Abstract
2-Aminopyridinomethyl pyrrolidines represent a class of highly potent and selective neuronal nitric oxide synthase inhibitors. Conditions for a Mitsunobu reaction of a naphthol and a hindered secondary alcohol were optimized to give good to excellent yields. A key step in the synthesis of these inhibitors is the deprotection of the benzyl group from the N-Boc and N-Bn double protected 2-aminopyridine ring at a late stage of the synthesis, which has been proven difficult in our previous syntheses. Acetic acid was found to facilitate the N-Bn deprotection.
Keywords: 2-Aminopyridinomethyl pyrrolidines, Mitsunobu reaction, N-Benzyl deprotection, Palladium-catalyzed hydrogenation
1. Introduction
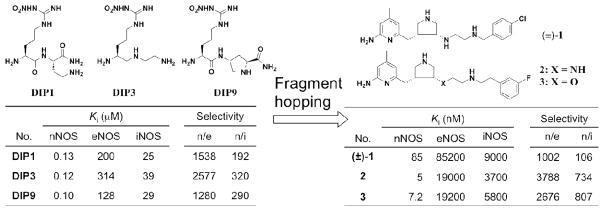
In our continued efforts to develop neuronal nitric oxide synthase (nNOS)-selective inhibitors,1 we established a new approach for fragment-based de novo design, called fragment hopping.2 The core of this approach is the derivation of the minimal pharmacophoric element for each pharmacophore. The minimal pharmacophoric element can be an atom, a cluster of atoms, a virtual graph or vectors. The new fragments that match the requirements of the minimal pharmacophoric elements are generated and hopped onto the corresponding position in the active site using LUDI fragment libraries. After linking the fragments by LUDI linker library, new inhibitors with novel scaffolds can be generated. The key features for both ligand binding affinity and isozyme selectivity can be included in the definition of minimal pharmacophoric elements, which leads to the generation of new inhibitors with greater isozyme selectivity. By using this new approach and starting with dipeptide inhibitors (e.g., DIP1, DIP3, DIP9, Figure 1), we were able to discover a series of novel highly potent and nNOS-selective small-molecule inhibitors with a 2-aminopyridinomethyl pyrrolidine scaffold (1–3 in Figure 1).2,3 The 2-aminopyridine group was used to mimic the amidino moiety of the nitroguanidino group of DIP1, DIP3 and DIP9. The pKa value of 2-amino-2,4-dimethylpyridine is 7.1.4 This fragment could act as a charge bioswtich. In the small intestine, the fragment could be in its neutral form, which is favorable for absorption; in the NOS active site, the local acidic environment would convert it into the protonated form, which is favorable for binding. The pyrrolidino fragment of 1 was generated as a substitute for the α-amino group of the dipeptide inhibitors that was responsible for the nNOS/eNOS (endothelial nitric oxide synthase) selectivity of DIP1, DIP3 and DIP9. First, the dipeptide inhibitors adopt distinctly different conformations in nNOS and eNOS.5 By replacing the inhibitor α-amino group with a rigid pyrrolidine secondary amine, the pyrrolidine amino group is locked in a conformation for enhanced interactions with nNOS D597 (the corresponding position in eNOS is N368), favoring nNOS selectivity. Second, a secondary amino group is more lipophilic and has less polar surface area compared with a primary amino group, which is better for membrane permeability. The introduction of halogen-substituted phenyl group increases inhibitor lipophilicity and also the selectivity between nNOS over iNOS (inducible nitric oxide synthase) because the corresponding pocket is L337 in nNOS and T121 in iNOS.6 Compounds 2 and 3 exhibit the highest in vitro nNOS inhibitory potency and nNOS selectivity over eNOS reported to date.7,8,9
Figure 1.
nNOS inhibitor design by fragment hopping
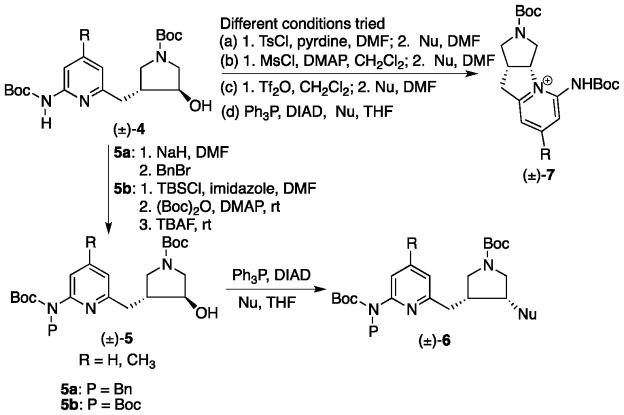
One key step in the synthesis of this series of inhibitors is the conversion of trans-alcohol 4 to cis-amines or cis-alcohols 5 (Scheme 1). It was found that mono-Boc protected 4′-[(6-aminopyridin-2-yl)methylpyrrolidin]-3′-ol (4) underwent an intramolecular cyclization to generate 7 instead of the desired addition by an external nucleophile in our original SN2 or Mitsunobu experiments (Scheme 1).2,3 Later, we found that double protection of the remote 2-amino group of the pyridine ring by a Boc/Bn or Boc/Boc combination can effectively block intramolecular cyclization.10 With dual protection, Mitsunobu reactions proceed smoothly to generate 6 (Scheme 1).
Scheme 1.
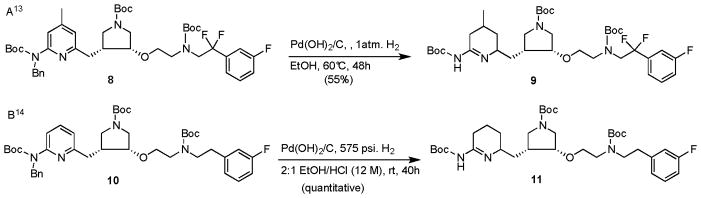
The Boc/Bn combination was then used as a standard protection strategy for the Mitsunobu chemistry of this class of inhibitors.3,8,11,12 Deprotection of the N-Bn group can be achieved by 20% Pd(OH)2/C, one atmosphere of hydrogen, 60 °C in EtOH for 24–48 h,3,8,11,12,13,14 or 20% Pd(OH)2/C, 2:1 EtOH/HCl (12 N), room temperature, 575 psi of hydrogen for 48 h.11 However, it was observed that the yields for deprotection of the N-Bn group at a late stage in the synthesis were not reproducible, leading to recovery of the starting material, generating new spots at the baseline of the TLC, or causing reduction of the pyridine ring, thereby generating 2-amino cyclic amidine derivatives 9 and 11 (Figure 2).13,14
Figure 2.
