SUMMARY
Interactions between the HDACI inhibitor belinostat and the proteasome inhibitor bortezomib were investigated in AML and ALL cells. Co-administration of sub-micromolar concentrations of belinostat with low nanomolar concentrations of bortezomib sharply increased apoptosis in both AML and ALL cell lines and primary blasts. Synergistic interactions were associated with interruption of both canonical and non-canonical NF-κB signaling pathways, e.g., accumulation of the phosphorylated (S32/S36) form of IκBα, diminished belinostat-mediated RelA/p65 hyperacetylation (K310), and reduced processing of p100 into p52. These events were accompanied by downregulation of NF-κB-dependent pro-survival proteins (e.g., XIAP, Bcl-xL). Moreover, belinostat/bortezomib co-exposure induced up-regulation of the BH3-only prodeath protein Bim. Significantly, shRNA knock-down of Bim substantially reduced the lethality of belinostat/bortezomib regimens. Administration of belinostat ± bortezomib also induced hyperacetylation (K40) of α-tubulin, indicating HDAC6 inhibition. Finally, in contrast to the pronounced lethality of belinostat/bortezomib toward primary leukemia blasts, equivalent treatment was relatively non-toxic to normal CD34+ cells. Together, these findings indicate that belinostat and bortezomib interact synergistically in both cultured and primary AML and ALL cells, and raise the possibilities that up-regulation of Bim and interference with NF-κB pathways contribute to this phenomenon. They also suggest that combined belinostat/bortezomib regimens warrant further attention in acute leukemias.
Keywords: AML, ALL, belinostat, bortezomib, NF-κB, Bim
INTRODUCTION
Histone deacetylase inhibitors (HDACIs) are prototypical epigenetic agents that alter chromatin structure and gene expression by modulating the reciprocal acetylation of lysine residues within histone tails by HDACs and histone acetyltransferases (HATs). In general, HDACIs induce histone acetylation and a more open chromatin structure conducive to the expression of differentiation- and death-associated genes 1. HDACIs preferentially induce cell death in transformed cells compared to their normal counterparts 2. HDACIs display differential specificities toward classes of HDACs or individual HDACs. For example, certain HDACIs such as fatty acids (sodium butyrate), benzamides (MS-275), or romidepsin (depsipeptide/FK288) primarily inhibit class I HDACs (e.g., HDAC 1, 2, and 3), while some HDACIs such as tubacin specifically target class II HDACs (e.g., HDAC6), 3. In this context, pan-HDACIs such as the hydroxamates (e.g., SAHA/vorinostat, LBH-589/panobinostat, PXD-101/belinostat) inhibit both class I and II HDACs 4. Notably, the pan-HDACI vorinostat has been approved for use in CTCL 5, and HDACIs have shown evidence of single agent activity in AML 6, 7
The mechanism(s) by which HDACIs kill leukemia cells remains uncertain. Diverse actions have been implicated including induction of oxidative injury, up-regulation of death receptors and pro-apoptotic proteins (e.g., Bim), and down-regulation of anti-apoptotic proteins 8. Because HDACs also mediate deacetylation of numerous non-histone proteins in addition to histones, exposure to HDACIs leads to hyperacetylation of diverse proteins, including chaperone proteins (e.g. Hsp90) and transcription factors (e.g. RelA/p65) 9. Notably, acetylation of RelA/p65 on lysine residues within the transactivation domain (e.g., K310) enhances the transcriptional activity of NF-κB 10. We have previously shown that pharmacologic IKK inhibitors diminish RelA/p65 acetylation and nuclear localization and in human leukemia cells exposed to HDACIs, resulting in a dramatic increase in lethality 11. Such findings raise the possibility that other agents capable of sparing IκBα from proteasomal degradation (e.g., proteasome inhibitors; PIs) might act similarly.
