Abstract
Adult cardiac muscle adapts to mechanical changes in the environment by growth and remodeling (G&R) via a variety of mechanisms. Hypertrophy develops when the heart is subjected to chronic mechanical overload. In ventricular pressure overload (e.g. due to aortic stenosis) the heart typically reacts by concentric hypertrophic growth, characterized by wall thickening due to myocyte radial growth when sarcomeres are added in parallel. In ventricular volume overload, an increase in filling pressure (e.g. due to mitral regurgitation) leads to eccentric hypertrophy as myocytes grow axially by adding sarcomeres in series leading to ventricular cavity enlargement that is typically accompanied by some wall thickening. The specific biomechanical stimuli that stimulate different modes of ventricular hypertrophy are still poorly understood. In a recent study, based on in-vitro studies in micropatterned myocyte cell cultures subjected to stretch, we proposed that cardiac myocytes grow longer to maintain a preferred sarcomere length in response to increased fiber strain and grow thicker to maintain interfilament lattice spacing in response to increased cross-fiber strain. Here, we test whether this growth law is able to predict concentric and eccentric hypertrophy in response to aortic stenosis and mitral valve regurgitation, respectively, in a computational model of the adult canine heart coupled to a closed loop model of circulatory hemodynamics. A non-linear finite element model of the beating canine ventricles coupled to the circulation was used. After inducing valve alterations, the ventricles were allowed to adapt in shape in response to mechanical stimuli over time. The proposed growth law was able to reproduce major acute and chronic physiological responses (structural and functional) when integrated with comprehensive models of the pressure-overloaded and volume-overloaded canine heart, coupled to a closed-loop circulation. We conclude that strain-based biomechanical stimuli can drive cardiac growth, including wall thickening during pressure overload.
1 Introduction
Mature cardiac tissue adapts to mechanical changes in the environment via growth and remodeling (G&R) including hypertrophy (cell growth), fibrosis (formation of extra connective tissue), extracellular matrix (ECM) restructuring, necrosis (cell death) and apoptosis (“programmed” cell death) (Goktepe et al., 2010; Rosenkranz, 2004). Perhaps one of the best-known occurrences of cardiac G&R is the enlarged athlete’s heart as a response to increased load. During exercise, large increases in cardiac output and heart rate are observed, but mean arterial pressure and end-diastolic pressure change very little (Zak, 1984). While the athlete’s heart is considered to be a benign form of hypertrophy, ventricular hypertrophy in the pressure- or volume-overloaded heart ultimately becomes maladaptive. When the heart is subjected to chronic overload conditions, hypertrophy may be compensatory initially, but eventually leads to ventricular dysfunction and ultimately congestive heart failure. In ventricular pressure overload, the left ventricle experiences an increase in resistance as it ejects blood into the aorta (afterload increase). Common causes of LV pressure overload are systemic hypertension or aortic stenosis to which the ventricles typically react by concentric hypertrophic growth, characterized by thickening of the wall associated with cardiac cell thickening when sarcomeres – the cardiac cell’s basic contractile units – are added in parallel (Gerdes, 1992). In volume overload, the heart experiences an increase in filling, or preload. Following ventricular volume overload – caused by for example a regurgitant mitral valve – eccentric hypertrophy ensues as cardiac cells grow axially by adding sarcomeres in series (Gerdes, 1992) leading to ventricular cavity enlargement that is typically accompanied by some wall thickening.
Biomechanical stimuli and neurohumoral factors are the two main triggers of cardiac G&R (Lorenz et al., 2009). Neurohumoral factors and their downstream signaling pathways that mediate cardiac hypertrophy are well known, but the specific biomechanical stimuli that stimulate different modes of ventricular hypertrophy are still poorly understood (Holmes, 2004; Lorenz et al., 2009; Omens, 1998). In the literature, various stimuli have been proposed including those that depend on: fiber strains at different stages in the cardiac cycle (Bupha-Intr et al., 2007; Guterl et al., 2007; Kroon et al., 2009); wall stress (Goktepe et al., 2010; Grossman et al., 1975); and regional strain-energy distributions (Vena et al., 2008). Of these, it has also been seen experimentally that hypertrophy and hypertrophic gene expression correlate better with strain than stress (Guterl et al., 2007; Omens, 1998). Strains have been proposed as stimuli for cardiac G&R as early as 1927 (Eyster et al., 1927). In cultured rat right ventricular papillary muscles, myocyte diameters increased independently of the level of stress with isometric contractions, whereas systolic shortening and/or diastolic lengthening regulated myocyte shape (Guterl et al., 2007). Grossman’s original hypothesis that hypertrophy is compensatory, maintaining normal cardiac function by normalizing wall stress (Grossman et al., 1975), has been challenged in the last decade (Opie et al., 2006). For example, in genetically engineered mice it has been shown that despite inadequate normalization of wall stress, cardiac function showed little deterioration in pressure-overloaded animals with moderate hypertrophy responses (Esposito et al., 2002). In wild-type mice however with similar pressure overload, a significant increase in chamber dimensions and progressive deterioration in cardiac function occurred. In volume overloaded rats, Herrmann et al (Herrmann et al., 2003) found that stress remained chronically elevated but diastolic strains were normalized by an increase in myocardial stiffness associated with an increase in collagen crosslinks.
Micropatterned neonatal rat cardiac cell cultures restore resting sarcomere length when they are subjected to uniaxial static stretch (Mansour et al., 2004). Cells added about one sarcomere per hour after a 10% stretch in the fiber direction, thereby restoring resting sarcomere length and thus compensating for the stretch applied. When stretch is applied transversely to the fiber axis, immunofluorescent labeling of hypertrophic marker atrial natriuretic factor is increased, phosphorylation of focal adhesion kinase is increased and contractile protein synthesis is up regulated, as observed in pressure-overload (Gopalan et al., 2003; Russell et al.; Simpson et al., 1999).
We have postulated in a previous study (Kerckhoffs, 2011) that cardiac cells would aim to maintain a preferred interfilament lattice spacing by adding sarcomeres in parallel in response to altered cellular cross-fiber strain. In support of this, Simpson et al (Simpson et al., 1999) argued that an increase in cross-fiber strain during systolic loading is a critical signal for promoting myocyte hypertrophy during the evolution of concentric hypertrophy. In their experiments, cross-fiber stretch of aligned myocytes was most effective at promoting the accumulation of contractile proteins and branching myofibrils, phenotypic characteristics that are typically used to describe cardiac myocytes during concentric hypertrophy. This is in addition to the assumption that myocytes maintain sarcomere length during axial loading by series addition of sarcomeres. The proposed growth law - which included mechanisms for both axial and radial cellular growth – reproduced physiological postnatal growth to a substantial extent in a computational model of the rat left ventricle (Kerckhoffs, 2011). In the present study, the aim was to test whether a single growth law based on these principles is able to predict concentric or eccentric remodeling in response to aortic stenosis (pressure overload) or mitral valve regurgitation (volume overload), respectively, in an integrative computational model of the adult canine ventricles coupled to a closed-loop model of the circulation.
2 Material and methods
A non-linear finite element model of the beating canine right and left ventricles with realistic fiber anatomy was used in this study. The finite element model was coupled to a lumped-parameter model of the circulation that also included the heart valves. After modeling valve pathologies, the ventricles were allowed to adapt in shape in response to the mechanical stimuli, growing to a final state with a new geometry and hemodynamics.
2.1 Material properties
Passive material properties were described by a strain energy law W, consisting of the transversely isotropic version Wpas proposed by Guccione (Guccione et al., 1991), and a slightly compressible part Wcomp (Doll and Schweizerhof, 2000):
| (1) |
| (2a) |
| (2b) |
| (3) |
Eff is Lagrangian strain in the fiber direction, Err is transmural radial strain transverse to the fiber, Ecc is cross-fiber strain perpendicular to the former two, and the remaining are associated shear strains. Cpas, Ccomp, bf, bt, and bfr are material parameters (Table 1) and F is the deformation gradient tensor.
Table 1.
Passive Material Properties
| Coefficient | Description | Value |
|---|---|---|
| Cpas [kPa] | Passive stress scaling constant | 0.44 |
| bf [−] | Parameter associated with fiber strain | 18.5 |
| bt [−] | Parameter associated with strain transmural to fiber and shear strain in radial-cross-fiber plane | 3.58 |
| bfr [−] | Parameter associated with shear strains in fiber-radial and fiber-crossfiber plane | 1.63 |
| Ccomp [kPa] | Bulk modulus | 350 |
The contractile properties were described by a Hill-type model (Kerckhoffs et al., 2010; Lumens et al., 2009), that was modified so that active tension does not increase indefinitely with sarcomere length. This imposes a limit on the total amount of work a fiber can generate. See the online supplement for details.
2.2 Growth Law
Growth was modeled by deforming the stress-free tissue configuration B0 to a grown configuration Bg, which is not necessarily stress-free (Goktepe et al., 2010; Kroon et al., 2009; Rodriguez et al., 1994). The growth model is based on a multiplicative decomposition of the deformation gradient F first proposed by Rodriguez et al (Rodriguez et al., 1994):
| (4) |
The growth deformation gradient Fg applies between B0 and an intermediate fictitious configuration B’g. The latter is a stress-free grown state where local kinematic compatibility conditions are not imposed. The deformation gradient Fe describes the deformation between B’g and Bg and the Cauchy stress σ(Fe) in the tissue only depends on this elastic deformation (Fg describes a plastic deformation).
2.2.1 Choice of biomechanical stimuli
The choice of biomechanical stimuli for cellular axial and radial growth were based on the following assumptions and experimental findings:
-
-
Mechanical stimuli are local: individual cardiac cells remodel, hence the stimulus should be sensed by each myocyte. We make the assumption that cells deform similarly with the surrounding tissue as seen experimentally by Rodriguez and Hunter (Rodriguez et al., 1992).
