Abstract
Background
Coumarin and their derivatives are important and useful compounds with diverse pharmacological properties. In the present study, we evaluated the in vitro cytotoxic activity of new acetoxycoumarin derivatives: 4-(7-methoxy-4-methyl-2-oxo-2H-chromen-3-yl)phenyl acetate (1), 4-(1-methyl-3-oxo-3H-benzo[f]chromen-2-yl)phenyl acetate (2), 4-(6-propionamido-4-methyl-2-oxo-2H-chromen-3-yl)phenyl acetate (3), 4-(7-acetoxy-2-oxo-4-phenyl-2H-chromen-3-yl)phenyl acetate (4), 4-(2-oxo-4-phenyl-2H-chromen-3-yl)phenyl acetate (5), 4-(6-bromo-2-oxo-4-phenyl-2H-chromen-3-yl)phenyl acetate (6), 4-(7-(diethylamino)-4-methyl-2-oxo-2H-chromen-3-yl)phenyl acetate (7), 4-(6,8-dibromo-4-methyl-2-oxo-2H-chromen-3-yl)phenyl acetate (8) against A549 human lung cancer, CRL 1548 rat liver cancer and CRL 1439 normal rat liver cells.
Materials and Methods
The cytotoxic activity was evaluated by crystal violet dye-binding assay. The effect of compounds 5 and 7 on different phases of the cell cycle was determined using flow cytometry.
Results
In the A549 lung cancer cell line, the 50% lethal dose (LD50) values for compounds 1–4, 6 and 8 were found to be >100 μM while those for 5 and 7 were 89.3 and 48.1 μM, respectively after 48 h treatment. In the CRL 1548 liver cancer cell line, only compound 7 showed toxicity, with an LD50 of 45.1 μM. Compounds 5 and 7 caused different cell phase arrest in lung and liver cancer cell lines.
Conclusion
The results indicate that 4-(7-(diethylamino)-4-methyl-2-oxo-2H-chromen-3-yl)phenyl acetate (7) had the highest cytotoxic activity in all of the examined cell lines.
Keywords: Acetoxycoumarins, cell viability, cell cycle, lethal dose
At present, cancer is one of the leading causes of death in the United States and in developed countries. Many efforts have been directed toward discovering anticancer agents endowed with cytotoxic action (1, 2). However, the non-selectivity and acute toxicity of many antitumor agents have been the major deterrent in their usage for treating human cancer, prompting the search for new chemopreventive and antitumor agents with improved tumor selectivity, efficiency and safety. One of the current methods for improving cancer therapy is protein acetylation, which involved the use of acetoxy drugs (e.g. polyphenolic acetates; PA) for catalyzing acetyl transferase (TAase) activity (3, 4).
Coumarin (2H-l-benzopyran-2-one) and its derivatives possess a wide range of various biological and pharmaceutical activities. They have wide range of applications as antitumor (5, 6), anti-HIV (7, 8), anticoagulant (9, 10), antimicrobial (11, 12), antioxidant (13, 14) and anti-inflammatory (15, 16) agents. The antitumor activities of a variety of coumarin compounds have been extensively examined (17–19). For example, they showed strong antiproliferative activity and induced apoptosis in various cancer cell lines such as A549 (lung), ACHN (renal), H727 (lung), MCF-7 (breast) and HL-60 (leukemia), in addition to prostate cancer, malignant melanoma, and metastatic renal cell carcinoma in clinical trials (20–24). A number of structurally different novel coumarin derivatives have been reported to display cytostatic and cytotoxic activities in both in vitro and in vivo assays (21, 25). Studies have also shown that the pattern of substitution on the basic coumarin core structure influences both its pharmacological and biochemical properties, including its therapeutic applications (19, 26). Recent investigations have demonstrated that the presence of microsomal TAase in liver catalyzed the transfer of acetyl groups from 7,8-diacetoxy-4-methylcoumarin (DAMC) to certain receptor proteins resulting in the modulation of their catalytic activities (27, 28). DAMC has also been reported to exhibit pro-oxidant effect in two human tumor cell lines (MDA-MB-468, breast and U-87 MG, glioma) (13). These studies and others strongly support the potential therapeutic applications of coumarin and its derivatives, making them attractive for further evaluation as novel therapeutic agents for cancer treatment.
