Abstract
Background and Purpose
CADASIL (cerebral autosomal dominant arteriopathy subcortical infarcts and leukoencephalopathy) is a genetic disorder hallmarked by ischemic stroke and vascular dementia. Characteristic pathological changes in the vasculature include thickening of small arteries and accumulation of heterogeneous material within the vessel wall. We tested whether endothelial von Willebrand factor (vWF) accumulates in CADASIL vessels and whether exposure of smooth muscle cells to vWF alters the expression of smooth muscle gene expression.
Methods
Brain sections obtained at autopsy from six North American CADASIL patients were examined using immunohistochemistry for vWF and IgG. Rat aortic smooth muscle cells (A7R5 cells) were tested for binding to infrared-tag labeled vWF. Finally, A7R5 cells were exposed to vWF, and expression of mature smooth muscle marker genes was analyzed by quantitative reverse transcriptase PCR.
Results
vWF is expressed in the penetrating arterial walls in all CADASIL samples. IgG, a marker of serum extravasation, was present only in a minority of arterial walls. vWF binds to smooth muscle cells in vitro, and low concentrations of vWF rapidly activate c-fos, EGR, TSP1, and c-myc while specifically inhibiting RNA encoding smooth muscle actin, calponin, and SM22.
Conclusions
These data demonstrate that vWF, likely produced by the endothelium, permeates the vessel wall of CADASIL brains. Exposure of smooth muscle cells to vWF results in reduction of specific RNAs required for normal vascular homeostasis. This is the first report of accumulation of a protein within CADASIL vessels that inhibits vascular gene expression and implicates a role for vWF beyond hemostasis.
Keywords: CADASIL, von Willebrand Factor (vWF), smooth muscle, small vessel disease, vascular permeability, immunoglobulin
Introduction
The monogenic cerebrovascular disorder CADASIL is the most common inherited cause of vascular dementia [1]. In CADASIL, causative mutations in NOTCH3 typically lead to an odd number of cysteine residues in the extracellular epidermal growth factor (EGF) domains. During development, NOTCH3 participates in cell lineage specification, and the media of arteries of mice deficient in Notch3 exhibit venous properties [2]; however, it is unknown whether mutant NOTCH3 causes CADASIL by a similar loss of function in human brain vessels or by a gain-of-function mechanism that results in vascular degeneration.
Phenotypically, CADASIL demonstrates substantial variability in age of onset and rate of progression, but patients commonly experience core clinical features include atypical migraine, mood disorders, and ischemic stroke, followed by vascular dementia and premature death [3]. Functional disability and cognitive deterioration of patients correlate with lacunar infarcts seen on MRI [4–7]. An improved understanding of the pathogenesis of small vessel disease leading to stroke in CADASIL may shed light on the molecular mechanisms of brain small vessel dysfunction and, in turn, suggest therapeutic targets.
Pathological hallmarks of CADASIL include thickening of penetrating vessels of the brain, smooth muscle cell loss, and replacement of vascular media with a disorganized extracellular matrix [1, 8–10]. This matrix is composed of a variety of elements that have been characterized previously with immunohistochemical techniques, including NOTCH3 [11–14, 9] and collagen [12–14, 9, 15, 16]. In addition, some investigators have reported increased fibrinogen, immunoglobulin, and fibronectin in CADASIL vessels (reviewed by Ruchoux [10]). Accumulation of these proteins likely results from smooth muscle dysfunction (eg. NOTCH3 buildup due to poor clearance), pathological endothelial barrier function (eg. fibrinogen and immunoglobulin extravasation) and vascular fibrosis (eg. collagen). Thus, proteins of the vessel wall described so far likely represent end-markers of the disease process, rather than molecules that trigger pathological functions.
Von Willebrand factor (vWF) is a large, multimeric protein expressed in platelets and endothelium. The two known functions of vWF are hemostatic [17]: 1) as an adhesive protein that mediates hemostasis through bridging interactions between the subendothelial collagen matrix and platelets and 2) as a carrier for Factor VIII. However, in experimental models of vascular injury, vWF expression has been observed in the media of the vessel wall after angioplasty [18, 19], collar induced atherosclerosis [20], carotid occlusion [21] and experimental fistula induction [22]. Furthermore, vWF staining has been observed in the intima of atherosclerotic carotid arteries in humans [23]. In this non-canonical location, the protein is inaccessible to circulating coagulation proteins and platelets. Therefore, it is possible that vWF deposited outside of the circulation exerts hemostasis-independent effects not yet characterized.
The objectives of this study are (1) to describe the distribution of vWF in small vessels in CADASIL and (2) to study the effects of vWF on genes required for maintenance of mature vascular smooth muscle.
Methods
Patient characteristics and tissue analysis
All CADASIL patients were diagnosed prior to death. Table 1 shows clinical information for the six CADASIL patients, each with independent heterozygous NOTCH3 mutations predicted to result in addition or deletion of a cysteine residue in an EGF repeat. One patient died of a deep frontal lobe intracranial hemorrhage with intraventricular extension. Brain specimens from twenty five control patients free of known neurological disease were obtained from the Maryland Brain Bank and the University of Michigan. Controls had no known brain disease, ranging from 23 to 89 years of age and included 7 women and 18 men. Tissue analyzed here includes CADASIL and control sections from the medial frontal lobe (both gray matter and adjacent white matter). Vascular abnormalities observed in CADASIL samples were verified by examination of occipital lobe sections from the same individuals.
Table 1.