-
-
Strain-based stimuli correlate better with G&R than stress-based variables (Guterl et al., 2007; Omens, 1998);
-
-
Stimuli should be independent of the hemodynamics of the cardiac cycle, in particular the timing of valve opening and closing: i.e. cells don’t “know” when valves open and close. In the pressure-volume loop, end-systolic and end-diastolic states may be distinctive points in the cardiac cycle, but regionally cardiac phase changes are much more subtle (e.g. in stress-strain loops (Kerckhoffs et al., 2005; Kerckhoffs et al., 2010)). Therefore, mechanical stimuli are likely to be found in extremes, amplitudes or means of strains (Holmes, 2004);
-
-
In vitro experiments with isolated neonatal cardiac myocytes have demonstrated that cardiac myocytes are able to distinguish between strains applied in the fiber vs. cross-fiber direction (Gopalan et al., 2003; Mansour et al., 2004; Russell et al.; Simpson et al., 1999);
-
-
Because cells undergo cyclic motion, we assume that the time scale of a homeostatic set point is associated with the cardiac cycle duration, e.g. the stimulus for fiber axial growth could be the deviation from mean or maximum fiber strain, evaluated over a cardiac cycle. Naturally, the rate of hypertrophic response is much slower than a cardiac cycle, and thus many beats with supra-normal fiber strain would be necessary to result in significant hypertrophy;
-
-
All adaptation mechanisms should be present under all overload conditions. For example, fibers mostly grow axially in volume overload, which leads to eccentric hypertrophy, whereas fibers mostly grow radially in pressure overload, leading to concentric hypertrophy. But under pressure overload, the mechanism of fiber axial growth should also be active and vice versa, in volume overload, the mechanism of fiber radial growth should be active.
The above criteria led to the choice of a stimulus sl for fiber axial growth driven by an imbalance in maximum fiber strain Eff with a set-point Eff,set whereas fiber radial growth was driven by a stimulus st of imbalance in the smallest maximum principal strain in the cellular cross-sectional plane with a set point value:
| (5) |
| (6) |
in which Ecross,max is the true algebraic maximum principal strain (not the maximum absolute magnitude) of the 2D cellular cross-sectional strain tensor Ecross:
| (7) |
where Ecc, Err and Ecr are cross-fiber strain, radial strain and cross-fiber radial shear, respectively.
Both the maximum fiber strain and principal cross-sectional strains were evaluated over 1 cardiac beat. The cumulative growth deformation gradient tensor was updated with the incremental growth deformation gradient tensor Fg,i (Kroon et al., 2009):
The deformation gradient tensors were defined with respect to the local fiber orientation (Fff in fiber direction, Fcc cross-fiber parallel to the wall and Frr radially), which allows for the definition of orthotropic (diagonal) cumulative and incremental growth tensors. The relation between components of Fg,i and the stimuli sl and st were described by sigmoids:
| (8) |
| (9) |
| (10) |
Here, Fg,i,ff describes growth in the fiber direction due to the addition of sarcomeres in series at step i, whereas for Fg,i,cc and Fg,i,rr it is assumed that sarcomeres are added equally in parallel in both cross-fiber directions, hence transversely isotropic growth. Because material has to be deposited in two directions, the square root is taken of the growth stretch ratio in both cross-fiber directions. Δtgrowth is the growth time step. For a description and values of the growth equation parameters, see Table 2. The relation between the incremental fiber axial growth Fg,i,ff and stimulus (Eq. 8) contains a small quiescent region around the zero stimulus. Simpson et al (Simpson et al., 1999) investigated protein metabolism in aligned myocytes stretched at varying degrees in the fiber and cross-fiber direction. A modest increase was observed in the total cellular concentration of myosin heavy chain and actin in cells stretched 2.5%. In Eq. 8, deviation of Fg,i,ff from ~0 occurs at 3% stretch above the set point (with 1% fiber axial growth per growth step). In experiments, cells responded to cross-fiber stretch as low as 0.5% (Simpson et al., 1999): hence, in our growth rule for fiber radial growth (Eqs. 9 and 10), there is no distinct quiescent region, albeit growth is slower around a zero stimulus than at higher values.
Table 2.
Growth Properties
| Coefficient | Description | Value |
|---|---|---|
| fff,max [1/day] | Maximum axial growth rate | 0.3 |
| ff | Factor affecting slope of sigmoid for cellular axial growth | 150 |
| sl,50 | Stimulus value when 50% of maximum axial growth is reached | 0.06 |
| Fff,50 | Cumulative fiber growth stretch ratio when 50% of maximum fiber length is reached | 1.35 |
| flength,slope | Factor affecting slope of axial growth evolution sigmoid | 40 |
| fcc,max [1/day] | Maximum radial growth rate | 0.1 |
| cf | Factor affecting slope of sigmoid for cellular radial growth | 75 |
| st,50 | Stimulus value when 50% of maximum radial growth is reached | 0.07 |
| Fcc,50 | Cumulative fiber growth stretch ratio when 50% of maximum fiber thickness is reached | 1.28 |
| Cthickness,slope | Factor affecting slope of radial growth evolution sigmoid | 60 |
All parameters, except fff,max and fcc,max, are dimensionless.
The evolution growth functions kff and kcc (Lubarda and Hoger, 2002) reduce the positive growth rates to zero when maximum cell length and thickness are reached (the latter two are quantified by the cumulative growth deformation tensor components , respectively, at step i):
| (11) |
| (12) |
The parameter values (flength,slope, Fff,50, cthickness,slope, Fcc,50) that characterize the growth evolution functions ensure a maximum cell axial growth ratio of about 1.54 (with a measured 200 µm maximum cell length, assuming a normal adult cell length of 130 µm)(Onodera et al., 1998) and a maximum cell radial growth ratio of 1.37 (with a measured 400 µm2 maximum cell cross-sectional area, assuming a normal adult cell cross-sectional area of 215 µm2)(Onodera et al., 1998). Note that the evolution growth functions are only used when the stimuli are positive: this will result in slow growth when maximum cell dimensions are being approached, but a normal shrink speed independent of cell dimensions.
2.3 Cardiovascular model
Normal heart
We have previously published a cardiovascular model of the normal canine heart, and employ that model here (Kerckhoffs et al., 2010). In brief, the model consisted of a 3D computational model that included the geometry and fiber architecture of the canine left and right ventricles, and coupling of the ventricular chambers to a closed circulation (Lumens et al., 2009). The latter was represented by a non-linear lumped parameter system model of the systemic and pulmonary circulation (the CircAdapt model (Arts et al., 2005)). The micro-adaptation algorithms of the CircAdapt model were not employed in this study. Peripheral resistance was adjusted to obtain the set cardiac output (1.8 liter/min). For each beat, active tension generation was started synchronously with a basic cycle length of 750 ms.
All parameter values and initial conditions of the circulation models are listed in the online supplement.
Pressure and volume overload models
The pressure overload model was represented by an aortic stenosis: this was achieved by changing the aortic valve opening area from 2.7 cm2 in the normal state to 0.15 cm2 in the diseased state. The volume overload model was represented by mitral regurgitation: this was achieved by changing the leaking area of the closed mitral valve from 5·10−6 cm2 in the normal state to 0.35 cm2 in the diseased state. In both overload models, active tension generation was started synchronously for each beat with a basic cycle length of 600 ms (Nagatomo et al., 1999).
3 Calculation
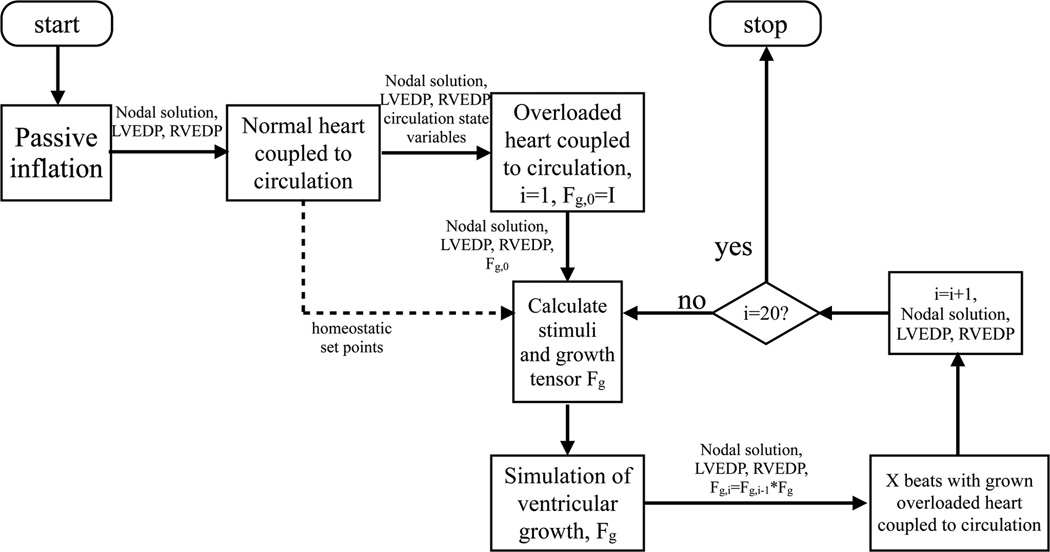
3.1 Solution Procedure
The FE anatomic model of the canine ventricles was discretized into 48 tricubic Hermite elements, with 1968 degrees of freedom. First, the left and right ventricles were inflated in 110 steps of 0.01 and 0.0018 kPa increments, respectively, to the initial end-diastolic pressures (decoupled from the circulatory model, see Figure 1]). At this point, the normal heart simulation was started without growth, fully coupled to the circulatory model, until reaching steady state (defined as relative difference in stroke volumes between LV and RV < 1%). For the last beat, maximum fiber strains and principal cross-sectional strains were recorded for all nodes. These values served as the set-points for the biomechanical stimuli in the overload simulations. The simulation was stopped at another end-diastolic state, which served as the new starting point for the overload simulations.
