Abstract
The aim of this project was to develop a method to assess fiber type specific protein content across the continuum of human skeletal muscle fibers. Individual vastus lateralis muscle fibers (n = 264) were clipped into two portions, one for SDS-PAGE fiber typing and one for Western blot protein identification. Following fiber type determination, fiber segments were combined into fiber type specific pools (~20 fibers/pool) and measured for total protein quantity, GAPDH, citrate synthase, and total p38 content. GAPDH content was 64%, 54%, 160%, and 138% more abundant in MHC I/IIa, MHC IIa, MHC IIa/IIx, and MHC IIx fibers when compared to MHC I. Inversely, citrate synthase content was 528%, 472%, 242%, and 47% more abundant in MHC I, MHC I/IIa, MHC IIa, and MHC IIa/IIx fibers when compared to MHC IIx. Total p38 content was 87% greater in MHC IIa versus MHC I fibers. These data and approach establish a reliable method for human skeletal muscle fiber type specific protein analysis. Initial results show particular proteins exist in a hierarchal fashion throughout the continuum of human skeletal muscle fiber types, further highlighting the necessity of fiber type specific analysis.
Keywords: Western blot, GAPDH, Citrate Synthase, p38
INTRODUCTION
Skeletal muscle is a complex multicellular tissue comprised of several muscle fiber types that have distinctly different functional and metabolic profiles [1, 2]. Muscle fiber type composition profoundly influences muscle performance, which has implications for human locomotion, athletic performance, and clinical conditions such as sarcopenia, muscle wasting, and muscle-associated metabolic disorders. Human muscle fiber types can be defined in various ways with the contractile protein myosin heavy chain (MHC), a common identifier. MHC is found in the forms of Type I, IIa, and IIx, and muscle fibers can contain only one (i.e., a pure fiber) or a combination (i.e., a hybrid fiber) of these isoforms, giving rise to the five most prevalent muscle fiber types in humans (I, I/IIa, IIa, IIa/IIx, IIx) [3, 4]. Once thought to be mostly static in adult humans [5, 6], the fiber type profile of human skeletal muscle has now been shown to have a high degree of plasticity, with a primary regulator of fiber type alterations being physical activity [7-15].
A major focus of our laboratory for the past 15 years has been to examine alterations in human single muscle fiber size, MHC composition, and contractile function with changes in physical activity patterns. To complement the fiber type specific nature of the contractile findings and to better understand muscle adaptation across the continuum of muscle fiber types, we have recently developed fiber type specific methods to measure mRNA at the individual transcript [16, 17] and transcriptome [18] levels, and stable isotopic methods to measure the rate of protein synthesis [19]. The purpose of this study was to extend these methods to include measurements of human muscle fiber type specific protein content. To define the method, we examined an anaerobic cytosolic enzyme (GAPDH), an aerobic mitochondrial enzyme (citrate synthase), and a regulatory protein (p38). We also examined single muscle fiber size and power to characterize the range in contractile performance among the fiber types studied for protein content. The methods outlined here can be adapted for an unlimited number of proteins found in skeletal muscle. Application of these methods will add to our understanding of skeletal muscle plasticity in a fiber specific manner and add insight into skeletal muscle health among active, inactive, and diseased states.
METHODS
Acquisition and Storage of Muscle Tissue
A vastus lateralis muscle biopsy [20] was self-obtained with implied consent by the senior investigator (44 y, 182 cm, 72 kg, VO2max = 4.4 L/min) who had >30 y of consistent training; ~6-8 hr/wk of moderate to vigorous cycling, swimming, and/or running. The tissue was separated into multiple ~15 mg pieces and 1) quickly frozen in liquid nitrogen and stored at -190°C for protein assessment or 2) placed in cold skinning solution (see below) and stored at -20°C for later analysis of single muscle fiber contractile performance.
Preservation of Muscle Tissue for Western Blotting
When ready to be processed, liquid nitrogen frozen muscle samples were placed into pre-chilled (30 min at -30°C) tubes containing 400 μl of RNAlater-ICE (Ambion, Austin, TX) and stored at -20°C for 48 hours. After the muscle tissue was equilibrated in RNAlater-ICE, it was placed into a second aliquot of 500 μl of RNAlater [21], and stored at -20°C until isolation of individual muscle fibers. RNAlater was chosen as the fiber isolation medium for the following reasons: 1) RNAlater preserves the stability of denatured proteins [22], making them suitable for Western blotting and 2) RNAlater does not disrupt the structure of tissue, thus tissue equilibrated in RNAlater can be removed from solution, handled at room temperature, and returned to RNAlater without degradation of molecular components [21, 23].
Single Fiber Isolation for Western Blot Analysis
A small bundle of muscle fibers (7-10 mm in length) was sectioned off the tissue sample in a Petri dish containing 1.5 ml of RNAlater. Individual muscle fibers were isolated with fine tweezers under a light microscope at room temperature [16, 17, 19]. As described previously [16], approximately one-quarter of each isolated fiber was clipped and placed into a tube with 40 μl of SDS sample buffer (1% SDS, 6 mg/ml EDTA, 0.06 M Tris, pH 6.8, 2 mg/ml bromophenol blue, 15% glycerol, and 5% β-mercaptoethanol) for myosin heavy chain (MHC) fiber type identification by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (see Single Muscle Fiber Myosin Heavy Chain Identification section). The remaining three-quarters of the isolated muscle fiber was placed into an individual well of a 96-well plate with 75 μl of RNAlater and stored at -20°C until MHC identification was completed.
Muscle Fiber Pooling and Homogenization
Two hundred and sixty four individual fibers were isolated, fiber typed based on MHC isoform composition (see Single Muscle Fiber Myosin Heavy Chain Identification section), and designated for Western blotting. Depending on availability, approximately 20 (range; 5-24) fibers of identical fiber type were then removed from the 96-well plate and washed with cold 1X PBS (Phosphate buffered saline; Sigma) in a Petri dish (5 min). This was done to remove any excess RNAlater as it exhibits poor compatibility with bicinchoninic acid (BCA) protein assays and substantially lowers the optical density of measured proteins. Washed fibers of identical fiber types (i.e. fiber-pool sample) were then collected with tweezers, placed in 60 μl of RIPA buffer (Pierce, Rockford, IL USA) with freshly added Halt™ Protease Inhibitor Cocktail (Pierce) and Phosphatase Inhibitor Cocktail (Pierce), homogenized for one minute with an electrically powered Teflon tip in a Teflon tube, and kept on ice.