CADASIL patient characteristics
| Patient | Age | Gender | NOTCH3 mutation | Symptoms |
|---|---|---|---|---|
| 1 | 58 | Male | Cys117Tyr | Dementia and ischemic stroke |
| 2 | 46 | Male | Arg90Cys | Dementia and ischemic stroke |
| 3 | 70 | Female | Cys1061Tyr | Dementia, ischemic stroke, ICH |
| 4 | 83 | Female | Arg332Cys | Dementia and ischemic stroke |
| 5 | 73 | Male | Asp353_Ser357dup | Dementia and ischemic stroke |
| 6 | 68 | Female | Arg141Cys | Dementia and ischemic stroke |
ICH = intracerebral hemorrhage
Immunohistochemistry
Formalin fixed tissues were embedded in paraffin, cut in 5um slices, deparaffinized, subjected to citrate-mediated antigen retrieval, and pretreated for 5 minutes with proteinase K. Immunostaining was performed using anti-vWF (DAKO Cat # A0082) and anti- IgG (human heavy chain; Ortho Diagnostics) using a DAKO autostainer with the EnVision detection system. Slides were counterstained with hematoxylin. For comparisons between vWF and IgG distribution patterns, we identified the same artery from serial sections of the same block.
Cell culture
Rat aortic smooth muscle cell line A7R5 was propagated in DMEM with 10% fetal bovine serum. For cell binding experiments, purified Factor VIII-free -vWF (Haematologic Technologies) or control protein Fc (R&D) was labeled with Alexa-700-succinimide (Invitrogen) and then purified from unreacted label by gel filtration through a spin column [24]. The labeled protein was then overlaid onto cells for 240 minutes at room temperature, washed three times, and the amount of labeled protein bound to the cells quantified on a Licor Odyssey flatbed scanner. In competition studies, unlabeled vWF or control Fc protein was co-incubated with labeled protein (10 µg/ml). For gene expression studies, cells were serum starved overnight and then exposed to 200 ng/mL purified vWF in serum free media. Cells were harvested for RNA purification after 30, 60, and 120 minutes of treatment with protein.
Quantitative real-time RT-PCR
Total RNA was purified from tissue culture cells with the RNeasy Mini Kit from Qiagen (Valencia, CA). RNA (1µg) was reverse transcribed into cDNA and then analyzed by real time quantitative polymerase chain reaction [25]. The mRNA expression was quantified using the comparative method, normalized to beta-actin and then referenced to control (cells without vWF treatment). Primer sequences for the tested genes are shown in Table 2.
Table 2.
Primer sequences for qPCR
| Gene | Primer Sequence (Sense) | Primer Sequence (Anti-sense) |
|---|---|---|
| SM22 | 5’-AAGAATGGCGTGATTCTGAGC-3’ | 5’-CTGCCTTCAAGAATTGAGCC-3’ |
| SMA | 5’-CTGGTATTGTGCTGGACTC -3’ | 5’-GAAGGAATAGCCACGCTCAG-3’ |
| Calponin | 5’-CAGTCAGCAGGGCATGACAG-3’ | 5’-AGTCATGCCAGCCTGGCTG-3’ |
| SM-MHC | 5’-ACTACAGAATGAAGTGGAGA-3’ | 5’-CAGGCTGTTCCTCTCATCT-3’ |
| c-fos | 5’-CAAAGTAGAGCAGCTATCTCCTGA-3’ | 5’-TTCTCGTCTTCAAGTTGATCTGTC-3’ |
| TSP1 | 5’-GTCGACGAGTGCAAAGAAGT-3’ | 5’-TAGCGTGTTCGACACCTCTG-3’ |
| c-myc | 5’-CAGCGACTCTGAAGAAGAACAAGA-3’ | 5’-CTGTGTGGAGGTTTGCTGTG-3’ |
| EGR | 5’-CTGACCACAGAGTCCTTTTCTGA-3’ | 5’-AAGTGTTGCCACTGTTGGGT-3’ |
Gene reporter assays
Reporter assays have been described previously [25]. Cells were transfected with fos-luciferase reporter in combination with a transfection control plasmid pRL-TK (Promega). After 24 hours, the cells were serum starved for 4 hours, followed by incubation for specified times with either buffer or purified vWF (200ng/ml). Cell lysates were assessed for reporter expression using a dual luciferase assay.
Statistics
Data are presented as mean ± SEM. Comparison of mean values between groups was performed using an unpaired, Student’s t test with alpha set at 0.05.
Results
Vascular vWF distribution in CADASIL
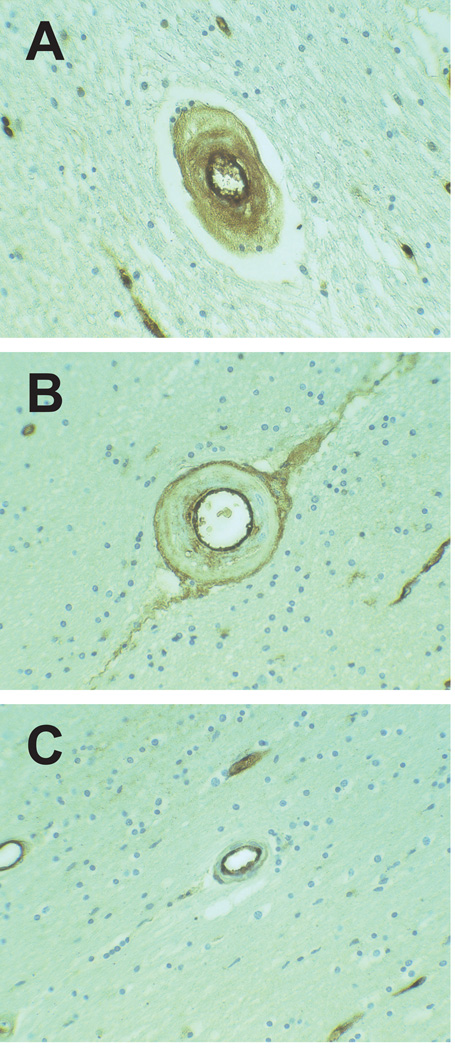
All CADASIL and control brain samples exhibited strong endothelial vWF staining in large and small vessels. In addition, three distinct patterns of vWF expression were identified in arteries of the white matter. First, fine, granular vWF immunoreactivity was observed within the entire width of thickened penetrating small arteries of the white matter (Figure 1A) in 6/6 CADASIL brains and in the majority of small arteries in each brain. This pattern was present sparsely in 2/25 control samples (a 25 year old with sickle cell disease, myocardial infarction and renal failure and a 67 year old with myocardial infarction). Second, a double barreled profile of vWF immunoreactivity was observed in thickened arteries with strong endothelial and adventitial staining and variable staining of the subintima and media; this pattern was observed in 2/6 of CADASIL samples and in 4/25 controls (Figure 1B). Third, in all samples, we observed thin-walled arteries containing vWF confined to the endothelium and subendothelium (Figure 1C).