Figure 1.
Flow chart of the calculations performed. LVEDP = left ventricular end-diastolic pressure; RVEDP = right ventricular end-diastolic pressure; Fg,i = cumulative growth tensor; Fg = incremental growth tensor; X=5.
Next, the overload simulations were started without growth using the appropriate alterations for the valves, and were run to a new steady state at end-diastole. This phase represents the initial hemodynamically overloaded heart before hypertrophic growth.
At this point, the ventricles were allowed to grow. First, stimuli and the resulting growth tensors thereof were calculated from the last (steady-state) cardiac cycle. Growth was introduced incrementally in the pressure- and volume-overloaded heart, decoupled from the circulation, at the last end-diastolic LV and RV pressures. Next, the simulation with the newly grown ventricles coupled to the circulation was restarted and continued for 5 cardiac cycles. This allowed the peripheral resistance to adjust to the new situation and to obtain a new acute steady-state. For the last of these 5 cardiac cycles, stimuli and growth tensors were again calculated and the procedure was repeated by inducing growth as mentioned earlier. A total of 20 growth steps were performed for both overload simulations. It should be emphasized that the parameter values of the aortic and mitral valve were the only differences between the two overload simulations.
After the simulations finished, unloaded geometries were obtained for each growth step by applying the calculated deformation growth tensors to the models’ unloaded geometry.
3.2 Numerical implementation
The simulations were solved with Continuity 6.4b (http://www.continuity.ucsd.edu) in parallel using 16 processors, on a Linux cluster with dual Intel XeonX5650 6-core 2.66Ghz processors.
A time step of 1 ms was used for the simulations of the cardiac cycles, whereas a growth time step Δtgrowth = 1 day was used for the growth simulations. However, this latter time step and its relation to the growth rate constants was later reassessed in comparison with experimental results. The nonlinear finite element models were solved with a modified Newton-Raphson iteration scheme. Integration was performed with 3×3×3 Gaussian quadrature points. Convergence was reached when both the sum of incremental displacements and the sum of the residuals were lower than 10−5 mm and 10−8 N, respectively. The Jacobian was calculated and factorized in the first iteration of a new time step and when the solution was diverging. The system of linear equations was solved with SuperLU (Li and Demmel, 2003).
4 Results
4.1 Model validation of acute changes
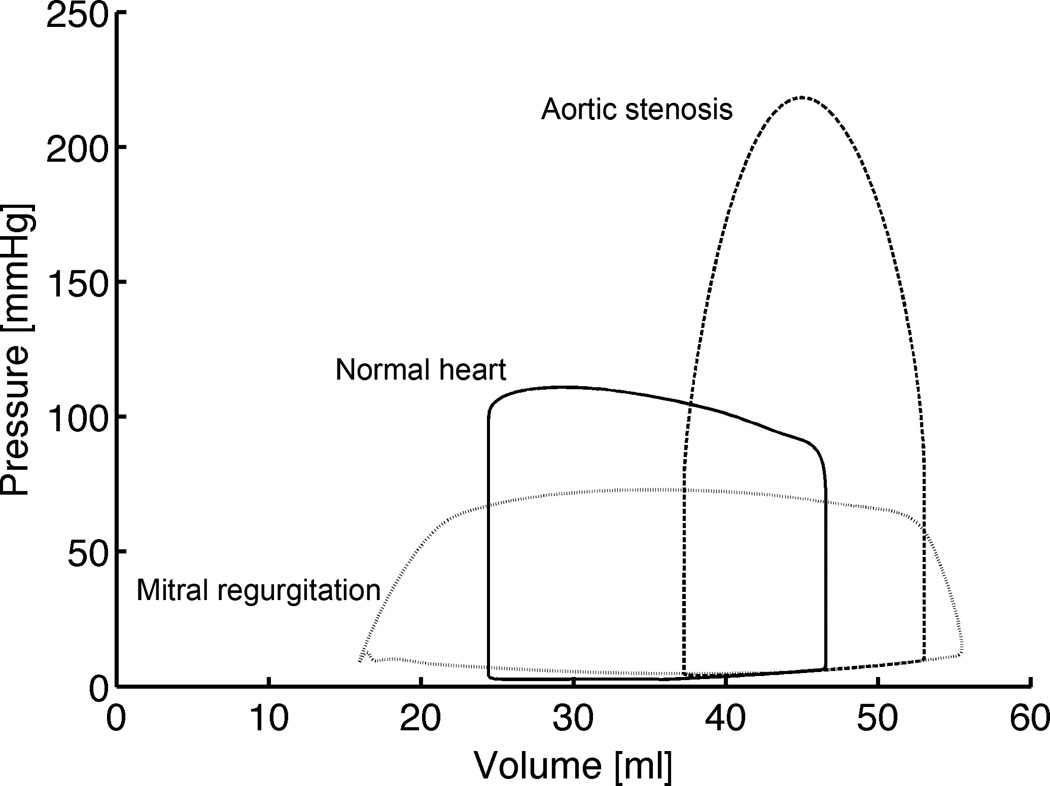
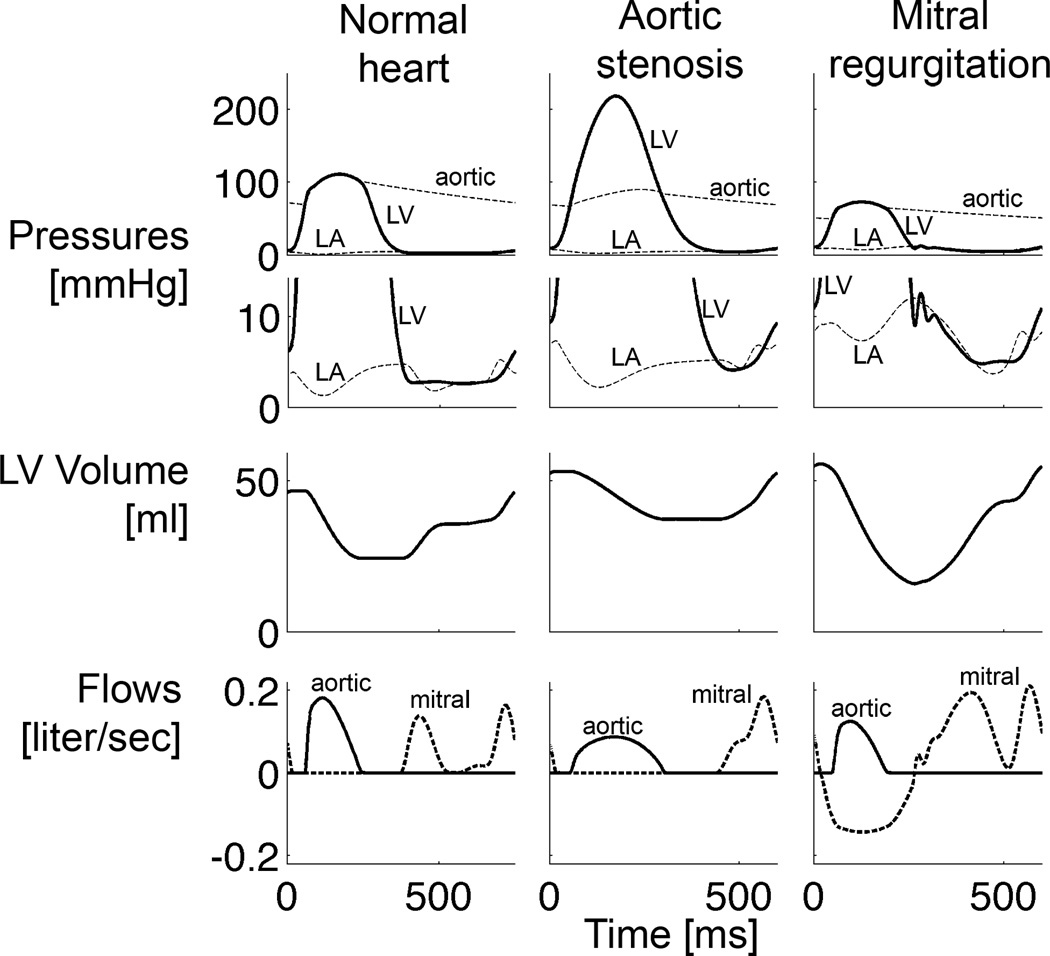
Changes in global hemodynamics for the simulations of the overloaded hearts, compared with normal, were in good general agreement with experiments (Figures 2 and 3, Table 3): End-diastolic pressures and volumes increased in the overloaded hearts, whereas forward ejection fraction decreased (22% and 30% during volume- and pressure-overload, respectively, vs. 48% in the normal heart). The volume-overloaded heart was characterized by the typical absence of isovolumic phases (Figure 2) and a large total ejection fraction of 71% with a mitral regurgitant fraction of 61% (i.e. 61% of blood volume in the LV at end-diastole flows back into the left atrium during the next contraction). EDP however only doubled, whereas it tripled in dog experiments (Dell'Italia et al., 1995). Duration of ejection was shorter than in the normal heart. A peak cavity pressure of 218 mmHg characterized the pressure-overloaded heart and duration of ejection prolonged compared with the normal heart, which is also in agreement with experiments (Katz et al., 1928).
Figure 2.
Steady-state pressure-volume loops of the normal heart, acute aortic stenosis and acute mitral regurgitation simulations (before growth).
Figure 3.
Steady-state left atrial (LA), left ventricular (LV) and aortic pressures, LV volume, aortic and mitral flows of the normal heart, acute aortic stenosis and acute mitral regurgitation simulations (before growth).
Table 3.