Protein Assay
Protein concentrations for each fiber-pool were determined by a BCA protein assay kit using a bovine serum albumin standard. After being stored on ice, a 20 μl aliquot from each fiber-pool sample was combined with 80 μl of RIPA buffer (plus inhibitors) (1:5 v/v), mixed thoroughly, and pipetted in triplicate (25 μl each) into a 96-well plate. Protein concentrations were read on a Wallac Victor 2 plate reader (Boston, MA, USA). As much of the remaining sample volume as possible was precisely pipetted and diluted with 2X blue buffer (2% SDS, 12 mg/ml EDTA, 0.12 M Tris (hydroxymethyl) aminomethane (pH 6.8), 2 mg/ml bromophenol blue, 15% glycerol and 10% b-mercaptoethanol) to a concentration of 0.5 μg/μl, and stored (-80°C).
MHC Verification of Pooled Muscle Fiber Samples
To verify the MHC isoform content in each fiber-pool, 10 μl of each sample [from BCA (1:5 v/v proteins/RIPA)] was combined with SDS sample buffer (1:10 v/v), run through SDS-PAGE, and assessed for MHC isoform composition based on migration distance (see Single Muscle Fiber Myosin Heavy Chain Identification section).
Western Blot Analysis
Western blot analyses were performed as previously described by our laboratory [24, 25]. Each Western blot sample (i.e. the samples which were assayed for protein concentration and then diluted with 2X blue buffer) was heated in a heating block for three minutes at 95°C prior to its first Western blot use. To determine the optimal amount of total protein to be loaded, a separate mixed muscle homogenate sample was used to perform load spectrum (from 20 μg down to 1 μg) experimentts on each protein of interest. The smallest amount of protein that produced the most consistent band was considered optimal. Equal amounts of total protein, as determined by using the BCA protein assay kit [1 μg for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 3 μg for Citrate Synthase (CS), and 5 μg for total p38] were then separated with a 10% gradient gel (Pierce) using SDS-PAGE for 75 minutes at 100V and transferred to a polyvinylidene fluoride (PVDF) membrane (Immobilon-P, Millipore, Bedford, MA) for 90 minutes at 40V at ~4°C. The membrane was blocked with 5% milk in Tris-buffered saline (pH 7.6) and 0.1% Tween- 20 (TBST) for one hour (gently rocking at room temperature), washed (3 × 5 min) in 1×TBST, and incubated with a primary antibody in 1×TBST for actin, (1:1000) (#4968, Cell Signaling), GAPDH (1:1000) (#2118, Cell Signaling) CS (1:1000) (ab96600, Abcam), or total p38 (1:1000) (#9211S, Cell Signaling) at 4°C overnight. Blots were washed (3 × 5 min in 1×TBST), incubated in an anti-rabbit horseradish peroxidase-conjugated- secondary antibody (1:4000) (#7074, Cell Signaling) mixed with 5% milk in 1×TBST for 1 hour, and washed again (4 × 10 min in 1×TBST) prior to being exposed for five minutes to either an enhanced chemiluminescent substrate (Amersham ECL Plus Western Blotting Detection System, GE) for CS and total p38 or a standard substrate (West Pico, Pierce) for GAPDH. Excess chemiluminescent substrate was removed and the blot was placed inside a plastic sheet protector to allow digital images of protein bands to be captured using a chemiluminescent imaging system (FluorChem SP, Alpha Innotech). Protein band density was analyzed using the spot density analysis software provided with the imaging system. A rectangular box was drawn around each protein band. The software generated a total value (integrated density value = IDV) of all pixel grey levels within each box. The total IDV was further adjusted by the size of the box and background, and represented the total protein signal in the band.
Molecular weight markers See Blue Plus 2 (MW range: 4-250 kDa) & Magic Mark (MW range: 20-220 kDa) (Invitrogen) were used throughout the study. Specifically, the See Blue Plus 2 marker was used as a visual guide during the gel run and transfer onto the PVDF membrane, while the Magic Mark marker was relied upon during the imaging phase since its protein molecular weights range (4-250 kDa) becomes visible with chemiluminescence. We relied upon the molecular weight markers 30 kDa, 40 kDa, 50 kDa, and 60 kDa to verify the identity of our proteins of interest (MW range: 37-52 kDa).
Considering small amounts of protein were to be loaded for all proteins of interest, we carefully evaluated Western blot detection and transfer capabilities. We chose to perform this evaluation (see results) with GAPDH since our load spectrum experiments suggested that 1 μg of total protein would be optimal for GAPDH analysis, and thus protein quantities in our samples would not be a limitation for this evaluation. Consistent sample loading was examined by loading 1 μg of total protein from a single MHC I and MHC IIa sample in quintuplicate and analyzing for GAPDH. To examine equal transferring across the PVDF membrane, the MHC I and MHC IIa samples were loaded at 1 and 2 μg of total protein in triplicate and analyzed for GAPDH. Detection accuracy (i.e. linearity) of our system was evaluated by plotting GAPDH signal (IDV) against a loading range consisting of five different amounts of total protein (0.125, 0.25, 0.5, 1.0, and 2.0 μg) from a MHC IIa sample, loaded in triplicate. Also, transfer accuracy of lower than 1 μg of total protein (0.125, 0.25, and 0.5 μg) loaded was examined in this linearity experiment.
The fiber type specific Western data presented here were based upon equal loading from protein assay results (see Results section). We also performed a series of experiments in which protein content was normalized to actin (data not shown), producing similar results to the equal protein loading approach. Although actin appears stable across all fiber types in the current study, it is our opinion that fiber type specific Western analysis may be performed without a control protein as long as equal protein loading and transfer is empirically evaluated. Considering protein expression differs between fiber types, age groups, and with interventions, finding a suitable control protein capable of covering all scenarios may be challenging.
Skinning, Relaxing and Activating Solutions for Single Fiber Contractile Experiments
Muscle fibers designated for contractile function measurements were placed in skinning solution containing (mM): 125 potassium propionate, 2.0 EGTA, 4.0 ATP, 1.0 MgCl2 and 20.0 imidazole (pH 7.0), and 50% (v/v) glycerol. Composition of relaxing and activating solutions were calculated using an interactive computer program described by Fabiato & Fabiato [26]. Solutions contained (mM): 7.0 EGTA, 20.0 idazole, 14.5 creatine phosphate, 1.0 free Mg2+, 4.0 free MgATP, KCl and KOH to produce an ionic strength of 180 mM and a pH of 7.0 and were adjusted for temperature, pH, and ionic strength using stability constants in the calculations [27]. Relaxing and activating solutions had free [Ca2+] of pCa 9.0 and pCa 4.5, respectively (where pCa = -log [Ca2+]).
Single Muscle Fiber Contractile Performance
The measurement of fiber size and corresponding single fiber contractile performance was carried out on single muscle fibers (n = 86) isolated from the muscle biopsy as we have previously described [28-31]. Following fiber isolation and measurement of diameter and power, each muscle fiber's MHC composition was determined as described below.
