Summary
Exposure to traumatic stressors typically causes lasting changes in emotionality and behavior. However, coping strategies have been shown to prevent and alleviate many stress consequences and the biological mechanisms that underlie coping are of great interest. Whereas the laboratory stressor inescapable tail-shock induces anxiety-like behaviors, here we demonstrate that permitting a rat to chew on a wooden dowel during administration of tail-shock prevented the development of anxiety like behaviors in the open field and juvenile social exploration tests. Uncontrollable stressors increase corticosterone and decrease thyroid hormone, and we hypothesized that coping would blunt these changes. While tail-shock did produce these effects, active coping did not alter hormone levels. The dissociation between behavioral resilience and circulating hormones is discussed with regard to the utility of these molecules as biomarkers for psychiatric disease.
Keywords: Chewing, Coping, Corticosterone, Thyroxine, Open-field test, Social Exploration, Rat
Stressors, defined as stimuli that are arousing, aversive, and uncontrollable or unpredictable (Kim and Diamond, 2002, Koolhaas et al., 2011), can induce multiple hormonal and behavioral responses. Additionally, stressor exposure is considered a precipitating factor for many illnesses (Juster et al., 2010) including mood disorders. Indeed, in animal models of mood disorders, particularly depression, stress paradigms are often the principal manipulation (Nestler and Hyman, 2010) and appropriately, these stress paradigms lead to adaptations in endocrine systems and behavior. Recently there has been a great deal of interest in determining processes that blunt the impact of stressors on both endocrine systems and behavior and the mechanisms by which they operate. One potential mechanism is that stress-blunting processes may alter behavior by acting through endocrine systems.
Active behavioral coping is the most potent stress-blunting process discovered to date. In humans, this strategy has been promoted for dealing with catastrophic events, managing stress-related conditions, such as post-traumatic stress disorder and overcoming negative consequences of loneliness and social isolation (Ledoux and Gorman, 2001, Cacioppo and Hawley, 2003, Olff et al., 2005). In the current animal studies, we used two models of active behavioral coping: chewing and behavioral control.
It has been suggested that allowing human and rodent subjects the opportunity to chew during stressor exposure is stress-blunting. In rodents, this has been done by providing an object, such as a wooden dowel that the subject will choose to chew (Hennessy and Foy, 1987, Berridge et al., 1999, Martijena et al., 2002, Hori et al., 2004, Kubo et al., 2009, Stalnaker et al., 2009, Smith, 2010, Johnson et al., 2011). A safety signal presented between stressors both reduces fear and the impact of stress on later anxiety (Christianson et al., 2011). As with safety signals, one interpretation of the chewing response is that it represents a displacement behavior (Tinbergen, 1974, Berridge et al., 1999) or a distractor and so may reduce fear during stress. Previous studies have demonstrated that chewing can blunt stress-induced changes in one endocrine axis, the hypothalamic-pituitary-adrenal (HPA axis), during stress (Hennessy and Foy, 1987, Hori et al., 2004). However, there is little, if any, previous work examining the effects of chewing during stress on subsequent stress-induced changes in behavior.
Behavioral control has been the most frequently studied preclinical model of active coping in rat. In the typical paradigm one set of subjects is allowed to control the termination of each of a series of tail-shocks by performing an operant response, such as turning a small wheel (termed an “escape response”). A second set of subjects is yoked to the escapable stress (ES) subjects. For these subjects each tail-shock begins at the same time as it does for its ES partner, but terminates whenever the ES subject responds. For these rats (IS for inescapable shock) turning the wheel has no consequence. Thus, the two groups of subjects are exposed to physically identical events, but the ES subjects have behavioral control over the duration of each shock but the IS group does not. Numerous studies have shown that the presence of control blunts or prevents many of the behavioral and neural consequences of the tail-shocks (Maier and Watkins, 2005).
Yet the presence of control, using the very same parameters that block the behavioral and neural effects of the stressor, has not been found to reduce the hypothalamic-pituitary-adrenal response to the tail-shock stressor. That is, both ES and IS produce the same increases in paraventricular nucleus corticotropin releasing factor (CRF) mRNA levels as well as other peptides (Helmreich et al., 1999), and the same increases in blood levels of ACTH and corticosterone (Maier et al., 1986). However, higher levels of plasma corticosterone have been reported in rats exposed to IS compared to ES when foot-shock paradigm is utilized (Swenson and Vogel, 1983), although not consistently (Mormede et al., 1988).