Acute hemodynamics (before growth) for the normal, pressure and volume overloaded hearts
| Normal | Aortic stenosis | Mitral regurgitation | ||||
|---|---|---|---|---|---|---|
| Simulation | Experiment | Simulation | Experiment | Simulation | Experiment | |
| Heart rate [bpm] | 80 | 87±6 (Nagatomo et al., 1999) |
100 | 104±3 (Nagatomo et al., 1999) |
100 | 111±8 (Kihara etal., 1988) |
| dP/dtmax [mmHg/sec] | 2231 | 1627±644 (Verbeek et al., 2003) |
2466 | 1468 | 1200±537 (Chen et al., 1991) |
|
| LVPmax [mmHg] | 111 | 123±7 (Nagatomo et al., 1999) |
218 | 197±5 (Nagatomo et al., 1999) |
73 | 105±28 (Chen etal., 1991) |
| LVEDP [mmHg] | 6 | 6.4±0.6 (Nagatomo et al., 1999)* 8±5 (Dell'Italia et al., 1995)* |
10 | 9.4±0.7 (Nagatomo et al., 1999)* |
12 | 23±5 (Young et al.,1996)*(Dell italia etal 1995)* |
| Systolic BP [mmHg] | 111 | 122±7 (Nagatomo et al., 1999) |
90 | 68±3 (Nagatomo et al., 1999) |
73 | |
| Diastolic BP [mmHg] | 69 | 86±5 (Nagatomo et al., 1999) |
67 | 55±2 (Nagatomo et al., 1999) |
50 | |
| forward SV [ml] | 22 | 26±5 (Young etal., 1996) 44±1.5 (Nagatomo et al., 1999) |
16 | 40±2 (Nagatomo et al., 1999) |
12 | |
| Mitral regurgitant fraction [%] | 0 | 0 | 61 | 56±18 (Dell'Italia et al., 1995) |
||
| total LVEF [%] | 48 | 47±6 (Young etal., 1996) 38 (Verbeek etal., 2003) |
30 | 71 | ||
Experimental values are meand±SD or meand±SE, followed by literature references.
Pulmonary artery wedge pressure instead of LVEDP. BP: aortic blood pressure; LVEDP: left ventricular end-diastolic pressure; LVEF: left ventricular ejection fraction; LVPmax: peak LV cavity pressure; SV: stroke volume
4.2 Model validation of chronic changes
4.2.1 Structure
In choosing a time to a growth step for the simulations, we compared the LV wall volume changes in our pressure-overload model with experimental values obtained at 5 days of aortic stenosis in dog (Nagatomo et al., 1999). In this experiment, LV mass was increased by 11.1% at day 5. In our pressure-overload simulation, LV wall volume was increased by 9.8% and 13.3% at growth steps 3 and 4, respectively. Hence, one simulation growth step encompassed about 1.5 days. This means that with Δtgrowth = 1.5 days, the maximum axial and radial growth rate constants fff,max and fcc,max become 0.2 and 0.067 days−1, respectively. Using this simple “time calibration” we further compared our results with those of experiments. With 20 growth steps, the total duration of growth encompassed about a month.
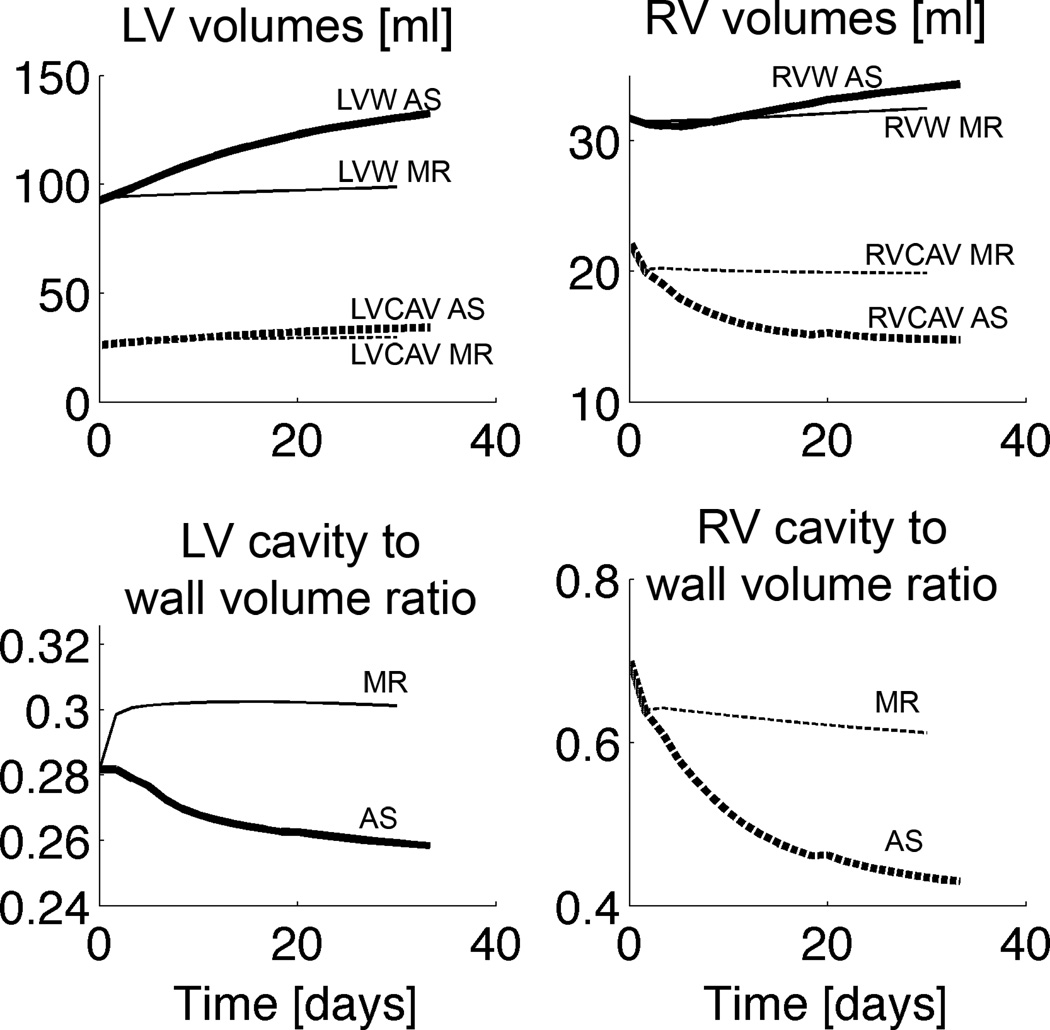
LV wall volume increased by 22% at day 10, which is similar to a 26.4% (p<0.05) increase in LV mass in a canine pressure overload experiment after 10 days of aortic stenosis (Nagatomo et al., 1999) (Figure 4). After about a month, LV wall volume in the model had increased by 42%. LV wall volume increased faster than LV cavity volume, exhibiting a concentric growth pattern, hence the LV wall volume to cavity volume decreased (Figure 4, also for the RV). The LV in the pressure overload simulation also showed dilation: LV unloaded volume increased by 15% at day 10. LV dilation has also been demonstrated in a canine pressure overload experiment in which LV end-diastolic diameter increased by 4% (p<0.01) after 9 days of aortic constriction (Sasayama et al., 1976). Even in reality, dilation appears to be a consistent factor in long-term G&R: for example, in the spontaneously hypertensive rat (SHR) fiber axial growth is a continuous process, surpassing fiber radial growth, leading to dilation in late stages of hypertrophy (Onodera et al., 1998).
Figure 4.
Unloaded cavity and wall volume changes during growth for the pressure (thick lines) and volume overload simulations (thin lines). AS aortic stenosis; MR mitral regurgitation; LVW left ventricular wall; LVCAV left ventricular cavity; RVW right ventricular wall; RVCAV right ventricular cavity
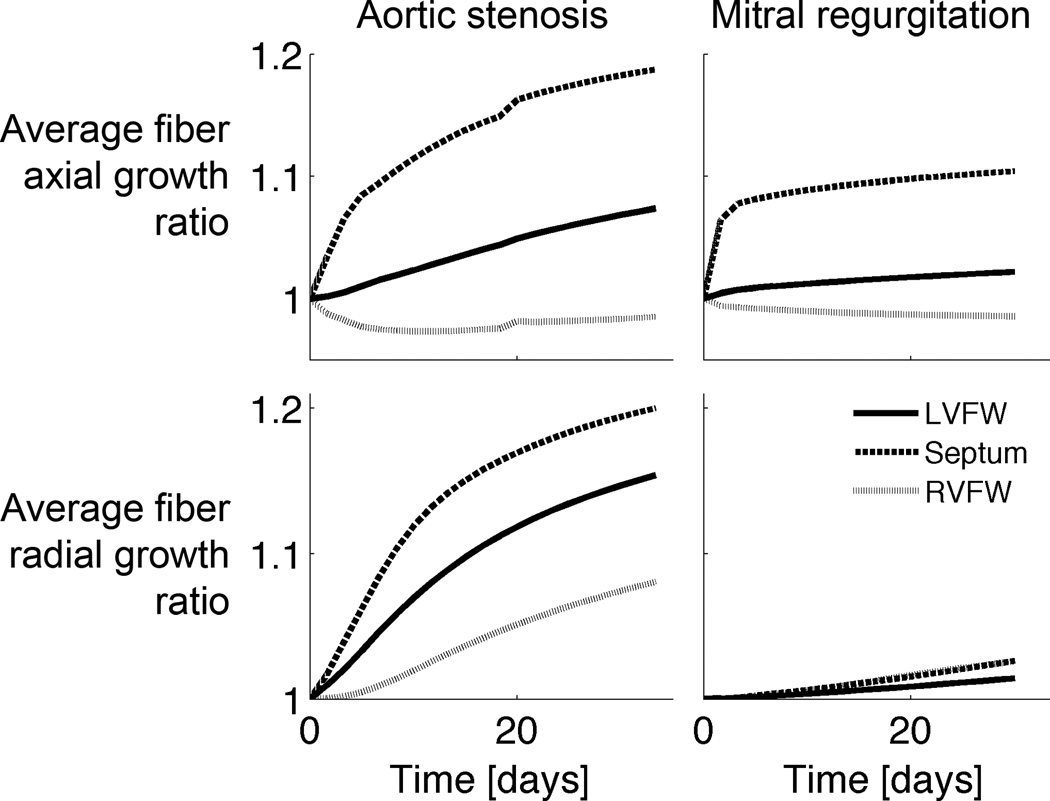
In the pressure overload simulation, average LVFW wall thickness increased by 8% and 12% at day 10 and 20, respectively (Figure 5), which is qualitatively similar to a 6% (p<0.001) and 11% (p<0.0001) increase in end-diastolic wall thickness in the aforementioned canine pressure overload experiment after 9 and 18 days of aortic constriction, respectively (Sasayama et al., 1976). The septum exhibited the largest average thickening (Figure 5), whereas the RVFW thickened by 8% after a month.