Single Muscle Fiber Myosin Heavy Chain Identification
The MHC isoform (fiber type) of each isolated muscle fiber (from both protein content and contractile performance experiments) was determined by SDS-PAGE (SE 600 series, Hoefer, San Francisco, CA) as previously described in detail by our laboratory [32]. The clipped portion (~2 mm) of each muscle fiber designated for Western blot analysis was solubilized in 40 μl of 1% SDS sample buffer. After contractile performance experiments, a ~3-4 mm segment of each fiber was solubilized in 80 μl of 1% SDS sample buffer. All samples were individually loaded (5 μl for Western blot samples and 2 μl for contractile performance samples) on a 3.5% stacking and 5% separating gel and run for 17 hours (overnight) at 4°C. Gels were then silver stained [33], allowing the MHC isoform profile (MHC I, I/IIa, IIa, IIa/IIx, IIx) of each muscle fiber to be determined according to migration distance.
Data Analysis
Results are reported as mean or mean ± SE when appropriate. The Western blot results are presented as Integrated Density Values (IDV) obtained for each protein band using spot density analysis software. Differences in protein content between fiber types are presented as the percent increase from the lowest value. Specifically, GAPDH and p38 are reported as the percent increase from MHC I while CS is reported as the percent increase from MHC IIx. When applicable, a coefficient of variation of <15% was accepted for the Western blot data analysis.
RESULTS
MHC Isoform Identification and Total Protein Concentration
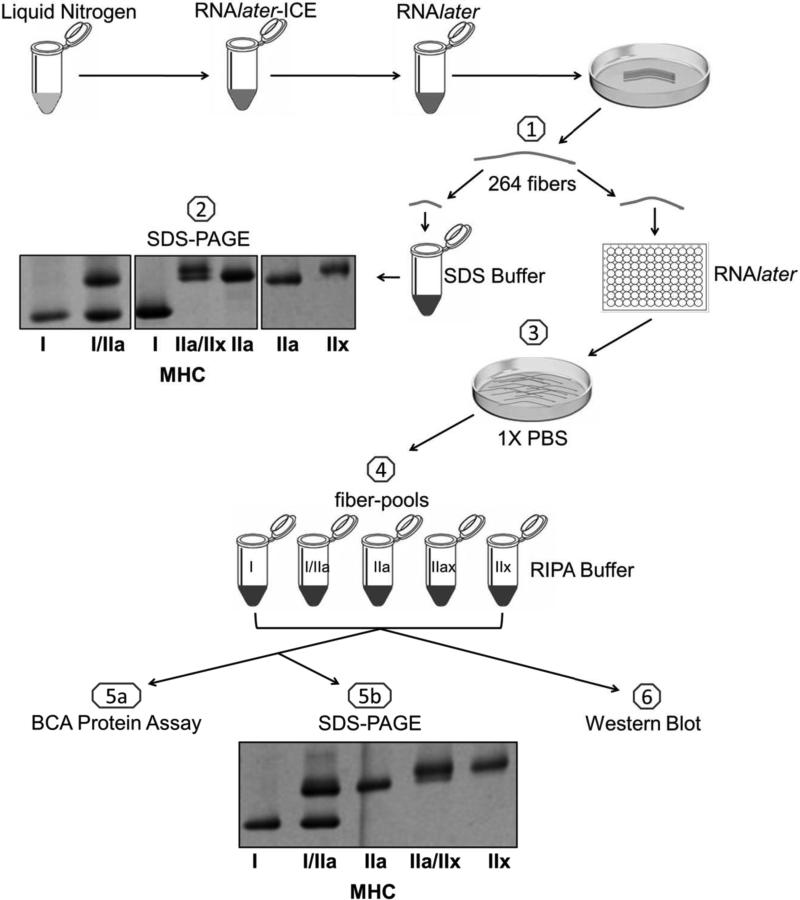
A schematic representation of study design is shown in Figure 1 (step 1-6). Isolation of muscle fibers designated for Western blotting is shown in step 1. Images of individual muscle fibers representing five different MHC isoform(s) are shown in step 2. Identified muscle fibers (n = 264: 135 MHC I, 5 MHC I/IIa, 89 MHC IIa, 24 MHC IIa/IIx, 11 MHC IIx) were washed in PBS (step 3) and then used to create six 20-fiber-pools of MHC I, four 20-fiber-pools of MHC IIa, and a single pool of 24 MHC IIa/IIx fibers. Since the number of MHC IIx and MHC I/IIa fibers in the muscle sample was limited, only one 11-fiber-pool of MHC IIx and one 5-fiber-pool of MHC I/IIa fibers was generated (step 4). The protein yield in the fiber-pools listed above ranged from 22 to 70 μg of total protein per 60 μl (step 5a). As shown in step 5b, fiber type pools were assembled correctly as assessed by SDS-PAGE. Densitometry of the hybrid pools in step 5b revealed the MHC I/IIa pool was 54% MHC I and 46% MHC IIa while the MHC IIa/IIx fiber-pool was 34% MHC IIa and 66% MHC IIx.
Figure 1.
Schematic representation of study design. 1) Two hundred and sixty four single human skeletal muscle fibers were isolated, clipped, and stored in SDS-Buffer and RNAlater. 2) All single fibers were subjected to SDS-PAGE with silver staining and classified based on migration distance. The image represents seven of these fibers. Next, single fibers of the same MHC composition were 3) briefly washed in cold 1X PBS and 4) combined into pools of 20 fibers for MHC I and MHC IIa; a pool of 11 for MHC IIx; a pool of 24 for MHC IIa/IIx and a pool of 5 for MHC IIx. 5a) The protein concentration of each fiber-pool was determined using the BCA protein assay kit. 5b) An aliquot from each fiber-pool was re-typed through SDS-PAGE to verify its MHC content. MHC = myosin heavy chain. 6) The remaining portion of each fiber-pool was utilized to assess protein content via Western blotting.
