomization was performed centrally via a web-based system according to a computer-generated randomization schedule prepared by Statistical Revelations Pty Ltd (http://www.statisticalrevelations.com.au/), with stratification according to center and the presence or absence of diabetic nephropathy (according to local clinical diagnosis). Patients in each trial arm received standard management, as per local PD unit protocols. Balance and stay.safe fluids were already commercially available in the participating countries at the commencement of this study such that all centers were already familiar with the training of patients in the administration of these solutions. The connectology was the same for both systems. Icodextrin was permitted in both the control and experimental groups. Each patient was followed for 24 months. The trial was open label.
Study Outcomes
The prespecified primary outcome measure was slope of decline over time of residual renal function, measured as the arithmetic mean of 24-hour urinary urea and creatinine clearances at 0, 3, 6, 9, 12, 18, and 24 months. Secondary outcomes included time from randomization to occurrence of anuria (urine volume <100 ml/d), indices of fluid balance (weight, BP, urine volume, peritoneal ultrafiltration volume, serum albumin, hemoglobin), peritonitis-free survival, technique survival, patient survival, and adverse events.
Statistical Analyses
Results were expressed as frequencies (percentages) for categorical variables, mean ± SD for continuous normally distributed variables, and median (range) for continuous non-normally distributed variables. For the primary outcome of slope of residual renal function decline over time, a mixed-effects general linear model was fitted, including treatment group, time, center, presence or absence of diabetic nephropathy, and PD modality (automated versus continuous ambulatory) as fixed-effects terms. Patient identification number was fitted as a “random” term in the model along with time and the intercept. In this way, the model provided estimates of the rate of decline in GFR (the slope) for each patient, allowing them also to have a different intercept (starting level). From these data, an overall estimate of the rate of decline in each treatment group was determined, corrected for the fixed-effects terms. The data were assumed to be normally distributed and to decline in a linear fashion. Differences in the rate of decline of residual renal function between the intervention and control groups were analyzed on an intention-to-treat basis. Time and treatment by time effects were tested using F tests based on the Kenward–Roger method.33 In view of the possibility of nonlinear decline in GFR, a model was also fitted that allowed the slope of the regression line to differ in the first year compared with the second year and then to allow these to also differ for the different treatments. A possible curvilinear decline was also investigated on a post hoc basis by fitting the same model to log(GFR) and by fitting an expanded model including a quadratic term to GFR. Mixed models assume missing at random patterns to cater for missed visits or withdrawal for any reason other than those related to treatment. It is recognized that withdrawals due to anuria are not missing at random and results should be interpreted in conjunction with other analyses, such as time to anuria. The balANZ trial aimed to recruit a total of 336 patients (168 in each group) on the basis of 80% power to detect a clinically important difference in the slope of residual renal function over time of 0.067 ml/min per 1.73 m2 per month, assuming a two-sided type 1 error of 5%. Commencing in November 2004, it was initially anticipated that trial recruitment would be completed by December 2008, with final follow-up completed by December 2010. As of October 1, 2008, 185 patients had been recruited into the study, accounting for 55% of the target. A decision was made by the Trial Management Committee to halt further recruitment on the basis of poor feasibility (i.e., insufficient recruitment rate to reach projected target within a reasonable time frame considering trial logistics and funding) and to continue the trial until the last patient enrolled had been followed for 2 years (September 1, 2010). Secondary time to event analyses (time to anuria, first peritonitis episode, technique failure or death) were performed by univariate Kaplan–Meier survival analyses and multivariate Cox proportional hazards model analyses with treatment group, center, presence of diabetic nephropathy, and baseline renal function as covariates. Data were analyzed by Statistical Revelations Pty Ltd. P<0.05 was considered significant.
Disclosures
D.W.J. is a consultant for Baxter Healthcare Pty Ltd and has previously received research funds from this company. He has also received speakers’ honoraria and research grants from Fresenius Medical Care. He has previously been a consultant to Gambro Pty Ltd. He is an International Society of Peritoneal Dialysis Councillor and is a current recipient of a Queensland Government Health Research Fellowship. F.G.B. is a consultant for Baxter and Fresenius. M.C. is an employee of Fresenius Medical Care. N.B. has previously received research funds from Roche; travel grants from Roche, Amgen, and Janssen-Cilag; and speaking honoraria from Roche. M.S. has participated in company-sponsored research for both Baxter and Fresenius; in the past, but not currently, M.S. has been a member of the clinical advisory boards of Baxter and Fresenius, and has attended sponsored meetings and received honoraria. All other authors have no conflicts of interest to declare.
Acknowledgments
The invaluable assistance provided by Caro Badcock from Statistical Revelations Pty Ltd with regard to all statistical analyses in this study is gratefully acknowledged. The invaluable assistance of Dr. Feidhlim Woods (former Fresenius employee and current Fresenius consultant) and Ms. Vanessa Wilson (current Fresenius employee) in providing advice regarding study design and coordination and critical review of the manuscript is gratefully acknowledged.