As part of our on-going investigation for anticancer agents, we herein report the in-vitro cytotoxic activity of new acetoxycoumarin drugs (1–8, Table I) against A549 human lung cancer, CRL 1548 rat liver cancer and CRL 1439 normal rat liver cells. Furthermore, effect of the active acetoxycoumarins on cell cycle progression using flow cytometry in the cancer cell lines was also studied.
Table I.
Cytotoxic activity (LD50, μM) of acetoxycoumarin derivatives (1–8) at 48 h treatment
| Compound | LD50 (Lethal Dose) (μM) | |||
|---|---|---|---|---|
| A549 Lung Cancer Cell | CRL1548 Liver Cancer Cell | CRL1439 Liver Normal Cell | ||
| 1 | 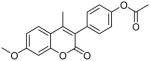 |
>100 | ND | ND |
| 2 | 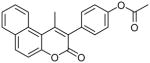 |
>100 | ND | ND |
| 3 | >100 | ND | ND | |
| 4 | 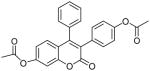 |
>100 | ND | ND |
| 5 | 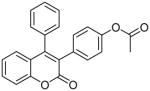 |
89.3 | >100 | >100 |
| 6 | 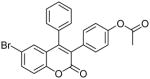 |
>100 | ND | ND |
| 7 | 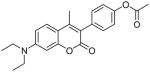 |
48.1 | 45.1 | 49.1 |
| 8 | 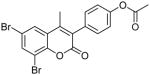 |
>100 | ND | ND |
The data represent the average of triplicate determinations at various concentrations
N/D means not determined
Materials and Methods
Chemicals
F12K medium, penicillin-streptomycin antibiotic solution (100X), fetal bovine serum (FBS), Trypsin-EDTA solution (1X), phosphate buffer (PBS), 50% glutaraldehyde, crystal violet and propidium iodide were obtained from Sigma-Aldrich Company (St. Louis, MO, USA). Monobasic and dibasic potassium phosphate, EDTA, D-glucose, Triton X-100 and ethanol were obtained from Thomas Scientific Company (Swedesboro, NJ, USA).
Cell line maintenance
Human A549 lung cancer, CRL 1538 rat hepatoma liver cancer and CRL 1439 normal rat liver cell lines were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured as per the guidelines supplied. The cells were maintained in F12K medium containing 100 units of penicillin/ml, 100 μg of streptomycin/ml, 2 mM L-glutamine and 10% FBS in T-75 cm2 flasks at 37°C in a 5% CO2 incubator.
Treatment of cells
For the evaluation of cell viability, the cells were plated at a density of 5×104 cells per well in polystyrene, flat-bottom 24-well microtiter plates (Corning Costar, Rochester, NY, USA) in F12K medium containing 10% FBS and allowed to stabilize overnight in a CO2 incubator at 37°C. The cells were then treated with compounds 1–8 at different concentrations (0–100 μM) in a final volume of 1 ml per well in triplicate wells for each treatment for 48 h at 37°C in a 5% CO2 incubator. All studies were repeated at least thrice. The cells at a density of 0.65×106 cells per T-25 flask (Corning Costar) in complete medium were plated for cell cycle analysis and allowed to stabilize overnight in a CO2 incubator at 37°C. The cells were then treated with compounds (5 or 7) at different concentrations (0, 20, 40 and 60 μM) in a final volume of 5 ml per flask in triplicate flasks for 48 h at 37°C in a 5% CO2 incubator.