Figure 1.
Novel patterns of vascular vWF distribution in brain vasculature. Transmural distribution of vWF was very common in CADASIL small vessels (A). Both CADASIL and control samples contained double-barreled vWF profiles; the example shown is from a CADASIL brain (B). vWF was confined to the endothelium in vessels of both CADASIL and control vessels (C). All vessels were magnified at 400x.
In addition, vWF immunohistochemistry revealed broad non-vascular distribution in both the gray and white matter. All CADASIL samples showed sporadic neuronal staining in the gray matter that was frequently associated with vessels and staining of microglia and astrocytes in the white matter. In some control samples, neurons, microglia, and astrocytes were also stained, though not as heavily.
IgG distribution in CADASIL arteries
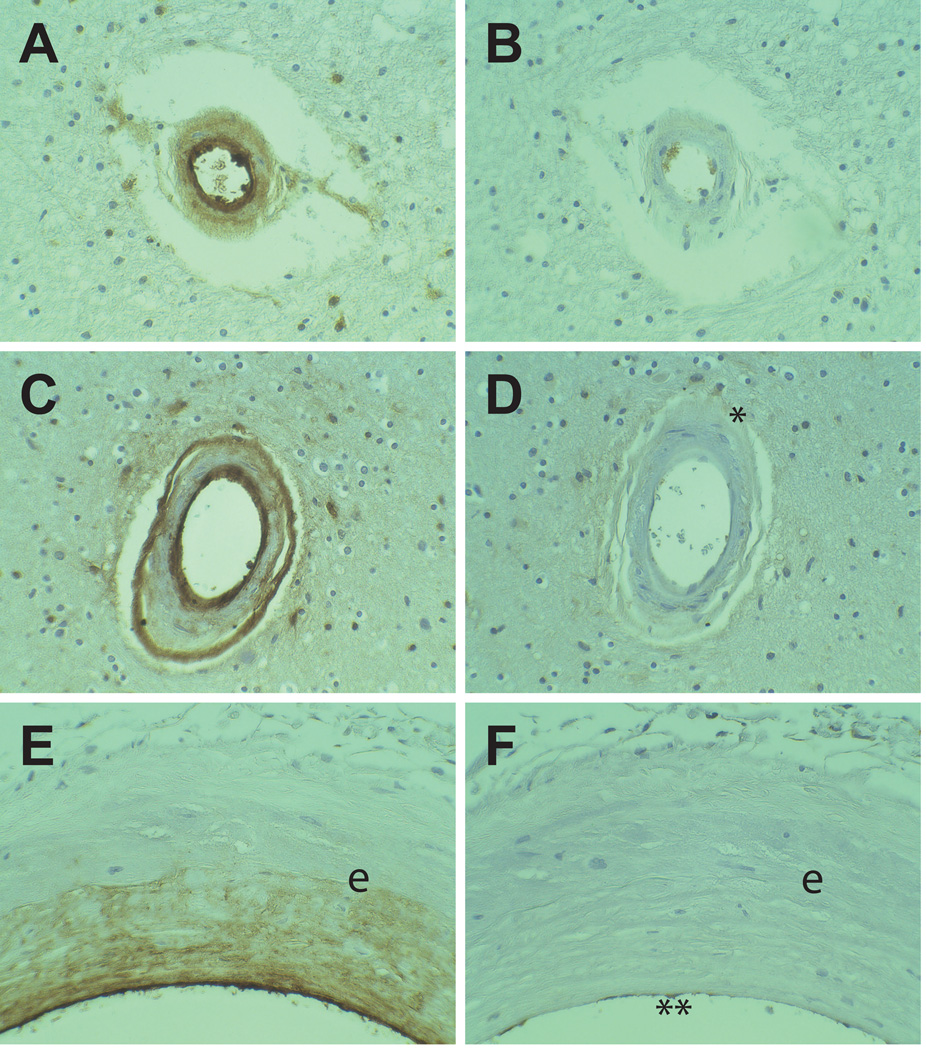
vWF is present in the serum and the endothelium. Therefore, vWF in the arteries could originate from either extravasation or local endothelial deposition into the arterial wall. To assess the role of serum protein extravasation in vWF deposition, the distribution of vWF and IgG was examined by immunohistochemistry in serial sections of CADASIL samples. IgG immunoreactivity was excluded from the vascular wall of most CADASIL arteries in the white matter exhibiting transmural vWF (Figures 2A–B). In arteries with a double-barreled distribution of vWF immunoreactivity, IgG was also excluded from the vessel, except for very faint endothelial and adventitial staining (Figure 2C–D). Small vessel exclusion of IgG in vWF-positive vessels was observed in all CADASIL patients.