Figure 5.
Average growth stretch ratios in the left ventricular free wall (LVFW), right ventricular free wall (RVFW) and septum for the pressure (aortic stenosis) and volume overload simulations (mitral regurgitation).
In the volume overload simulation, dilation occurred rapidly early on (with the septum dilating the fastest), but slowed down at around the 5th day (Figures 4 and 5). After a month, average cellular axial growth was 2%, 11% and -1% in the LVFW, septum and RVFW, respectively. Average wall thickening was 2% in the LVFW and 3% in the RVFW and septum.
After a month, LV wall volume increased by 7% and LV unloaded cavity volume by 14%, thus exhibiting an eccentric growth pattern (Figure 4). In a volume overload experiment, LV mass increased by 19–27% after 5 months of mitral regurgitation (Dell'Italia et al., 1995; Young et al., 1996). RV wall volume increased by only 3%, comparable to experiments, in which RV mass was not significantly changed (Young et al., 1996). LV end-diastolic volume (LVEDV) increased by 37%, compared with the normal heart, whereas in a volume overload experiment, LVEDV increased by 74% after 5–6 months of mitral regurgitation (Young et al., 1996). The LV wall volume to cavity volume increased rapidly early on but stayed about constant thereafter (Figure 4).
4.2.2 Function
After 10 days, LVEDP decreased from 10 to 8 mmHg which is qualitatively similar to the −10% change in the canine pressure overload model after 10 days of aortic stenosis (Nagatomo et al., 1999) (Table 4). Large differences existed however in the blood pressures between simulation and experiment (Table 4).
Table 4.
Hemodynamics for the pressure and volume overloaded hearts
| Aortic stenosis | Mitral regurgitation | |||||||
|---|---|---|---|---|---|---|---|---|
| Simulation (10 Days) |
Experiment (Nagatomo etal., 1999) (10 days) |
Simulation (1 month) |
Experiment (Young et al., 1996) (5–6 months) |
|||||
| dP/dtmax [mmHg/sec] | 2155 | (−13%) | 1455 | (−1%) | ||||
| LVPmax [mmHg] | 221 | (+1%) | 212±16 | (+8%) | 75 | (0%) | ||
| LVEDP [mmHg] | 8.4 | (−16%) | 8.4* | (−10%) | 10 | (−16%) | 15±10* | (−35%) |
| Systolic BP [mmHg] | 91 | (+1%) | 129±9 | (+90%) | 75 | (+2%) | ||
| Diastolic BP [mmHg] | 65 | (0%) | 89±5 | (+62%) | 51 | (+2%) | ||
| forward SV [ml] | 15.9 | (−1%) | 34.6±5 | (−13.5%) | 12 | (−45%)# | 21.5 | (−19%)# |
| Mitral regurgitant fraction [%] | 0 | 61 | 61±15 | |||||
| total LVEF [%] | 26 | (−13%) | 70 | (+46%)# | 56±4 | (+19%)# | ||
Experimental values are meand±SD or meand±SE. Cells show absolute values and percentage change compared with the acute overload cases between parentheses, unless noted otherwise.
Pulmonary artery wedge pressure instead of LVEDP.
Compared with normal. BP: aortic blood pressure; LVEDP: left ventricular end-diastolic pressure; LVEF: left ventricular ejection fraction; LVPmax: peak LV cavity pressure; SV: stroke volume
In the volume overload model, dp/dtmax remained similar at 1455 mmHg/sec. LVEDP and total ejection fraction decreased from 12 to 10 mmHg and from 71 to 70%. These changes are in good agreement with experiments (Dell'Italia et al., 1995; Young et al., 1996) (Table 4).
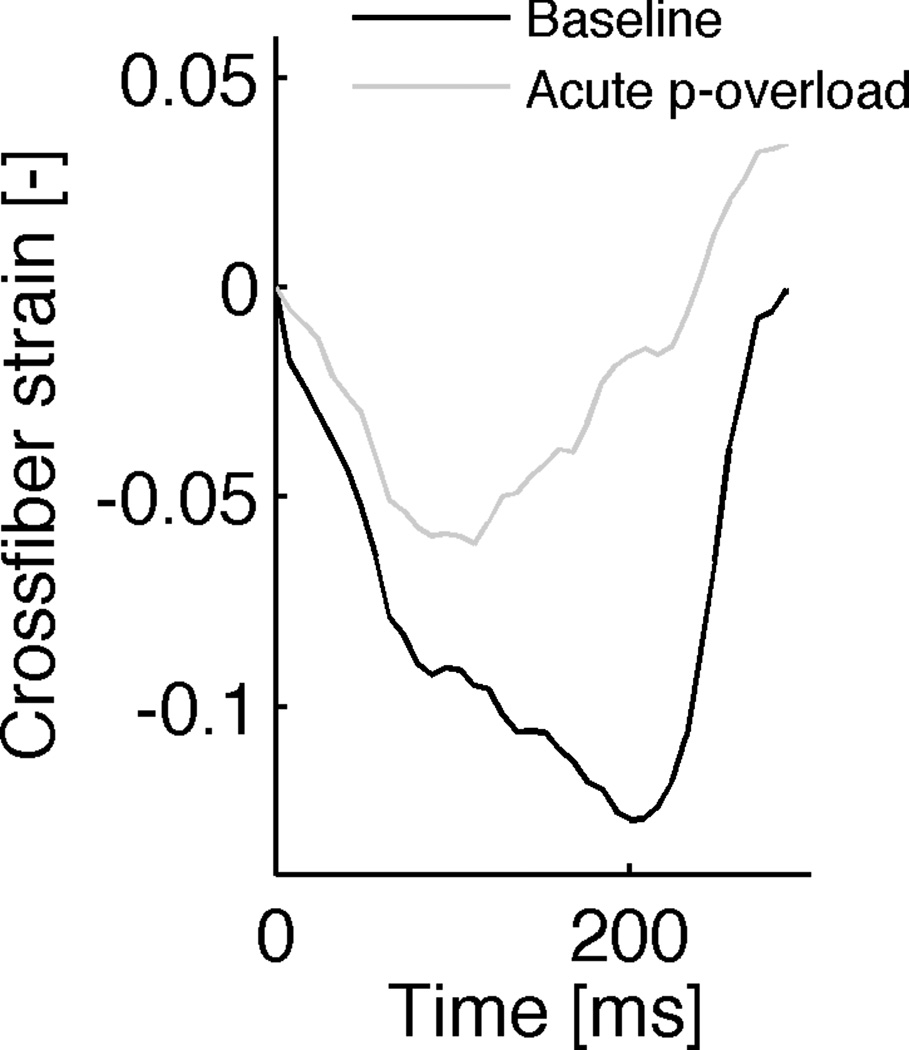
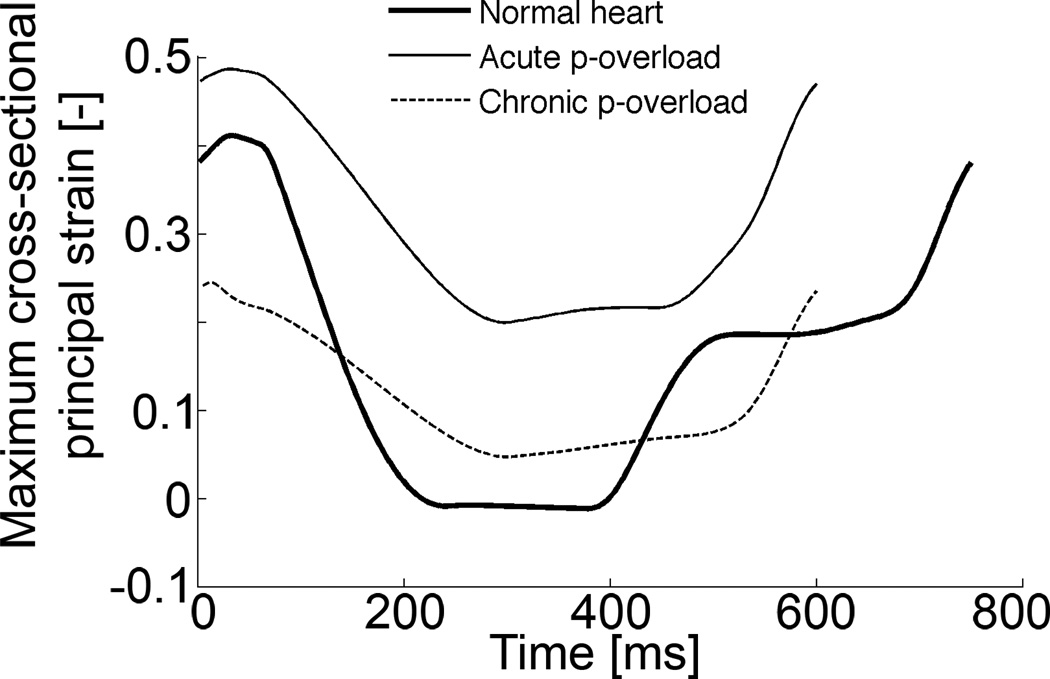
During systole, maximum cross-sectional principal strain decreased in the simulations of the normal heart. The same systolic downward trend of the maximum cross-sectional principal strain was observed in the simulation of aortic stenosis, but the magnitude of the peak negative strain in the pressure overload experiment was less than that in the normal heart (Figure 6, right panel). Both strains reached a minimum during systole: the differences between these minima (between those from the aortic constriction simulation and the normal heart) are the stimuli for fiber radial growth. These strain courses are very similar to cross-fiber strains measured previously in the dog (Figure 6, left panel).