In addition to the HPA axis, the hypothalamic-pituitary-thyroid (HPT) axis is also stress responsive; both acute and chronic stressors lead to decreases in circulating thyroid hormones in male rats (Langer et al., 1983, Helmreich et al., 2005, Helmreich and Tylee, 2011). The term thyroid hormones includes 3,5,3′,5′-tetraiodothyronine (T4) and 3,5,3′-triiodothyronine (T3). T4 is converted to T3 by the activity of deiodinase enzymes located within most target tissues, including the central nervous system. In humans, dysregulation of thyroid hormones has long been associated with mood disorders, particularly depression (Joffe and Sokolov, 1994) as have glucocorticoids (McEwen, 2005). It is possible that stressor control might influence the HPT response to the stressor, even though it did not influence the HPA response. This possibility was examined here. In addition, there have not been any previous studies determining the effects of ‘chewing’ on stress-induced changes in thyroid hormone levels. Importantly, both thyroid hormones and glucocorticoids have membrane receptors in addition to genomic receptors, allowing both rapid and delayed effects on cellular function (Davis et al., 2008, Groeneweg et al., 2011).
To address the question of whether active-behavioral coping leads to acute changes in stress-regulated hormone systems (the HPA and HPT) that are correlated with acute stress-induced changes in behavior, we used two behavioral measures: exploration in a novel open field and a juvenile social exploration test. Both of these tests have been utilized as tests of rat ‘emotionality’ and, importantly, both are influenced by stress (Ramos and Mormede, 1998, Christianson et al., 2008b). Previous work has demonstrated that behavioral control blunts the impact of stress on social exploration (Christianson et al., 2008b).
In sum, the goals of the current series of experiments were to determine whether active behavioral coping during stress alters stress-induced changes in tests of rat emotionality and, second, whether active behavioral coping during stress changes the endocrine responses to the stress. The over-arching goal is to determine if changes in these specific endocrine systems are correlated with changes in behavior, with the view that this may provide information regarding mechanisms regulating behavior.
Materials and Methods
Overview
These experiments were conducted at two sites: The University of Rochester, Rochester NY, and the University of Colorado, Boulder CO. At both institutions the university committee on animal use approved all experimental protocols and all animals were treated in accordance with the NIH Guide for Care and Use of Laboratory Animals. The experiments were divided between the two institutions as follows: experiments utilizing the chewing paradigm, with acoustic analysis of chew behavior, open field testing and peripheral corticosterone and T4 measures were conducted at Rochester; experiments utilizing the chew paradigm with measures of juvenile social exploration and experiments utilizing controllable/uncontrollable stress were conducted at Colorado. At both institutions, blood samples for hormone measurements were collected via tail-nick at the times indicated.
Adult male Sprague-Dawley rats (Charles River Laboratories at Rochester and from Harlan Labs, IN at Colorado), 250–310g at the beginning of the stress sessions were used. Animals were housed with ad libitum access to food and water in groups of 2–4 rats in standard plastic cages on a 12:12h light/dark schedule. Testing began at least 5 days after arrival.
Behavioral Procedures
Stress and Chewing Behavior
Prior studies of uncontrollable stress effects on behavioral and endocrine measures have utilized 100 trials of inescapable tail-shocks delivered on a variable interval schedule ranging from 5 to 115 sec, with a mean duration of 60 seconds (Helmreich et al., 1999, Christianson et al., 2008b). In the current experiments rats received either a single session of 100 trials of 5 sec inescapable tail-shocks (S) or the same treatment with a wooden stick added to the chamber to permit chewing as an active coping behavior (SC). This manipulation was conducted at both the University of Rochester and Colorado. At Rochester stress occurred in commercially available tail-shock chambers (Med Associates, St. Albans VT) measuring 12 × 8 × 14 cm with grid floors, and shocks (1.0 mA) were delivered to the tail via disposable finger electrodes (Discount Disposables: Mansfield R & D, St. Albans VT) augmented with electrolyte paste. In Colorado stress occurred in acrylic tubes described previously (Christianson et al., 2008b); shocks (1.6 mA) were delivered to the tail by copper electrodes augmented with electrolyte paste. At both sites shock was administered inside a sound attenuating chamber with a fan and houselight and was controlled by computers directing scrambled shock generators (Rochester-Med Associates; Colorado-Coulbourn Instruments, Whitehall, PA).
In the stress-chew (SC) condition, a chew stick (Rochester: a fruitwood stick covered with crunchy bark, Big Branch Bites, Super Pet, Elkgrove Village IL; Colorado: 1cm square basswood dowel) was secured directly behind the subject in the Rochester lab or directly in front of the subject’s mouth in the Colorado lab. These technical differences were the result of independent pilot studies identifying the most reliable chewing behavior in the different chambers. In some experiments a group of rats (R; lightly restrained) were placed in a tail-shock chamber or in acrylic tubes, but were not shocked. The manipulation lasted approximately 90 min (0930 to 1100 h); animals were returned to their home cage immediately at the end of the session. An additional experiment was conducted in the Colorado laboratory in which rats received inescapable stress or exactly equal escapable stress (ES). In the ES condition rats were able to control the offset of tail-shock by turning a wheel inside the restraint apparatus. Procedures were identical to those previously reported (Christianson et al., 2008a).