The boronic anhydride proteasome antagonist bortezomib (Velcade) is a reversible inhibitor of the 26S proteasome that has been approved for the treatment of refractory multiple myeloma and mantle cell lymphoma 12. The basis for bortezomib lethality is also uncertain, but may involve inhibition of IκBα proteasomal degradation and NF-κB activation 13, among numerous other possible mechanisms. Notably, PIs, like HDACIs, have also been shown to target transformed cells preferentially 14. In preclinical studies, PIs such as bortezomib, as a single agent, induce apoptosis in human acute myeloid leukemia (AML) cells at low nM concentrations 15 and have also been shown to target leukemia stem cells 16. However, while bortezomib has shown only modest activity as monotherapy in AML, suggestions of improved anti-leukemic efficacy when combined with established cytotoxic therapy have been reported 17. Significantly, in vivo administration of bortezomib inhibits NF-κB activity in leukemic blasts 18 and attenuates 20S proteasome activity e.g., to ~50% at 24 hr in leukemic cells 19.
There is abundant evidence that HDACIs and PIs interact to induce cell death in human malignant cells, including those of hematopoietic origin. For example, we and other groups have reported that HDACIs, including belinostat, interact synergistically with proteasome inhibitors (e.g., bortezomib) in human multiple myeloma 20–22, CLL 23, and lymphoma cells 24, 25. Synergistic interactions have also been observed in Bcr/abl+ human leukemia cells 26. Moreover, several mechanisms have been invoked to explain this synergism. For example, PIs may mimic the actions of IKK inhibitors by sparing IκBα from proteasomal degradation, thereby preventing HDACI-mediated RelA/p65 acetylation and activation, leading to enhanced cell death through potentiation of oxidative injury 11, 22. On the other hand, HDACIs inhibit the microtubuleassociated deacetylase HDAC6, and thereby preventing recruitment of misfolded cargo proteins to dynein motors, resulting in aggresome dysfunction and cell death 27. Finally, combined HDAC and proteasome inhibition may result in accumulation of misfolded proteins, leading to ER stress-related lethality 28.
Currently, available evidence concerning synergism between belinostat and bortezomib has largely involved indolent B- and T-cell malignancies 20, 23, 24. In contrast, interactions between these agents in acute myeloid and lymphoid leukemia (ALL) cells have not yet been explored. Here we report that co-administration of bortezomib and belinostat at very low (nM) concentrations induces apoptosis in both cultured and primary AML and ALL (including T-cell and B-cell ALL) cells in a highly synergistic manner. These events are associated with inhibition of both the canonical and non-canonical NF-κB pathways and down-regulation of NF-κB-dependent anti-apoptotic proteins. Furthermore, the present findings indicate that up-regulation of Bim (and particularly the BimEL isoform) plays a significant functional role in interactions between these agents in both AML and ALL cells. Together, these findings provide a rationale for exploring strategies combining bortezomib and belinostat, in treatment of AML, T-cell ALL, and B-cell ALL.
MATERIALS AND METHODS
Human acute leukemia cell lines
Human AML (U937, AML, M4-M5 with t[10;11][p13;q14]), acute promyelocytic leukemia/APL (HL-60, M2 with loss and several rearrangements involving chromosome 5), AML bearing the FLT3-ITD mutation (MV4-11, biphenotypic B-AML), and T-cell ALL (Jurkat) cell lines were purchased from ATCC (American Type Culture Collection, Rockville, MD) and maintained in RPMI medium containing 10% FBS as previously described 11. The human B-cell ALL (SEM, pre-B cell with chromosomal translocation t[4;11][q21;q23]) cell line was obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and cultured in Iscove’s MDM (IMDM) medium containing 10% FBS. All experiments were performed utilizing logarithmically growing cells (3–6 × 105 cells/ml).