Figure 6.
LV midwall cross-fiber strains as a function of time for 1 cardiac cycle from a dog experiment, performed in our lab (left) (Howard et al., 2011) and maximum cross-sectional principal strain from current simulations (right). Activation is at zero ms. Cross-fiber strain decreases during systole in the normal heart (baseline) and during aortic constriction (acute p-overload), but the magnitude of the peak negative strain in the pressure overload experiment is less than that in the normal heart. Wall thickening through growth in the chronic pressure-overload simulation (right panel, after 18 growth steps) leads to normalization of the smallest strain value. Strain reference in the simulation was the unloaded state. Strain reference in the experiments was end-diastole and end-diastolic pressure for baseline and p-overload were matched.
5 Discussion
We have presented a strain-based cardiac growth law that includes fiber axial and radial cellular growth and tested it in whole heart simulations subjected to chronic pressure and volume overload. The main findings of this study were that: (1) a single growth law is able to reproduce most observed physiological responses, including both acute and chronic changes in structure and function, when integrated with two comprehensive computational models of the pressure-overloaded (by aortic stenosis) and volume-overloaded (by mitral regurgitation) canine heart, coupled to a closed-loop circulation and (2) a strain-based stimulus can drive wall thickening during pressure-overload as opposed to the more commonly proposed stress-based stimuli (Goktepe et al., 2010; Rausch et al., 2011). This may reconcile previously contradictory in vivo and in vitro findings.
How do the biomechanical stimuli lead to the observed structural responses? Diastolic fiber strain – referred to the unloaded state – has previously been proposed and modeled as a stimulus for fiber axial growth (Arts et al., 2005; Emery and Omens, 1997; Goktepe et al., 2010; Omens, 1998). In addition, it has been shown experimentally that isolated cells grow axially in response to stretch applied parallel to their fiber axis (Mansour et al., 2004). Hence, during volume-overload (but also during pressure-overload), an increased preload increases end-diastolic volume and thus stretches fibers high enough above the set-points, triggering fiber axial growth throughout the LV myocardium. This leads to eccentric growth on the chamber level, since the fibers lie mostly in the plane of the ventricular wall. But fiber radial growth is likely regulated by a radially-aligned structure: Russell and investigators (Russell et al., 2010) have suggested a possible mechanical cross-fiber strain sensor located at the Z-disc. Previously, we have presented a stimulus for fiber radial growth, based on the deviation of diastolic cross-fiber strain – also referred to the unloaded state – from zero (Kerckhoffs, 2011). This growth law was tested in a model of normal post-natal rat growth, loaded passively at end-diastole. To be able to test the growth law in a simulation of the beating heart, in the current study, the growth law was modified, such that fiber radial growth was induced by the deviation of the smallest maximum principal strain in the cellular cross-sectional plane. The largest difference with the previous study (Kerckhoffs, 2011) was the timing of the radial growth stimulus within the cardiac cycle (diastole vs. smallest value which occurs around end-systole). In a normal cardiac cycle at end-diastole, cells in the myocardium are stretched in the fiber direction and take on a more ellipsoidal cross-sectional shape (assuming unloaded fibers are circular in cross-section). In this short-axis cross-section, cells are compressed in cardiac radial direction (in the direction from endo- to epicardium) and stretched in the perpendicular direction (in-plane cross-fiber). The latter direction is closely aligned with the maximum principal cross-sectional strain. When cells are activated in systole, they shorten in the fiber direction, thicken radially and also shorten in cross-fiber direction (Figure 6).
When the LV is subjected to an increased afterload, cells shorten and thicken less and cross-fiber strain remains higher than in the normal heart (Figure 6). Taking the smallest value (of maximum cross-sectional principal strain) in the normal heart as a homeostatic set-point for fiber radial growth, the deviation from this value in the pressure overload simulation leads to a positive stimulus of fiber radial growth and tends to normalize cross-fiber strain back to the value in the normal heart (Figure 6). This normalization occurs because the thicker fibers reach a lower maximum principal cross-sectional strain at end-diastole. Experimental evidence for this phenomenon however is still lacking.
The predictive power of the proposed multiscale model of cardiac mechanics and growth needs further testing. Experiments with ventricular pacing have shown acute redistribution of coronary perfusion and contraction (Prinzen et al., 1992; Waldman and Covell, 1987; Wyman et al., 1999): early in the cardiac cycle, myofibers shorten quickly against a low load in early-activated regions, while stretching fibers in late-activated regions. Later in the cycle, early-activated fibers are stretched whereas late-activated fibers contract against increased load. Long-term pacing leads to chronic asymmetric remodeling: the wall in early-activated regions become thinner whereas in late activated regions, the walls become thicker (Prinzen et al., 1995; van Oosterhout et al., 1998). The ability of the model to predict these measured regionally varying growth patterns would be a stringent test.
Although we make the assumption that a stimulus for cellular radial growth is associated with 1) a cellular cross-sectional strain and 2) that cross-fiber strain relates to interfilament lattice spacing change in vivo, there are other possible explanations of these mechanisms. With respect to the first point, several other stimuli candidates have been proposed in the literature (Holmes, 2004), including both stress and strain based growth laws. In a passive elastic tissue, one could achieve the equivalent results with either formulation. In the heart or other muscular tissues, it is theoretically possible to distinguish experimentally stress from strain during contraction. But we do not have sufficient knowledge of the molecular mechano-transductive complex of the myocyte to make this distinction. Most classical studies of ventricular hypertrophy have focused on systolic stress as a driver for concentric hypertrophy and diastolic stress (or strain) as the signal for eccentric hypertrophy (Grossman et al., 1975). But the results of in vivo experiments with genetically engineered mice and myocyte stretch studies have called these assumptions into question, as pointed out in the Introduction. With respect to the second point, ventricular deformation studies have shown that wall thickening during systole is dominated by interlaminar shearing (Costa et al., 1999; Legrice et al., 1995; Waldman et al., 1988). But since these studies measure strain at the tissue level, the contributions of cell cross-sectional shape change to wall thickening in vivo are not known.
Patient-specific modeling is the development of computational models of human pathophysiology that are individualized to patient-specific data with the goal to understand mechanisms of disease, monitor patients and evaluate treatment options specifically for a patient (Kerckhoffs, 2010; Neal and Kerckhoffs, 2010; Sachse and Seemann, 2009; Taylor and Figueroa, 2009; Trayanova, 2011). To date, virtually all patient-specific model results are evaluated acutely, thus ignoring chronic effects (for an exception, see (Lumens et al., 2010)). But validated comprehensive patient-specific models of cardiac (electro-) mechanics combined with growth and remodeling will become invaluable in predicting long-term effects of medical devices, surgeries and therapies on the heart. They will also provide a framework to gain an understanding of reverse remodeling mechanisms, for example in valve replacement/repair (Enriquez-Sarano et al., 1995; Webb et al., 2007), cardiac resynchronization therapy (Kass, 2005), cardiac restraint device therapy (Acker, 2005; Klodell Jr et al., 2008) and cardiac assist device therapy (Rogers et al., 2010; Trumble et al., 2011).
In the current study, we modeled the progression to compensated hypertrophy. In the future, the proposed models can serve as a framework for implementing increased mechanistic detail such as defective mechanotransduction pathways (Heineke and Molkentin, 2006). This will allow for in silico investigation of effects of altered remodeling pathways from the cell to the systems level on decompensated heart failure at multiple levels as well.
5.1 Limitations
5.1.1 Numerical Implementation
Because of the large computational effort (each growth simulation requires about 3 weeks of computation time) it is not straightforward to perform a full-scale sensitivity analysis. However, a parameter variation analysis has been performed in our computational study of normal post-natal growth in the rat (Kerckhoffs, 2011). In the latter study, the ratio of the growth rate constants for fiber axial and radial growth was determined, such that normal post-natal growth of the LV radius and wall thickness matched those measured in experiments. This ratio has been replicated here in the parameters fff,max and fcc,max.
For the modeling of growth we followed the same approach as in Kroon et al (Kroon et al., 2009) in which the ordinary differential equation for volume rate change is linearized with respect to the volume at the start of the growth increment. For other numerical schemes we refer the reader to Ambrosi et al (2011).
5.1.2 Growth law
In the growth law, maximum cellular dimensions were limited by the growth evolution functions (Eqs. 11–12), but no limit was imposed on minimum dimensions. Cardiac myocytes may also have minimum dimensions, but these are unknown (Baskin and Taegtmeyer, 2011). Shrinking of myocytes however – which would be important in the modeling of reverse remodeling – does not play a role in the current study.
The results of a normal heart simulation were used to obtain the set points for the growth law, derived from the assumption that the normal ventricular model is at growth equilibrium prior to the hemodynamic overload. This approach makes the set points location-dependent, but all myocytes are possibly subjected to the same functional requirements. Hence, an interesting future study to consider is the simulation of a normal heart with uniform set points for fiber radial and axial growth. This will result in the development of residual stresses in the normal heart model (Kerckhoffs, 2011), which were also absent in the normal heart model in the current study.