Evaluation of cell viability
At the end of the incubation period, the viability was evaluated by dye uptake assay according to our previous report (20). The lethal dose of the compound, i.e. the dose of tested compound where 50% cell death is observed compared to the untreated control (LD50) was calculated according to the method of Ipsen and Feigl (29).
Cell cycle analysis by flow cytometry
The effect of compounds 5 and 7 on cell cycle phases in A549 (lung) and CRL 1548 (liver) cancer cell lines were studied using a C6 Accuri flow cytometer (Accuri Cytometers, Ann Arbor, MI, USA) according to our previously reported method (20).
Statistical analysis
The viability and cell cycle analysis results are presented as the mean± standard deviation (n=9). All data for treated cells are presented as percentage values in comparison to the untreated control (100%). The data were analyzed for significance by one-way ANOVA, and then compared by Dunnett’s multiple comparison tests, using GraphPad Prism Software, version 3.0 (GraphPad Software, Inc., San Diego, CA, USA). Differences from the respective untreated control were considered statistically significant when p<0.05.
Results
Cytotoxicity
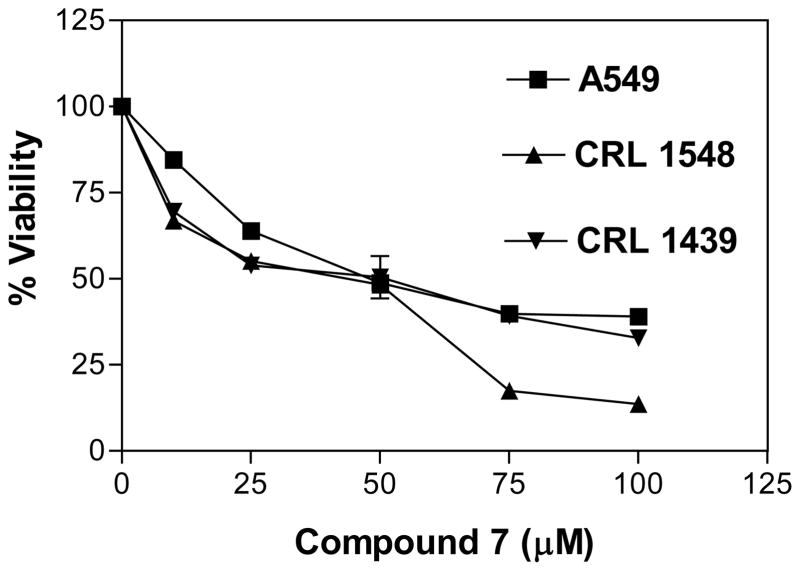
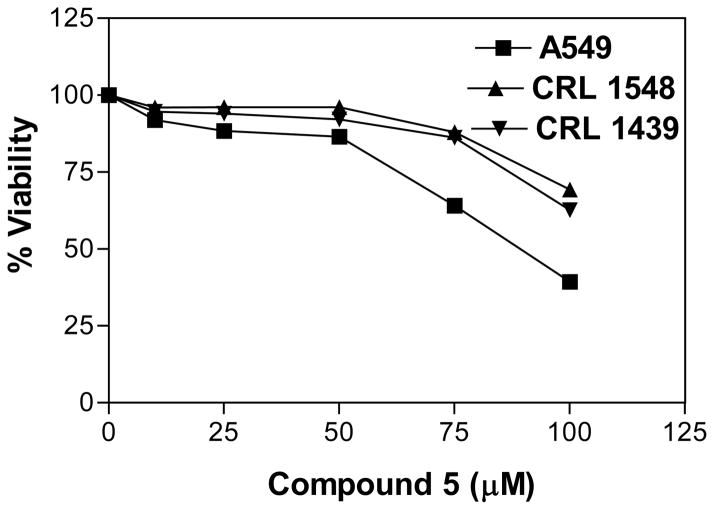
The in vitro cytotoxic activity of compounds 1–8 was evaluated at different concentrations (0, 25, 50, 75 and 100 μM) against A549 lung cancer, CRL 1548 liver cancer and CRL 1439 normal liver cells. The LD50 values for all tested compounds in the above cell lines are shown in Table I. Compounds showing the cytotoxic activity (LD50 < 100 μM) against A549 lung cancer cell line were considered as active compounds and tested against both CRL 1548 cancer liver and CRL 1439 normal liver cell lines for selectivity. Compound 7 showed higher toxicity (LD50 of 48.1, 45.1 and 49.6 μM, respectively) against A549, CRL 1548 and CRL 1439 cell lines (Figure 1), while compound 5 showed lower toxicity (LD50 of 89.3 μM) against A549 cell line and no toxicity (LD50 of >100 μM, inactive) against CRL 1439 and CRL 1548 cell lines (Figure 2).