Fiber Type Specific Protein Content
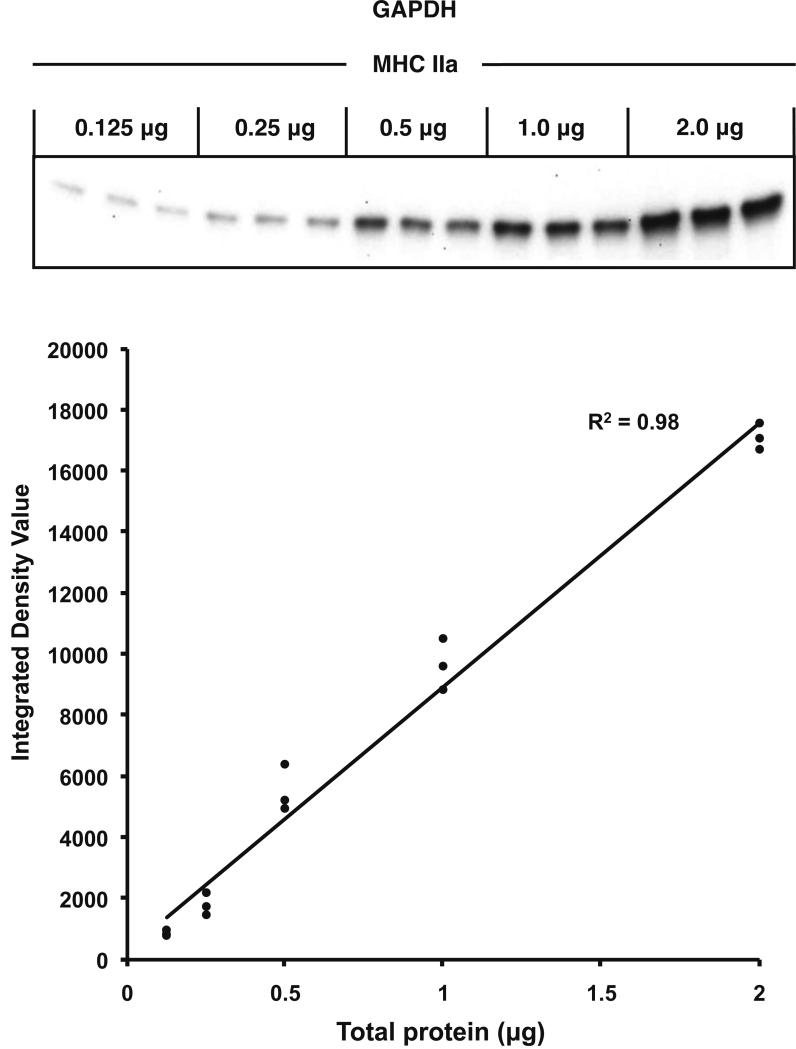
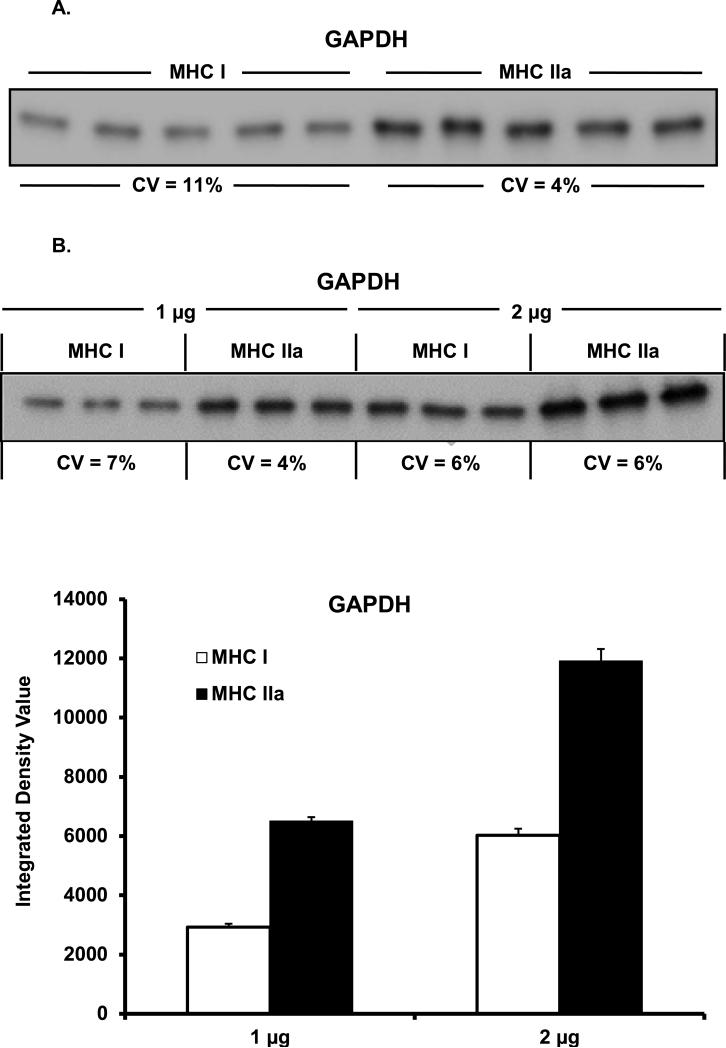
Given the relatively small amounts of protein in the fiber-pool samples, our Western blot system accuracy was examined. Linearity of our system was demonstrated by loading five different amounts of total protein from a MHC IIa sample, ranging from 0.125 μg to 2.0 μg. As can be seen in Figure 2, linearity of GAPDH content was confirmed (R2 = 0.98). Consistent loading of small amounts of protein was confirmed by loading 1 μg of total protein from a single MHC I and MHC IIa sample in quintuplicate and analyzed for GAPDH abundance. As shown in Figure 3A, consistent loading was achieved as the coefficient of variation was 11% and 4% for the MHC I and MHC IIa pools, respectively. The integrated density value (IDV) showed 96% greater content of GAPDH in MHC IIa (15,337 ± 298 IDV) fibers when compared to MHC I (7,826 ± 392 IDV) fibers.
Figure 2.
Verification of Western blot system linearity. Linearity of our system was examined by loading five different amounts of total protein (0.125, 0.25, 0.5, 1.0, and 2.0 μg) in triplicate from a MHC IIa sample, and evaluated for GAPDH content. The GAPDH signal (Integrated Density Value = IDV) was plotted against corresponding amounts of total protein.
Figure 3A & B.
Western blot GAPDH load and transfer evaluation. (A) Single MHC I and MHC IIa samples were loaded (1 μg total protein) in quintuplicate (CV = 11% and 4%, respectively). MHC IIa fibers displayed 96% more GAPDH when compared to MHC I fibers. (B) Single MHC I and MHC IIa samples were loaded in triplicate at 1 μg and 2 μg of total protein and assessed for GAPDH content. GAPDH protein content doubled when 2 μg was compared to 1 μg. MHC IIa fibers again displayed 123% and 98% more GAPDH when compared to MHC I fibers at 1 and 2 μg, respectively. MHC = myosin heavy chain. CV = coefficient of variation.
Consistent transferring of small amounts of protein across the PVDF membrane was verified by loading 1 and 2 μg of total protein from MHC I and MHC IIa sample in triplicate and analyzing for GAPDH content (Figure 3B) [(1μg MHC I; 2,920 ± 124 IDV, CV = 7% and MHC IIa; 6,502 ± 134 IDV, CV = 4%) (2 μg MHC I; 6,029 ± 214 IDV, CV = 6% and MHC IIa; 11,907 ± 413 IDV, CV = 6%)]. GAPDH content doubled in both MHC I and MHC IIa when the amount of sample loaded doubled from 1 μg to 2 μg, suggesting consistent transferring. Similar to the previous experiments, a difference in GAPDH content was observed between MHC I and MHC IIa of 123% (1 μg) and 98% (2 μg).