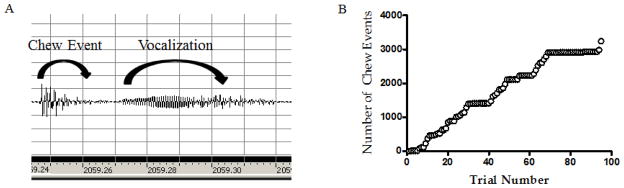
In the Rochester lab, chewing behavior was quantified from audio recordings inside the chamber obtained with a digital audio recorder (Olympus DS-50). Audio files were filtered using GoldWave software and acoustic wavelet analysis was performed using a MatLab algorithm. This analysis quantified the total number of chew events during the stress session, allowing for examination of relationships between the amount of chewing, the timing of the chew events, neuroendocrine responses and behavioral outcomes (Fig. 1). At the Colorado laboratory chewing was confirmed by visual inspection of the dowel during and after stress.
Figure 1.
For chew paradigm experiments conducted at Rochester, acoustic wavelet analysis was used to quantify chewing behavior throughout the stress session. Examples of output from an individual animal’s analysis are shown. Audio recordings were filtered using Goldwave Software and chew events were discriminated from other acoustic events (A) and quantified using a MATLAB algorithm written by S.T. Govindarajan and Dr. W. E. O’Neill (University of Rochester). This quantification allowed cumulative record of chew events across the stress session to be plotted (B).
Open Field Tests
Exploratory behavior before and after the stressor was assessed using the open field test (arena: 81 × 81 cm) under dim lighting (20 lux; (Ramos and Mormede, 1998)). Individual subjects were placed in the center of the arena and behavior was recorded for 3 min by a digital camcorder suspended above. The arena was cleaned with diluted Roccal-D solution between subjects. Total distance traveled and time spent in the center of the arena (60 × 60 cm) were computed using CleverSys TopScan 2.0 tracking software, and the change in time spent in the center (post-stress minus pre-stress) was calculated.
Social Exploration Tests
Social behavior after the stress was determined in a 3 min social exploration test exactly as previously described (Christianson et al., 2008a). Briefly, each rat was allocated a standard plastic tub cage with shaved wood bedding and a wire lid in a designated testing room. Rats were placed in the test cage and 60 min later a naïve juvenile (28–31 day old) was added. An observer who was blind to the experimental treatment measured the duration of exploratory behaviors, including sniffing, pinning and allogrooming initiated by the adult rat to the juvenile.
Blood sampling and Hormone Assays
Peripheral blood samples were collected by tail-nick. Briefly, rats were gently restrained, the tail sanitized with 70% ethanol and 1mm of the tail was cut with a sterile scalpel. Approximately 100 μL of peripheral blood were collected and allowed to clot at room temperature, centrifuged, and the plasma fraction was subsequently separated and frozen at −80 °C until assay. Peripheral levels of corticosterone and Total T4 were determined using radioimmunoassay kits from MP Biomedicals/Pharmaceuticals (Solon, OH) using the specified protocols. All samples for each experiment were run in a single assay. Inter- and intra-assay variability are reported from information supplied by MP Biomedicals: corticosterone intra-assay variability = 4.4–10.3%, inter-assay variability = 6.5–7.1%; Total T4 intra-assay variability = 3.3–8.1%, inter-assay variability = 5.3–11.4%
Experimental Procedures
Overview
In the Rochester laboratory, separate cohorts of rats were sampled for peripheral corticosterone or T4 measurements. These rats were also used for open field testing. In the Colorado laboratory, a third cohort was used for social exploration tests and a fourth for stressor controllability effects on T4.
Stress and chewing effects on corticosterone and open field behavior
For the assessment of plasma corticosterone levels, subjects (n=33; 11 per group) were assigned to inescapable stress (S), stress with chewing (SC) or chamber restraint without tail-shock (R) groups. Baseline open field behavior was assessed in all subjects prior to stress. Baseline blood samples were collected via tail-nick immediately prior to the stress session (0 min) and additional samples were collected 100, 140 and 200 min post-stress initiation. Post-stress open field testing occurred 240 min after stressor initiation (1300 h). A technical failure produced a 24-hr light cycle for a subset of the animals; their data were initially examined separately.
Stress and chewing effects on T4 and open field behavior
For the assessment of plasma T4 levels, subjects (n=40; 10 per group) were assigned to similar S, SC, and R conditions as described above. Additionally a chamber restraint with chewing (RC) condition was included. In SC and RC conditions, chew sticks were mounted directly in front of and behind the subjects. On the day before the stress session, baseline blood samples were collected via tail-nick (1200 h) and baseline open field behavior was assessed (1300 h). On the day of stress, post-stress blood samples were collected at 180 min after stressor initiation (1200 h), based on previous studies examining acute stress-induced fluctuations in T4 (Helmreich and Tylee, 2011). Open field testing took place approximately 240 min post-stressor initiation (1300 h).
Stress and Chewing Effects on Social Exploration
For the assessment of social exploration rats (n=8–10 per group) were assigned to inescapable stress (S), stress with chew (SC), restraint chew (RC) or left in the homecage (HC). Restraint alone was not included because we have previously demonstrated that restraint alone does not influence later social exploration (Christianson et al., 2008b, Christianson et al., 2009) Social exploration tests were conducted 24h after stress.
