Abstract
Studies have indicated that personality may be associated with inflammatory markers such as Interleukin (IL) - 6. One pathway between personality and IL-6 may be health behaviors and conditions resulting in inflammation, while an alternate pathway involves activation of stress-response systems. In a clinical trial sample of 200 older adults, we examined associations between personality traits at baseline and 3 measures of IL-6 spanning 34 weeks of follow-up. Results indicated that IL-6 remained very stable over time, and that higher Conscientiousness and Openness were associated with lower IL-6 across the entire 34 week period. Goal striving was the active subcomponent of Conscientiousness, while aesthetic interests was the active subcomponent of Openness in IL-6 associations. Common health behaviors and chronic illness accounted for only a portion of these effects, suggesting that other behavioral and/or physiological processes may also predispose some persons to inflammation. Personality phenotype may provide useful prognostic information for inflammation. Older adults lower in Conscientiousness and Openness constitute a target population for anti-inflammatory interventions.
Interleukin (IL)-6 is a low-molecular weight pro-inflammatory cytokine strongly predictive of mortality in older persons (De Martinis and et al. 2005; Grunewald and Singer 2006) and useful clinically as a general indicator of systemic burden and mortality risk (Gruenewald, Seeman et al. 2006). However, IL-6 appears to play a causal role in further health decline by leading to dysregulation of adaptive immune function (Rabin 2007), and is closely tied to depression (Howren, Lamkin et al. 2009). IL-6 is produced acutely in response to stress-related activation of the hypothalamic-pituitary-adrenal (HPA) axis, and remains chronically elevated in response to inflammatory illnesses, prolonged health-damaging behaviors such as smoking, and persistent stress-related HPA axis and sympathetic nervous system (SNS) overactivity (Rabin 2007). Older persons represent an important population in which to study IL-6, because the aging of the immune system leads to greater susceptibility to disease in elders.
Some aspects of personality have been reportedly linked with circulating IL-6 levels. The Big-5 personality model (Goldberg 1993) has been used as a framework for studying such associations. The Big 5 comprise Neuroticism (emotional stability and negative moods), Extraversion (sociability, vitality, and positive mood), Openness (cognitive and behavioral flexibility, culture, and intellect), Agreeableness (trust, compliance, and compassion), and Conscientiousness (diligence, goal-striving, self-discipline). Neuroticism has been linked to inflammatory markers in both an older cohort over-sampled for major depression (Bouhuys, Flentge et al. 2004) and an Italian population cohort (Sutin, Terracciano et al. 2009). In the latter study, the association was accounted for primarily by the angry hostility and vulnerability aspects of Neuroticism (vulnerability reflects poor self-esteem and shame). Studies also suggest that facets of Extraversion, such as positive emotions in healthy younger adults (Doyle, Gentile et al. 2006), optimism in a midlife sample (Roy, Diez-Roux et al. 2010), and activity (i.e., busy engagement with life) in midlife medically burdened adults (Chapman, Khan et al. 2009; Sutin, Terracciano et al. 2009) are associated with inflammatory cytokines. One study found lower IL-6 in people who were more Conscientiousness (Sutin, Terracciano et al. 2009), and another found lower C-reactive protein (CRP) in African Americans, but not Caucasians, higher in Openness to Experience (Jonassaint, Boyle et al. 2010).
Two other aspects of personality have also been linked to inflammatory markers. Type D is defined by a combination of high negative affectivity or elevated Neuroticism, and social inhibition, an aspect of low Extraversion (De Fruyt and Denollet 2002). Hostility reflects a combination of low levels of Agreeableness, mixed with angry affect, an element of elevated Neuroticism (Dembroski and Costa 1987). In coronary patients, Type D has been linked to higher platelet Tumor Necrosis Factor (TNF)-α receptors cross-sectionally (Denollet, Conraads et al. 2003; Conraads, Denollet et al. 2006) and 1 year later (Denollet, Schiffer et al. 2009). The magnitude of the cross-sectional difference in TNF-α between individuals categorized as Type D is equivalent to that observed in individuals differing by 18 years of age (Denollet, Vrints et al. 2008), suggesting a potential role of personality in accelerated aging. Hostility has been linked to higher TNF-α and Interferon (IFN)-γ in military personnel (Mommersteeg, Vermetten et al. 2008) and higher levels of matrix metalloproteinasemetallopreinase (mmp-9), a degrading enzyme associated with inflammation (Garvin, Nilsson et al. 2009), in a European cross-sectional population study. In healthy adults 40 and younger, hostility or composite measures including hostility have been linked cross-sectionally to TNF-α (Miller, Cohen et al. 1999; Suarez, Lewis et al. 2002). Links between hostility and IL-6 have also been reported (Suarez 2003; Sjogren, Leanderson et al. 2006).
At least two explanations can account for these associations. First, personality is a strong predictor of health behaviors such as smoking (Terracciano and Costa 2004), diet and exercise (Goldberg and Stycker 2002; Rhodes and Courneya 2003), alcohol consumption (Anton and Miller 2005; Murray, Barnes et al. 2005), and risky behaviors (Bogg and Roberts 2004). These behaviors may lead directly to an inflammatory response or indirectly via general illness burden (Chapman, Lyness et al. 2007) or elevated body mass index (Chapman, Fiscella et al. 2009). A second pathway involves the psychophysiology of stress. Personality tendencies may lead to self-selection into stressful situations and environments (Caspi and Roberts 2001), variation in the perception of stress (De Jong, van Sonderen et al. 1999), and differential coping effectiveness, possibly an index of HPA activation (Carver and Connor-Smith 2010).