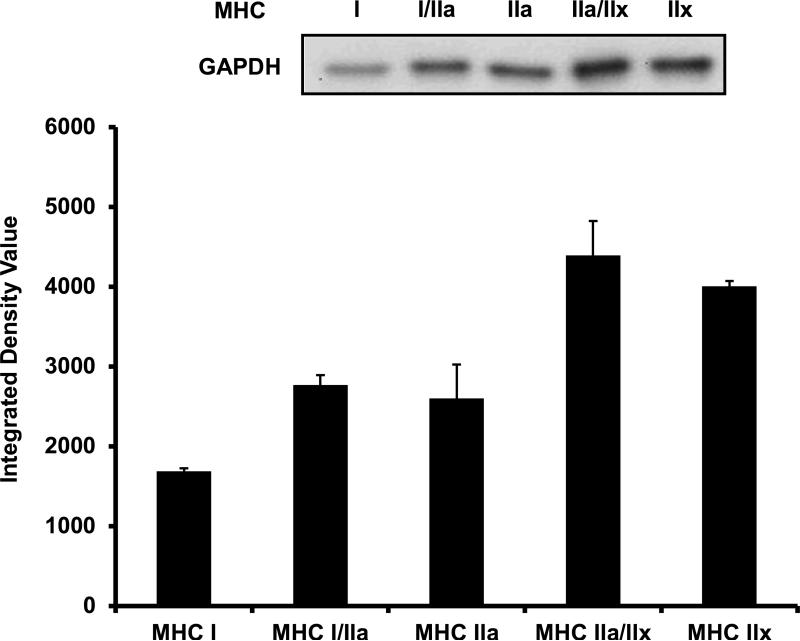
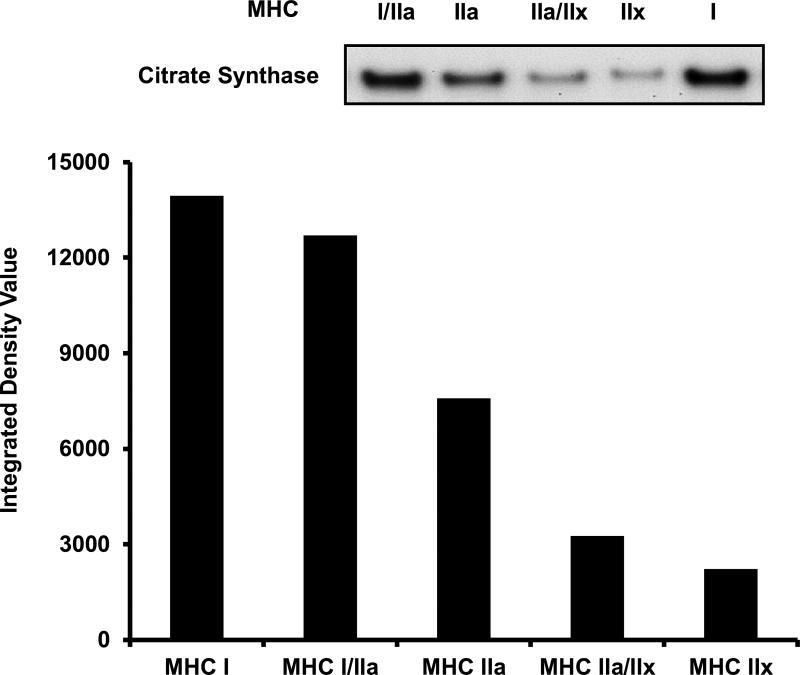
Next, we examined GAPDH and CS protein content throughout the continuum of skeletal muscle fiber types. In 1 μg of total protein, GAPDH content displayed 64%, 54%, 160%, and 138% greater abundance in MHC I/IIa (2,765 ± 125 IDV), MHC IIa (2,599 ± 429 IDV), MHC IIa/IIx (4,390 ± 430 IDV), and MHC IIx (4,004 ± 69 IDV) fibers when compared to MHC I (1,686 ± 41 IDV) fibers, respectively (Figure 4). When CS content in 3 μg of total protein was measured across the fiber type continuum, MHC I (13,946 IDV), MHC I/IIa (12,699 IDV), MHC IIa (7,588 IDV), and MHC IIa/IIx (3,263 IDV) fibers displayed 528%, 472%, 242%, and 47% greater CS content when compared to MHC IIx (2,221 IDV) fibers, respectively (Figure 5).
Figure 4.
GAPDH content across the continuum of human skeletal muscle fiber types. Lanes 1-5 were loaded with 1 μg of total protein as follows: MHC I, MHC I/IIa, MHC IIa, MHC IIa/IIx, and MHC IIx. The loading scheme was repeated in lanes 6-10 (not shown) and the densitometry value for each isoform was averaged. MHC I/IIa, MHC IIa, MHC IIa/IIx, and MHC IIx displayed 64%, 54%, 160%, and 138% greater content of GAPDH when compared to MHC I, respectively. MHC = myosin heavy chain.
Figure 5.
CS content across the continuum of human skeletal muscle fiber types. Lanes were loaded with 3 μg of total protein as follows: MHC I/IIa, MHC IIa, MHC IIa/IIx, MHC IIx, and MHC I. MHC I, MHC I/IIa, MHC IIa, and MHC IIa/IIx displayed 528%, 472%, 242%, and 47% greater content of CS when compared to MHC IIx, respectively. Due to sample limitation, only one CS fiber type continuum experiment was performed and therefore SE values are not available. MHC = myosin heavy chain. CS = Citrate Synthase.
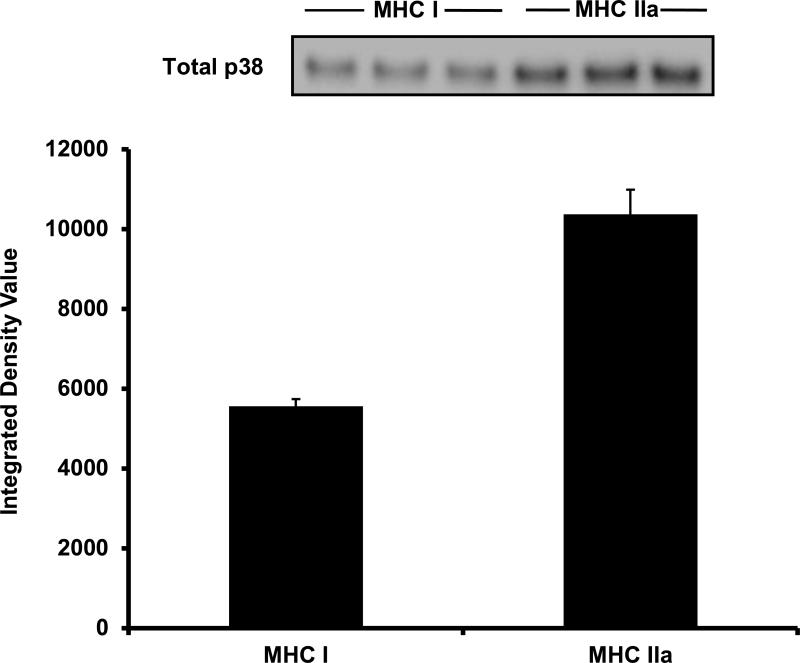
The final goal was to expand our analysis to include a protein of lower abundance than GAPDH and CS. Due to sample limitation, total p38 content in 5 μg of total protein was analyzed only in a single MHC I and MHC IIa sample. Much like GAPDH, p38 showed 87% greater content in MHC IIa (IDV = 10,366 ± 616, CV = 9%) fibers when compared to MHC I (IDV = 5,557 ± 186, CV = 1%) fibers (Figure 6).