Debenzylation of Boc/Bn or Boc/Boc-diprotected analogs 7 and 9
The Boc/Boc combination was then pursued as an alternative protection strategy for the Mitsunobu reactions.10 Although this strategy requires two more steps (protection of the 3-hydroxyl group in 4 with TBS, and then deprotection of TBS with TBAF after the second Boc group is added to the 2-amino group of the pyridine ring), it was found that each step can be achieved in high yields (> 95%), and the deprotection of the Boc group is facile.10 This approach has been used in the synthesis of derivatives in this class.15 However, the second N-Boc group on the same amine is labile to various bases. Our recent study demonstrated that di-Boc deprotected 4′-[(6-aminopyridin-2-yl)methylpyrrolidin]-3′-ol (5b) can cause a fast N→O Boc migration via a base-generated alkoxide.16 The mechanism of the migration is intramolecular, implicating an unusual nine-membered cyclic transition state.
2. Results and Discussion
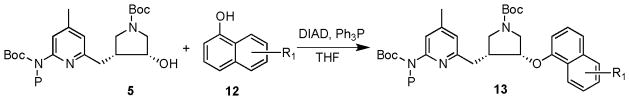
In our ongoing search for new nNOS-selective inhibitors based on scaffolds 1 and 2, we were interested in synthesizing more lipophilic derivatives (13, Scheme 2) using a Mitsunobu reaction. Although quite a few successful examples have been reported for the reaction between a secondary alcohol and a phenolic group,17,18,19 the reaction between a secondary alcohol and a sterically hindered phenolic hydroxyl group is challenging. Reaction times usually require 3 to 7 days or the reaction concentration of the starting material has to be increased to 3 M and then subjected to sonication.20 In our study, a model reaction using 5 (R = Me; P = Boc or SEM) and α-naphthol as the substrate was conducted to identify optimal reaction conditions, as summarized in Supplementary Table 1. No product was generated, as detected by TLC, by varying the protecting group (P) from Boc to [(trimethylsilyl)ethoxy]methyl (SEM). No product was generated when the concentration of the starting material was increased to 0.06 M, DIAD was changed to DEAD, or the reaction time was extended to 3 days. When the reaction mixture was heated to reflux for 24 h18,21 or a base (Et3N) was added, as suggested in the literature,22 the product was detected by TLC, but significant amounts of impurities were generated, which made silica gel column purification extremely difficult.
Scheme 2.
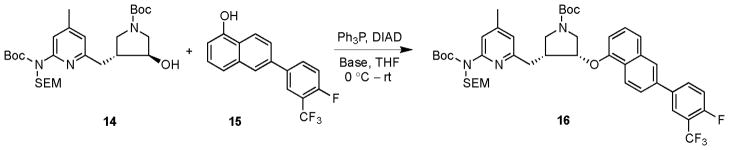
Recognizing that the base might be able to play an important role in facilitating the reaction,23,24,25 different bases were tried with another set of starting materials (enantiomer 14 with 15 in Scheme 3). One reason to use 15 is that the electron-withdrawing effect of the 4-fluoro-3-trifluoromethylphenyl group might enhance the acidity of the α-naphthol. However, without a base, no product was detectable by TLC after the reaction mixture was stirred for 26 h at room temperature (other reaction conditions remain the same as entry 1 in Supplementary Data Table 1). When five equivalents of Et3N were added to the reaction mixture, desired product 16 was isolated by silica gel column chromatography in a 19% yield after 18 h of stirring at room temperature. The structure of the product was confirmed by NMR and high-resolution mass spectrometries. Other bases were tried to see whether or not the reaction yield could be increased. Hünig’s base (N,N-diisopropylethylamine) or 4-methylmorpholine afforded better yields (25% and 23%, respectively), while 2,6-lutidine offered a worse result (< 10% yield). One general problem observed in the base-promoted Mitsunobu reaction was that more impurities were generated, which made product purification extremely difficult.
Scheme 3.
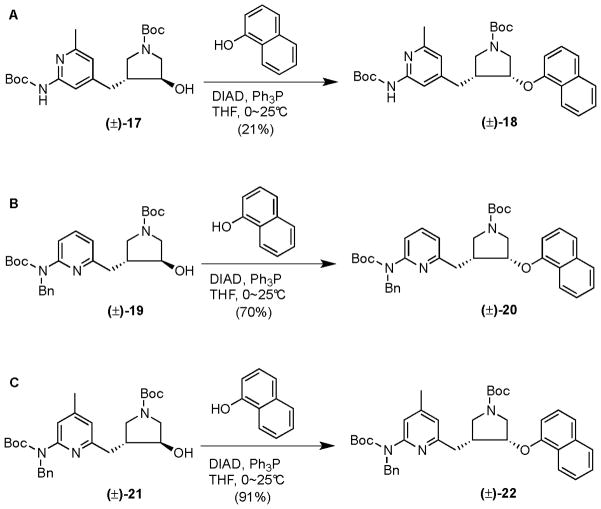
Having experienced the difficulty with the Mitsunobu reactions using the Boc/Boc or Boc/SEM protecting strategy, we decided to return to the Boc/Bn protecting strategy, because the lesson we learned was protection of the 2-amino group can affect remote SN2 reactions. A related compound (17, Scheme 4),3 did not undergo the previously observed intramolecular cyclization in the Mitsunobu reaction because of ring strain; instead, the Mitsunobu reaction proceeded smoothly in a 52% yield when diphenylphosphorylazide (DPPA) was used as the nucleophile, as reported in our previous study.9 Compound 17 was, therefore, used in a Mitsunobu reaction with α-naphthol. As shown in Scheme 4A, this reaction was surprisingly clean. When 0.02 M of 17 was allowed to react with 0.03 M of α-naphthol in anhydrous THF at room temperature for 14 h, only two products were generated. Desired product 18 was easily isolated by silica gel column chromatography in a 21% yield. The major product (in a 66% yield) was suspected to be a self-assembled oligomer via an intermolecular attack by the pyridine nitrogen of one starting material on the pyrrolidine hydroxyl group of the other starting material, although no definitive data were obtained to substantiate that hypothesis. When 19 was allowed to react with α-naphthol by the same protocol, the yield of product was much higher (Scheme 4B); desired product 20 was isolated in a 70% yield by silica gel column chromatography. When 21 was subjected to the Mitsunobu reaction with α-naphthol by the same protocol, a 91% yield of product 22 was generated after silica gel column chromatography (Scheme 4C). This protocol (0.02 M of 21 allowed to react with 0.03 M of α-naphthol, 0.03 M of DIAD, and 0.03 M of Ph3P in anhydrous THF from 0 °C to room temperature for 14 h) was used as the standard protocol for the Mitsunobu reaction of this series of compounds.
Scheme 4.
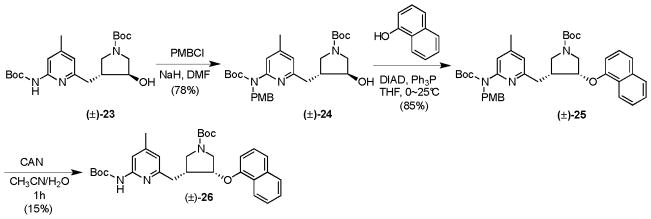
Encouraged by the results from the Boc/Bn protecting group strategy, the para-methoxybenzyl (PMB) protecting group was also tried. Compound 23 was deprotonated with NaH in DMF and then allowed to react with PMBCl to generate 24 in a 78%, which was then subjected to the Mitsunobu reaction by the protocol discussed above to successfully generate 25 in an 85% yield (Scheme 5).