Although suggestive, research to date has tended to focus on younger and middle aged populations. It is unclear whether these findings would accurately characterize older persons. The extent to which personality is associated with IL-6 levels longitudinally is also less studied. Finally, comparatively few studies have also looked at personality comprehensively, using the Big 5. The purpose of the present study was to examine the association of all aspects of the Big 5 model with the 34-week course of IL-6 in a clinical trial sample of older adults recruited from the community. We modeled these associations with and without adjustment for health behaviors, chronic conditions, and BMI in order to explore whether personality phenotype operates solely through health behaviors and conditions or whether unexamined mediators can account for the associations.
Methods
Participants
Participants were 200 older adults recruited from the community by newspaper advertisements and flyers, as part of a randomized controlled trial on Mindfulness Based Stress Reduction (MBSR) to enhance adaptive immune response (immunoglobulins G and M) to challenge. Exclusion criteria included frank psychosis, acute substance intoxication, or indications of major cognitive impairment on screening. Randomization was to either MBSR or a wait-list control. IL-6 was not a primary intervention outcome, and it was assayed at weeks 8 (immediately post-intervention), 11, and 34, but not at baseline (pre-intervention). Sample attrition was minimal (<3%) over the course of the study.
Procedure
After recruitment, participants were randomized to either the MBSR or control conditions (100 in each). All participants were included in the present analysis, while treatment arm was examined as a both a potential confounder and effect modifier of hypothesized personality effects. All participants completed personality and other assessments at baseline. Next, participants assigned to the MBSR condition completed the intervention program. Space and focus precludes a detailed description of MBSR (Kabat-Zinn 1990). Briefly, it was conducted in once-weekly groups for 8 weeks, and focused on meditation and relaxation techniques, yoga, and self-awareness of one’s mood, cognitive activity, and physiological sensations. At the conclusion of the intervention (8 weeks after baseline), participants in both conditions received an immune challenge (an injection of a novel protein antigen, keyhole limpet hemocyanin (KLH), a pathogen known to stimulate a humoral adaptive immune response). Different doses of KLH (<200mg vs. >200mg) were including to see if the intervention effect on adaptive immune response was observed only at one dosage. KLH dosage was examined as both a possible confounder and effect modifier in the present analysis.
Assessment of IL-6 and Personality
Blood collection at 8, 11, and 34 weeks used conventional venipuncture and vacutainer tubes (BD Diagnostics, Franklin Lakes, NJ). The timing occurred randomly throughout the day, meaning random variation was added both within and between-persons. This random variation would be expected to increase standard error estimates, meaning hypothesis tests are conservative—that is, extra error variance reduces, not increases the probability of observing a significant association. Blood was centrifuged and serum was stored at −80°C until assayed. Serum levels of IL-6 were assayed with enzyme-linked immunoassay (ELISA) using high-sensitivity kits purchased from R&D Systems, Inc. (Minneapolis, MN). IL-6 OptEIA kits were used according to manufacturer’s guidelines via ELISAprotocols with the standard curve run on each 96-well assay plate. Samples were run in duplicate. Absorbance was read at 490 nm with 650 nm wavelength correction within 30 minutes after development using an automated Opsys MR Microplate Reader (Thermo Labsystems, Chantilly, Virginia). The minimum detectable limit for IL-6 is 0.039 pg/ml. The intra-assay variability is approximately 4% and the inter-assay CV is <5%.
Subjects were enrolled and completed the intervention and follow-ups in several waves from March 2006 to May 2009, meaning that particular phases of the study were not systematically tied to particular times of the year. At baseline, demographic data was also collected via interview. Personality was measured by the NEO-FFI (Costa and McCrae 1992). This 60-item instrument produces scores on the Big 5 dimensions, which were converted to T-scores according to the national norms (Costa and McCrae 1992). Cronbach’s alphas for the Big 5 domain scales ranged from .70 to .85. The NEO-FFI also produces 2–3 subcomponents for each Big 5 dimension (Saucier 1998; Chapman 2007), which were used in supplementary analyses to identify specific aspects of Big 5 dimensions associated with IL-6. Cronbach’s alpha for the subcomponents ranged from .55 to .85, with the exception of the Openness subcomponent Unconventionality, which showed a typically low alpha of .40, as in prior work (Saucier 1998; Chapman 2007), and was not used.
Covariates
The following health conditions and behaviors were assessed at baseline for covariate adjustment purposes: BMI in kg/m2, based on self-reported height and weight; a count of self-report of the following conditions: hypertension, myocardial infarction, coronary heart failure, arthritis, diabetes, stroke, cancer, chronic back pain, ulcer, liver problems, and persistent angina (based on subject responses to the stem “Has a doctor ever told you have ______”, with clarification and definition of the names of the conditions when necessary); frequency of moderate to vigorous physical activity over the past month, based on the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (Stewart, Mills et al. 1997); smoking status (current or quit less than 6 months ago, vs. never or quit more than six months ago); and alcohol usage based on the past month (for each of white wine, red wine, regular beer, light beer, and liquor, rating beverage consumption as 0 = never, 1 = < 1/month, 2 = 1–3 glasses/month, 3 = 1 glass/week, 4 = 2–4 glasses/week, 5 = 5–6 glasses/week, 6 = 1 glass/day, 7 = 2–3 glasses/day; scored as the sum of ratings for each drink type). Participants were also asked whether or not they were on a low-fat, low cholesterol, or diabetes-friendly diet. Finally, depression was assessed as an additional adjustment variable for sensitivity analyses using the Center for Epidemiologic Studies Depression Scale, Revised (CESD-R; (Eaton 2004)).