Figure 6.
Total p38 content in MHC I and MHC IIa fiber-pools. In 5 μg of total protein, MHC IIa fibers displayed 87% more total p38 when compared to MHC I fibers. MHC = Myosin heavy chain.
Single Fiber Size and Contractile Performance
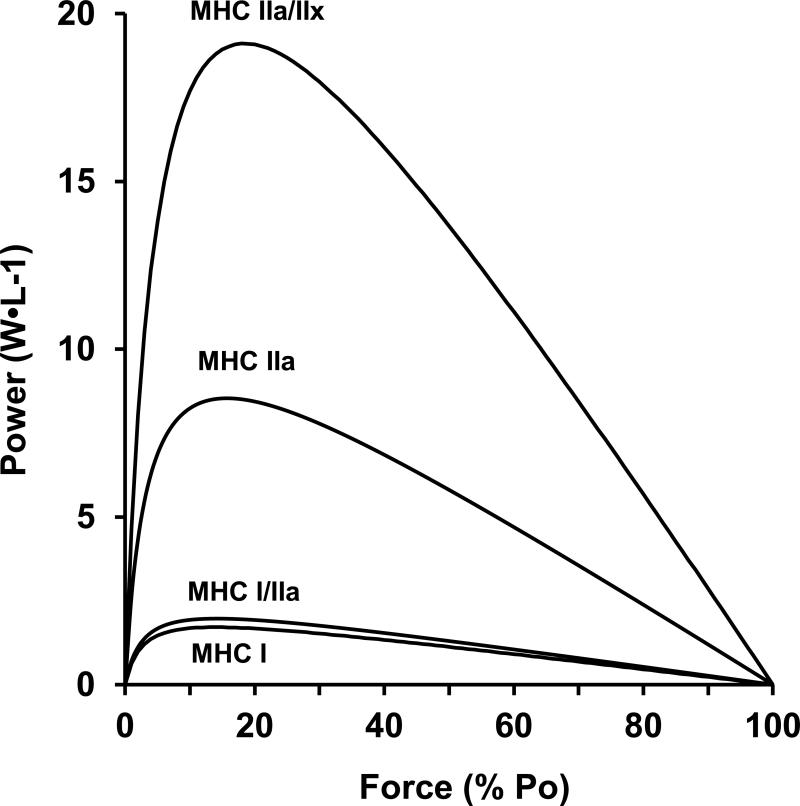
Eighty-six fibers were examined to determine contractile performance. Diameter and fiber type distribution are presented in Table 1 and power (normalized to cell volume) is depicted in Figure 7. Power increased 2, 6, and 12 fold for MHC I/IIa, MHC IIa, and MHC IIa/IIx fibers, respectively, when compared to MHC I fibers. No pure MHC IIx fibers were studied.
Table 1.
Single muscle fiber size.
| Fiber type | n (# of fibers) | Fiber CSA (μm2) |
|---|---|---|
| MHC I | 39 | 8872 ± 350 |
| MHC I/IIa | 6 | 8175 ± 497 |
| MHC IIa | 33 | 10,441 ± 481 |
| MHC IIa/IIx | 8 | 6009 ± 642 |
Data are presented as mean ± SE. MHC = Myosin heavy chain. CSA = Cross sectional area.
Figure 7.
Individual human skeletal muscle fibers were studied for their contractile performance. Normalized power was 2, 6, and 12 fold greater in MHC I/IIa, MHC IIa, and MHC IIa/IIx fibers when compared to MHC I fibers. No MHC IIx fibers were studied. MHC = myosin heavy chain.
DISCUSSION
We sought to develop an approach to examine fiber type specific protein content from human muscle biopsy samples. Of consideration was an approach that would accurately identify fiber types across the continuum, have the ability to assess a large number of proteins from a given fiber type, and be reasonable from both a time and cost perspective when applying to human based exercise studies. The primary findings were: 1) fiber type specific protein content can be measured via Western blotting in conjunction with SDS-PAGE fiber typing across the continuum of human skeletal muscle fiber types (MHC I, I/IIa, IIa, IIa/IIx, IIx), 2) further support for the method outlined here was the expected findings of anaerobic (GAPDH), aerobic (citrate synthase), and regulatory (p38) protein content in slow (MHC I) and fast (MHC IIa) muscle fiber types, and 3) for the first time, hybrid (I/IIa, IIa/IIx) and the MHC IIx fibers were shown to have metabolic protein content that generally existed in a hierarchal fashion along the continuum of human muscle fiber types.
The method we present here was shown to be consistent and adaptable for applications that require low amounts of protein. Support for this assay approach with human muscle biopsy samples comes from the consistent protein load and transfer studies (1 vs. 2 μg) and low coefficient of variation among the multiple loading paradigms employed in this investigation. For fiber typing we chose to use SDS-PAGE due to our familiarity with the technique and to decrease the likelihood of fiber misclassification compared to other approaches for identifying fiber types in humans [7, 32, 34]. The SDS-PAGE approach to fiber typing enables a large volume of muscle fibers (200-400) to be examined relatively rapidly (<24 h) with identification of the five main fiber types in human skeletal muscle (MHC I, I/IIa, IIa, IIa/IIx, IIx) without the need for fiber type specific antibodies.
The fiber-pooling approach in the current study generated enough total protein to allow the use of a commercially available protein assay kit. This is highly favorable for Western blot analysis as it enables a known amount of total protein to be loaded and serve as a control, and thereby eliminates the need for a control/housekeeping protein, which may be challenging to define for different fiber types, age groups, and interventions. The protein assay analysis also enabled us to perform a loading spectrum step for each protein of interest to establish the optimal amount of protein to be loaded for the Western blot analysis. The load spectrum is important since proteins have a wide range of molecular weight and abundance in human skeletal muscle. This was highlighted in the current investigation, as each protein of interest required a different optimal loading amount for the Western blot analysis (GAPDH = 1 μg, CS = 3 μg, p38 = 5 μg). The load spectrum step optimizes loading for Western blot analysis, which increases Western blotting accuracy [35] and saves samples.