Stressor controllability effects on T4
To document the effects of the IS/ES tail-shock paradigm on thyroid hormones, we assessed plasma T4 in a cohort of rats (n=7/condition) exposed to escapable stress (ES), inescapable stress (IS) restraint (R) or home cage (HC). A single blood sample was taken 240 min post-stress initiation.
Statistical Analysis
Statistical analyses were conducted using Graphpad Prism 5.0 software. Separate two-way ANOVA were used to assess the effects of different light cycles (12, 24 hr) and experimental conditions (S, SC, R) on open field behavior and corticosterone measures. These data were initially analyzed separately, but we found no significant difference in comparison with animals housed under the standard light/dark cycle and we therefore pooled the data. After pooling the data, one-way ANOVA was used to assess the effects of experimental conditions at each sampling time point. For the experiment that included a restraint-chew condition, two-way ANOVA was used to assess the effects of stress and chewing manipulations on open field behavior and peripheral T4. One-way ANOVA was used to assess the effects of experimental condition on social exploration behaviors. For the experiment examining stressor controllability, one-way ANOVA was used to assess between-groups differences in peripheral T4. All post-hoc tests were conducted with the Fisher’s Least Significant Difference using SPSS software.
Results
Behavior
Among rats subjected to the stress-chew paradigm followed by social exploration testing, 3 out of 12 did not engage in chewing behavior and their data were excluded, resulting in groups of 8–10 rats in each condition. Notably, rats appeared only to gnaw on the wooden dowel and did not appear to ingest much, if any, of the wood. This suggests that the behavior is more akin to a displacement behavior than a consummatory behavior. As in our previous studies, inescapable stress reduced time spent in social exploration and, interestingly, the opportunity to chew completely prevented this effect (Fig. 2A). A one-way ANOVA revealed a significant main effect (F(3, 34) = 4.56, P < .01), and post-hoc analyses showed that the stress group (S) spent less time in social exploration than the SC and R groups
Figure 2.
A. Subjects (n=8–10 per group) were exposed to inescapable stress (S) and some subjects had the opportunity to chew a wooden dowel during stress exposure (SC). Social exploration of a juvenile conspecific was measured 24 hr post-manipulation. S subjects had significantly reduced social exploration, compared to SC and home cage (HC) groups. B. Subjects (n=11 per group) were exposed to inescapable stress (S) and some subjects had the opportunity to chew a wooden stick during stress exposure (SC). Subjects were tested in an open field arena before and after the experimental manipulations. Post-hoc tests confirmed that the S subjects had more negative change scores compared with SC and R groups. C. Subjects (n=10 per group) were exposed to inescapable stress (S) and some subjects had the opportunity to chew a wooden stick during stress exposure (SC). Restraint (R) and restraint-chew (RC) condition involved placing subjects in the chamber without tail-shocks. Subjects were tested in an open field arena before and after the experimental manipulation. A two-way ANOVA (stress x chew) indicated a main effect the opportunity to chew on the change observed in the amount of time subjects spent in the center of the arena. Post-hoc tests indicated that SC subjects were significantly different from S and C subjects; groups sharing the same small letter indicator were not significantly different from each other.
The opportunity to chew also altered behavior assessed in the open field test; in both cohorts tested, the opportunity to chew during treatment was associated with enhanced exploration of the center of the arena post-treatment (Figs. 2B and 2C). In the first cohort (Fig. 2B), one-way ANOVA revealed a significant main effect of condition on the change in the amount of time spent in the arena’s center (F(2, 32) = 5.77, p < .01); specifically, S animals spent significantly less time in the center of the arena post-stress than the SC group or the R group. Between-groups differences were not observed with respect to the change in total distance traveled in the open field. Among SC subjects, the number of chewing events was not significantly correlated with behavior measures.
In a second cohort that included a restraint-chew condition, the opportunity to chew was again associated with enhanced exploration of the center of the arena, irrespective of whether subjects received inescapable tail-shocks (Fig. 2C). Two-way ANOVA revealed a significant main effect of chew opportunity on the change in time spent in the center of the arena (F(3, 39) = 8.24, p < .01); the interaction term between stress and chew was non-significant. As in the first cohort, between-groups differences were not observed in total distance traveled in the open field nor was the number of chewing events significantly correlated with behavior measures.
Hormones
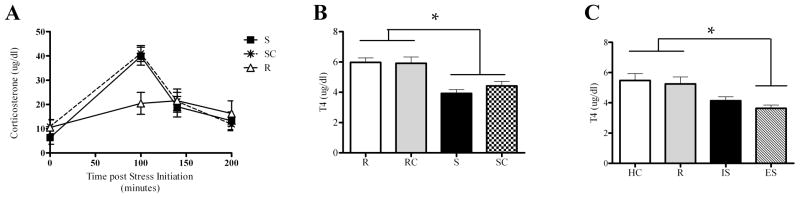
Inescapable tail-shock was associated with a robust corticosterone response that was not altered by the opportunity to chew (Fig. 3A). Specifically, using the pooled light-cycle data, one-way ANOVAs revealed a significant main effect of condition on 100 min corticosterone (F(2, 32) = 18.81, p < .001) and the change in corticosterone from 0 to 100 min (F(2, 32) = 23.95, p < .001). Post-hoc tests indicated that S and SC subjects had significantly higher 100 min corticosterone and change in corticosterone from 0 to 100 min than did R subjects but S and SC groups were not significantly different from each other. Within the SC group the number of chew events was not significantly correlated with hormone measures.