Analysis
We modeled the association between personality at study entry and IL-6 at 8, 11, and 34 weeks using Generalized Estimating Equations (GEE). GEE is a method for analyzing clustered or longitudinal measurements, similar to repeated measures ANOVA, but allows for flexible treatment of non-normal data and makes no assumptions about the form of error distributions (Hanley, Negassa et al. 2003). Thus it is able to provide standard errors and p-values robust to violation of parametric assumptions made by other longitudinal techniques such as mixed effect or hierarchical linear models. All models were fit with an exchangeable working correlation matrix, log link and Gaussian distribution; analysis using a Gamma distribution and log link revealed similar findings and are not reported here.
Model 1 included all Big 5 dimensions, adjusting for demographic covariates (age, sex, college education or higher) and study design covariates (treatment arm, and the week 8 KLH dose administered at 8 weeks (200 mg or greater vs. <200 mg)). These factors were conceptualized as either unignorable features of the study requiring control for conservative estimates, or as demographic confounders. Personality traits were scaled by 2 SD units, in order to facilitate interpretation of differences between high and low levels of the trait. This is similar to a z-score approach, in which continuous variables are differenced from their mean and scaled by one standard deviation, so that the regression coefficient reflects the difference between persons 1 standard deviation apart (i.e., the 84th percentile, or +1 SD, vs. the 50th percentile, or mean). In scaling by two standard deviations and differencing scores from −1SD, coefficients therefore reflected the difference in log IL-6 between someone at the 84th percentile (+1 SD) vs. 16th percentile (−1 SD) levels of the trait, at each IL-6 measurement. Linear transformations of any kind do not change p-values, and are used only to make regression coefficients reflect differences between persons meaningfully different on the predictor. Note also that because all repeated measures of IL-6 are included, main effects indicate the association between a variable assessed at study entry and average IL-6 across the study period. Model 2 added health behaviors (alcohol score, smoking status, low fat/diabetic diet, frequency of moderate or vigorous activity over the past month), BMI, and health conditions as covariates. The change in parameter estimates for the Big 5 between model 1 and model 2 therefore provided an index of the extent to which personality associations were explained by health behaviors and conditions. We considered these covariates as potential mediators of personality effects on IL-6, although the distinction is conceptual rather than literal as covariates and personality were assessed contemporaneously.
We used a flexible approach for assessing change over time in IL-6—separate slope terms (called regression splines) captured change from weeks 8 to 11, and then from weeks 11 to 34. This allowed us to determine if IL-6 went up then down, or down then up, rather than assuming that change would go strictly in one direction across the study period. To examine whether the Big 5 were associated with rate of change between either weeks 8 to 11 or 11 to 34, we tested interaction terms between each Big 5 dimension and each spline term. The clinical relevance and magnitude of personality effects were examined by first comparing personality-IL-6 associations to age-IL-6 associations. This was done first by examining the regression coefficients for personality and age, to determine the age difference at baseline necessary to produce an IL-6 difference comparable to −1 vs. +1 SD of the personality trait in question. Because differences in personality are difficult to intuitively understand, this anchored the magnitude of the personality effect to a metric more familiar to most, age differences. Second, we formed a global personality risk index based on the summed T-scores of significant personality predictors (that is, simply adding the T-scores of significant traits to create a single composite inflammatory risk score for personality). Psychometrically, additive combinations of 2 of the Big 5 represent a vector at a 45 degree angle to 2 orthogonal factor axes; theoretically these represent personality “styles” reflecting behavioral tendencies linked to both Big 5 dimensions (Costa and Piedmont 2003). We then identified the cut point on this inflammatory risk score at which IL-6 levels exceeded the threshold of 3.19 pg/ml shown to double mortality probability in older persons (Harris and Wallace 1999).
Supplementary analyses replaced Big 5 domains with their subcomponents, to identify specific aspects of the domain driving the IL-6 association. Finally, sensitivity analyses examined whether personality and treatment arm interacted, and whether any other factors drove observed fluctuations in IL-6 across the study period. We also examined whether additional adjustment for depression (also scaled by 2 SD units) altered observed Neuroticism associations. All analyses were performed with Stata 11 Special Edition (College Station, Texas).