Figure 2.
Comparison between vWF and IgG immunoreactivity CADASIL brains. Serial sections from CADASIL brains were stained for vWF (A, C, E) and IgG (B, D, F). Small penetrating white matter arteries (A–B) with transmural deposition of vWF did not contain IgG. Arteries which exhibited a double barreled vWF staining pattern (C–D) faintly stained for IgG in the adventitia (*). Meningeal arteries (E–F) demonstrated heavy intimal deposition of vWF without IgG labeling. Endothlium of the arteries contained both vWF and IgG (marked ** in (F)). The elastic lamina is marked (e). All vessels were magnified at 400x.
The independent distribution of vWF and IgG was most clearly seen in large meningeal arteries (Figure 2E–F). In large CADASIL arteries, vWF was heavily deposited in the endothelium and the pathologically thickened intima, with scant accumulation in the media. A thin layer of IgG staining was observed within the endothelium. However, IgG was absent from the subintima and media. In controls, the intima was only sporadically thickened, and vWF was not seen in normal intima.
Outside of the vascular system, staining for IgG was present in only scattered cells compared to vWF, though there was abundant expression in neurons, microglia, and astrocytes in regions of each section which exhibited high cellular vWF staining. This was seen in both CADASIL and control brains and has previously been described [26, 27].
vWF binds to smooth muscle cells and alters transcriptional responses
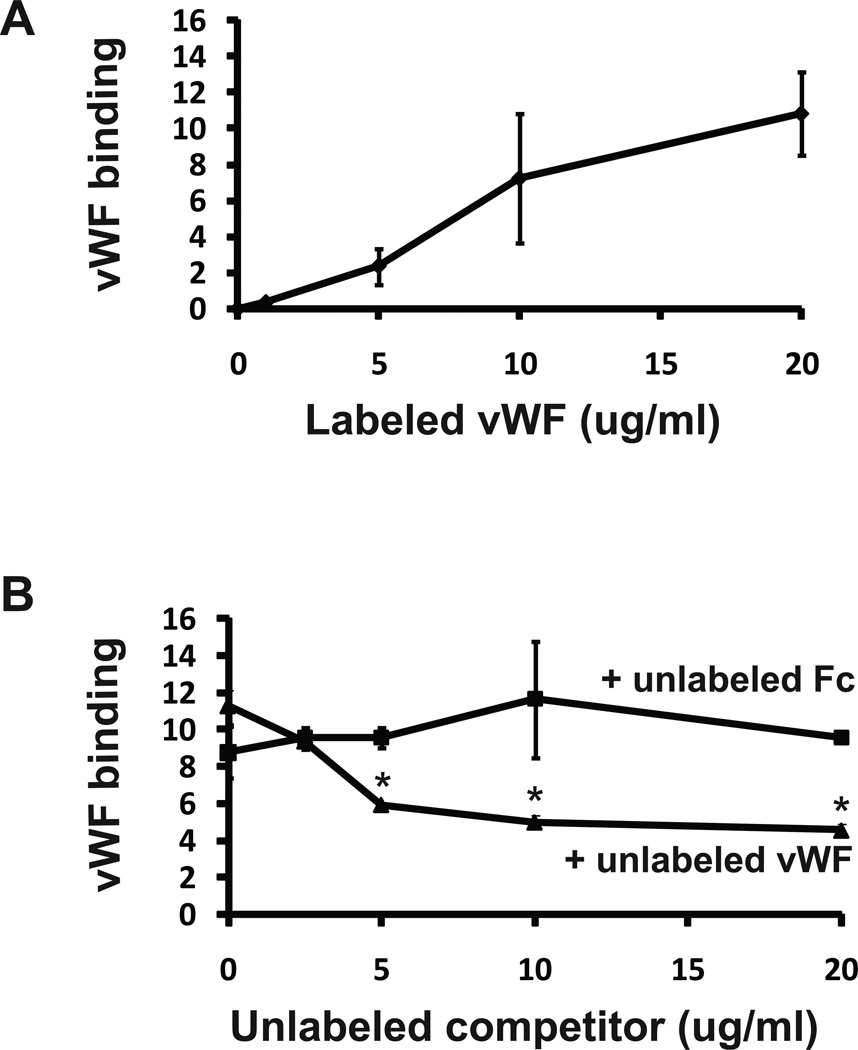
To investigate the possible biological functions of vWF in the vascular media, we assessed the effects of vWF on smooth muscle cells in culture. Labeled vWF avidly bound to rat aortic smooth muscle cells (A7R5 cells) (Figure 3A). Unlabeled vWF competed for label binding, but a control protein (Fc fragment) did not affect vWF cell binding (Figure 3B), suggesting that vWF binds to a specific receptor on the cell surface.
Figure 3.
vWF binding to smooth muscle cells. Rat aortic smooth muscle cells (A7R5) were incubated with purified, infrared-tagged human vWF at varying concentrations to assess binding. Binding to cells increased in a dose dependent fashion (A). Unlabeled vWF, but not recombinant Fc protein, competed against 10ug/ml labeled vWF for cell binding (B). * denotes differences between vWF competition and control competition values (p < 0.05).