Figure 3.
A. Peripheral blood samples were obtained from subjects exposed to the stress-chew paradigm (the same animals described in Figure 2B). Tail-shock stress caused a significant increase in plasma corticosterone that was not significantly different in animals that chewed a wooden stick during tail-shock. B. Peripheral blood samples were obtained from subjects exposed to the stress-chew paradigm (the same animals described in Figure 2C). Plasma T4 levels were measured in samples collected 180 m post-stressor initiation. A two-way ANOVA indicated a significant main effect of the stress manipulation on T4; post-hoc tests confirmed that S and SC groups were significantly different from than R and RC groups, but were not different from each other. C. Peripheral blood samples were obtained from subjects (n=8 per group) exposed to the following conditions within the wheel-turn stressor controllability paradigm: inescapable stress (IS), escapable stress (ES), restraint (R) or home cage (HC). Peripheral T4 levels were measured in samples collected 240 min hours post-stressor initiation. One-way ANOVA indicated a main effect of condition on T4; post-hoc tests indicated that IS and ES subjects had significantly lower T4 than R and HC groups but were not different from each other.
Inescapable tail-shock caused a decrease in peripheral T4, and this decrease was not altered by the opportunity to chew (Fig. 3B). Specifically, a two-way ANOVA revealed a significant main effect of stress on T4 (F(3, 39) = 31.08, p < .001), and post-hoc tests indicated that the S and SC groups were significantly different from the R and RC groups, but not different from each other. There was no main effect of the opportunity to chew on T4. Among SC and RC subjects, the number of chew events was not significantly correlated with hormone measures.
In the IS/ES stress paradigm, inescapable tail-shock again led to an apparent decrease in peripheral T4 levels, and this effect was not altered by the opportunity to control the stressor (Fig. 3C). A one-way ANOVA revealed a significant main effect of condition on change in T4 (F(3, 22) = 6.22, p < .01); post-hoc tests indicated that IS and ES subjects were significantly different from HC and R groups, but were not different from each other.
Discussion
The overarching goal of this series of experiments was to determine whether behavioral coping procedures would produce parallel changes in stress-regulated endocrine systems and behavior. We used two acute, active behavioral stress-coping paradigms; chewing, which has not been extensively characterized and behavioral control (IS/ES), which has been extensively characterized. In both paradigms, the physical stimulus (tail-shock) was identical for the subjects that were or were not provided with a putative coping response (either stick-chewing or wheel-turning to terminate the shock). We found that active behavioral coping did prevent stress-induced changes in behavior (this was not previously known for chewing), indicating that they were effective paradigms, but active behavioral coping did not alter stress-induced changes in the peripheral markers of HPA or HPT axis activity. This dissociation between the measured hormones and behavior suggest that the systems are not directly linked, and that changes in circulating levels of corticosterone or thyroid hormones do not directly result in changes in behavior. Changes in hormones do indicate that the animal received a physical stressor, but do not reflect subsequent changes in anxiety-like behavior. One resulting conclusion is that peripheral levels of these hormones may not be useful biomarkers for some of the ultimate consequences resulting from stress (Woodson et al., 2003). That said, it is important to note that we have only monitored open field and social exploration behaviors; changes in peripheral corticosterone and T4 may indeed be correlated with changes in other behaviors (Rodgers et al., 1999).
Stressor-induced hormonal changes have been conceptualized in several different ways. Hormones such as HPA and HPT products have sometimes been thought to participate in causation of the behavioral effects of stressors via their action at central receptors (Mong and Pfaff, 2004). However the finding that behavioral coping strategies, which block the effects of the stressor on behavior, do not alter hormonal profiles offers no encouragement for such a view. Alternatively, stress hormones could operate in the brain as permissive factors (Korte, 2001) and interact with other central factors to induce changes in behavior. For example, glucocorticoids are important for memory consolidation, but operate through an interaction with NE within the amygdala (Roozendaal et al., 2009). Previous work has also implicated glucocorticoids in the development of learned helplessness (Edwards et al., 1990, Baez et al., 1996, Kademain et al., 2005) although the results are not consistent. For example, lesion or inhibition of the HPA axis failed to prevent the effect of tail-shocks on the shuttle box test (MacLennan et al., 1982).