Primary AML and ALL blasts
Bone marrow (BM) or peripheral blood (PB) samples were obtained with informed consent, in accordance with the Declaration of Helsinki, from patients with histologically documented AML or ALL undergoing routine diagnostic procedures with approval from the institutional review board of Virginia Commonwealth University. AML samples were FAB sub-type M2; ALL samples were L3 and Ph1 chromosome-negative. Mononuclear cells were isolated by centrifugation at 400g for 30 min over Histopaque-1077 (Sigma Diagnostics, St. Louis, MO). Viability of the cells was regularly > 95% by trypan blue exclusion; all samples consisted of > 70% blasts. Cells were incubated in RPMI 1640 medium containing 10% FBS, and all experiments were performed at density of 1 × 106 cells/ml as described previously 29. The experiments generally involved a 24 hr-treatment interval to minimize spontaneous cell death (e.g., < 10%). Normal human cord blood (CB) CD34+ cells were isolated as previously described 30.
Reagents
The proteasome inhibitor bortezomib and the pan-HDAC inhibitor belinostat were provided by Millennium (Cambridge, MA) and TopoTarget (Copenhagen, Denmark), respectively. These agents were dissolved in DMSO, and stored at −20°C. Stock solutions of these agents were subsequently diluted with serum-free RPMI medium prior to use. In all experiments, the final concentration of D MSO did not exceed 0.1%.
RNA interference with short hair-pin shRNA (shRNA)
SureSilencing shRNA plasmids (neomycin resistance) were purchased from SABioscience (Frederick, MD), including shBim (human BCL2L11, GAGACGAGTTTAACGCTTACT) and negative control shRNA (shNC, GGAATCTCATTCGATGCATAC) 29. U937 and Jurkat were stably transfected with these constructs using the Amaxa Nucleofector device with Cell Line Specific Nucleofector Kits (Amaxa GmbH, Cologne, Germany) as per the manufacturer’s instructions, and clones with down-regulated Bim expression, detected by Western blot, were continuously cultured under selection with G418 (U937, 400 μg/ml; Jurkat, 800 μg/ml).
Assessment of cell death
The extent of apoptosis was evaluated by flow cytometric analysis utilizing annexin V-FITC/PI or DiOC6/7-AAD staining as described previously 29. Briefly, 1×106 cells were stained with annexin V-FITC (BD PharMingen) and 5 μg/ml propidium iodide (PI; Sigma) in 1x binding buffer for 15 minutes at room temperature in the dark. Samples were then analyzed by flow cytometry within 1 hr to determine the percentage of cells displaying annexin V positivity. In some cases, mitochondrial injury and cell death was assessed by double staining with 40nM 3,3-dihexyloxacarbocyanine (DiOC6; Molecular Probes Inc., Eugene, OR) and 0.5μg/ml 7-AAD (Sigma) in PBS at 37°C for 20 min, and then analyzed using Becton-Dickinson FACScan (Becton-Dickinson, San Jose, CA). For primary samples, apoptosis was also examined by assessing Wright-Giemsa stained cytospin slides under light microscopy (Olympus BX40, with an objective lens Plan 40x/0.65; Olympus America Inc, Center Valley, PA). The images were acquired by a CE digital camera using RS Image Version 1.7.3. software (Roper Scientific Photometrics, Tucson, AZ).
Western blot analysis
Samples from whole-cell lysates were prepared, and 30 μg of protein per condition were subjected to Western blot analysis as previously described in detail 31. The blots were probed with the appropriate dilution of primary antibody as below. Where indicated, blots were reprobed with anti-β-actin (Sigma) or anti-α-tubulin (Oncogene Inc., San Diego, CA) to ensure equal loading and transfer of proteins. The primary antibodies included: anti-acetylated (acetyl K310) p65 (Abcam, Cambridge, MA); anti-phospho-IκBα (S32/36), anti-p100/p52, anti-Bcl-xL, anti-cleaved PARP (Cell Signaling, Beverly, MA); anti-p65 (Santa Cruz Biotech, Santa Cruz, CA); anti-survivin (R&D Systems, Minneapolis, MN); anti-PARP (Biomol, Plymouth Meeting, PA); anti-acetylated α-tubulin (K40; Sigma); anti-XIAP (BD Transduction Lab, San Diego, CA); anti-Bim (Calbiochem, San Diego, CA).