Scheme 5.
However, deprotection of the N-PMB group again proved to be difficult. Various catalytic hydrogenation reactions were tried, as shown in Supplementary Scheme 1. When 10% palladium on activated carbon was used as the catalyst, 25 was not converted to the deprotected product under a hydrogen atmosphere at room temperature; only starting material was recovered. When 20% Pd(OH)2/C was used as the catalyst, 25 was not converted to the product (26) at room temperature or 40 °C. Less than 10% of 26 was generated when the reaction mixture was stirred under a hydrogen atmosphere at 60 °C for 24 h. The N-PMB group of 25 also could not be removed with the oxidative reagent 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ); all of the starting material was recovered by silica gel column chromatography after the reaction mixture was stirred at room temperature for 24 h. On the other hand, 25 undergoes a rapid and complete reaction in 1 h using ceric ammonium nitrate (CAN) as the oxidant. In the CAN reaction, however, many impurities were generated, which made the separation of the product very difficult; compound 26 was obtained in a yield of 15% after silica gel column chromatography. Another reason why more impurities were generated in the CAN reaction and the yield of the product was so low is because of the electron-rich character of the α-naphthol ether substructure in the product.
DDQ oxidation can be a much cleaner reaction, as it was reported that the 2,4-dimethoxybenzyl protecting group of Boc-protected amines can be easily deprotected by DDQ.26 An alternative protection and deprotection strategy was proposed, as shown in Supplementary Scheme 2. However, the synthesis of 2,4-dimethoxybenzyl halides could not be achieved after several attempts because of the electron-rich benzene.
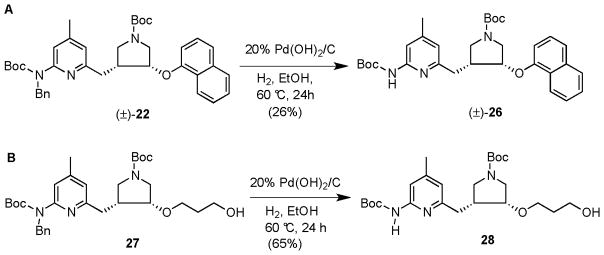
Therefore, deprotection of the N-benzyl group of 22 was attempted again. It was found that when 45 mg of 20% Pd(OH)2/C was used with 0.15 mmol of 22 in 10 mL of EtOH, and the reaction was stirred at 60 °C for 24 h under a hydrogen atmosphere, all of the starting material disappeared, but the yield of 26 was only 26% after silica gel column chromatography (Scheme 6A). Other by-products, which would not migrate even when 100% EtOAc was used as eluent, stayed at the baseline. Another series of compounds was subjected to debenzylation conditions, as shown in Scheme 6B. One mmole of 27 in 60 mL of EtOH was allowed to hydrogenate with 300 mg of 20% Pd(OH)2/C at one atmosphere of hydrogen. A similar result was observed, namely, that 27 was totally consumed in the reaction, but only 65% of 28 was obtained after silica gel column purification. This reaction was repeated three times, and the yield was consistently about 65%.
Scheme 6.
After consideration of the reaction mechanism, an acid additive, such as acetic acid, was thought to be helpful for the reaction because hydrogenolysis of more polarized σ bonds is easier than nonpolarized bonds.27 Protonation of the pyridine ring can polarize the Bn-N bond and make the Bn-N bond more electrophilic for surface hydride attack in hydrogenolysis. When variable equivalents of acetic acid (0.5, 1, 1.5 or 2 equivalents) were added to the reaction mixture as an additive, and the other reaction conditions were kept the same as those in Scheme 6, the yields of products in all reactions were constant and dramatically increased compared with those without acetic acid. The yield of product for starting material 22 was increased to over 60%, while the yield of product from 27 was increased to 89%. The time to complete the reaction was shortened to 14 h. This reaction was repeated twice for 27, and the yields were consistently high. Compound 27 was further used to optimize the reaction conditions. It was found that when the quantity of 20% Pd(OH)2/C was cut in half (150 mg per 1 mmol of 27), the reaction gave the same yield. However, if the quantity of 20% Pd(OH)2/C, was reduced to 100 mg/per 1 mmol of starting material, or the reaction was left at room temperature or maintained at 40 °C, the yields of product decreased. The reaction time of ≥14 hours also was important. Reaction times of 14 or 18 hours afforded complete conversion of the starting material with similar yields, while reaction times of 6 or 10 hours left significant amounts of starting material, as monitored by NMR spectrometry. A possible mechanism that explains the role of the acetic acid is shown in Supplementary Scheme 3.
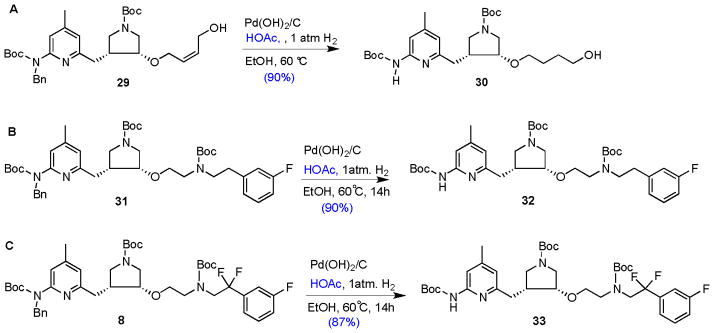
Additional substrates gave similarly high yields with the newly developed protocol (1 mmol substrate in 60 mL of EtOH containing 1.5 mmol of acetic acid allowed to hydrogenate with 150 mg of 20% Pd(OH)2/C at one atmosphere hydrogen and 60 °C for 14 h), as shown in Scheme 7. Compound 29 can be smoothly converted to 30 in a yield of 90%. The reaction was run three times, and the yield was reproducible. Compound 31 was converted to 32 in a yield of 90%, and 8 was converted to 33 in a yield of 87%. Reactions of 8 and 31 were repeated twice, and the yields were reproducible. By using this new protocol, none of the 2-amino cyclic amidine products (see Figure 2) was observed with any of the substrates (8, 22, 27, 29, and 31) tested so far, which had been a serious problem with molecules subjected to this synthetic route.
Scheme 7.
3. Conclusions
Reaction conditions for a Mitsunobu reaction of α-naphthol with a hindered secondary alcohol have been optimized. A variety of reaction conditions were tried for debenzylation of an N-tert-butyloxycarbonyl (N-Boc) and N-benzyl (N-Bn) double protected 2-aminopyridine, and it was shown that low yields of debenzylated product were obtained unless 1.5 equivalent of acetic acid is added during hydrogenolysis.