Results
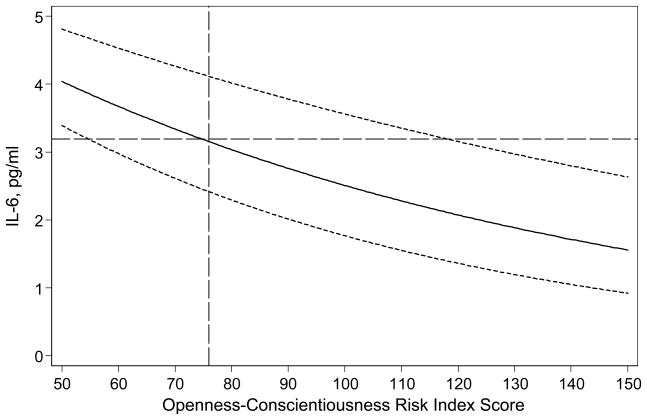
The demographics of study participants are shown in Table 1. The sample was well educated, and overwhelmingly white. Zero order correlations between each of the Big 5 and IL-6 over follow-up (fit with GEE to adjust for repeated measures) were: Neuroticism, .02 (p = .782); Extraversion, −.01 (p = .810); Openness, −.13 (p = .025); Agreeableness, −.06 (p = .253); Conscientiousness, −.07 (p = .158). Adjusting each of the Big 5 for age and sex, revealed similar results. Entering all of the Big 5 simultaneously and adjusting for age and sex (Table 2, Model 1) shows more pronounced associations for Openness and Conscientiousness, which persisted when adjusting for several other demographic and study design features (Table 2, Model 2). Adjusting each single Big 5 dimension for demographics only, Openness (B = −.178, p = .098) and Conscientiousness (B = −.124, p = .089) trended toward association with log IL-6 over the study period. When all of the Big 5 dimensions were entered simultaneously (Model 2), higher Conscientiousness and Openness were both significantly associated with lower levels of log IL-6 across the study period. Figure 1 shows the association of average IL-6 over the study period with the Conscientiousness+Openness prognostic index (p = .006): a combined T-score of 76 on these two personality domains marked the point at which IL-6 levels reached the threshold of 3.19 pg/ml, at which mortality probability doubles for older persons (Harris et al., 1999). The model coefficients in Table 2 (model 2) indicated that the magnitude of the association between IL-6 and a 2 SD difference in Openness was roughly equivalent to (−.216 coefficient for Conscientiousness divided by .025 coefficient for age =) about 8.4 years of age. The magnitude of the Conscientiousness association was roughly equivalent to about 6.3 years of age.
Table 1.
Descriptive Statistics for 200 Study Participants
| Variable | Mean/N | SD/% |
|---|---|---|
| Demographics | ||
| Age | 72.8 | 6.7 |
| Male | 77 | 38.3% |
| Female | 124 | 61.7% |
| College or Greater Education | 142 | 71% |
| White | 199 | 99% |
| Big 5 Personality Dimensions (T-scores) | ||
| Neuroticism | 44.0 | 10.7 |
| Extraversion | 51.9 | 10.8 |
| Openness | 53.9 | 10.3 |
| Agreeableness | 57.4 | 11.2 |
| Conscientiousness | 50.3 | 11.6 |
| Health Factors | ||
| Chronic Condition Count | 2.0 | 1.5 |
| BMI | 26.6 | 4.7 |
| Monthly Frequency, Moderate or Vigorous Activity | 18.7 | 8.8 |
| Smoking: Current/last 6 months | 9 | 4.5% |
| Low Fat or Diabetic Diet | 55 | 27.6% |
| Normal diet | 144 | 72.4% |
| Weekly Alcohol Score | 9.6 | 4.2 |
| Design Factors | ||
| MBSR Arm | 100 | 50% |
| >= 200 mg KLH Challenge (vs. < 200) | 57 | 28.5% |
| IL-6 Measurements (pg/ml) | ||
| Week 8 | 2.5 | 2.1 |
| Week 11 | 2.7 | 2.2 |
| Week 34 | 2.6 | 2.3 |
Notes: T-scores represent psychometric scaling to the normative sample for the personality instrument (Costa & McCrae, 1992). Normative sample mean T-score = 50 for each Big 5 dimension, with standard deviation of 10. Weekly alcohol score derived from the sum of responses to five different types of drinks (red wine, white wine, liquor, light beer, regular beer), each with a response scale of 0 = never, 1 = less than 1/month 2 = 1–3 glasses/month, 3 = 1 glass week, 4 = 2–4 glasses/week, 5 = 5–6 glasses/week, 6 = 1 glass/day, 7 = 2–3 glasses/day.
Table 2.
Associations of Personality with Log IL-6
| Predictor | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| B | p | B | p | B | p | |
| Big 5 Personality Factors | ||||||
| Neuroticism | .009 | .920 | −0.023 | 0.808 | −0.042 | 0.666 |
| Extraversion | .116 | .212 | 0.07 | 0.430 | 0.114 | 0.194 |
| Openness | −.208 | .029 | −0.216 | 0.034 | −0.196 | 0.039 |
| Agreeableness | −.078 | .420 | −0.073 | 0.451 | −0.077 | 0.368 |
| Conscientiousness | −.156 | .048 | −0.161 | 0.035 | −0.167 | 0.028 |
| Demographic and Design Factors | ||||||
| Age, years | .023 | .002 | 0.025 | 0.001 | 0.028 | 0.000 |
| Female vs. Male | −.171 | .093 | −0.138 | 0.186 | −0.187 | 0.055 |
| Bachelors Education vs. < Bachelors | 0.089 | 0.429 | 0.043 | 0.698 | ||
| Treatment | 0.118 | 0.251 | 0.09 | 0.351 | ||
| Klh 200 mg + vs. < 200 mg | 0.003 | 0.969 | −0.015 | 0.860 | ||
| Change, weeks 8–11 | 0.023 | 0.184 | 0.014 | 0.430 | ||
| Change, weeks 11–34 | 0 | 0.963 | 0 | 0.912 | ||
| Health Factors | ||||||
| BMI, kg/m2 | 0.018 | 0.048 | ||||
| Chronic Condition Count | −0.006 | 0.721 | ||||
| Alcohol Score | −0.026 | 0.030 | ||||
| Smoking | −0.166 | 0.523 | ||||
| Physical Activity Frequency | −0.003 | 0.546 | ||||
| Low fat vs. standard diet | 0.056 | 0.516 | ||||
| Intercept (Average log IL-6 across measurements) | −0.733 | 0.20 | −1.118 | 0.115 | ||
Note: B = difference in all measurements of log IL associated with a 1-unit difference predictor. Personality scaled so coefficients reflect the difference in log IL-6 across study period between a person at the 84th, vs. 16th percentile of each Big 5 dimension. Terms for time reflect weekly rate of change over designated period for log IL-6.