Another consideration for our approach was to develop a method that would yield enough total protein to allow for assessment of multiple proteins for a given fiber type. The fiber-pooling model accomplished this goal. This is important when examining numerous proteins in a particular pathway or any type of analysis that may require several proteins to be analyzed. Compared to an approach of analyzing one muscle fiber at a time, fiber pooling helps reduce the volume of Western blot work, which saves time and resources. It should be noted that obtaining 20 (or more) fibers of a given MHC type is not always possible (i.e. targeting less abundant isoforms in limited tissue samples). However, this is not a major limitation as the MHC I/IIa and MHC IIx pools analyzed here only contained 5 and 11 fibers, respectively, but still generated enough total protein to assess some proteins of interest. The fiber-pooling model outlined here is also advantageous for researchers conducting human based experiments. In these types of experiments where multiple treatment groups and pre-to-post interventions are common, examination of protein content across the continuum of fiber types can be easily managed when using the fiber-pooling model.
Several laboratories have used alternative techniques (e.g. fluorescent staining or immunohistochemistry) to assess metabolic [36-38] and size regulating [39-44] proteins in human MHC I and IIa fibers. The methodology presented here was the first to employ SDS-PAGE fiber typing and the first to assess protein content in hybrid (MHC I/IIa and MHC IIa/IIx) and pure MHC IIx fibers via Western blots. The inclusion of the hybrid muscle fibers is a valuable aspect of our model as they are prevalent in most human populations and are particularly responsive to chronic perturbation (e.g., aging, unloading, exercise) [11, 13, 31, 32]. Conversely, the MHC IIx fibers in human muscle are generally rare (<2%) in active healthy individuals [7, 34, 45], but can become more prominent in inactive and diseased states [15]. When assessing various aspects of skeletal muscle health and plasticity, the continuum of fiber types should be considered to better understand muscle adaptation.
Application of this technique indicates both metabolic (GAPDH and CS) and signaling proteins (p38) exist in a hierarchal manner across all fiber types. GAPDH is a major facilitator of glycolysis and thus not surprisingly, was more abundant in the fast-twitch fibers. Previous literature supports this contention as GAPDH mRNA abundance [46], activity [47], and protein content exist preferentially in fast-twitch fibers in active [48-50], inactive [49, 50], diseased [51], and aging [46, 47] humans. Inversely, CS content (a common marker of mitochondrial abundance and oxidative capacity) was greatest in slow-twitch fibers. These data complement the well accepted notion that glycolytic enzymes are more abundant in fast-twitch fibers and oxidative enzymes are more abundant in slow-twitch fibers [1]. However, for the first time this concept is extended to include hybrid fiber types. We also found a signaling protein (p38) to be more abundant in fast-twitch fibers. Future research will be required to determine if this is consistent in the human vastus lateralis or if factors such as the subjects’ long history of aerobic endurance exercise influenced the findings.
In summary, we present a new methodology for the assessment of human skeletal muscle fiber type specific protein content that is compatible with SDS-PAGE fiber typing, a standard protein assay kit, and Western blotting. The initial results indicate a hierarchal relationship in specific protein content and contractile function across the entire continuum of human skeletal muscle fiber types. This technique will allow future research to determine changes in protein content in response to various perturbations (e.g., aging, disease, exercise) in MHC I and MHC IIa as well as the understudied hybrid and MHC IIx fibers, providing a more complete profile of skeletal muscle health in humans.
ACKNOWLEDGMENTS
This investigation was supported by National Aeronautics and Space Administration grant NNJ06HF59G (S. Trappe) and National Institutes of Health grant AG038576 (S. Trappe).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Saltin B, Gollnick P. Handbook of Physiology. Skeletal Muscle. Am. Physiol. Soc.; Bethesda, MD: 1983. Skeletal muscle adaptability: significance for metabolism and performance. [Google Scholar]
- 2.Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol. 1999;9:87–95. doi: 10.1016/s1050-6411(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 3.Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:C1723–8. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- 4.Billeter R, Heizmann CW, Howald H, Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem. 1981;116:389–95. doi: 10.1111/j.1432-1033.1981.tb05347.x. [DOI] [PubMed] [Google Scholar]
- 5.Gollnick PD, Armstrong RB, Saltin B, Saubert C.W.t., Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34:107–11. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- 6.Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–44. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- 7.Andersen JL, Klitgaard H, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of sprinters: influence of training. Acta Physiol Scand. 1994;151:135–42. doi: 10.1111/j.1748-1716.1994.tb09730.x. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher P, Trappe S, Harber M, Creer A, Mazzetti S, Trappe T, Alkner B, Tesch P. Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol Scand. 2005;185:61–9. doi: 10.1111/j.1365-201X.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 9.Klitgaard H, Mantoni M, Schiaffino S, Ausoni S, Gorza L, Laurent-Winter C, Schnohr P, Saltin B. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand. 1990;140:41–54. doi: 10.1111/j.1748-1716.1990.tb08974.x. [DOI] [PubMed] [Google Scholar]
- 10.Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, Saltin B. Ageing alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiol Scand. 1990;140:55–62. doi: 10.1111/j.1748-1716.1990.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 11.Trappe S, Harber M, Creer A, Gallagher P, Slivka D, Minchev K, Whitsett D. Single muscle fiber adaptations with marathon training. J Appl Physiol. 2006;101:721–7. doi: 10.1152/japplphysiol.01595.2005. [DOI] [PubMed] [Google Scholar]
- 12.Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol. 2009;106:1159–68. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 13.Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol. 2001;91:1955–61. doi: 10.1152/jappl.2001.91.5.1955. [DOI] [PubMed] [Google Scholar]
- 14.Zhou MY, Klitgaard H, Saltin B, Roy RR, Edgerton VR, Gollnick PD. Myosin heavy chain isoforms of human muscle after short-term spaceflight. J Appl Physiol. 1995;78:1740–4. doi: 10.1152/jappl.1995.78.5.1740. [DOI] [PubMed] [Google Scholar]
- 15.Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D. Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol. 2007;102:340–9. doi: 10.1152/japplphysiol.00609.2006. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Jemiolo B, Trappe S. Proteolytic mRNA expression in response to acute resistance exercise in human single skeletal muscle fibers. J Appl Physiol. 2006;101:1442–50. doi: 10.1152/japplphysiol.00438.2006. [DOI] [PubMed] [Google Scholar]
- 17.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun. 2004;320:1043–50. doi: 10.1016/j.bbrc.2004.05.223. [DOI] [PubMed] [Google Scholar]
- 18.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe SW. Transcriptome Signature of Resistance Exercise Adaptations: Mixed Muscle and Fiber Type Specific Profiles in Young and Old Adults. J Appl Physiol. 2012 doi: 10.1152/japplphysiol.00435.2011. doi:10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickinson JM, Lee JD, Sullivan BE, Harber MP, Trappe SW, Trappe TA. A new method to study in vivo protein synthesis in slow- and fast-twitch muscle fibers and initial measurements in humans. J Appl Physiol. 2010;108:1410–6. doi: 10.1152/japplphysiol.00905.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest Suppl. 1962:1–110. [Google Scholar]
- 21.Ambion RNAlater tissue collection: RNA stabilization solution. http://wwwambioncom/techlib/prot/bp_7020pdf.