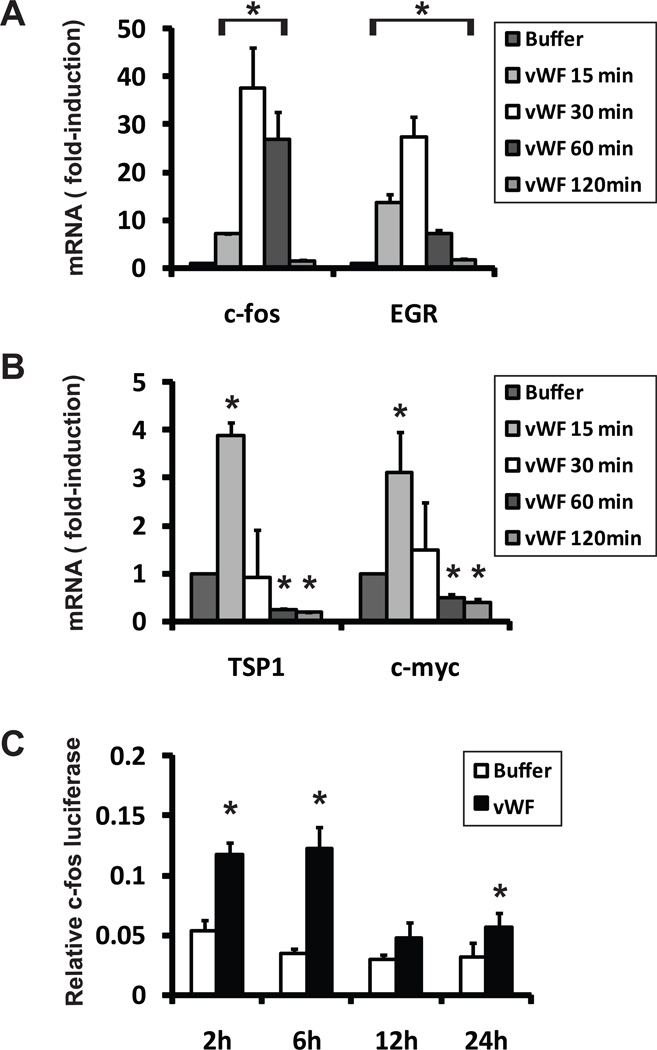
Previous studies have demonstrated that vWF stimulates smooth muscle cell proliferation, suggesting that vWF could stimulate cell signaling programs. A dose response curve using concentrations of vWF well-below serum concentrations indicated that 200ng/ml vWF induced cell proliferation of A7R5 cells, suggesting that minor concentrations of the protein are sufficient to trigger biological effects. To investigate this possibility, we added low concentrations of purified vWF (200ng/ml) to A7R5 cells for brief periods of time and measured gene responses. Multiple genes previously associated with growth factor stimulation were robustly upregulated in vWF cells, including c-FOS, TSP1, c-JUN, and EGR (Figure 4A–B). The promoter of c-FOS [28] was also rapidly activated by addition of vWF (Figure 4C).
Figure 4.
vWF rapidly activates immediate early genes in smooth muscle cells. A7R5 cells were pulsed with either vWF (200ng/ml) or buffer. RNA encoding four immediate early genes was rapidly upregulated (A–B). TSP1 and c-myc were moderately upregulated at 15 minutes and significantly repressed at 60 and 120 minutes. (C) Transcription of a cloned c-Fos promoter fused to the genetic reporter luciferase was also significantly acutely upregulated. * denotes differences compared to buffer control (p < 0.05).
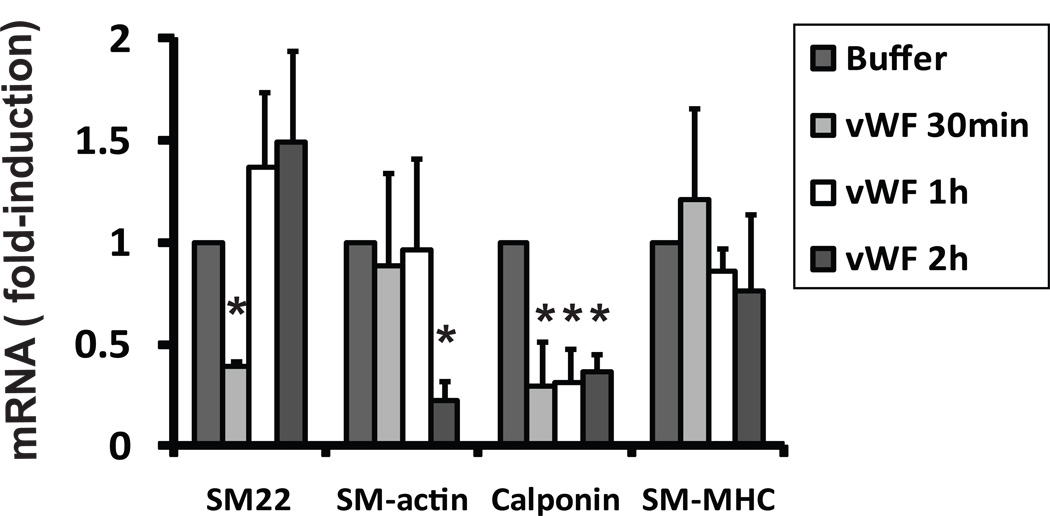
The rapid stimulation of immediate early genes is a response shared by engagement of multiple receptors and is common to numerous cell types. To determine the potential effects of vWF on genes specific to maintaining the differentiated smooth muscle phenotype, we also measured levels of smooth muscle genes after addition of vWF to A7R5 cells (Figure 5). Purified vWF robustly reduced the expression of SM22 at 30 minutes. vWF also reduced the expression of smooth muscle actin RNA after 120 minutes. Calponin RNA was significantly reduced at all time points examined. Smooth muscle myosin heavy chain RNA did not change after exposure to vWF. No visual evidence of cell death was observed after exposure to vWF, nor did we find significant differences in beta-actin RNA or Renilla luciferase activity after treatment.