NF-κB activity analysis
U937 cells were stably transfected with NF-κB TransLucent Reporter Vector (NF-κB/Luc; Panomics, Redwood City, CA). Luciferase assays were performed using a Luciferase Reporter Assay Kit (BD Clontech, Polo Alto, CA). Relative luciferase activities were normalized to total protein. RelA-specific DNA binding activity was measured by using Nuclear Extract Kit and TransAM™ NF-κB p65 Chemi Kit (Active Motif, Carlsbad, CA). For both assays, NF-κB activity was expressed as the fold-increase relative to untreated controls.
Statistical analysis
For analysis of cell death and NF-κB activity, values represent the means ± SD for at least three separate experiments performed in triplicate (for cultured cell lines) or at least one experiment depending upon cell availability (for primary samples). The significance of differences between experimental variables was determined using the Student’s t-test. Analysis of synergism was performed according to Median Dose Effect analysis using the software program Calcusyn (Biosoft, Ferguson, MO) 32.
RESULTS
Belinostat interacts synergistically with bortezomib in various human acute leukemia cells
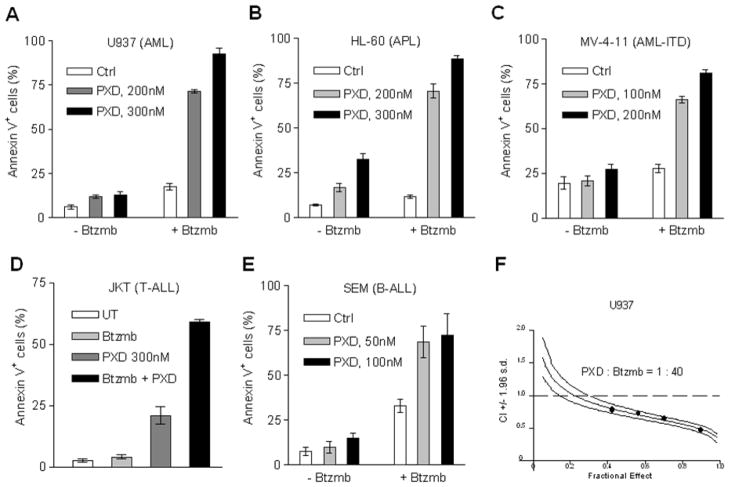
To determine whether synergism between bortezomib and belinostat occurred in acute myeloid and lymphoid leukemia cells, as previously observed in indolent B-cell malignancies 20, 23, various leukemia cell lines, including U937/AML (Fig 1A), HL-60/APL (Fig 1B), MV-4-11/AML bearing the FLT3-ITD mutation (Fig 1C), Jurkat/T-cell ALL (Fig 1D), and SEM/B-cell ALL cells (Fig 1E), were exposed (24 hr) to sub-nanomolar concentrations (50–300 nM) of belinostat in the presence or absence of low concentrations (3–5 nM) of bortezomib, after which apoptosis was monitored by annexin V/PI staining and flow cytometry. In all cases, single agent administration was associated with minimal to modest toxicity, whereas combined exposure resulted in a pronounced increase in apoptosis in each leukemia cell type. Median Dose Effect analysis of apoptosis in U937 cells exposed to belinostat and bortezomib administered at a fixed concentration ratio (40:1) yielded CI values < 1.0, corresponding to a synergistic interaction (Fig 1F). Similar results were obtained with the other leukemia cell types (data not shown). Alternative schedules (e.g., bortezomib prior to or following belinostat did not result in greater cell killing compared to simultaneous treatment, data not shown). These findings indicate that very low nanomolar concentrations of bortezomib markedly potentiate the lethality of sub-micromolar concentrations of belinostat in diverse acute myeloid and lymphoid leukemia cell types.