Figure 1.
Effect of compound 7 on A549, CRL 1548 and CRL 1439 cell viability. The cells at a initial density of 0.5×104 per well were treated with compound 7 in a final volume of 1 ml F12K complete medium containing 10% FCS for 48 h. Data are represented as the mean and SEM (error bars) for n=9.
Figure 2.
Effect of compound 5 on A549, CRL 1548 and CRL 1439 cell viability. The cells at a initial density of 0.5×104 per well were treated with compound 5 in a final volume of 1 ml F12K complete medium containing 10% FCS for 48 h. Data are represented as the mean and SEM (error bars) for n=9.
Cell cycle distribution
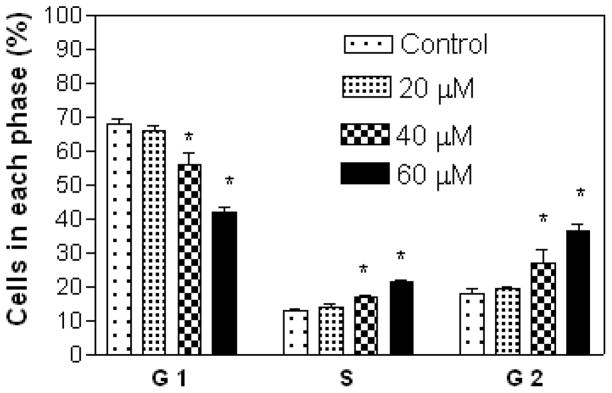
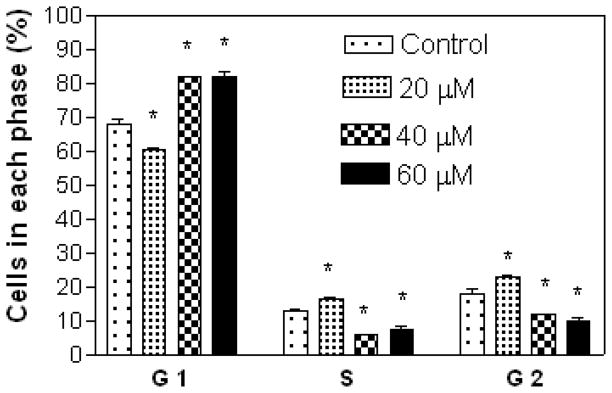
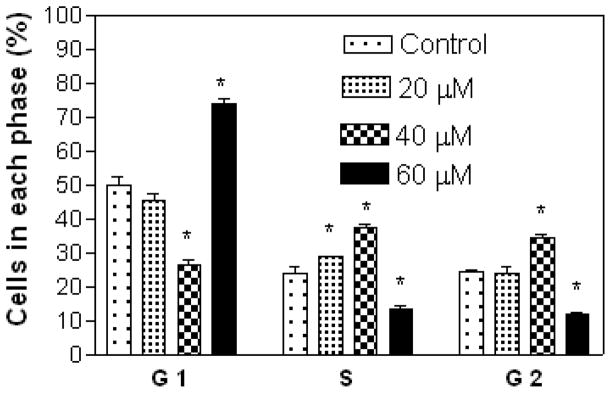
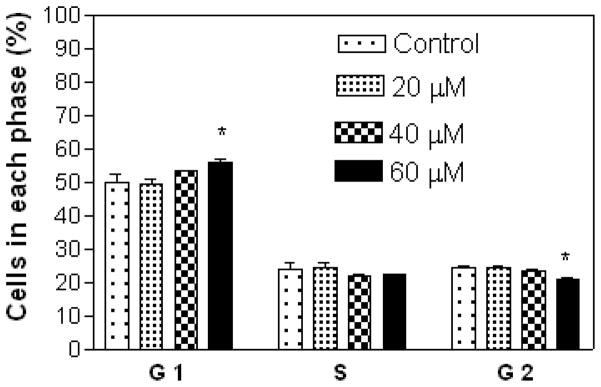
Results show that A549 cells were arrested in the S and G2 phases with increasing concentration of compound 7 (Figure 3), while treatment with compound 5 at 20 μM (lower concentration) showed cell arrest in the S and G2 phases, and at 40 and 60 μM (higher concentrations) in the G1 phase (Figure 4). Compound 7 at 20 μM arrested CRL 1548 cells in the S phase, at 40 μM in the S, and G2 phases and at 60 μM in the G1 phase (Figure 5), while for compound 5, cells were arrested in the G1 phase with increasing concentration (Figure 6).
Figure 3.
Effect of compound 7 on A549 lung cancer cell cycle distribution. The cells at an initial density of 0.65×106/ml per T-25 flask in F12K complete medium containing 10% FCS were treated with compound 7 for 48 h. Data are represented as the mean and SEM (error bars) for n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
Figure 4.
Effect of compound 5 on A549 lung cancer cell cycle distribution. The cells at an initial density of 0.65×106/ml per T-25 flask in F12K complete medium containing 10% FCS were treated with compound 5 for 48 h. Data are represented as the mean and SEM (error bars) for n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
Figure 5.