Figure 1.
Note. Association between IL-6 across all measurements and personality risk index (Openness + Conscientious T-scores). IL-6 threshold of 3.19, at which mortality probability doubles in older adults (Harris et al., 1999) denoted with horizontal dashed line. Personality risk index score of 76, at which IL-6 reaches threshold, marked by vertical dashed line. Dotted lines denote +/− 1 standard error of estimate.
With respect to change over time, log IL-6 appeared very stable throughout the follow-up period. The spline terms indicated no significant mean-level shifts from weeks 8 to 11 or 11 to 34. An autoregressive working correlation matrix also indicated moderate to strong rank-order stability in IL-6 (r = .51 between adjacent measurements). No substantial effects of personality on rate of change were observed.
Table 2, Model 3 shows additional adjustment for health behaviors and conditions conceptualized as possible mediators. Adding these variables to the model accounted for less than 10% of the associations between both Openness and Conscientiousness and log IL-6 across the study period. Among covariates, age, alcohol score, BMI, and, marginally, sex, were all associated with higher levels of IL-6 over the study period. Personality associations did not vary across intervention arm, and no other health factors were independently associated with IL-6 over the study period.
Analyses of Big 5 subcomponents revealed that the Openness effect was mainly driven by aesthetic interests (B = −.242, p = .019), while the Conscientiousness effect was driven primarily by goal striving (B = −.203, p = .013). These supplementary analyses used the same covariates as the main analyses (Model 2). An additional analyses adjusting Model 2 for CESD-R scores revealed a significant association between depression and log IL-6 across follow-up (B = .174, p = .028).
Discussion
We examined whether the Big 5 personality dimensions were associated with IL-6 longitudinally in older persons, and the extent to which health behaviors and conditions might explain this association. While most prior studies on Type D hostility in young and midlife samples, our findings suggest that Openness and Conscientiousness are important correlates of inflammation in an older community sample. Only recently have studies of inflammation examined the full Big 5. Two reports have found associations between inflammatory markers and Conscientiousness and Openness: higher Conscientiousness was associated with lower IL-6 in an Italian population sample (Sutin, Terracciano et al. 2009), and higher Openness was associated with lower CRP among middle aged blacks (Jonassaint, Boyle et al. 2010).
Adjustment for common health behaviors and health conditions did not explain the effects observed here. This suggests that the associations may reflect the role of unmeasured health behaviors or differences in chronic stress physiology associated with these personality dimensions. From a neuroimmune interaction perspective, Conscientiousness may be linked to serotonergic systems (DeYoung 2006), and to frontal-lobe inhibitory mechanisms (DeYoung, Hirsh et al. 2010). In turn, these may help regulate emotion, thereby reducing the activation of the amygdale-HPA axis pathway. A lifetime of goal pursuit involves many self-induced challenges, and things normally perceived as stressful may be experienced in a more positive, or controllable, manner by these individuals. Openness is correlated with orbitofrontal cortex function in older persons (Sutin, Beason-Held et al. 2009). This region is responsible for cognitive and behavioral flexibility that may lead to more adaptive perceptions of and response to stress. Orbitofrontal regions are also known to inhibit amygdale activation, reducing HPA axis response (Costa-Pinto and Palermo-Neto 2010). These hypotheses reflect popular foci of the literature and must be regarded as only a few of several possibilities, however. Findings could reflect a wide variety of other behavioral and/or physiological pathways (including the sympatho-vagal system), and the present results suggest associations to be explained, rather than implicating one mechanism more strongly than another.
In addition to acting as a “transducer” of environmental stress into neuroendocrine response, personality traits may be linked to IL-6 in another way. Eysenck (Eysenck and Eysenck 1985), who focused on Neuroticism and Extraversion, believed that personality developed in tandem with the some elements of the nervous system. The sympatho-vagal system was thought to be similarly intertwined with personality. In other words, personality traits may represent phenotypic “signals” for low thresholds in activation of these stress response systems. To the extent that this hypothesis is true, one would expect to find polymorphisms linked to HPA axis or sympatho-vagal system dysregulation also linked with personality phenotype. This intriguing possibility warrants further study in its own right.
Few studies have examined the association between personality and IL-6 longitudinally. In our cohort, mean levels of IL-6 did not change substantially over the study period. Further, personality did not appear meaningfully linked to any individual variation in IL-6 change. This stability over the short term is beneficial among more Open and Conscientious persons, because their IL-6 remains relatively low, but disadvantageous for less Open and Conscientious people, because their circulating IL-6 remains consistently high.