4. Experimental Section
4.1 General Methods, Reagents, and Materials
All reagents were used without further purification unless stated otherwise. 1H NMR and 13C NMR spectra were recorded in CDCl3. Chemical shifts are reported as values in parts per million (ppm) and the reference resonance peaks set at 0 ppm [TMS(CDCl3)] for 1H NMR spectra and 77.23 ppm (CDCl3) for 13C NMR spectra. Thin-layer chromatography was carried out on pre-coated silica gel 60 F254 plates with visualization accomplished with phosphomolybdic acid or ninhydrin spray reagent or with a UV–visible lamp. Column chromatography was performed with silica gel 60 (230–400 mesh). Tetrahydrofuran (THF) and ethyl ether were redistilled under nitrogen using sodium and benzophenone as the indicator. Dichloromethane was redistilled from CaH2 under nitrogen. Other dry solvents were directly purchased from the companies.
4.2 General Procedure for the Mitsunobu Reaction
To a solution of triphenylphosphine (Ph3P, 0.079 g, 0.3 mmol), the starting alcohol (0.2 mmol), and α-naphthol (0.043 g, 0.3 mmol) in dry THF (10 mL), was added dropwise diisopropyl azodicarboxylate (DIAD, 0.061 g, 0.060 mL, 0.3 mmol) at 0 ºC. The solution was stirred at 0 ºC for 1 h and then at room temperature overnight (13 h). The reaction mixture was concentrated in vacuo, and the crude residue was purified by silica gel column chromatography to yield the product.
4.2.1 (±)-tert-butyl 3-{{2′-[(tert-butoxycarbonyl)amino]-6′-methylpyridin-4′-yl}methyl}-4-hydroxypyrrolidine-1-carboxylate (17)
1H NMR (CDCl3, 500 MHz): δ 7.649-7.582 (m, 2H), 6.639 (s, 1H), 4.062 (m, 1H), 3.684-3.497 (m, 2H), 3.286-3.234 (m, 1H), 3.100 (m, 1H), 2.769-2.730 (m, 1H), 2.518-2.378 (m, 5H), 1.496 (s, 9H), 1.448 (s, 9H). 13C NMR (CDCl3, 125.7 MHz): δ 156.923 (1C), 154.830 (1C), 152.644 (1C), (151.766+151.689) (1C), 151.515 (1C), 118.711 (1C), 109.476 (1C), 80.971 (1C), (79.698+79.629) (1C), (74.414+73.833) (1C), (52.682+52.431) (1C), (49.061+48.891) (1C), (46.957+46.419) (1C), 37.180 (1C), 28.619 (3C), 28.394 (3C), 23.999 (1C). MS (ESI, CH2Cl2): [C21H33N3O5] m/z 407.9 ([M+H]+).
4.2.2 (±)-tert-Butyl 3-{{2′-[(tert-butoxycarbonyl)amino]-6′-methylpyridin-4′-yl}methyl}-4-(naphthalen-1-yloxy)pyrrolidine-1-carboxylate (18)
Column chromatography (hexanes: EtOAc = 7:3). 1H NMR (CDCl3, 400MHz): δ 8.300-8.271 (m, 1H), 7.965 (m, 1H), 7.828-7.802 (m, 1H), 7.677-7.667 (m, 1H), 7.548-7.499 (m, 2H), 7.475-7.434 (m, 1H), 7.365-7.307 (m, 1H), 6.712-6.660 (m, 1H), 6.583 (s, 1H), 4.848-4.800 (m, 1H), 3.811-3.485 (m, 4H), 3.123-3.056 (m, 1H), 2.973-2.907 (m, 1H), 2.773-2.692 (m, 1H), 2.244 (s, 3H), 1.471 (s, 9H), (1.453+1.379) (m, 9H). 13C NMR (CDCl3, 125.7 MHz): δ 156.86 (1C), (154.40+154.35) (1C), 152.55 (1C), 152.41 (1C), 152.34 (1C), 151.57 (1C), 151.42 (1C), 151.39 (1C), (134.76) (1C), 134.72 (1C), (127.66+127.56) (1C), (126.68+126.49) (1C), (126.24+126.18) (1C), (125.64+125.59) (1C), 125.38 (1C), (121.94+121.78) (1C), 120.90 (1C), 118.85 (1C), 108.86 (1C), (106.00 +105.86) (1C), (80.83+80.79) (1C), (79.82+79.61) (1C), (76.77+76.10) (1C), (51.49+51.03) (1C), (49.48+49.13) (1C), (44.93+42.26) (1C), (33.03+32.92) (1C), (28.48+28.39+28.24) (6C), 23.77 (1C). HRMS (ESI, CH3OH) Calcd for C31H40N3O5, 533.2890; Found, 533.2887.
4.2.3 (±)-tert-Butyl 3-{{6-[benzyl(tert-butoxycarbonyl)amino]pyridin-2-yl}methyl}-4-hydroxypyrrolidine-1-carboxylate (19)
1H NMR (CDCl3, 500 MHz): δ 7.572-7.458 (m, 2H), 7.291-7.192 (m, 5H), 6.880-6.853 (m, 1H), 5.184-5.159 (m, 2H), 4.045 (m, 1H), 3.716-3.532 (m, 2H), 3.213-3.154 (m, 1H), 3.115-3.057 (m, 1H), 2.938-2.728 (m, 2H), 2.458-2.403 (m, 1H), 1.449-1.438 (m, 18H). 13C NMR (CDCl3, 125.7 MHz): δ (157.61+157.59) (1C), (154.58+154.55) (1C), (154.25+154.21) (1C), 153.82 (1C), (139.27+139.20) (1C), (137.94+137.85) (1C), 128.23 (2C), 127.94 (2C), 126.77 (1C), (119.42+119.17) (1C), (117.77+117.56) (1C), 81.55 (1C), 79.33 (1C), (75.06+74.33) (1C), (52.59+52.17+51.61) (1C), (50.42+50.25+49.59+49.18) (1C), (45.28+44.22) (1C), 39.29 (1C), 28.48 (3C), 28.14 (3C). HRMS (ESI, CH3OH) Calcd for C27H38N3O5, 483.2733; Found, 483.2733.
4.2.4. (±)-tert-Butyl 3-{{6′-[benzyl(tert-butoxycarbonyl)amino]pyridin-2′-yl}methyl}-4-(naphthalen-1-yloxy)pyrrolidine-1-carboxylate (20)
Column chromatography (CH2Cl2: EtOAc = 8.5:1.5). 1H NMR (CDCl3, 400MHz): δ 8.276-8.260 (m, 1H), 7.882 (m, 1H), 7.572-7.232 (m, 11H), 6.736 (m, 1H), 6.409-6.392 (m, 1H), 5.163 (s, 2H), 4.999-4.989 (m, 1H), 3.744-3.354 (m, 4H), 3.205 (m, 1H), 3.017 (m, 1H), 2.887-2.831 (m, 1H), 1.441-1.348 (m, 18H). 13C NMR (CDCl3, 125.7 MHz): δ 157.28 (1C), 154.35 (1C), 154.29 (1C), 153.78 (1C), 152.72 (1C), 139.74 (1C), 139.65 (1C), 137.54 (1C), 131.14 (1C), 128.16 (2C), 128.13 (1C), 127.23 (1C), 126.85 (2C), 126.78 (1C), 126.63 (1C), 126.52 (1C), 125.71 (1C), 125.49 (1C), 122.35 (1C), (118.63) (1C), 116.62 (1C), 81.41 (1C), 79.52 (1C), (70.80+69.91) (1C), (51.34+49.85) (1C), (49.55+49.14) (1C), (42.94+42.23) (1C), 34.58 (1C), (28.49+28.43+28.15) (6C). HRMS (ESI, CH3OH) Calcd for C37H43N3O5, 609.3203; Found, 609.3212.