Figure 1. Bortezomib synergistically potentiates belinostat lethality in multiple AML and ALL cell lines.
(A-E) Logarithmically-growing U937 (A), HL-60 (B), MV-4-11 (C), Jurkat (D), and SEM (E) cells were exposed (24 hr) to the indicated concentrations of belinostat (PXD) ± bortezomib (SEM, 3 nM; other lines, 5 nM), after which the percentage of apoptotic cells was determined by annexin V/PI staining and flow cytometry. The results represent the means ± S.D. for experiments performed in triplicate on three separate occasions. AML cell lines were exposed (24 hr) to PTL (U937 and HL-60, 5 μM and 7.5 μM; MV-4-11, 5 μM; NB4, 3 μM) ± vorinostat (V, U937, 1.5 μM; HL-60 and NB4, 1 μM; MV-4-11, 0.5μM) or LBH589 (L, U937, 15 nM; NB4, 7.5 nM; HL-60, 5 nM; MV-4-11, 3 nM). After drug treatment, apoptosis was monitored by annexin V staining and flow cytometry. (F) Median Dose Effect analysis was employed to characterize interactions between belinostat and bortezomib in U937 cells, administered over a range of concentrations at a fixed ratio (40:1) for 24 hr, reflected by apoptosis induction (annexin V staining). Combination Index (CI) values < 1.0 correspond to a synergistic interaction.
Co-administration of bortezomib and belinostat interrupts NF-κB signaling pathways, and attenuates HDAC6-mediated α-tubulin acetylation in human acute leukemia cells
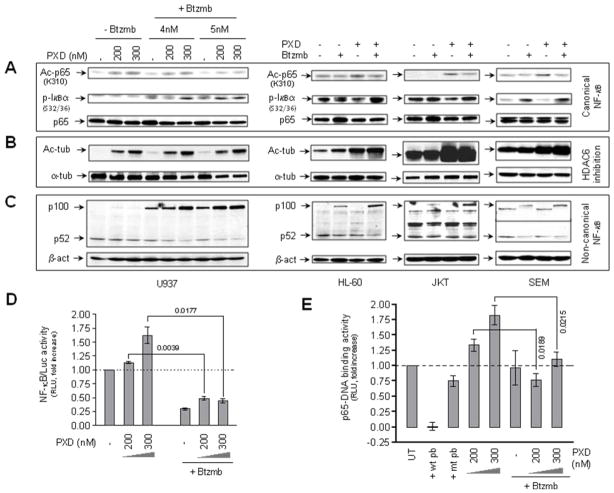
In light of previous findings that HDACIs (e.g., vorinostat and romidepsin) induce NF-κB activation via promoting RelA/p65 acetylation in human leukemia cells 23, 33, the canonical NF-κB singaling pathway was then examined in human acute myeloid and lymphoid leukemia cell lines treated with belinostat ± bortezomib. As shown in Fig 2A, exposure of U937, HL-60, Jurkat, or SEM cells to belinostat (100–300 nM) clearly increased K310 acetylation of RelA/p65, possibly through inhibition of the nuclear class I HDAC3 34. This effect was discernibly attenuated by co-administration of bortezomib (3–5 nM, Fig 2A). In accord with these findings, co-administration of bortezomib resulted in increased expression of the S32/36 phosphorylated form of IκBα (Fig 2A), an NF-κB inhibitory protein that sequesters RelA/p65 in cytoplasm, presumably due to blockade of proteasome-mediated IκBα degradation following S32/36 phosphorylation 35.. On the other hand, consistent with the reported effects of other pan-HDACIs 21, exposure of U937, HL-60, Jurkat, and SEM cells to belinostat also resulted in a marked increase in K40 acetylation of α-tubulin (Fig 2B), indicating inhibition of the cytoplamic class II HDAC6 36. However, in contrast to attenuation of belinostat-mediated RelA/p65 K310 acetylation, co-administration of bortezomib did not affect α-tubulin K40 acetylation in human acute leukemia cells (Fig 2B). Moreover, because proteasome function may also be involved in regulation of the non-canonical NF-κB signaling pathway 37, effects of bortezomib on processing of the precursor p100 (NF-κB2) into the active form p52, a hallmark of activation of this pathway 38, were also examined in acute leukemia cells exposed to belinostat. As shown in Fig 2C, although changes varied in diverse cell types, treatment with bortezomib alone resulted in increased levels of p100 in