Our Openness-Conscientiousness risk index reflects standing on the personality style represented by these two traits dealing mostly with learning (Costa and Piedmont 2003). Those with the highest levels of IL-6 represented a style lacking in both the interest and discipline to acquire new knowledge and skills (Costa and Piedmont 2003), which may cause general adaptive difficulties. With respect to other dimensions of the Big 5, one prior study using the Big 5 framework found associations between Neuroticism and IL-6 (Sutin, Terracciano et al. 2009) that were not apparent in our sample. That study was a cross-sectional analysis of an Italian population cohort varying widely in age, however. The Neuroticism association was due at least in part to the “angry hostility” facet on the 240-item NEO-PI R. Only 1 item from this facet is included on the briefer NEO-FFI used in this study. Another study of older adults found an association between IL-6 and Neuroticism on the Eysenck Personality Inventory (Bouhuys, Flentge et al. 2004), but 50% of the sample had significant depressive symptoms. The relative lack of association between IL-6 and Neuroticism in our sample therefore likely reflects differences from previous studies in measurement, population, or both.
Similarly, the Agreeableness scale on the NEO inventories captures primarily acquiescent vs. adversarial behavior, rather than hostile affect (which is a component of Neuroticism in the full NEO-PI-R). The active ingredient of hostility scales previously associated with IL-6 may be the angry affect absent from the Agreeableness domain on the NEO-FFI. Extraversion findings have been somewhat mixed, with some studies finding some Extraversion constructs like positive affect (Cohen, Doyle et al. 2003) or sociability (Cohen, Doyle et al. 2003) relevant to IL-6, while others have found more of a role of the “surgency” or vitality aspect of this Big 5 domain, with only marginal or non-significant associations between the overall Extraversion construct and IL-6 (Chapman, Khan et al. 2009; Sutin, Terracciano et al. 2009). In this older sample, no significant associations with Extraversion were noted. This underscores the possibility that traits related to Extraversion may be linked to IL-6 in only some samples.
Our findings have several research, clinical, and public health implications. From a psychoneuroimmunological standpoint, they shed light on types of older persons more and less susceptible to IL-6 elevations. In turn, we can generate hypotheses about the mechanisms linking personality phenotype to inflammation. Clinically, older persons who are uninterested in setting and pursuing personal goals, and/or who have no enjoyment for arts and culture, may bear closer monitoring because elevated inflammation can lead to the progression or exacerbation of many chronic diseases. Epidemiologic data suggest such persons show reduced 5-year survival rates (Harris and Wallace 1999; Gruenewald, Seeman et al. 2006). These patients may benefit from psychosocial interventions that help them build a more fulfilling and meaningful life. The magnitude of IL-6 differences between different personality phenotypes was on par with age differences of 6 to 8 years, suggesting that individual differences in psychosocial factors should not be ignored in clinical risk evaluation. From a public health perspective, findings suggest that personality variation in the population may be implicated in conditions with inflammatory components. Identifying high-risk segments of the older public might facilitate targeted anti-inflammatory interventions during a life phase when immune function is both critical and in decline.
Debates currently exist over the malleability of personality tendencies. One promising approach that avoids attempting to alter personality itself involves targeting individuals with maladaptive personality configurations (identified through brief screens in primary care). These persons can then be assessed more definitively and, if they are indeed high-risk personality configurations, subjected to closer clinical monitoring and possibly intervention. A heavy emphasis is emerging on tailoring behavioral interventions in particular to the attitudes and behavioral tendencies of those most in need of them (Noar SM 2007). Personalized medicine (Ginsburg and Willard 2009) might incorporate phenotypic personality information as well as genotypic information on disease proneness. While genetic markers provide insightful biological information on specific disease risk, personality phenotype provides general information on general behavior and susceptibility to the physiological effects of stress.
Study findings must be interpreted in light of a balanced understanding of strengths and limitations. Our sample was from a randomized controlled trial for a psychosocial intervention, and thus represented a sample size common to clinical trials but small from an epidemiologic perspective. However, the intervention itself was not associated with IL-6 trajectories, nor did personality effects vary across intervention arms. Sample size is also relative, and it is not clear to what extent null findings represent insufficient power versus actual population differences. Another limitation was that the sample was primarily white and well-educated. Future studies will benefit from the inclusion of more diverse samples. IL-6 was also measured randomly throughout the day. The statistical effect of this random variation is increased error variance, meaning that analyses were more likely to miss a personality effect than to find a spurious one. With specific time-of-day measurement, it would be possible to adjust for this in analyses; no such measurements were available for this analysis. BMI was based on self-reported height and weight. To the extent that personality was associated with systematic bias in reports, it is possible that personality effects were either under or overestimated. Future work eliminating this random noise by restricting time of measurement may enhance power. Medication use was not assessed, and this could also influence circulating IL-6. Finally, we did not measure pre-intervention levels of IL-6. Thus, baseline personality associations with IL-6 levels across the study period may reflect the association of baseline personality and baseline IL-6, the latter of which simply remains stable over time. Study strengths included in-depth personality information provided by the Big 5 model; a longitudinal design; extensive covariate coverage permitting adjustment for a variety of inflammatory health factors; and an older community sample representing a population in which, to our knowledge, these questions have not yet been addressed.