4.2.5. (±)-tert-butyl 3-{{6′-[benzyl(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-hydroxypyrrolidine-1-carboxylate (21)
1H NMR (CDCl3, 500 MHz): δ 7.338-7.178 (m, 6H), 6.713-6.696 (m, 1H), 5.168-5.079 (m, 2H), (4.164+3.869) (brs, 1H), 4.061-4.042 (m, 1H), 3.726-3.528 (m, 2H), 3.211-3.144 (m, 1H), 3.094-3.044 (m, 1H), 2.812-2.765 (m, 2H), 2.488-2.364 (m, 1H), 2.296-2.289 (m, 3H), 1.452-1.416 (m, 18H). 13C NMR (CDCl3, 125.7 MHz): δ 157.398 (1C), (154.727+154.666) (1C), 154.405 (1C), 154.016 (1C), (149.463+149.341) (1C), (139.530+139.445) (1C), 128.340 (2C), 127.180 (1C), 126.871 (1C), (120.914+120.696) (1C), (118.577+118.358) (1C), 81.516 (1C), 79.416 (1C), (75.287+74.577) (1C), (52.798+52.367) (1C), (50.619+50.461) (1C), (49.817+49.404) (1C), (45.403+44.638) (1C), 39.368 (1C), 28.634 (3C), 28.294 (3C), 21.257 (1C). MS (ESI, CH3OH): [C28H39N3O5] m/z 498.4 ([M+H]+); m/z 995.2 ([2M+H]+).
4.2.6. (±)-tert-Butyl 3-{{6′-[benzyl(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-(naphthalen-1-yloxy)pyrrolidine-1-carboxylate (22)
Column chromatography (hexanes: Me2CO = 7:3). 1H NMR (CDCl3, 400MHz): δ 8.263 (m, 1H), 7.931 (m, 1H), 7.606-7.202 (m, 10H), 6.574 (m, 1H), 6.402-6.393 (m, 1H), 5.160 (s, 2H), 4.996 (m, 1H), 4.689 (m, 1H), 3.722-3.440 (m, 3H), 3.143 (m, 1H), 2.975-2.842 (m, 2H), 2.127 (m, 3H), 1.560-1.415 (m, 18H). 13C NMR (CDCl3, 125.7 MHz): δ 157.025 (1C), 156.376 (2C), 154.513 (1C), 153.974 (1C), 152.854 (1C), 148.771 (1C), 139.999 (1C), 131.320 (1C), 128.265 (2C), 127.501 (1C), 127.008 (1C), 126.923 (1C), 126.720 (1C), 126.622 (1C), 125.799 (1C), 125.595 (1C), 122.480 (2C), 120.214 (1C), 117.243 (1C), 105.106 (1C), 81.382 (1C), 79.617 (1C), (70.798+70.068) (1C), (51.561+51.217+50.028) (1C), (49.731+49.324) (1C), (43.022+42.466) (1C), 34.636 (1C), (28.635+28.571) (3C), 28.296 (3C), (22.146+22.087) (1C). HRMS (ESI, CH3OH) Calcd for C38H45N3O5, 623.3359; Found, 623.3358.
4.2.7. (±)-tert-butyl 3-{{6′-[(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-hydroxypyrrolidine-1-carboxylate (23)
1H NMR (CDCl3, 500 MHz): δ (8.032-7.994+7.787-7.757) (m, 1H), 7.662 (s, 1H), 6.664-6.650 (m, 1H), 5.338 (brs, 1H), 4.111-4.104 (m, 1H), 3.766-3.574 (m, 2H), 3.260-3.043 (m, 2H), 2.843-2.721 (m, 2H), 2.475-2.381 (m, 1H), 2.307 (s, 3H), 1.479 (s, 9H), 1.446 (s, 9H). 13C NMR (CDCl3, 125.7 MHz): δ (157.457+157.395) (1C), (154.618+154.521) (1C), (152.478+152.428) (1C), (151.573+151.507) (1C), 150.644 (1C), 119.342 (1C), 110.734 (1C), (80.936+80.863) (1C), (79.377+79.338) (1C), (74.982+74.371) (1C), (52.566+52.257) (1C), (49.700+49.189) (1C), (45.220+44.802) (1C), (39.161+39.049) (1C), 28.495 (3C), 28.220 (3C), 21.349 (1C). MS (ESI, CH2Cl2): [C21H33N3O5] m/z 407.9 ([M+H]+).
4.2.8. (±)-tert-butyl 3-{{6′-[(tert-butoxycarbonyl)(4″-methoxybenzyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-hydroxypyrrolidine-1-carboxylate (24)
1H NMR (CDCl3, 500 MHz): δ 7.270-7.223 (m, 1H), 7.185-7.171 (2H, m), 6.792-6.779 (m, 2H), 6.691 (m, 1H), 5.065 (s, 2H), 4.113-4.041 (m, 1H), 3.720-3.710 (m, 3H), 3.694-3.551 (m, 2H), 3.224-3.179 (m, 1H), 3.110-3.100 (m, 1H), 2.846-2.834 (m, 1H), 2.684-2.603 (m, 1H), 2.480-2.441 (m, 1H), 2.254 (s, 3H), 1.431-1.422 (m, 18H). 13C NMR (CDCl3, 125.7 MHz): δ 158.343 (1C), 157.290 (1C), 154.502 (1C), 154.201 (1C), 153.708 (1C), (148.891+148.785) (1C), (131.275+131.173) (1C), 128.538 (2C), (120.470+120.309) (1C), (118.344+118.123) (1C), 113.413 (2C), 81.019 (1C), 79.088 (1C), (74.662+73.953) (1C), 54.962 (1C), (52.572+52.139) (1C), (49.640+49.504) (1C), (49.423+49.037) (1C), (45.417+44.696) (1C), 39.072 (1C), 28.357 (3C), 28.081 (3C), 20.917 (1C). MS (ESI, CH3OH): [C29H41N3O6] m/z 528.4 ([M+H]+). HRMS (ESI, CH3OH) Calcd for C29H41N3O6, 527.2995; Found, 527.3000.