Stressor-induced hormonal changes have also been thought of as biomarkers that can be useful non-invasive tools for both characterizing and identifying complex brain processes (Piazza et al., 2010). However, the results of the current study suggest that measurement of one or two biomarkers may not accurately predict the animal’s current status. Measurement of multiple biomarkers from an individual may prove to be more beneficial and informative (Schmidt et al., 2011). Additionally, peripheral biomarkers do not inform as to what is occurring centrally, at the molecular and/or receptor level, where hormonal information is transduced into cellular processes. Moreover, the strength of the hormone signal can be fine-tuned at the cellular level (Bagamasbad and Denver, 2011).
It should be noted that previous work has demonstrated that chewing can alter HPA and PVN responses to immobilization stress (Hori et al., 2004, Hori et al., 2005). The more intense stressor used in the current studies, which has been shown to induce marked behavioral changes, is probably of greater magnitude and potency, in addition to being a different modality, than the stressors used in the previous studies. These factors may contribute to the discrepancies among the studies. Stressor magnitude may also address difference between previous foot-shock and current tail-shock HPT results. Helmreich et al (Helmreich et al., 2006) previously reported that inescapable foot-shock stress caused a greater decrease in peripheral T3 levels than did escapable foot-shock stress, but these results were not replicated in the current studies using the tail-shock paradigm. In addition to using a lower amperage stimulus than the tail-shock studies, the foot-shock chambers also allowed the animals to perform behaviors to decrease stimulus intensity that are not available with tail-shock.
In the current study, we also noted inconsistencies in the impact of stress on open-field behavior. In one cohort, tail-shock stress caused a greater decrease in time spent in the center than mild restraint, while in another cohort the changes caused by the two treatments were of similar magnitude. This discrepancy may have been caused by differences between the cohorts in the protocol for pre-stress testing. Irrespective of these differences, chewing was still able to blunt the effect of the stressor (either tail-shock or restraint) on open-field behavior and, importantly, these changes in behavior were not paralleled by changes in circulating corticosterone or T4.
The present focus on thyroid hormones during active behavioral coping is partially based on similarities between the consequences of hypothyroidism and inescapable stress. For example, stress-induced gastric ulceration occurs at a higher frequency in animals experiencing inescapable stress compared to animals experiencing escapable stress (Maier, 1993), and hypothyroidism is also associated with an increased prevalence of stress-induced gastric ulcers (Money et al., 1986). Also, administration of T3 after inescapable stress prevents the development of learned helplessness, suggesting that a decrease in thyroid hormone levels caused by the shock session maybe partly responsible for the development of learned helplessness (Martin et al., 1985). Classically, TH hormone effects are thought to take place within the time frame of days to weeks, not within the minutes to hours time-points measured in the current study. However, more recent evidence suggests that changes in TH can have short-term consequences, including within the CNS (Kong et al., 2004, Sui et al., 2006, Davis and Tremont, 2007, Caria et al., 2009).
In conclusion, the results of the current experiments further substantiate the use of chewing or gnawing by rodents as an active behavioral coping paradigm to blunt the effects of stress on anxiety-related behaviors. Furthermore, we observed dissociation between the effects of stress on behavior endpoints versus the effects of stress on hormonal endpoints. This dissociation strengthens the conclusion that the two endpoints are not causally linked. Although the results obtained from the two paradigms used in the current study provide consistent results, it is possible that the use of more chronic stress paradigms and/or different behavioral measures may yield different phenomena. These questions can be addressed by future experiments.
Acknowledgments
This work was supported by the Department of Psychiatry at the University of Rochester, and grant MH080789 to DLH, MH050479 to SFM and MH082453 to JPC.
We would like to thank the vivarium staff at the University of Rochester for their excellent animal care. This work was supported by the Department of Psychiatry at the University of Rochester, and grant MH080789 to DLH, MH050479 to SFM and MH082453 to JPC. Special thanks to S. T. Govindarajan and W.E. O’Neill (University of Rochester) for developing the Matlab sound analysis routines. The authors appreciate the thoughtful comments from Dr. J. Fudge, R. Ader and J. Moynihan. At Rochester, the late Dr. Ader was instrumental in the early stages of setting up the chew paradigm.
Footnotes
Contributors
Authors D.L. Helmreich and J.P. Christianson designed the study, collected, and analyzed the data. DLH was assisted by D. Tylee and K. Becoats. JPC was assisted by K.H. Kubala. S.T. Govindarajan, under the guidance of W.E. O’Neill, developed the chew analysis routines at Rochester. L. Watkins and S.F. Maier maintain the laboratory at the University of Colorado. DLH, JPC, and DT all made significant contributions to the writing of the manuscript. All authors approved the final manuscript.