In summary, findings indicated a substantial role for personality in inflammation. Further studies may enhance our understanding of this phenomenon by more directly examining the neuroimmune pathways responsible for Big-5-inflammation associations, and by examining the dynamics of inflammation in response to different kinds of psychological and biological stressors.
Acknowledgments
Support: Dr. Chapman and Dr. Seplaki are supported by Mentored Research Scientist Development Awards from the National Institute on Aging (Chapman: K08AG031328; Seplaki: K01AG031332.) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. Study Funding came from R01AG025474 (Moynihan, PI) and 1R24AG031089 (Moynihan, PI).
Footnotes
Disclosures: No members of the authorship team have any real or perceived conflicts of interest to disclose. This study received no funding from the manufacturers of KLH.
References
- Anton SD, Miller PM. Do negative emotions predict alcohol consumption, saturated fat intake, and physical activity in older adults? Behavior modification. 2005;29(4):677–688. doi: 10.1177/0145445503261164. [DOI] [PubMed] [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and Health-Related Behaviors: A Meta-Analysis of the Leading Behavioral Contributors to Mortality. Psychological bulletin. 2004;130(6):887–919. doi: 10.1037/0033-2909.130.6.887. Ameran Psyhooga Assn. [DOI] [PubMed] [Google Scholar]
- Bouhuys AL, Flentge F, et al. Potential psychosocial mechanisms linking depression to immune function in elderly subjects. Psychiatry Res. 2004;127(3):237–245. doi: 10.1016/j.psychres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Carver CS, Connor-Smith J. Personality and coping. Annual Review of Psychology. 2010;61:679–704. doi: 10.1146/annurev.psych.093008.100352. [DOI] [PubMed] [Google Scholar]
- Caspi A, Roberts BW. Personality development across the life course: The argument for change and continuity. Psychological Inquiry. 2001;12(2):49–66. [Google Scholar]
- Chapman BP. Bandwidth and fidelity on the NEO-Five Factor Inventory: replicability and reliability of Saucier’s (1998) item cluster subcomponents. J Pers Assess. 2007;88(2):220–234. doi: 10.1080/00223890701268082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Fiscella K, et al. Can the influence of childhood socioeconomic status on men’s and women’s adult body mass be explained by adult socioeconomic status or personality? Findings from a national sample. Health Psychol. 2009;28(4):419–427. doi: 10.1037/a0015212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Khan A, et al. Gender, race/ethnicity, personality, and interleukin-6 in urban primary care patients. Brain Behav Immun. 2009;23(5):636–642. doi: 10.1016/j.bbi.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Lyness JM, et al. Personality and medical illness burden among older adults in primary care. Psychosom Med. 2007;69(3):277–282. doi: 10.1097/PSY.0b013e3180313975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, et al. Sociability and susceptibility to the common cold. Psychological Science. 2003;14(5):389–395. doi: 10.1111/1467-9280.01452. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, et al. Emotional style and susceptibility to the common cold. Psychosomatic medicine. 2003;65(4):652–657. doi: 10.1097/01.psy.0000077508.57784.da. [DOI] [PubMed] [Google Scholar]
- Conraads VM, Denollet J, et al. Type D personality is associated with increased levels of tumour necrosis factor (TNF)-alpha and TNF-alpha receptors in chronic heart failure. International journal of cardiology. 2006;113(1):34–38. doi: 10.1016/j.ijcard.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Costa-Pinto FA, Palermo-Neto J. Neuroimmune interactions in stress. Neuroimmunomodulation. 2010;17(3):196–199. doi: 10.1159/000258722. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. Revised NEO Personality Inventory and NEO Five Factor Inventory: Professional Manual. Odessa, FL: Psychological Assessment; 1992. [Google Scholar]
- Costa PT, Piedmont RL. In: Multivariate Assessment: NEO-PI R Profiles of Madeline G. Paradigms of Personality Assessment. Wiggins JS, editor. New York, Guilford: 2003. pp. 262–280. [Google Scholar]
- De Fruyt F, Denollet J. Type D personality: A five-factor model perspective. Psychology & Health. 2002;17(5):671–683. [Google Scholar]
- De Jong GM, van Sonderen E, et al. A comprehensive model of stress - The roles of experienced stress and neuroticism in explaining the stress-distress relationship. Psychotherapy and psychosomatics. 1999;68(6):290–298. doi: 10.1159/000012346. [DOI] [PubMed] [Google Scholar]
- De Martinis MFCMD, et al. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. Febs Letters. 2005;579(10):2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Dembroski TM, Costa PT., Jr Coronary prone behavior: components of the type A pattern and hostility. J Pers. 1987;55(2):211–235. doi: 10.1111/j.1467-6494.1987.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Denollet J, V, Conraads M, et al. Cytokines and immune activation in systolic heart failure: the role of Type D personality. Brain Behav Immun. 2003;17(4):304–309. doi: 10.1016/s0889-1591(03)00060-6. [DOI] [PubMed] [Google Scholar]
- Denollet J, Schiffer AA, et al. Usefulness of Type D personality and kidney dysfunction as predictors of interpatient variability in inflammatory activation in chronic heart failure. Am J Cardiol. 2009;103(3):399–404. doi: 10.1016/j.amjcard.2008.09.096. [DOI] [PubMed] [Google Scholar]
- Denollet J, Vrints CJ, et al. Comparing Type D personality and older age as correlates of tumor necrosis factor-alpha dysregulation in chronic heart failure. Brain Behav Immun. 2008;22(5):736–743. doi: 10.1016/j.bbi.2007.10.015. [DOI] [PubMed] [Google Scholar]