4.2.9. (±)-tert-Butyl 3-{{6′-[(tert-butoxycarbonyl)(4″-methoxybenzyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-(naphthalen-1-yloxy)pyrrolidine-1-carboxylate (25)
Column chromatography (hexanes: Me2CO = 7:3). 1H NMR (CDCl3, 400MHz): δ 8.277-8.263 (m, 1H), 7.907 (m, 1H), 7.557-7.232 (m, 5H), 7.163-7.149 (m, 2H), 6.797-6.785 (m, 2H), 6.582 (m, 1H), 6.457-6.441 (m, 1H), 5.770 (s, 2H), 4.992 (m, 1H), 4.736 (m, 1H), 3.769 (s, 3H), 3.728-3.454 (m, 3H), 3.155 (m, 1H), 2.998-2.880 (m, 2H), 2.124 (m, 3H), 1.432-1.265 (m, 18H). 13C NMR (CDCl3, 125.7 MHz): δ 158.35 (1C), 158.28 (1C), 156.93 (1C), 156.13 (1C), 154.39 (1C), 153.90 (1C), 148.63 (1C), 131.80 (1C), 131.70 (1C), 131.15 (1C), (128.38+128.29) (2C), 127.43 (1C), 126.56 (1C), 125.71 (1C), 125.49 (1C), 122.38 (1C), 120.13 (2C), 117.50 (1C), 113.44 (2C), 104.94 (1C), 81.17 (1C), 79.55 (1C), (70.75+69.96) (1C), 55.23 (1C), (51.42+51.00) (1C), (49.64+49.30+49.22) (1C), 43.06 (1C), 34.636 (1C), (28.48+28.42) (6C), 22.01 (1C). HRMS (ESI, CH3OH) Calcd for C39H47N3O6, 653.3465; Found, 653.3464.
4.3 General Procedure for the Debenzylation Reaction
To a solution of the starting material (1 mmol) in EtOH (60 mL) was added HOAc (1.5 mmol) at room temperature. The solution was then treated with 20% wt Pd(OH)2 on carbon (150 mg). The reaction mixture was stirred at 60 ºC under an atmosphere of hydrogen for 14 h. The catalyst was filtered through Celite. The Celite pad was washed with EtOH (30 mL × 2). The combined filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography to afford the desired product.
4.3.1. (±)-tert-Butyl 3-{{6′-[(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-(naphthalen-1-yloxy)pyrrolidine-1-carboxylate (26)
Column chromatography (hexanes: EtOAc = 7:3). 1H NMR (CDCl3, 400MHz): δ 8.313-8.297 (m, 1H), 7.892 (m, 1H), 7.568-7.509 (m, 4H), 7.187 (m, 2H), 6.613-6.575 (m, 2H), 5.005-4.865 (m, 2H), 3.798-3.492 (m, 3H), 3.106-2.892 (m, 3H), 2.152 (m, 3H), 1.520 (s, 9H), 1.449-1.394 (m, 9H). 13C NMR (CDCl3, 100.7 MHz): δ 157.712 (1C), 156.417 (1C), 154.584 (1C), 153.061 (1C), 152.599 (1C), 151.653 (1C), 150.244 (1C), 131.438 (1C), 127.621 (2C), 126.772 (1C), 125.954 (1C), 125.727 (1C), 122.743 (2C), 119.471 (1C), (110.519+110.451) (1C), 105.187 (1C), 80.996 (1C), 79.776 (1C), (70.998+70.127) (1C), (51.632+51.298) (1C), (49.693+49.428) (1C), (43.634+43.088) (1C), 35.234 (1C), (28.683+28.607) (3C), 28.455 (3C), 22.192 (1C). HRMS (ESI, CH3OH) Calcd for C31H39N3O5, 533.2890; Found, 533.2886.
4.3.2. (3R,4R)-tert-Butyl 3-{{6′-[benzyl(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-(3′-hydroxypropoxy)pyrrolidine-1-carboxylate (27)
1H NMR (CDCl3, 400MHz): δ 7.389-7.353 (m, 1H), 7.303-7.174 (m, 5H), 6.710 (s, 1H), 5.161 (s, 2H), 3.696-3.536 (m, 5H), 3.444-3.370 (m, 1H), 3.334-3.260 (m, 1H), 3.217-3.047 (m, 2H), 2.924-2.870 (m, 1H), 2.728-2.676 (m, 1H), 2.595-2.532 (m, 1H), 2.298-2.284(m, 3H), 1.760-1.751 (m, 2H), 1.459-1.442 (m, 9H), 1.413 (s, 9H,). 13C NMR (CDCl3, 100.7 MHz): δ (157.850+157.755) (1C), (155.023+154.620) (1C), 154.429 (1C), 153.895 (1C), 148.658 (1C), (139.781+139.751) (1C), 128.113 (2C), (127.065+126.977) (2C), (126.619+126.553) (1C), 120.239 (1C), (117.441+117.360) (1C), (81.214+81.163) (1C), 79.347 (1C), (79.222+78.519) (1C), (67.445+67.254) (1C), 60.677 (1C), (50.833+50.305) (1C), 50.086 (1C), (49.361+48.884) (1C), (42.908+42.227) (1C), 34.551 (1C), (32.397+32.295) (1C), 28.537 (3C), 28.200 (3C), 21.147 (1C). MS (ESI, CH3OH): [C31H45N3O6] m/z 556.0 ([M+H]+); m/z 578.1 ([M+Na]+). HRMS (ESI, CH3OH) Calcd for C31H46N3O6, 555.3308; Found, 555.3313.
4.3.3. (3R,4R)-tert-Butyl 3-{{6′-[(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-(3′-hydroxypropoxy)pyrrolidine-1-carboxylate (28)
Column chromatography (cylcohexanes: EtOAc = 6:4). 1H NMR (CDCl3, 400MHz): δ 7.907 (s, 1H), 7.663-7.653 (m, 1H), 6.674 (s, 1H), 4.335 (brs, 1H), 3.889-3.879 (m, 1H), 3.745-3.707 (m, 1H), 3.649-3.605 (m, 2H), 3.560-3.369 (m, 3H), 3.258-3.101 (m, 2H), 2.907-2.852 (m, 1H), 2.719-2.657 (m, 1H), 2.496-2.457 (m, 1H), 2.310-2.300 (m, 3H), 1.822 (m, 2H), 1.511 (s, 9H), 1.458 (s, 9H). 13C NMR (CDCl3, 100.7 MHz): δ (158.055+157.945) (1C), (154.722+154.466) (1C), 152.496 (1C), 151.566 (1C), 150.035 (1C), 118.833 (1C), (110.292+110.241) (1C), (80.533+80.497) (1C), (79.149+79.061) (1C), (78.724+77.691) (1C), (66.456+66.361) (1C), (59.417+59.344) (1C), (50.664+50.196) (1C), (49.185+48.767) (1C), (44.255+43.508) (1C), (34.551+34.287) (1C), (32.177+32.075) (1C), 28.376 (3C), 28.120 (3C), 21.184 (1C). MS (ESI, CH3OH): [C24H39N3O6] m/z 466.3 ([M+H]+); m/z 488.2 ([M+Na]+); m/z 952.8 ([2M+Na]+). HRMS (ESI, CH3OH) Calcd for C24H40N3O6, 465.2839; Found, 465.2839.