Conflict of Interest
We have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baez M, Siriczman I, Volosin M. Corticosterone is involved in foot shock-induced inactivity in rats. Physiology & behavior. 1996;60:795–801. doi: 10.1016/0031-9384(96)00025-x. [DOI] [PubMed] [Google Scholar]
- Bagamasbad P, Denver RJ. Mechanisms and significance of nuclear receptor auto- and cross-regulation. Gen Comp Endocrinol. 2011;170:3–17. doi: 10.1016/j.ygcen.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge C, Mitton E, Clark W, Roth R. Engagement in a non-escape (displacement) behavior elicits a selective and lateralized suppression of frontal cortical dopaminergic utilization in stress. Synapse. 1999;32:187–197. doi: 10.1002/(SICI)1098-2396(19990601)32:3<187::AID-SYN5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Hawley L. Social isolation and health, with an emphasis on underlying mechanisms. Perspectives in Biology and Medicine. 2003;46:S39–S52. [PubMed] [Google Scholar]
- Caria MA, Dratman MB, Kow LM, Mameli O, Pavlides C. Thyroid hormone action: nongenomic modulation of neuronal excitability in the hippocampus. J Neuroendocrinol. 2009;21:98–107. doi: 10.1111/j.1365-2826.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Christianson JP, Benison AM, Jennings J, Sandsmark EK, Amat J, Kaufman RD, Baratta MV, Paul ED, Campeau S, Watkins LR, Barth DS, Maier SF. The sensory insular cortex mediates the stress-buffering effects of safety signals but not behavioral control. J Neurosci. 2008a;28:13703–13711. doi: 10.1523/JNEUROSCI.4270-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Jennings JH, Ragole T, Flyer JG, Benison AM, Barth DS, Watkins LR, Maier SF. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol Psychiatry. 2011;70:458–464. doi: 10.1016/j.biopsych.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008b;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;1 doi: 10.1080/10253890802510302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD, Tremont G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 2007;32:49–65. [PubMed] [Google Scholar]
- Davis PJ, Leonard JL, Davis FB. Mechanisms of nongenomic actions of thyroid hormone. Front Neuroendocrinol. 2008;29:211–218. doi: 10.1016/j.yfrne.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Edwards E, Harkins K, Wright G, Henn F. Effects of Bilateral Adrenalectomy on the Induction of Learned Helplessness Behavior. Neuropsychopharmacology. 1990;3:109–114. [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J Endocrinol. 2011;209:153–167. doi: 10.1530/JOE-10-0472. [DOI] [PubMed] [Google Scholar]
- Helmreich D, Crouch M, Dorr N, Parfitt D. Peripheral triiodothyronine (T3) levels during escapable and inescapable shock. Physiology and Behavior. 2006;87:114–119. doi: 10.1016/j.physbeh.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Helmreich D, Parfitt D, Lu X-Y, Akil H, Watson S. Relation between the hypothalamic-pituitary-thyroid (HPT) axis and the hypothalamic-pituitary-adrenal (HPA) axis during repeated stress. Neuroendocrinology. 2005;81:183–192. doi: 10.1159/000087001. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Tylee D. Thyroid hormone regulation by stress and behavioral differences in adult male rats. Horm Behav. 2011;60:284–291. doi: 10.1016/j.yhbeh.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmreich DL, Watkins LR, Deak T, Maier SF, Akil H, Watson SJ. The effect of stressor controllability on stress-induced neuropeptide mRNA expression within the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 1999;11:121–128. doi: 10.1046/j.1365-2826.1999.00300.x. [DOI] [PubMed] [Google Scholar]
- Hennessy M, Foy T. Nonedible material elicits chewing and reduces the plasma corticosterone response during novelty exposure in mice. Behavioral Neuroscience. 1987;101:237–245. doi: 10.1037//0735-7044.101.2.237. [DOI] [PubMed] [Google Scholar]
- Hori N, Lee M, Sasaguri K, Ishii H, Kamei M, Kimoto K, Toyoda M, Sato S. Supression of stress-induced nNOS expression in the rat hypothalamus by biting. Journal of Dental Research. 2005;84:624–628. doi: 10.1177/154405910508400708. [DOI] [PubMed] [Google Scholar]
- Hori N, Yuyama N, Tamura K. Biting suppresses stress-induced expression of corticotropin-releasing factor (CRF) in the rat hypothalamus. J Dent Res. 2004;83:124–128. doi: 10.1177/154405910408300208. [DOI] [PubMed] [Google Scholar]
- Joffe RT, Sokolov ST. Thyroid hormones, the brain, and affective disorders. Crit Rev Neurobiol. 1994;8:45–63. [PubMed] [Google Scholar]
- Johnson AJ, Jenks R, Miles C, Albert M, Cox M. Chewing gum moderates multi-task induced shifts in stress, mood, and alertness. A re-examination. Appetite. 2011;56:408–411. doi: 10.1016/j.appet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and biobehavioral reviews. 2010;35:2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Kademain S, Bignante A, Lardone P, McEwen B, Volosin M. Biphasic Effects of Adrenal Steroids on Learned Helplessness Behavior Induced by Inescapable Shock. Neuropsychopharmacology. 2005;30:58–66. doi: 10.1038/sj.npp.1300577. [DOI] [PubMed] [Google Scholar]