The balANZ trial was funded by Fresenius Medical Care Australia. The steering committee, which included two Fresenius employees (Margaret Clarke and Vanessa Wilson) and one former Fresenius employee (Feidhlim Woods), conceived, designed, and supervised the trial and the statistical analysis plan. Site investigators and their locally employed study nurses collected the patient data at each site. Regular site visits and source data checks were performed to confirm adherence to the protocol and the veracity of the data obtained. D.W.J. wrote the first draft of the manuscript; subsequent drafts were reviewed by all members of the steering committee. The members of the steering committee attest that the study was performed in accordance with the protocol and the statistical analysis plan and vouch for the accuracy and completeness of the reported analyses. D.W.J. confirms that he had full access to the data in the study and had final responsibility for the decision to submit for publication.
The collaborators (balANZ investigators) from each site are as follows. Australian centers: G. Rangan and L. Liew, Blacktown Hospital, Sydney, New South Wales; U. Steinwandel, Fremantle Hospital, Fremantle, Western Australia; L. Garvey, John Hunter Hospital, Newcastle, New South Wales; M. Gilbert, Liverpool Hospital, Sydney, New South Wales; I. Abraham and J. Nandkumar, Monash Medical Centre, Melbourne, Victoria; A. Coburn and V. Bali, Princess Alexandra Hospital, Brisbane, Queensland; S. McDonald, S. Frasca, M. Hockley, and C. Russ, The Queen Elizabeth Hospital, Adelaide, South Australia; K. Bannister, M. Hockley, and K. Pirone, Royal Adelaide Hospital, South Australia; L. Williams, Royal Brisbane Hospital, Brisbane, Queensland; K. Warr and G. Smith, Perth, Western Australia; S. Pellicano, Sir Charles Gairdner Hospital, Perth, Western Australia; and E. O’Flaherty, St. Vincent’s Hospital, Melbourne, Victoria. New Zealand centers: L. Reed and L. Anderson, Dunedin Hospital, Dunedin; and B. Jagannathan and P. Nicholls, Middlemore Hospital, Auckland. Singapore centers: C.K. Tam, Singapore General Hospital, Singapore; and R. Lee, Tang Tock Seng Hospital, Singapore.
Footnotes
D.W.J. and F.G.B. contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Krediet RT: 30 years of peritoneal dialysis development: The past and the future. Perit Dial Int 27[Suppl 2]: S35–S41, 2007 [PubMed] [Google Scholar]
- 2.Lameire N, Van Biesen W: Epidemiology of peritoneal dialysis: A story of believers and nonbelievers. Nat Rev Nephrol 6: 75–82, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Rumpsfeld M, McDonald SP, Johnson DW: Peritoneal small solute clearance is nonlinearly related to patient survival in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 29: 637–646, 2009 [PubMed] [Google Scholar]
- 5.Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, Nolph KD, Pollock CA, Prowant B, Farrell PC: The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 37: 598–604, 1991 [PubMed] [Google Scholar]
- 6.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vonesh EF, Snyder JJ, Foley RN, Collins AJ: Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney Int Suppl 103: S3–S11, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, Passlick-Deetjen J, Euro Balance Trial Group : The Euro-Balance Trial: The effect of a new biocompatible peritoneal dialysis fluid (balance) on the peritoneal membrane. Kidney Int 66: 408–418, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Park HC, Seo BJ, Do JY, Yun SR, Song HY, Kim YH, Kim YL, Kim DJ, Kim YS, Ahn C, Kim MJ, Shin SK: Superior patient survival for continuous ambulatory peritoneal dialysis patients treated with a peritoneal dialysis fluid with neutral pH and low glucose degradation product concentration (Balance). Perit Dial Int 25: 248–255, 2005 [PubMed] [Google Scholar]
- 10.Lee HY, Choi HY, Park HC, Seo BJ, Do JY, Yun SR, Song HY, Kim YH, Kim YL, Kim DJ, Kim YS, Kim MJ, Shin SK: Changing prescribing practice in CAPD patients in Korea: Increased utilization of low GDP solutions improves patient outcome. Nephrol Dial Transplant 21: 2893–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Johnson DW, Williams JD: Impact of peritoneal dialysis solutions on outcomes. In: Evidence-Based Nephrology, edited by Molony DA, Craig JC, Oxford, UK, Blackwell, doi:10.1002/9781444303391.ch46 [Google Scholar]
- 12.Kim S, Oh J, Kim S, Chung W, Ahn C, Kim SG, Oh KH: Benefits of biocompatible PD fluid for preservation of residual renal function in incident CAPD patients: A 1-year study. Nephrol Dial Transplant 24: 2899–2908, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Haag-Weber M, Krämer R, Haake R, Islam MS, Prischl F, Haug U, Nabut JL, Deppisch R, behalf of the DIUREST Study Group : Low-GDP fluid (Gambrosol trio) attenuates decline of residual renal function in PD patients: A prospective randomized study. Nephrol Dial Transplant 25: 2288–2296, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Weiss L, Stegmayr B, Malmsten G, Tejde M, Hadimeri H, Siegert CE, Ahlmén J, Larsson R, Ingman B, Simonsen O, van Hamersvelt HW, Johansson AC, Hylander B, Mayr M, Nilsson PH, Andersson PO, De los Ríos T: Biocompatibility and tolerability of a purely bicarbonate-buffered peritoneal dialysis solution. Perit Dial Int 29: 647–655, 2009 [PubMed] [Google Scholar]