5.1.3 Acute circulatory control
Overall, the acute changes (< 1 minute) in hemodynamics for the simulations of the overloaded hearts, compared with normal, were in good agreement with experiments. Peak LV cavity pressure in the volume-overload simulation however was lower than measured in experiments, which is most likely due to the absence of several fast baro-reflex responses in the models. For example, myocardial contractility was held constant throughout all simulations, but in reality increases due to beta-adrenergic stimulation when a drop in aortic pressure (or time-derivative thereof) is sensed by stretch-sensitive (baro-)receptors in the proximal aorta. Heart rate typically also increases. We prescribed a higher heart rate (from 80 to 100 bpm) as performed in canine pressure-overload experiments (Nagatomo et al., 1999). Increase in venous tone in order to increase venous return was another baro-reflex mechanism that was not included. We did include however the regulation of cardiac output by peripheral resistance which added to the increased preload in both overload simulations. Inclusion of these acute reflexes in future studies may result in a better starting point for the chronic growth simulations. For example, end-diastolic pressure in the acute volume-overload simulation remained rather low, compared with experiments, which led to a reduced stimulus for fiber axial growth and as a result, repressed dilation.
5.1.4 Long-term circulatory control
There are many other long-term adaptation mechanisms that were not included in the current study. For example, the atria in the heart (Kihara et al., 1988) and arteries and veins in the circulation also adapt their dimensions to altered hemodynamics (Arts et al., 2005). The absence of circulation adaptation may explain the discrepancy with experiments, observed in the blood pressures in the chronic pressure-overload simulation. A particular mechanism that was not included in the model is blood volume control, which in the CircAdapt model of the circulation is regulated by mean aortic pressure. In a preliminary simulation with variable blood volume, the volume-overload model exhibited much more physiological eccentric growth than in the present results. However, the pressure-overloaded heart also became exposed to a large preload and showed eccentric hypertrophy. Mean aortic pressure however is only one of many mechanisms of circulatory control. For example, there is an opposing mechanism of blood volume control by atrial stretch and ANP production, which was also not included in the model. Most likely, to achieve appropriate hypertrophy, a balance is needed between chronic and acute circulatory control on one side with ventricular growth laws on the other. Another possible explanation for the eccentric LV growth in response to blood volume change is that the fiber axial growth rate constant may have been too large: i.e. under pathological conditions, concentric hypertrophy seems to be a faster type of growth than eccentric (Moalic et al., 1981), whereas isolated myocytes show attenuated responses to stretch in the fiber direction compared with cross-fiber stretch (Simpson et al., 1999). Hence, further computational studies with long-term control mechanisms are needed in order to investigate these interactions between circulatory control and ventricular growth.
Other chronic changes currently not included that warrant future studies are changes in contractility, sensitivity to beta-adrenergic stimulation, fibrosis, extracellular matrix restructuring, necrosis and apoptosis.
Supplementary Material
Acknowledgments
We thank Prof. Theo Arts for kindly providing the code for the CircAdapt model.
Funding Sources
The study has been supported by the National Biomedical Computation Resource (NIH grant P41 RR08605) (to A.D.M) and NIH grant R01 HL96544 (to A.D.M.). This investigation was conducted in part using a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR-017588-01 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acker MA. Clinical Results with the Acorn Cardiac Restraint Device with and without Mitral Valve Surgery. Seminars in Thoracic and Cardiovascular Surgery. 2005;17:361–363. doi: 10.1053/j.semtcvs.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Ambrosi D, Ateshian GA, Arruda EM, Cowin SC, Dumais J, Goriely A, Holzapfel GA, Humphrey JD, Kemkemer R, Kuhl E, Olberding JE, Taber LA, Garikipati K. Perspectives on biological growth and remodeling. J Mech Phys Solids. 2011;59:863–883. doi: 10.1016/j.jmps.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts T, Delhaas T, Bovendeerd P, Verbeek X, Prinzen FW. Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. Am. J. Physiol.-Heart Circ. Physiol. 2005;288:H1943–H1954. doi: 10.1152/ajpheart.00444.2004. [DOI] [PubMed] [Google Scholar]
- Baskin KK, Taegtmeyer H. Taking pressure off the heart: the ins and outs of atrophic remodelling. Cardiovasc. Res. 2011;90:243–250. doi: 10.1093/cvr/cvr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bupha-Intr T, Holmes JW, Janssen PML. Induction of hypertrophy in vitro by mechanical loading in adult rabbit myocardium. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H3759–H3767. doi: 10.1152/ajpheart.01267.2006. [DOI] [PubMed] [Google Scholar]
- Chen C, Rodriguez L, Guerrero J, Marshall S, Levine R, Weyman A, Thomas J. Noninvasive estimation of the instantaneous first derivative of left ventricular pressure using continuous-wave Doppler echocardiography. Circulation. 1991;83:2101–2110. doi: 10.1161/01.cir.83.6.2101. [DOI] [PubMed] [Google Scholar]
- Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am. J. Physiol.-Heart Circ. Physiol. 1999;276:H595–H607. doi: 10.1152/ajpheart.1999.276.2.H595. [DOI] [PubMed] [Google Scholar]
- Dell'Italia LJ, Meng QC, Balcells E, Straeter-Knowlen IM, Hankes GH, Dillon R, Cartee RE, Orr R, Bishop SP, Oparil S, et al. Increased ACE and chymase-like activity in cardiac tissue of dogs with chronic mitral regurgitation. American Journal of Physiology - Heart and Circulatory Physiology. 1995;269:H2065–H2073. doi: 10.1152/ajpheart.1995.269.6.H2065. [DOI] [PubMed] [Google Scholar]
- Doll S, Schweizerhof K. On the Development of Volumetric Strain Energy Functions. Journal of Applied Mechanics. 2000;67:17–21. [Google Scholar]
- Emery JL, Omens JH. Mechanical regulation of myocardial growth during volume-overload hypertrophy in the rat. Am. J. Physiol.-Heart Circ. Physiol. 1997;42:H1198–H1204. doi: 10.1152/ajpheart.1997.273.3.H1198. [DOI] [PubMed] [Google Scholar]
- Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve Repair Improves the Outcome of Surgery for Mitral Regurgitation : A Multivariate Analysis. Circulation. 1995;91:1022–1028. doi: 10.1161/01.cir.91.4.1022. [DOI] [PubMed] [Google Scholar]
- Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic Alterations That Inhibit In Vivo Pressure-Overload Hypertrophy Prevent Cardiac Dysfunction Despite Increased Wall Stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- Eyster JAE, Meek WJ, Hodges FJ. Cardiac changes subsequent to aortic lesions. Arch Intern Med. 1927;39:536–549. [Google Scholar]
- Gerdes AM. The use of isolated myocytes to evaluate myocardial remodeling. Trends in Cardiovascular Medicine. 1992;2:152–155. doi: 10.1016/1050-1738(92)90023-L. [DOI] [PubMed] [Google Scholar]
- Goktepe S, Abilez OJ, Parker KK, Kuhl E. A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis. J. Theor. Biol. 2010;265:433–442. doi: 10.1016/j.jtbi.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, Omens JH, McCulloch AD. Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnology and Bioengineering. 2003;81:578–587. doi: 10.1002/bit.10506. [DOI] [PubMed] [Google Scholar]
- Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione JM, McCulloch AD, Waldman LK. Passive Material Properties of Intact Ventricular Myocardium Determined from a Cylindrical Model. J. Biomech. Eng.-Trans. ASME. 1991;113:42–55. doi: 10.1115/1.2894084. [DOI] [PubMed] [Google Scholar]
- Guterl KA, Haggart CR, Janssen PM, Holmes JW. Isometric contraction induces rapid myocyte remodeling in cultured rat right ventricular papillary muscles. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293:H3707–H3712. doi: 10.1152/ajpheart.00296.2007. [DOI] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Herrmann KL, McCulloch AD, Omens JH. Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy. American Journal of Physiology - Heart and Circulatory Physiology. 2003;284:H1277–H1284. doi: 10.1152/ajpheart.00168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JW. Candidate mechanical stimuli for hypertrophy during volume overload. J. Appl. Physiol. 2004;97:1453–1460. doi: 10.1152/japplphysiol.00834.2003. [DOI] [PubMed] [Google Scholar]
- Howard EJ, Covell JW, Mulligan LJ, McCulloch AD, Omens JH, Kerckhoffs RCP. Improvement in pump function with endocardial biventricular pacing increases with activation time at the left ventricular pacing site in failing canine hearts. American Journal of Physiology - Heart and Circulatory Physiology. 2011 doi: 10.1152/ajpheart.00295.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kass DA. Cardiac Resynchronization Therapy. J. Cardiovasc. Electrophysiol. 2005;16:S35–S41. doi: 10.1111/j.1540-8167.2005.50136.x. [DOI] [PubMed] [Google Scholar]
- Katz LN, Ralli EP, Cheer S-N. The cardiodynamic changes in the aorta and left ventricle due to stenosis of the aorta. J. Clin. Invest. 1928;5:205–227. doi: 10.1172/JCI100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs RCP. Patient specific modeling of the cardiovascular system: Technology-driven personalized medicine. New York, NY: Springer; 2010. p. 265. [Google Scholar]