Effect of compound 7 on CRL 1548 liver cancer cell cycle distribution. The cells at an initial density of 0.65×106/ml per T-25 flask in F12K complete medium containing 10% FCS were treated with compound 7 for 48 h. Data are represented as the mean and SEM (error bars) for n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
Figure 6.
Effect of compound 5 on CRL 1548 liver cancer cell cycle distribution. The cells at an initial density of 0.65×106/ml per T-25 flask in F12K complete medium containing 10% FCS were treated with compound 5 for 48 h. Data are represented as the mean and SEM (error bars) for n=3. *Statistically significant difference from the control (p<0.05) using Dunnett’s multiple comparison test.
Discussion
The cytotoxic activity of the different acetoxycoumarin analogs (1–8) was evaluated in A549 lung cancer, CRL 1548 liver cancer, and CRL 1439 normal liver cells by a simple and reproducible crystal violet dye-staining assay (20). Compounds 1–8 bear a structural resemblance to DAMC in that they possess a coumarin core pharmacophore with an acetoxy functional group. The in vitro cytotoxicity results indicate that compound 7 was highly toxic to all the cells studied here, indicating that the target of action may be the same, although further studies are needed to confirm species or tissue specificity. On the other hand, the less toxic compound 5 exhibited tissue specific toxicity by showing higher toxicity in the A549 lung cancer cells in comparison to the CRL 1548 liver cancer cells. Previous study has shown how tissue specific toxicity by chemo preventive agents may help to identify potential toxicity problems, which are a major problem in the development of anticancer drugs (31). In this investigation, compounds 5 and 7 show less and non-specific toxicity in the examined cell lines, thus indicating how the pattern of substitution on the basic coumarin chemical structure changes cytotoxic and tumor-specific potential. Furthermore, it also shows the groups that could be necessary for cytotoxic activity of acetoxycoumarins towards cancer cell lines.