- 15.Fan SL, Pile T, Punzalan S, Raftery MJ, Yaqoob MM: Randomized controlled study of biocompatible peritoneal dialysis solutions: Effect on residual renal function. Kidney Int 73: 200–206, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Szeto CC, Chow KM, Lam CW, Leung CB, Kwan BC, Chung KY, Law MC, Li PK: Clinical biocompatibility of a neutral peritoneal dialysis solution with minimal glucose-degradation products—a 1-year randomized control trial. Nephrol Dial Transplant 22: 552–559, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, Kim BS, Park HC, Choi KH, Ha SK, Han DS, Lee HY: The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: An open randomized prospective trial. Perit Dial Int 28: 174–182, 2008 [PubMed] [Google Scholar]
- 18.Tranaeus A, The Bicarbonate/Lactate Study Group : A long-term study of a bicarbonate/lactate-based peritoneal dialysis solution—clinical benefits. Perit Dial Int 20: 516–523, 2000 [PubMed] [Google Scholar]
- 19.le Poole CY, van Ittersum FJ, Weijmer MC, Valentijn RM, ter Wee PM: Clinical effects of a peritoneal dialysis regimen low in glucose in new peritoneal dialysis patients: A randomized crossover study. Adv Perit Dial 20: 170–176, 2004 [PubMed] [Google Scholar]
- 20.Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, Kawaguchi Y, Kawanishi H, Korbet S, Krediet R, Lindholm B, Oreopoulos D, Rippe B, Selgas R, International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis : Evaluation and management of ultrafiltration problems in peritoneal dialysis. Perit Dial Int 20[Suppl 4]: S5–S21, 2000 [PubMed] [Google Scholar]
- 21.Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, Price V, Ramalakshmi S, Szeto CC: ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 31: 614–630, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Misra M, Vonesh E, Van Stone JC, Moore HL, Prowant B, Nolph KD: Effect of cause and time of dropout on the residual GFR: A comparative analysis of the decline of GFR on dialysis. Kidney Int 59: 754–763, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, Hollett P: Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int 23: 276–283, 2003 [PubMed] [Google Scholar]
- 24.Shin SK, Noh H, Kang SW, Seo BJ, Lee IH, Song HY, Choi KH, Ha SK, Lee HY, Han DS: Risk factors influencing the decline of residual renal function in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 19: 138–142, 1999 [PubMed] [Google Scholar]
- 25.Zeier M, Schwenger V, Deppisch R, Haug U, Weigel K, Bahner U, Wanner C, Schneider H, Henle T, Ritz E: Glucose degradation products in PD fluids: Do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int 63: 298–305, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Justo P, Sanz AB, Egido J, Ortiz A: 3,4-Dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes 54: 2424–2429, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Hoff CM: In vitro biocompatibility performance of Physioneal. Kidney Int Suppl 88: S57–S74, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Johnson DW, Clarke M, Wilson V, Woods F, Brown FG: Rationale and design of the balANZ trial: A randomised controlled trial of low GDP, neutral pH versus standard peritoneal dialysis solution for the preservation of residual renal function. BMC Nephrol 11: 25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badve SV, Hawley CM, McDonald SP, Brown FG, Boudville NC, Wiggins KJ, Bannister KM, Johnson DW: Use of aminoglycosides for peritoneal dialysis-associated peritonitis does not affect residual renal function. Nephrol Dial Transplant 27: 381–387, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Shemin D, Maaz D, St Pierre D, Kahn SI, Chazan JA: Effect of aminoglycoside use on residual renal function in peritoneal dialysis patients. Am J Kidney Dis 34: 14–20, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Montenegro J, Saracho R, Gallardo I, Martínez I, Muñoz R, Quintanilla N: Use of pure bicarbonate-buffered peritoneal dialysis fluid reduces the incidence of CAPD peritonitis. Nephrol Dial Transplant 22: 1703–1708, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Furkert J, Zeier M, Schwenger V: Effects of peritoneal dialysis solutions low in GDPs on peritonitis and exit-site infection rates. Perit Dial Int 28: 637–640, 2008 [PubMed] [Google Scholar]
- 33.Kenward MG, Roger JH: Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53: 983–997, 1997 [PubMed] [Google Scholar]