- Kerckhoffs RCP. Computational Modeling of Cardiac Growth in the Post-Natal Rat with a Strain-Based Growth Law. J Biomech. 2011 doi: 10.1016/j.jbiomech.2011.11.028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs RCP, Faris O, Bovendeerd PHM, Prinzen FW, Smits K, McVeigh ER, Arts T. Electromechanics of paced left ventricle simulated by straightforward mathematical model: comparison with experiments. Am. J. Physiol.-Heart Circ. Physiol. 2005;289:H1889–H1897. doi: 10.1152/ajpheart.00340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerckhoffs RCP, Omens JH, McCulloch AD, Mulligan LJ. Ventricular dilation and electrical dyssynchrony synergistically increase regional mechanical non-uniformity but not mechanical dyssynchrony: A computational model. Circulation: Heart Failure. 2010;3:528–536. doi: 10.1161/CIRCHEARTFAILURE.109.862144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Sasayama S, Miyazaki S, Onodera T, Susawa T, Nakamura Y, Fujiwara H, Kawai C. Role of the left atrium in adaptation of the heart to chronic mitral regurgitation in conscious dogs. Circ.Res. 1988;62:543–553. doi: 10.1161/01.res.62.3.543. [DOI] [PubMed] [Google Scholar]
- Klodell CT, Jr, Aranda JM, Jr, McGiffin DC, Rayburn BK, Sun B, Abraham WT, Pae WE, Jr, Boehmer JP, Klein H, Huth C. Worldwide surgical experience with the Paracor HeartNet cardiac restraint device. The Journal of Thoracic and Cardiovascular Surgery. 2008;135:188–195. doi: 10.1016/j.jtcvs.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Kroon W, Delhaas T, Arts T, Bovendeerd P. Computational modeling of volumetric soft tissue growth: application to the cardiac left ventricle. Biomechanics and Modeling in Mechanobiology. 2009;8:301–309. doi: 10.1007/s10237-008-0136-z. [DOI] [PubMed] [Google Scholar]
- Legrice IJ, Takayama Y, Covell JW. Transverse-Shear Along Myocardial Cleavage Planes Provides a Mechanism for Normal Systolic Wall Thickening. Circ.Res. 1995;77:182–193. doi: 10.1161/01.res.77.1.182. [DOI] [PubMed] [Google Scholar]
- Li XS, Demmel JW. SuperLU_DIST: A scalable distributed-memory sparse direct solver for unsymmetric linear systems. ACM T. Math. Software. 2003;29:110–140. [Google Scholar]
- Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: Targeting Raf/MEK/ERK1/2-signaling. The International Journal of Biochemistry & Cell Biology. 2009;41:2351–2355. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lubarda VA, Hoger A. On the mechanics of solids with a growing mass. International Journal of Solids and Structures. 2002;39:4627–4664. [Google Scholar]
- Lumens J, Blanchard DG, Arts T, Mahmud E, Delhaas T. Left ventricular underfilling and not septal bulging dominates abnormal left ventricular filling hemodynamics in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1083–H1091. doi: 10.1152/ajpheart.00607.2010. [DOI] [PubMed] [Google Scholar]
- Lumens J, Delhaas T, Kirn B, Arts T. Three-Wall Segment (TriSeg) Model Describing Mechanics and Hemodynamics of Ventricular Interaction. Ann. Biomed. Eng. 2009;37:2234–2255. doi: 10.1007/s10439-009-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour H, de Tombe PP, Samarel AM, Russell B. Restoration of Resting Sarcomere Length After Uniaxial Static Strain Is Regulated by Protein Kinase C{epsilon} and Focal Adhesion Kinase. Circ Res. 2004;94:642–649. doi: 10.1161/01.RES.0000121101.32286.C8. [DOI] [PubMed] [Google Scholar]
- Moalic JM, Bercovici J, Swynghedauw B. Protein synthesis during systolic and diastolic cardiac overloading in rats: a comparative study. Cardiovasc Res. 1981;15:515–521. doi: 10.1093/cvr/15.9.515. [DOI] [PubMed] [Google Scholar]
- Nagatomo Y, Carabello BA, Hamawaki M, Nemoto S, Matsuo T, McDermott PJ. Translational mechanisms accelerate the rate of protein synthesis during canine pressure-overload hypertrophy. American Journal of Physiology - Heart and Circulatory Physiology. 1999;277:H2176–H2184. doi: 10.1152/ajpheart.1999.277.6.H2176. [DOI] [PubMed] [Google Scholar]
- Neal ML, Kerckhoffs R. Current progress in patient-specific modeling. Brief Bioinform. 2010;11:111–126. doi: 10.1093/bib/bbp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omens JH. Stress and strain as regulators of myocardial growth. Progress in Biophysics and Molecular Biology. 1998;69:559–572. doi: 10.1016/s0079-6107(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Onodera T, Tamura T, Said S, McCune SA, Gerdes AM. Maladaptive Remodeling of Cardiac Myocyte Shape Begins Long Before Failure in Hypertension. Hypertension. 1998;32:753–757. doi: 10.1161/01.hyp.32.4.753. [DOI] [PubMed] [Google Scholar]
- Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodeling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- Prinzen FW, Cheriex EC, Delhaas T, Vanoosterhout MFM, Wellens HJJ, Reneman RS. Asymmetric Thickness of the Left-Ventricular Wall Resulting from Asynchronous Electric Activation - a Study in Dogs with Ventricular Pacing and in Patients with Left-Bundle-Branch Block. Am. Heart J. 1995;130:1045–1053. doi: 10.1016/0002-8703(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Prinzen FW, Delhaas T, Arts T, Reneman RS. Effect of Asynchronous Electrical Activation on Regional Contractile Work in the Canine Left-Ventricle. Faseb J. 1992;6:A1232–A1232. [Google Scholar]
- Rausch M, Dam A, Göktepe S, Abilez O, Kuhl E. Computational modeling of growth: systemic and pulmonary hypertension in the heart. Biomechanics and modeling in mechanobiology. 2011 doi: 10.1007/s10237-010-0275-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EK, Hoger A, McCulloch AD. Stress-dependent finite growth in soft elastic tissues. J. Biomech. 1994;27:455–467. doi: 10.1016/0021-9290(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez EK, Hunter WC, Royce MJ, Leppo MK, Douglas AS, Weisman HF. A method to reconstruct myocardial sarcomere lengths and orientations at transmural sites in beating canine hearts. American Journal of Physiology - Heart and Circulatory Physiology. 1992;263:H293–H306. doi: 10.1152/ajpheart.1992.263.1.H293. [DOI] [PubMed] [Google Scholar]
- Rogers JG, Aaronson KD, Boyle AJ, Russell SD, Milano CA, Pagani FD, Edwards BS, Park S, John R, Conte JV, Farrar DJ, Slaughter MS. Continuous Flow Left Ventricular Assist Device Improves Functional Capacity and Quality of Life of Advanced Heart Failure Patients. J. Am. Coll. Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Russell B, Curtis MW, Koshman YE, Samarel AM. Mechanical stress-induced sarcomere assembly for cardiac muscle growth in length and width. J. Mol. Cell. Cardiol. 2010;48:817–823. doi: 10.1016/j.yjmcc.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse FB, Seemann G. Special issue: Functional imaging and modelling of the heart. Med Image Anal. 2009;13:345. doi: 10.1016/j.media.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Sasayama S, Ross J, Jr, Franklin D, Bloor C, Bishop S, Dilley R. Adaptations of the left ventricle to chronic pressure overload. Circ Res. 1976;38:172–178. doi: 10.1161/01.res.38.3.172. [DOI] [PubMed] [Google Scholar]
- Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of Cardiac Myocyte Protein Turnover and Myofibrillar Structure In Vitro by Specific Directions of Stretch. Circ.Res. 1999;85:e59–e69. doi: 10.1161/01.res.85.10.e59. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Figueroa CA. Patient-Specific Modeling of Cardiovascular Mechanics. Annu. Rev. Biomed. Eng. 2009;11:109–134. doi: 10.1146/annurev.bioeng.10.061807.160521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayanova NA. Whole-Heart Modeling: Applications to Cardiac Electrophysiology and Electromechanics. Circ Res. 2011;108:113–128. doi: 10.1161/CIRCRESAHA.110.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble DR, McGregor W, Kerckhoffs RCP, Waldman LK. Cardiac Assist with a Twist: Apical Torsion as a Means to Improve Failing Heart Function. J. Biomech. 2011 doi: 10.1115/1.4005169. in press. [DOI] [PubMed] [Google Scholar]
- van Oosterhout MFM, Prinzen FW, Arts T, Schreuder JJ, Vanagt WYR, Cleutjens JPM, Reneman RS. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation. 1998;98:588–595. doi: 10.1161/01.cir.98.6.588. [DOI] [PubMed] [Google Scholar]
- Vena P, Gastaldi D, Socci L, Pennati G. An anisotropic model for tissue growth and remodeling during early development of cerebral aneurysms. Computational Materials Science. 2008;43:565–577. [Google Scholar]
- Verbeek X, Vernooy K, Peschar M, Cornelussen RNM, Prinzen FW. Intra-ventricular resynchronization for optimal left ventricular function during pacing in experimental left bundle branch block. J. Am. Coll. Cardiol. 2003;42:558–567. doi: 10.1016/s0735-1097(03)00641-7. [DOI] [PubMed] [Google Scholar]
- Waldman LK, Covell JW. Effects of Ventricular Pacing on Finite Deformation in Canine Left-Ventricles. Am. J. Physiol. 1987;252:H1023–H1030. doi: 10.1152/ajpheart.1987.252.5.H1023. [DOI] [PubMed] [Google Scholar]
- Waldman LK, Nosan D, Villarreal F, Covell JW. Relation between Transmural Deformation and Local Myofiber Direction in Canine Left-Ventricle. Circ.Res. 1988;63:550–562. doi: 10.1161/01.res.63.3.550. [DOI] [PubMed] [Google Scholar]
- Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, Sinhal A, Carere RG, Munt B, Ricci D, Ye J, Cheung A, Lichtenstein SV. Percutaneous Transarterial Aortic Valve Replacement in Selected High-Risk Patients With Aortic Stenosis. Circulation. 2007;116:755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- Wyman BT, Hunter WC, Prinzen FW, McVeigh E. Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am. J. Physio.-Heart Circ. Physiol. 1999;276:H881–H891. doi: 10.1152/ajpheart.1999.276.3.H881. [DOI] [PubMed] [Google Scholar]
- Young AA, Orr R, Smaill BH, Dell'Italia LJ. Three-dimensional changes in left and right ventricular geometry in chronic mitral regurgitation. Am J Physiol. 1996;271:H2689–H2700. doi: 10.1152/ajpheart.1996.271.6.H2689. [DOI] [PubMed] [Google Scholar]
- Zak R. Growth of the heart in health and disease. New York: Raven Press; 1984. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.