4.3.4. (3R,4R)-tert-Butyl 3-{{6′-[benzyl(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-[((Z)-4′-hydroxybut-2′-en-1′-yl)oxy]pyrrolidine-1-carboxylate (29)
1H NMR (CDCl3, 500MHz): δ 7.401-7.368 (m, 1H), 7.292-7.163 (m, 5H), 6.699 (s, 1H), 5.799-5.699 (m, 1H) 5.640-5.573 (m, 1H), 5.158 (s, 2H), 4.139-4.061 (m, 2H), 4.011-3.929 (m, 1H), 3.809-3.532 (m, 2H), 3.456-3.368 (m, 2H), 3.209-3.063 (m, 2H), 2.941-2.876 (m, 1H), 2.751-2.588 (m, 2H), 2.303-2.289 (m, 3H), 1.454-1.435 (m, 9H), 1.406 (s, 9H). 13C NMR (CDCl3, 125.7 MHz): δ (157.860+157.809) (1C), (154.923+154.630) (1C), (154.600+154.564) (1C), 153.868 (1C), (148.594+148.536) (1C), (139.878+139.841) (1C), (132.260+132.143) (1C), 128.136 (2C), (127.419+127.213) (1C), 127.052 (2C), (126.649+126.576) (1C), 120.306 (1C), (117.208+117.149) (1C), (81.332+81.288) (1C), (79.332+79.222) (1C), (78.212+77.589) (1C), (69.195+69.158) (1C), 62.617 (1C), (51.037+50.561) (1C), (50.107+50.063) (1C), (49.440+48.993) (1C), (42.628+41.903) (1C), 34.513 (1C), 28.587 (3C), 28.221 (3C), 21.189 (1C). MS (ESI, CH3OH): [C32H45N3O6] m/z 568.1 ([M+H]+). HRMS (ESI, CH3OH) Calcd for C32H46N3O6, 567.3308; Found, 567.3311.
4.3.5. (3R,4R)-tert-Butyl 3-{{6′-[(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-(4′-hydroxybutoxy)pyrrolidine-1-carboxylate (30)
Column chromatography (hexanes: EtOAc = 7:3). 1H NMR (CDCl3, 500MHz): δ 7.627-7.583 (m, 2H), 6.660-6.649 (m, 1H), 3.757-3.397 (m, 6H), 3.289-3.254 (m, 2H), 3.192-3.091 (m, 1H), 2.920-2.866 (m, 1H), 2.712-2.537 (m, 2H), 2.312-2.294 (m, 3H), 1.666 (m, 4H), 1.517 (s, 9H), 1.452 (s, 9H). 13C NMR (CDCl3, 125.7 MHz): δ (158.33+158.25) (1C), (155.01+154.76) (1C), 152.66 (1C), 151.62 (1C), 150.14 (1C), 119.32 (1C), (110.40+110.35) (1C), (80.92+80.84) (1C), (79.44+79.35) (1C), (79.35+78.35) (1C), (69.31+69.14) (1C), 62.53 (1C), (50.81+50.41) (1C), (49.34+48.95) (1C), (43.51+42.82) (1C), (34.68+34.55) (1C), 29.79 (1C), 28.63 (3C), 28.37 (3C), (26.55+26.53) (1C), (21.44+21.42) (1C). MS (ESI, CH3OH): [C25H41N3O6] m/z 502.3 ([M+Na]+). HRMS (ESI, CH3OH) Calcd for C25H41N3O5, 479.2995; Found, 479.2998.
4.3.6. (3R,4R)-tert-Butyl 3-{{6′-[benzyl(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-{2′-[(tert-butoxycarbonyl)(3″-fluorophenethyl)amino]ethoxy}pyrrolidine-1-carboxylate (31)
The NMR spectra are the same as those reported in the previous study.8 1H NMR (CDCl3, 500 MHz): δ 7.426-7.395 (m, 1H), 7.237-7.178 (m, 6H), 6.986-6.857 (m, 3H), 6.634-6.609 (m, 1H), 5.163 (s, 2H), 3.646-3.165 (m, 10H), 3.086-3.022 (m, 1H), 2.893-2.779 (m, 3H), 2.692-2.649 (m, 1H), 2.558 (m, 1H), 2.291-2.273(m, 3H), 1.445-1.409 (m, 27H). 13C NMR (CDCl3, 125.7 MHz): δ (163.967+162.012) (1C), (157.756+157.665) (1C), (155.419+155.279) (1C), (154.812+154.623) (1C), (154.514+154.447) (1C), 153.974 (1C), 148.655 (1C), 142.013 (1C), 139.973 (1C), 129.997 (1C), 128.176 (2C), (127.120+127.035) (2C), (126.670+126.591) (1C), (124.685+124.667) (1C), 120.058 (1C), (117.174+117.126) (1C), (115.875+115.711) (1C), (113.337+113.246+113.173+113.076) (1C), (81.298+81.243) (1C), (79.725+79.203+78.881) (3C), (68.232+68.056) (1C), (50.934+50.430) (1C), (50.273+50.194) (1C), 50.024 (1C), (49.386+48.937) (1C), (47.971+47.468) (1C), (42.890+42.762+42.282) (1C), (35.003+34.711+34.341) (2C), 28.591 (3C), 28.488 (3C), 28.275 (3C), 21.239 (1C). MS (ESI, CH3OH): [C43H59FN4O7] m/z 763.5 ([M+H]+); m/z 785.5 ([M+Na]+); m/z 1546.8 ([2M+Na]+).
4.3.7. (3R,4R)-tert-Butyl 3-{2′-[(tert-butoxycarbonyl)(3″-fluorophenethyl)amino]ethoxy}-4-{{6′-[(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}pyrrolidine-1-carboxylate (32)
The NMR spectra are the same as those reported in the previous study.8 1H NMR (CDCl3, 500 MHz): δ 7.618-7.600 (m, 1H), 7.471-7.457 (m, 1H), 7.254-7.242 (m, 1H), 7.002-6.874 (m, 3H), 6.620-6.591 (m, 1H), 3.781-3.737 (m, 1H), 3.602-3.261 (m, 9H), 3.160-3.062 (m, 1H), 2.859-2.819 (m, 3H), 2.703-2.541 (m, 2H), 2.301-2.279 (m, 3H), 1.512 (s, 9H), 1.442 (m, 18H). 13C NMR (CDCl3, 125.7 MHz): δ (163.870+161.915) (1C), (158.218+158.169) (1C), (155.382+155.334+155.170) (1C), (154.696+154.544) (1C), 152.492 (1C), (151.527+151.515) (1C), 149.894 (1C), (141.958+141.898) (1C), (129.979+129.906) (1C), (124.600+124.582) (1C), (119.117+119.063) (1C), (115.790+115.626) (1C), (113.240+113.149+113.070+112.979) (1C), (110.235+110.168) (1C), (80.721+80.660) (1C), (79.616+79.586) (1C), (79.750+79.221+79.149+78.651) (2C), (68.250+68.159+67.977) (1C), (50.922+50.485) (1C), (50.212+50.072+49.981) (1C), (49.216+48.864) (1C), (47.941+47.449) (1C), (43.333+43.175+42.707+42.556) (1C), (34.936+34.736+34.547+34.432+34.268) (2C), 28.482 (3C), 28.385 (3C), 28.263 (3C), 21.281 (1C). MS (ESI, CH3OH): [C36H53FN4O7] m/z 673.8 ([M+H]+); m/z 695.6 ([M+Na]+); m/z 1367.5([2M+Na]+).