Figure 5.
vWF represses levels of mRNA for smooth muscle genes SM22, SM-actin, calponin, and SM-MHC. A7R5 cells were serum starved and then exposed to 200ng/ml vWF or buffer. Total RNA was harvested at 30, 60, and 120 minutes and RNA levels corresponding to specified genes were measured by q-RT-PCR. Beta-actin controls were used to normalize expression levels of genes shown. * denotes differences compared to buffer control (p < 0.05).
Discussion
Studies of isolated cases of CADASIL have disclosed a number of molecular components of the thickened media of small arteries. These components are thought to be consequences of the pathophysiological processes that lead to vessel disease, such as impaired protein clearance and fibrosis. We now show in a series of six North American CADASIL brains that vWF avidly accumulates within the entire thickness of small vessels. Although vWF is classically considered a hemostatic protein, we demonstrate that at relatively low concentrations, this protein can activate immediate early gene transcription and suppress transcripts of genes that are essential to the differentiated smooth muscle cell. Thus, vWF may be the first identified component of small vessel disease that activates a vascular pathogenic pathway.
vWF pattern in small vessels of CADASIL
This demonstration of localized vWF in CADASIL small vessels provides new evidence that vWF permeates sites that are directly responsible for stroke pathogenesis in CADASIL.
How does vWF gain access into the arteries? Native vWF is a large multimer with a molecular weight that may exceed 20 MDa, a size that is two orders of magnitude larger than that of IgG, which was excluded from small vessel walls. As such, it is unlikely that serum-derived vWF enters the artery through a permeable endothelial barrier. Despite some reports, most studies indicate that very little serum protein escapes into the wall of vessels in CADASIL [29, 30, 14, 31] or in animal disease models [32]. Thus, we hypothesize that the bulk of the small vessel vWF originates locally from the endothelium that has been shown to synthesize and secrete vWF in both the apical and basal directions.
Consistent with this model, we observe a gradient of deposition of vWF in many CADASIL vessels, with the highest levels closer to the endothelial cell layer. Moreover, small vessels, in which the endothelium is in close proximity to the vascular media, showed the highest propensity for transmural vWF deposition. In contrast, large vessels, in which the endothelium is separated from smooth muscle cells by a thickened intima, frequently had intimal vWF, but rarely displayed vWF in the media.
vWF is normally confined to endothelial cells and their basement membrane where it forms complexes with extracellular matrix proteins such as collagen [33]. Ultrastructural studies have demonstrated widening of cell-cell distances between smooth muscle cells in CADASIL [34, 35]. As such, potential explanations for the penetration of vWF through the vascular media may include compromise of the basement membrane and intercellular matrix between smooth muscle cells.
We surmise that the double-barreled vWF distribution may be a manifestation of endothelial expression of vWF combined with deposition of serum products in the adventitia of the vessel, since a small amount of IgG was noted in the adventitia of many vessels. vWF deposition on the outer edge of arteries is of uncertain significance, since selective adventitial staining was seen frequently in large vessels of control brains and inconsistently in CADASIL small vessels of the white matter.
Potential role in vascular pathology
Our studies demonstrate that vWF is capable of suppressing multiple genes that normally define the mature smooth muscle cell. Low concentrations of vWF (we used 2% of the reported serum concentration) have an acute and robust effect on three of four smooth muscle genes we examined. On the basis of vWF cell-binding (Figure 3) and the rapidity of the immediate early response (Figure 4), it is likely that a receptor dependent mechanism in smooth muscle cells may alter signaling mechanisms required for the normal maintenance of smooth muscle RNA levels. A relationship between vWF and Notch proteins has not yet been established, but given the importance of Notch in smooth muscle biology, Notch is a viable candidate receptor for vWF.
These studies demonstrate that when vWF is found in the vessel wall, it likely exerts potent functional effects beyond hemostasis. The findings are consistent with the observation that vWF can stimulate smooth muscle proliferation in vitro [21]. Previous studies have demonstrated that after vascular injury, vWF can extend into the intima of large peripheral vessels [18, 20, 19, 22, 21]. Since vWF is expressed in a diverse array of conditions that stimulate intimal hyperplasia and functionally participates in experimental vascular remodeling [36, 37], its function within the vessel wall in other clinically important conditions that result in arteriosclerosis, such as atherosclerosis, hypertensive arterial disease, and post-stent vascular hyperplasia, deserves further exploration.
The presence of vWF in pathological small vessels in a limited number of control patients (invariably in thickened small vessels) suggests that its transmural deposition may participate not only in CADASIL, but in other causes of cerebral small vessel disease. We restricted the control group to patients that did not have known neurological disease, which may have led to an underestimate of the incidence of vWF deposition in the general population. Future studies on patients with known common small vessel disease (without NOTCH3 mutations) are warranted to determine whether vWF deposition in the vascular media affects the large population of individuals with ischemic white matter disease. Finally, we note that multiple studies have correlated high vWF levels with risk of ischemic stroke. Although it has been assumed that stroke risk is related to functions of vWF in thrombosis [38–40], our studies suggest that nonhemostatic vWF mechanisms of cerebrovascular disease warrant additional consideration.
Conclusion
In summary, we demonstrate that small arteries in CADASIL are the target of transmural vWF deposition, likely originating from local endothelial synthesis. Moreover, we show novel biological functions of vWF acting on smooth muscle cells which may lead to cellular pathology. Future studies will be needed to mechanistically link vWF cellular function and vascular pathology in CADASIL; this question will need to be validated directly in animal strains which develop CADASIL pathology and in which vWF levels can be manipulated. Although a number of murine models are available which express a range of vWF levels, the arteriosclerotic pathology in human CADASIL has not been fully replicated in mice [32, 35]. We are currently attempting to develop novel animal models in which small vessel pathology, including vessel thickening and vWF accumulation, is fully recapitulated.