Both compounds (5 and 7) caused cell cycle arrest at different phases in the A549 lung and CRL 1548 liver cancerous cell lines. For example, compound 7 showed concentration-dependent cell cycle arrest in the CRL 1548 liver cancer cell line, while inducing cell cycle arrest at the same phase (S/G2 phase) with increasing concentration in A549 lung cancer cell line. This cytotoxic effect contradicts earlier findings that coumarin and derivatives inhibit cell growth by inducing cell cycle arrest in the G1 phase in A549 lung carcinoma cell line (32), thus indicating a different mode of cell death or target action for different coumarin derivatives. However, compound 5 showed cell cycle arrest at the same phase (G1 phase) with increasing concentrations in CRL 1548 liver cancer cell line, while showing concentration-dependent cell cycle arrest in A549 lung cancer cell line which is consistent with our earlier report involving triphenylethylene-type coumarin compounds (20).
Conclusion
The in vitro cytotoxic activity of acetoxycoumarin derivatives was studied in lung cancer, liver cancer and normal liver cell lines. Compound 7 exhibits cytotoxic activity against A549 lung cancer, CRL 1548 liver cancer and CRL 1439 normal liver cell lines, while compound 5 showed cytotoxic activity only against the A549 lung cancer cell line and no toxicity (inactive) against CRL 1439 normal liver and CRL 1548 liver cancer cell lines. Compound 5 and 7 also showed cell cycle arrest at different phases in A549 lung and CRL 1548 liver cancer cell lines. On the basis of these results, compound 7 could be considered as attractive leads in the future development of potential anticancer agents.
Acknowledgments
Faculty Research Development Funds (NIH/NCRR/RCMI grant G12 RR0 3020) is gratefully acknowledged for financial support. The Authors also gratefully acknowledge Dr. David H. Powell of the Mass Spectrometry Unit, Department of Chemistry at University of Florida for assistance with mass spectra analysis.
References
- 1.Sloane D. Cancer epidemiology in the United States: racial, social, and economic factors. Methods Mol Biol. 2009;471:65–83. doi: 10.1007/978-1-59745-416-2_4. [DOI] [PubMed] [Google Scholar]
- 2.Hotta K, Ueoka H. New cytotoxic agents: a review of the literature. Crit Rev Oncol Hematol. 2005;55(1):45–65. doi: 10.1016/j.critrevonc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Raj HG, Singh BK, Kohli E, Dwarkanath BS, Jain SC, Rastogi RC, Kumar A, Adhikari JS, Watterson AC, Olsen CE, Parmar VS. Acetoxy drug: protein transacetylase: A novel enzyme mediating protein acetylation by polyphenolic peracetates. Pure Appl Chem. 2005;77(1):245–250. [Google Scholar]
- 4.Dwarakanath BS, Verma A, Bhatt AN, Parmar VS, Raj HG. Targeting protein acetylation for improving cancer therapy. Indian J Med Res. 2008;128 (1):13–21. [PubMed] [Google Scholar]
- 5.Madari H, Panda D, Wilson L, Jacobs RS. Dicoumarol: a unique microtubule stabilizing natural product that is synergistic with Taxol. Cancer Res. 2003;63(6):1214–1220. [PubMed] [Google Scholar]
- 6.Kostova I. Synthetic and natural coumarins as cytotoxic agents. Curr Med Chem Anticancer Agents. 2005;5(1):29–46. doi: 10.2174/1568011053352550. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi Y, Xie L, Cosentino LM, Lee KH. Anti-AIDS agents-XXVIII. Synthesis and anti-HIV activity of methoxy substituted 3′,4′-di-O-(−)-camphanoyl-(+)-cis khellactone (DCK) analogues. Bioorg Med Chem Lett. 1997;7:2573–2578. doi: 10.1016/s0960-894x(98)00367-9. [DOI] [PubMed] [Google Scholar]