- Kim J, Diamond D. The stressed hippocampus, synaptic plasticity and lost memories. Nature Reviews:Neuroscience. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kong WM, Martin NM, Smith KL, Gardiner JV, Connoley IP, Stephens DA, Dhillo WS, Ghatei MA, Small CJ, Bloom SR. Triiodothyronine stimulates food intake via the hypothalamic ventromedial nucleus independent of changes in energy expenditure. Endocrinology. 2004;145:5252–5258. doi: 10.1210/en.2004-0545. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neuroscience and biobehavioral reviews. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Kubo KY, Sasaguri K, Ono Y, Yamamoto T, Takahashi T, Watanabe K, Karasawa N, Onozuka M. Chewing under restraint stress inhibits the stress-induced suppression of cell birth in the dentate gyrus of aged SAMP8 mice. Neurosci Lett. 2009;466:109–113. doi: 10.1016/j.neulet.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Langer P, Foldes O, Kvetnansky R, Culman J, Torda T, El Daher F. Pituitary-thyroid function during acute immobilization stress in rats. Exp Clinical Endocrinol. 1983;82:51–60. doi: 10.1055/s-0029-1210255. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Gorman J. A Call to Action: Overcoming Anxiety Through Active Coping. American Journal of Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Drugan RC, Hyson RL, Maier SF, Madden Jt, Barchas JD. Dissociation of long-term analgesia and the shuttle box escape deficit caused by inescapable shock. J Comp Physiol Psychol. 1982;96:904–912. [PubMed] [Google Scholar]
- Maier S. Learned Helplessness: Relationships with fear and anxiety. In: Stanford C, Salmon P, editors. Stress: From synapse to syndrome. London: Academic Press; 1993. pp. 207–243. [Google Scholar]
- Maier S, Ryan S, Barksdale C, Kalin N. Stressor Controllability and the Pituitary-Adrenal System. Behavioral Neuroscience. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Martijena I, Manzanares P, Lacerra C, Molina V. Gabaergic modulation of the stress response in frontal cortex and amygdala. Synapse. 2002;45:86–94. doi: 10.1002/syn.10085. [DOI] [PubMed] [Google Scholar]
- Martin P, Brochet D, Soubrie P, Simon P. Triiodothyronine-induced reversal of learned helplessness in rats. Biol Psychiatry. 1985;20:1023–1025. doi: 10.1016/0006-3223(85)90202-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Money S, Cheron R, Jaffe B, Zinner M. The effects of thyroid hormones on the formation of stress ulcers in the rat. J Surgical Res. 1986;40:176–180. doi: 10.1016/0022-4804(86)90120-4. [DOI] [PubMed] [Google Scholar]
- Mong JA, Pfaff DW. Hormonal symphony: steroid orchestration of gene modules for sociosexual behaviors. Mol Psychiatry. 2004;9:550–556. doi: 10.1038/sj.mp.4001493. [DOI] [PubMed] [Google Scholar]
- Mormede P, Dantzer R, Michaud B, Kelley KW, Le Moal M. Influence of stressor predictability and behavioral control on lymphocyte reactivity, antibody responses and neuroendocrine activation in rats. Physiol Behav. 1988;43:577–583. doi: 10.1016/0031-9384(88)90211-9. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Langeland W, Gersons BP. The psychobiology of PTSD: coping with trauma. Psychoneuroendocrinology. 2005;30:974–982. doi: 10.1016/j.psyneuen.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci. 2010;65:513–525. doi: 10.1093/geronb/gbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Mormede P. Stress and emotionality: a multidimensional and genetic approach. Neuroscience and biobehavioral reviews. 1998;22:33–57. doi: 10.1016/s0149-7634(97)00001-8. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiology & behavior. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Effects of chewing gum on cognitive function, mood and physiology in stressed and non-stressed volunteers. Nutr Neurosci. 2010;13:7–16. doi: 10.1179/147683010X12611460763526. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Espana RA, Berridge CW. Coping behavior causes asymmetric changes in neuronal activation in the prefrontal cortex and amygdala. Synapse. 2009;63:82–85. doi: 10.1002/syn.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui L, Wang F, Liu F, Wang J, Li BM. Dorsal hippocampal administration of triiodothyronine enhances long-term memory for trace cued and delay contextual fear conditioning in rats. J Neuroendocrinol. 2006;18:811–819. doi: 10.1111/j.1365-2826.2006.01480.x. [DOI] [PubMed] [Google Scholar]
- Swenson R, Vogel W. Plasma Catecholamine and Corticosterone as Well as Brain Catecholamine Changes During Coping in Rats Exposed to Stressful Footshock. Pharmacology Biochemistry and Behavior. 1983;18:689–693. doi: 10.1016/0091-3057(83)90007-2. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The study of instinct. New York: Oxford University Press; 1974. [Google Scholar]
- Woodson JC, Macintosh D, Fleshner M, Diamond DM. Emotion-induced amnesia in rats: working memory-specific impairment, corticosterone-memory correlation, and fear versus arousal effects on memory. Learn Mem. 2003;10:326–336. doi: 10.1101/lm.62903. [DOI] [PMC free article] [PubMed] [Google Scholar]