Acknowledgments
We thank the patients and families who donated tissues for this study. We also acknowledge the generous resources provided by University of Michigan Alzheimers Disease Research Center (funded by NIH P50 AG008761-20-SI), who provided control tissues. Other human tissues were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, contract HHSN275200900011C, Ref. No. NO1-HD-9-0011. Shannon Dunn contributed to c-Fos luciferase studies.
Sources of Funding: This study was supported by grants NS054724 and NS062816 from NIH-NINDS and VA Merit award 5I01BX000375.
Footnotes
Author Contributions: X.Z. performed in vitro studies, H.M. analyzed the data, M.B. analyzed the data, J.S. analyzed the data and provided key molecular reagents, E.J.R., B.E.M., B.S.L., and B.B.W. provided pathological samples and edited the manuscript. M.M.W. designed the study, collected and analyzed the data, and wrote the manuscript.
References
- 1.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8(7):643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 2.Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18(22):2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desmond DW, Moroney JT, Lynch T, Chan S, Chin SS, Mohr JP. The natural history of CADASIL: a pooled analysis of previously published cases. Stroke. 1999;30(6):1230–1233. doi: 10.1161/01.str.30.6.1230. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan A, Gschwendtner A, Guichard JP, Buffon F, Cumurciuc R, O'Sullivan M, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007;69(2):172–179. doi: 10.1212/01.wnl.0000265221.05610.70. [DOI] [PubMed] [Google Scholar]
- 5.Liem MK, Lesnik Oberstein SA, Haan J, van der Neut IL, Ferrari MD, van Buchem MA, et al. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology. 2009;72(2):143–148. doi: 10.1212/01.wnl.0000339038.65508.96. [DOI] [PubMed] [Google Scholar]
- 6.Liem MK, van der Grond J, Haan J, van den Boom R, Ferrari MD, Knaap YM, et al. Lacunar infarcts are the main correlate with cognitive dysfunction in CADASIL. Stroke. 2007;38(3):923–928. doi: 10.1161/01.STR.0000257968.24015.bf. [DOI] [PubMed] [Google Scholar]
- 7.Jouvent E, Viswanathan A, Mangin JF, O'Sullivan M, Guichard JP, Gschwendtner A, et al. Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL. Stroke. 2007;38(6):1786–1790. doi: 10.1161/STROKEAHA.106.478263. [DOI] [PubMed] [Google Scholar]
- 8.Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. 2002;12(3):371–384. doi: 10.1111/j.1750-3639.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14(4):358–364. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruchoux MM, Maurage CA. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56(9):947–964. [PubMed] [Google Scholar]
- 11.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105(5):597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low WC, Junna M, Borjesson-Hanson A, Morris CM, Moss TH, Stevens DL, et al. Hereditary multi-infarct dementia of the Swedish type is a novel disorder different from NOTCH3 causing CADASIL. Brain. 2007;130(Pt 2):357–367. doi: 10.1093/brain/awl360. [DOI] [PubMed] [Google Scholar]
- 13.Miao Q, Kalimo H, Bogdanovic N, Kostulas K, Borjesson-Hanson A, Viitanen M. Cerebral arteriolar pathology in a 32-year-old patient with CADASIL. Neuropathol Appl Neurobiol. 2006;32(4):455–458. doi: 10.1111/j.1365-2990.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 14.Miao Q, Paloneva T, Tuisku S, Roine S, Poyhonen M, Viitanen M, et al. Arterioles of the lenticular nucleus in CADASIL. Stroke. 2006;37(9):2242–2247. doi: 10.1161/01.STR.0000236838.84150.c2. [DOI] [PubMed] [Google Scholar]
- 15.Oide T, Nakayama H, Yanagawa S, Ito N, Ikeda S, Arima K. Extensive loss of arterial medial smooth muscle cells and mural extracellular matrix in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) Neuropathology. 2008;28(2):132–142. doi: 10.1111/j.1440-1789.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- 16.Szpak GM, Lewandowska E, Wierzba-Bobrowicz T, Bertrand E, Pasennik E, Mendel T, et al. Small cerebral vessel disease in familial amyloid and non-amyloid angiopathies: FAD-PS-1 (P117L) mutation and CADASIL. Immunohistochemical and ultrastructural studies. Folia Neuropathol. 2007;45(4):192–204. [PubMed] [Google Scholar]
- 17.Sadler JE. von Willebrand factor: two sides of a coin. J Thromb Haemost. 2005;3(8):1702–1709. doi: 10.1111/j.1538-7836.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- 18.Bosmans JM, Kockx MM, Vrints CJ, Bult H, De Meyer GR, Herman AG. Fibrin(ogen) and von Willebrand factor deposition are associated with intimal thickening after balloon angioplasty of the rabbit carotid artery. Arterioscler Thromb Vasc Biol. 1997;17(4):634–645. doi: 10.1161/01.atv.17.4.634. [DOI] [PubMed] [Google Scholar]
- 19.Giddings JC, Banning AP, Ralis H, Lewis MJ. Redistribution of von Willebrand factor in porcine carotid arteries after balloon angioplasty. Arterioscler Thromb Vasc Biol. 1997;17(10):1872–1878. doi: 10.1161/01.atv.17.10.1872. [DOI] [PubMed] [Google Scholar]