- 8.Shikishima Y, Takaishi Y, Honda G, Ito M, Takfda Y, Kodzhimatov OK, Ashurmetov O, Lee KH. Chemical constituents of Prangos tschiniganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem Pharm Bull (Tokyo) 2001;49 (7):877–880. doi: 10.1248/cpb.49.877. [DOI] [PubMed] [Google Scholar]
- 9.Manolov I, Maichle-Moessmer C, Danchev N. Synthesis, structure, toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur J Med Chem. 2006;41(7):882–890. doi: 10.1016/j.ejmech.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Jung J, Kin J, Park O. Simple and cost effective syntheses of 4-hydroxycoumarin. Synth Commun. 1999;29:3587–3595. [Google Scholar]
- 11.Ostrov DA, Hernandez Prada JA, Corsino PE, Finton KA, Le N, Rowe TC. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob Agents Chemother. 2007;51(10):3688–3698. doi: 10.1128/AAC.00392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musiciki B, Periers AM, Laurin P, Ferroud D, Benedetti Y, Lachaud S, Chatreaux F, Haesslein JL, LLtis A, Pierre C, Khider J, Tessol N, Airault M, Demassey J, Dupuis-Hamelin C, Lassaigne P, Bonnefoy A, Vicat P, Klich M. Improved antibacterial activities of coumarin antibiotics bearing 5′,5′-dialkylnoviose: biological activity of RU 79115. Bioorg Med Chem Lett. 2000;10:1695–1699. doi: 10.1016/s0960-894x(00)00304-8. [DOI] [PubMed] [Google Scholar]
- 13.Koshy L, Dwarakanath BS, Raj HG, Chandra R, Mathew TL. Suicidal oxidative stress induced by certain antioxidants. Indian J Exp Biol. 2003;41(11):1273–1278. [PubMed] [Google Scholar]
- 14.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr Pharm Des. 2004;10 (30):3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 15.Ghate M, Manohar D, Kulkarni V, Shobha R, Kattimani SY. Synthesis of vanillin ethers from 4-(bromomethyl) coumarins as anti-inflammatory agents. Eur J Med Chem. 2003;38 (3):297–302. doi: 10.1016/s0223-5234(03)00016-3. [DOI] [PubMed] [Google Scholar]
- 16.Kontogiorgis CA, Hadjipavlou-Litina DJ. Synthesis and antiinflammatory activity of coumarin derivatives. J Med Chem. 2005;48 (20):6400–6408. doi: 10.1021/jm0580149. [DOI] [PubMed] [Google Scholar]
- 17.Baba M, Jin Y, Mizuno A, Suzuki H, Okada Y, Takasuka N, Tokuda H, Nishino H, Okuyama T. Studies on cancer chemoprevention by traditional folk medicines XXIV. Inhibitory effect of a coumarin derivative, 7-isopentenyloxycoumarin, against tumor-promotion. Biol Pharm Bull. 2002;25 (2):244–246. doi: 10.1248/bpb.25.244. [DOI] [PubMed] [Google Scholar]
- 18.Thornes D, Daly L, Lynch G, Browne H, Tanner A, Keane F, O’Loughlin S, Corrigan T, Daly P, Edwards G. Prevention of early recurrence of high-risk malignant melanoma by coumarin. Irish Melanoma Group. Eur J Surg Oncol. 1989;15 (5):431–435. [PubMed] [Google Scholar]
- 19.Musa MA, Cooperwood JS, Khan MO. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem. 2008;15(26):2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musa MA, Badisa VL, Latinwo LM, Waryoba C, Ugochukwu N. In vitro cytotoxicity of benzopyranone derivatives with basic side chain against human lung cell lines. Anticancer Res. 2010;30 (11):4613–4617. [PMC free article] [PubMed] [Google Scholar]