4.3.8. (3R,4R)-tert-butyl 3-{{6′-[benzyl(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}-4-{2′-{(tert-butoxycarbonyl)[2″,2″-difluoro-2″-(3‴-fluorophenyl)ethyl]amino}ethoxy}pyrrolidine-1-carboxylate (8)
The NMR spectra are the same as those reported in the earlier study.11
4.3.9. (3R,4R)-tert-Butyl 3-{2′-{(tert-butoxycarbonyl)[2″,2″-difluoro-2″-(3‴-fluorophenyl)ethyl]amino}ethoxy}-4-{{6′-[(tert-butoxycarbonyl)amino]-4′-methylpyridin-2′-yl}methyl}pyrrolidine-1-carboxylate (33)
The NMR spectra are the same as those reported in the previous study.11
Supplementary Material
Acknowledgments
The authors are grateful to the National Institutes of Health (GM049725) for financial support of this research.
Footnotes
Supplementary Data Available. Supplementary Table 1, Supplementary Schemes 1–3, and 1H NMR, and 13C NMR spectra for 17–32. This material is can be found online at doi:10.1016/j.tet.xxxxxxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silverman RB. Acc Chem Res. 2009;42:439–451. doi: 10.1021/ar800201v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji H, Stanton BZ, Igarashi J, Li H, Martásek P, Roman LJ, Poulos TL, Silverman RB. J Am Chem Soc. 2008;130:3900–3914. doi: 10.1021/ja0772041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji H, Li H, Martásek P, Roman LJ, Poulos TL, Silverman RB. J Med Chem. 2009;52:779–797. doi: 10.1021/jm801220a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paudler WW, Blewitt HL. J Org Chem. 1966;31:1295–1298. [Google Scholar]
- 5.Flinspach ML, Li H, Jamal J, Yang W, Huang H, Hah JM, Gómez-Vidal JA, Litzinger EA, Silverman RB, Poulos TL. Nat Struct Mol Biol. 2004;11:54–59. doi: 10.1038/nsmb704. [DOI] [PubMed] [Google Scholar]
- 6.Ji H, Li H, Flinspach M, Poulos TL, Silverman RB. J Med Chem. 2003;46:5700–5711. doi: 10.1021/jm030301u. [DOI] [PubMed] [Google Scholar]
- 7.Delker SL, Ji H, Li H, Jamal J, Fang J, Xue F, Silverman RB, Poulos TL. J Am Chem Soc. 2010;132:5437–5442. doi: 10.1021/ja910228a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji H, Delker SL, Li H, Martásek P, Roman LJ, Poulos TL, Silverman RB. J Med Chem. 2010;53:7804–7824. doi: 10.1021/jm100947x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji H, Tan S, Igarashi J, Li H, Derrick M, Martásek P, Roman LJ, Vásquez-Vivar J, Poulos TL, Silverman RB. Ann Neurol. 2009;65:209–217. doi: 10.1002/ana.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawton GR, Ji H, Silverman RB. Tetrahedron Lett. 2006;47:6113–6115. [Google Scholar]
- 11.Lawton GR, Ralay Ranaivo H, Chico LK, Ji H, Xue F, Martásek P, Roman LJ, Watterson DM, Silverman RB. Bioorg Med Chem. 2009;17:2371–2380. doi: 10.1016/j.bmc.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman RB, Lawton GR, Ralay Ranaivo H, Chico LK, Seo J, Watterson DM. Bioorg Med Chem. 2009;17:7593–7605. doi: 10.1016/j.bmc.2009.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue F, Li H, Delker SL, Fang J, Martásek P, Roman LJ, Poulos TL, Silverman RB. J Am Chem Soc. 2010;132:14229–14238. doi: 10.1021/ja106175q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue F, Li H, Fang J, Roman LJ, Martásek P, Poulos TL, Silverman RB. Bioorg Med Chem Lett. 2010;20:6258–6261. doi: 10.1016/j.bmcl.2010.08.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue F, Huang J, Ji H, Fang J, Li H, Martásek P, Roman LJ, Poulos TL, Silverman RB. Bioorg Med Chem. 2010;18:6526–6537. doi: 10.1016/j.bmc.2010.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue F, Silverman RB. Tetrahedron Lett. 2010;51(18):2536–2538. doi: 10.1016/j.tetlet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furuta K, Wang GX, Minami T, Nishizawa M, Ito S, Suzuki M. Tetrahedron Lett. 2004;45:3933–3936. [Google Scholar]
- 18.Chao J, Israiel M, Zheng J, Aki C. Tetrahedron Lett. 2007;48:791–794. [Google Scholar]
- 19.Chappie TA, Humphrey JM, Allen MP, Estep KG, Fox CB, Lebel LA, Liras S, Marr ES, Menniti FS, Pandit J, Schmidt CJ, Tu M, Williams RD, Yang FV. J Med Chem. 2007;50:182–185. doi: 10.1021/jm060653b. [DOI] [PubMed] [Google Scholar]
- 20.Lepore SD, He Y. J Org Chem. 2003;68:8261–8263. doi: 10.1021/jo0345751. [DOI] [PubMed] [Google Scholar]
- 21.Zong K, Groenendaal L, Reynolds JR. Tetrahedron Lett. 2006;47:3521–3523. [Google Scholar]
- 22.Manivel P, Rai NP, Jayashankara PV, Arunachalam PN. Tetrahedron Lett. 2007;48:2701–2705. [Google Scholar]
- 23.Richter LS, Gadek TR. Tetrahedron Lett. 1994;35:4705–4706. [Google Scholar]
- 24.Wipf P, Weiner WS. J Org Chem. 1999;64:5321–5324. doi: 10.1021/jo990352s. [DOI] [PubMed] [Google Scholar]
- 25.Lizaraburu ME, Shuttleworth SJ. Tetrahedron Lett. 2002;43:2157–2159. [Google Scholar]
- 26.Dagoneau C, Denis JN, Vallée Y. Synthesis. 1999;(5):602–604. [Google Scholar]
- 27.Smith GV, Notheisz F. Hydrogenolysis. Chapter 4. Academic Press; San Diego, CA: 1999. Heterogeneous Catalysis in Organic Chemistry; pp. 119–218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.