- 22.De Paepe ME, Mao Q, Huang C, Zhu D, Jackson CL, Hansen K. Postmortem RNA and protein stability in perinatal human lungs. Diagn Mol Pathol. 2002;11:170–6. doi: 10.1097/00019606-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Kasahara T, Miyazaki T, Nitta H, Ono A, Miyagishima T, Nagao T, Urushidani T. Evaluation of methods for duration of preservation of RNA quality in rat liver used for transcriptome analysis. J Toxicol Sci. 2006;31:509–19. doi: 10.2131/jts.31.509. [DOI] [PubMed] [Google Scholar]
- 24.Williamson DL, Raue U, Slivka DR, Trappe S. Resistance exercise, skeletal muscle FOXO3A, and 85-year-old women. J Gerontol A Biol Sci Med Sci. 2010;65:335–43. doi: 10.1093/gerona/glq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547:977–87. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 27.Godt RE, Lindley BD. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982;80:279–97. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luden N, Hayes E, Galpin A, Minchev K, Jemiolo B, Raue U, Trappe TA, Harber MP, Bowers T, Trappe S. Myocellular basis for tapering in competitive distance runners. J Appl Physiol. 2010;108:1501–9. doi: 10.1152/japplphysiol.00045.2010. [DOI] [PubMed] [Google Scholar]
- 29.Trappe S, Costill D, Thomas R. Effect of swim taper on whole muscle and single muscle fiber contractile properties. Med Sci Sports Exerc. 2000;32:48–56. [PubMed] [Google Scholar]
- 30.Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. J Physiol. 2003;552:47–58. doi: 10.1113/jphysiol.2003.044966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol. 2004;557:501–13. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson DL, Godard MP, Porter DA, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain coexpression in single muscle fibers from older men. J Appl Physiol. 2000;88:627–33. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- 33.Giulian GG, Moss RL, Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983;129:277–87. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- 34.Harber MP, Gallagher PM, Trautmann J, Trappe SW. Myosin heavy chain composition of single muscle fibers in male distance runners. Int J Sports Med. 2002;23:484–8. doi: 10.1055/s-2002-35067. [DOI] [PubMed] [Google Scholar]
- 35.Mollica JP, Oakhill JS, Lamb GD, Murphy RM. Are genuine changes in protein expression being overlooked? Reassessing Western blotting. Anal Biochem. 2009;386:270–5. doi: 10.1016/j.ab.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fiber-type dependence of AMPK-alpha1 and -beta1. J Appl Physiol. 2011;110:820–5. doi: 10.1152/japplphysiol.01082.2010. [DOI] [PubMed] [Google Scholar]
- 37.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–81. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 38.Russell AP, Somm E, Praz M, Crettenand A, Hartley O, Melotti A, Giacobino JP, Muzzin P, Gobelet C, Deriaz O. UCP3 protein regulation in human skeletal muscle fibre types I, IIa and IIx is dependent on exercise intensity. J Physiol. 2003;550:855–61. doi: 10.1113/jphysiol.2003.040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koopman R, Pennings B, Zorenc AH, van Loon LJ. Protein ingestion further augments S6K1 phosphorylation in skeletal muscle following resistance type exercise in males. J Nutr. 2007;137:1880–6. doi: 10.1093/jn/137.8.1880. [DOI] [PubMed] [Google Scholar]
- 40.Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab. 2006;290:E1245–52. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- 41.Lee-Young RS, Canny BJ, Myers DE, McConell GK. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol. 2009;107:283–9. doi: 10.1152/japplphysiol.91208.2008. [DOI] [PubMed] [Google Scholar]
- 42.Rose AJ, Bisiani B, Vistisen B, Kiens B, Richter EA. Skeletal muscle eEF2 and 4EBP1 phosphorylation during endurance exercise is dependent on intensity and muscle fiber type. Am J Physiol Regul Integr Comp Physiol. 2009;296:R326–33. doi: 10.1152/ajpregu.90806.2008. [DOI] [PubMed] [Google Scholar]
- 43.Stuart CA, Howell ME, Baker JD, Dykes RJ, Duffourc MM, Ramsey MW, Stone MH. Cycle training increased GLUT4 and activation of mammalian target of rapamycin in fast twitch muscle fibers. Med Sci Sports Exerc. 2010;42:96–106. doi: 10.1249/MSS.0b013e3181ad7f36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tannerstedt J, Apro W, Blomstrand E. Maximal lengthening contractions induce different signaling responses in the type I and type II fibers of human skeletal muscle. J Appl Physiol. 2009;106:1412–8. doi: 10.1152/japplphysiol.91243.2008. [DOI] [PubMed] [Google Scholar]
- 45.Andersen JL, Klitgaard H, Bangsbo J, Saltin B. Myosin heavy chain isoforms in single fibres from m. vastus lateralis of soccer players: effects of strength-training. Acta Physiol Scand. 1994;150:21–6. doi: 10.1111/j.1748-1716.1994.tb09655.x. [DOI] [PubMed] [Google Scholar]
- 46.Welle S, Bhatt K, Thornton CA. High-abundance mRNAs in human muscle: comparison between young and old. J Appl Physiol. 2000;89:297–304. doi: 10.1152/jappl.2000.89.1.297. [DOI] [PubMed] [Google Scholar]
- 47.Hunter GR, Newcomer BR, Weinsier RL, Karapondo DL, Larson-Meyer DE, Joanisse DR, Bamman MM. Age is independently related to muscle metabolic capacity in premenopausal women. J Appl Physiol. 2002;93:70–6. doi: 10.1152/japplphysiol.01239.2001. [DOI] [PubMed] [Google Scholar]
- 48.Nuhr M, Crevenna R, Gohlsch B, Bittner C, Pleiner J, Wiesinger G, Fialka-Moser V, Quittan M, Pette D. Functional and biochemical properties of chronically stimulated human skeletal muscle. Eur J Appl Physiol. 2003;89:202–8. doi: 10.1007/s00421-003-0792-8. [DOI] [PubMed] [Google Scholar]
- 49.Essen-Gustavsson B, Henriksson J. Enzyme levels in pools of microdissected human muscle fibres of identified type. Adaptive response to exercise. Acta Physiol Scand. 1984;120:505–15. doi: 10.1111/j.1748-1716.1984.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 50.Yoshioka M, Tanaka H, Shono N, Shindo M, St-Amand J. Gene expression profile of sprinter's muscle. Int J Sports Med. 2007;28:1053–8. doi: 10.1055/s-2007-965117. [DOI] [PubMed] [Google Scholar]
- 51.Hittel DS, Hathout Y, Hoffman EP, Houmard JA. Proteome analysis of skeletal muscle from obese and morbidly obese women. Diabetes. 2005;54:1283–8. doi: 10.2337/diabetes.54.5.1283. [DOI] [PubMed] [Google Scholar]