- 21.Stanchev S, Momekov G, Jensen F, Manolov I. Synthesis, computational study and cytotoxic activity of new 4-hydroxycoumarin derivatives. Eur J Med Chem. 2008;43(4):694–706. doi: 10.1016/j.ejmech.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Thornes RD, Daly L, Lynch G, Breslin B, Browne H, Browne HY, Corrigan T, Daly P, Edwards G, Gaffney E, Henley J, Healy F, Keane F, Lennon F, McMurray N, O’Loughlin S, Shine M, Tanner A. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J Cancer Res Clin Oncol. 1994;120 (Suppl):S32–34. doi: 10.1007/BF01377122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohler JL, Gomella LG, Crawford ED, Glode LM, Zippe CD, Fair WR, Marshall ME. Phase II evaluation of coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma. Prostate. 1992;20 (2):123–131. doi: 10.1002/pros.2990200208. [DOI] [PubMed] [Google Scholar]
- 24.Marshall ME, Butler K, Fried A. Phase I evaluation of coumarin (1,2-benzopyrone) and cimetidine in patients with advanced malignancies. Mol Biother. 1991;3(3):170–178. [PubMed] [Google Scholar]
- 25.Egan D, James P, Cooke D, O’Kennedy R. Studies on the cytostatic and cytotoxic effects and mode of action of 8-nitro-7-hydroxycoumarin. Cancer Lett. 1997;118(2):201–211. doi: 10.1016/s0304-3835(97)00331-5. [DOI] [PubMed] [Google Scholar]
- 26.Carotti A, Carrieri A, Chimichi S, Boccalini M, Cosimelli B, Gnerre C, Carrupt PA, Testa B. Natural and synthetic geiparvarins are strong and selective MAO-B inhibitors. Synthesis and SAR studies. Bioorg Med Chem Lett. 2002;12 (24):3551–3555. doi: 10.1016/s0960-894x(02)00798-9. [DOI] [PubMed] [Google Scholar]
- 27.Raj HG, Parmar VS, Jain SC, Goel S, Singh A, Gupta K, Rohil V, Tyagi YK, Jha HN, Olsen CE, Wengel J. Mechanism of biochemical action of substituted 4-methylbenzopyran-2-ones. Part II: Mechanism-based inhibition of rat liver microsome-mediated aflatoxin B1-DNA binding by the candidate antimutagen 7,8-diacetoxy-4-methylcoumarin. Bioorg Med Chem. 1998;6 (10):1895–1904. doi: 10.1016/s0968-0896(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 28.Khurana P, Kumari R, Vohra P, Kumar A, Seema, Gupta G, Raj HG, Dwarakanath BS, Parmar VS, Saluja D, Bose M, Vij A, Chaudhary NK, Adhikari JS, Tyagi YK, Kohli E. Acetoxy drug: protein transacetylase catalyzed activation of human platelet nitric oxide synthase by polyphenolic peracetates. Bioorg Med Chem. 2006;14(2):575–583. doi: 10.1016/j.bmc.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Ipsen J, Feigl P. Bancroft’s Introduction to Biostatistics. 2. Harper and Row; New York: 1970. p. 164. [Google Scholar]
- 30.Badisa RB, Tzakou O, Couladis M, Pilarinou E. Cytotoxic activities of some Greek Labiatae herbs. Phytother Res. 2003;17:472–476. doi: 10.1002/ptr.1175. [DOI] [PubMed] [Google Scholar]
- 31.Elmore E, Luc TT, Steele VE, Redpath JL. Comparative tissue-specific toxicities of 20 cancer preventive agents using cultured cells from 8 different normal human epithelia. In Vitr Mol Toxicol. 2001;14(3):191–207. doi: 10.1089/109793301753407957. [DOI] [PubMed] [Google Scholar]
- 32.Goel A, Prasad AK, Parmar VS, Ghosh B, Saini N. 7,8-Dihydroxy-4-methylcoumarin induces apoptosis of human lung adenocarcinoma cells by ROS-independent mitochondrial pathway through partial inhibition of ERK/MAPK signaling. FEBS Lett. 2007;581(13):2447–2454. doi: 10.1016/j.febslet.2007.04.052. [DOI] [PubMed] [Google Scholar]