- DeYoung CG. Higher-order factors of the Big Five in a multi-informant sample. Journal of Personality and Social Psychology. 2006;91(6):1138–1151. doi: 10.1037/0022-3514.91.6.1138. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, et al. Testing predictions from personality neuroscience: brain structure and the big five. Psychol Sci. 2010;21(6):820–828. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WJ, Gentile DA, et al. Emotional style, nasal cytokines, and illness expression after experimental rhinovirus exposure. Brain, behavior, and immunity. 2006;20(2):175–181. doi: 10.1016/j.bbi.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Muntaner C, Smith C, Tien A, Ybarra M. Center for Epidemiologic Studies Depression Scale: Review and Revision (CESD and CESDR) In: Maruish M, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. Hillsdale, NJ: L. Earlbaum Associates; 2004. p. 3. [Google Scholar]
- Eysenck HJ, Eysenck MW. Personality and Individual Differences: A Natural Science Approach. New York: Plenum; 1985. [Google Scholar]
- Garvin P, Nilsson L, et al. Plasma levels of matrix metalloproteinase-9 are independently associated with psychosocial factors in a middle-aged normal population. Psychosomatic medicine. 2009;71(3):292–300. doi: 10.1097/PSY.0b013e3181960e7f. [DOI] [PubMed] [Google Scholar]
- Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009;154(6):277–287. doi: 10.1016/j.trsl.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Goldberg LR. The structure of phenotypic personality traits. American Psychologist. 1993;48(1):26–34. doi: 10.1037//0003-066x.48.1.26. Ameran Psyhooga Assn. [DOI] [PubMed] [Google Scholar]
- Goldberg LR, Stycker LA. Personality traits and eating habits: The assessment of food preferences in a large community sample. Personality and Individual Differences. 2002;32(1):49–65. [Google Scholar]
- Gruenewald TL, Seeman TE, et al. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci U S A. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald TLSTERCDKAS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proceedings of the National Academy of Sciences. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley JA, Negassa A, et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- Harris TBFLTRPCMCWSEWHHHCHJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. The American Journal of Medicine. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, et al. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Jonassaint CR, Boyle SH, et al. Personality and inflammation: the protective effect of openness to experience. Ethn Dis. 2010;20(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain and illness. New York: Delacorte; 1990. [Google Scholar]
- Miller GE, Cohen S, et al. Personality and tonic cardiovascular, neuroendocrine, and immune parameters. Brain Behav Immun. 1999;13(2):109–123. doi: 10.1006/brbi.1998.0545. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PM, Vermetten E, et al. Hostility is related to clusters of T-cell cytokines and chemokines in healthy men. Psychoneuroendocrinology. 2008;33(8):1041–1050. doi: 10.1016/j.psyneuen.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Murray RP, Barnes GE, et al. Does personality mediate the relation between alcohol consumption and cardiovascular disease morbidity and mortality? Addictive Behaviors. 2005;30(3):475–488. doi: 10.1016/j.addbeh.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Noar SM, BC, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychological bulletin. 2007;133(4):673–693. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- Rabin BS. Introduction to immunology and immune-endocrine interactions. In: Vedhara IMK, editor. Human Psychoneuroimmunology. Oxford: Oxford University Press; 2007. pp. 1–24. [Google Scholar]
- Rhodes RE, Courneya KS. Relationships between personality, an extended theory of planned behaviour model and exercise behaviour. British Journal of Health Psychology. 2003;8 doi: 10.1348/135910703762879183. [DOI] [PubMed] [Google Scholar]; Journal Article. :19–36. [Google Scholar]
- Roy B, Diez-Roux AV, et al. Association of Optimism and Pessimism With Inflammation and Hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA) Psychosomatic medicine(Journal Article) 2010 doi: 10.1097/PSY.0b013e3181cb981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier G. Replicable item-cluster subcomponents in the NEO five-factor inventory. Journal of personality assessment. 1998;70(2):263–276. doi: 10.1207/s15327752jpa7002_6. [DOI] [PubMed] [Google Scholar]
- Sjogren E, Leanderson P, et al. Interleukin-6 levels in relation to psychosocial factors: studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain, behavior, and immunity. 2006;20(3):270–278. doi: 10.1016/j.bbi.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, et al. Evaluation of CHAMPS, a physical activity promotion program for older adults. Ann Behav Med. 1997;19(4):353–361. doi: 10.1007/BF02895154. [DOI] [PubMed] [Google Scholar]
- Suarez EC. Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosomatic medicine. 2003;65(4):523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, et al. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain, behavior, and immunity. 2002;16(6):675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Beason-Held LL, et al. Sex differences in resting-state neural correlates of openness to experience among older adults. Cerebral Cortex. 2009;19(12):2797–2802. doi: 10.1093/cercor/bhp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, et al. High Neuroticism and low Conscientiousness are associated with interleukin-6. Psychol Med. 2009:1–9. doi: 10.1017/S0033291709992029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano A, Costa PT., Jr Smoking and the Five-Factor Model of personality. Addiction. 2004;99(4):472–481. doi: 10.1111/j.1360-0443.2004.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]