- 20.De Meyer GR, Hoylaerts MF, Kockx MM, Yamamoto H, Herman AG, Bult H. Intimal deposition of functional von Willebrand factor in atherogenesis. Arterioscler Thromb Vasc Biol. 1999;19(10):2524–2534. doi: 10.1161/01.atv.19.10.2524. [DOI] [PubMed] [Google Scholar]
- 21.Qin F, Impeduglia T, Schaffer P, Dardik H. Overexpression of von Willebrand factor is an independent risk factor for pathogenesis of intimal hyperplasia: preliminary studies. J Vasc Surg. 2003;37(2):433–439. doi: 10.1067/mva.2003.63. [DOI] [PubMed] [Google Scholar]
- 22.Qin F, Dardik H, Pangilinan A, Robinson J, Chuy J, Wengerter K. Remodeling and suppression of intimal hyperplasia of vascular grafts with a distal arteriovenous fistula in a rat model. J Vasc Surg. 2001;34(4):701–706. doi: 10.1067/mva.2001.116804. [DOI] [PubMed] [Google Scholar]
- 23.Tohgi H, Utsugisawa K, Yoshimura M, Nagane Y, Ukitsu M. Local variation in expression of pro- and antithrombotic factors in vascular endothelium of human autopsy brain. Acta Neuropathol. 1999;98(2):111–118. doi: 10.1007/s004010051058. [DOI] [PubMed] [Google Scholar]
- 24.Meng H, Zhang X, Lee SJ, Strickland DK, Lawrence DA, Wang MM. Low density lipoprotein receptor-related protein-1 (LRP1) regulates thrombospondin-2 (TSP2) enhancement of Notch3 signaling. J Biol Chem. 2010;285(30):23047–23055. doi: 10.1074/jbc.M110.144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng H, Zhang X, Hankenson KD, Wang MM. Thrombospondin 2 potentiates notch3/jagged1 signaling. J Biol Chem. 2009;284(12):7866–7874. doi: 10.1074/jbc.M803650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alafuzoff I, Adolfsson R, Bucht G, Winblad B. Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J Neurol Sci. 1983;60(3):465–472. doi: 10.1016/0022-510x(83)90157-0. [DOI] [PubMed] [Google Scholar]
- 27.Alafuzoff I, Adolfsson R, Grundke-Iqbal I, Winblad B. Blood-brain barrier in Alzheimer dementia and in non-demented elderly. An immunocytochemical study. Acta Neuropathol. 1987;73(2):160–166. doi: 10.1007/BF00693782. [DOI] [PubMed] [Google Scholar]
- 28.Cui TX, Piwien-Pilipuk G, Huo JS, Kaplani J, Kwok R, Schwartz J. Endogenous CCAAT/enhancer binding protein beta and p300 are both regulated by growth hormone to mediate transcriptional activation. Mol Endocrinol. 2005;19(8):2175–2186. doi: 10.1210/me.2004-0502. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann M, Ebke M, Yuan Y, Bruck W, Mugler M, Schwendemann G. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL): a morphological study of a German family. Acta Neuropathol. 1996;92(4):341–350. doi: 10.1007/s004010050528. [DOI] [PubMed] [Google Scholar]
- 30.Gray F, Robert F, Labrecque R, Chretien F, Baudrimont M, Fallet-Bianco C, et al. Autosomal dominant arteriopathic leuko-encephalopathy and Alzheimer's disease. Neuropathol Appl Neurobiol. 1994;20(1):22–30. doi: 10.1111/j.1365-2990.1994.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 31.Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol. 1995;89(6):500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- 32.Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120(2):433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rand JH, Wu XX, Potter BJ, Uson RR, Gordon RE. Co-localization of von Willebrand factor and type VI collagen in human vascular subendothelium. Am J Pathol. 1993;142(3):843–850. [PMC free article] [PubMed] [Google Scholar]
- 34.Brulin P, Godfraind C, Leteurtre E, Ruchoux MM. Morphometric analysis of ultrastructural vascular changes in CADASIL: analysis of 50 skin biopsy specimens and pathogenic implications. Acta Neuropathol. 2002;104(3):241–248. doi: 10.1007/s00401-002-0530-z. doi:10.1007/s00401-002-0530-z. [DOI] [PubMed] [Google Scholar]
- 35.Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, et al. Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol. 2003;162(1):329–342. doi: 10.1016/S0002-9440(10)63824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kageyama S, Yamamoto H, Yoshimoto R. Anti-human von willebrand factor monoclonal antibody AJvW-2 prevents thrombus deposition and neointima formation after balloon injury in guinea pigs. Arterioscler Thromb Vasc Biol. 2000;20(10):2303–2308. doi: 10.1161/01.atv.20.10.2303. [DOI] [PubMed] [Google Scholar]
- 37.Methia N, Andre P, Denis CV, Economopoulos M, Wagner DD. Localized reduction of atherosclerosis in von Willebrand factor-deficient mice. Blood. 2001;98(5):1424–1428. doi: 10.1182/blood.v98.5.1424. [DOI] [PubMed] [Google Scholar]
- 38.Bongers TN, de Maat MP, van Goor ML, Bhagwanbali V, van Vliet HH, Gomez Garcia EB, et al. High von Willebrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS13, inflammation, and genetic variability. Stroke. 2006;37(11):2672–2677. doi: 10.1161/01.STR.0000244767.39962.f7. [DOI] [PubMed] [Google Scholar]
- 39.Folsom AR, Rosamond WD, Shahar E, Cooper LS, Aleksic N, Nieto FJ, et al. Prospective study of markers of hemostatic function with risk of ischemic stroke. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1999;100(7):736–742. doi: 10.1161/01.cir.100.7.736. [DOI] [PubMed] [Google Scholar]
- 40.Qizilbash N, Duffy S, Prentice CR, Boothby M, Warlow C. Von Willebrand factor and risk of ischemic stroke. Neurology. 1997;49(6):1552–1556. doi: 10.1212/wnl.49.6.1552. [DOI] [PubMed] [Google Scholar]