U937, HL-60, Jurkat, and SEM cells, accompanied by a slight but discernible reduction in p52 levels. Notably, these events (particularly p100 accumulation) were clearly enhanced by co-administration of belinostat (Fig 2C). Finally, a RelA/p65-DNA binding assay and an NF-κB luciferase reporter assay were employed to determine whether co-administration of bortezomib affects transcriptional activity of NF-κB in human acute leukemia cells exposed to belinostat. As shown in Fig 2D and 2E, whereas exposure to belinostat increased activity of both RelA/p65-DNA binding and NF-κB luciferase reporter in U937 cells, co-administration of bortezomib significantly abrogated these events. Together, these findings suggest that bortezomib attenuates both canonical and non-canonical NF-κB signaling pathways in acute myeloid and lymphoid leukemia cells exposed to belinostat.
Figure 2. Exposure to belinostat ± bortezomib results in α-tubulin hyperacetylation, and inhibition of both canonical and non-canonical NF-κB pathways in AML and ALL cell lines.
(A-C) U937, HL-60, Jurkat, and SEM cells were exposed (24 hr) to belinostat (U937, 200 nM and 300 nM; HL-60 and Jurkat, 300 nM; SEM, 100 nM) ± bortezomib (U937, 4 nM and 5 nM; HL-60 and Jurkat, 5 nM; SEM, 4 nM), after which cells were lysed and subjected to Western blot analysis to assess total and lysine 310 acetylated RelA/p65, serine 32/36 phosphorylated form of IκBα (A), total and lysine 40 acetylated α-tubulin (B), and expression of the precursor p100 and its active form p52 (C). Each lane was loaded with 30 μg of protein; blots were subsequently stripped and reprobed for expression β-actin to ensure equivalent loading and transfer of protein. Results of a representative experiment are shown; two additional studies yielded equivalent results. (D) U937 cells were stably transfected with NF-κB luciferase reporter as described in “Materials and Methods”. Cells were exposed to 200 nM or 300 nM belinostat with or without 5 nM bortezomib for 24 hr, after which cells were lysed and subjected to luciferase activity analysis. Relative luciferase activity was determined following normalization of values to total protein. NF-κB activity (reflected by RLU, relative light unit) was expressed as the fold-increase relative to values for untreated controls. (E) U937 cells were exposed to 200 nM or 300 nM belinostat in the presence or absence of 5 nM bortezomib, after which nuclear extracts were prepared and subjected to a RelA/p65-specific NF-κB-DNA binding assay as described in “Materials and Methods”. Wild-type (wt) and mutated (mt) consensus oligonucleotides were used as competitors for RelA/p65-DNA binding in order to demonstrate assay specificity. RelA/p65-DNA binding activity (reflected by relative light unit/RLU) was expressed as fold increase relative to untreated controls. For panels 2D and 2E, results represent the means ± S.D. for triplicate determinations performed on three separate occasions. Values shown represent P values for the significance of differences between cells exposed to belinostat alone versus those exposed to belinostat and bortezomib.
Co-administration of bortezomib and belinostat leads to down-regulation of NF-κB-dependent anti-apoptotic proteins in human acute leukemia cells
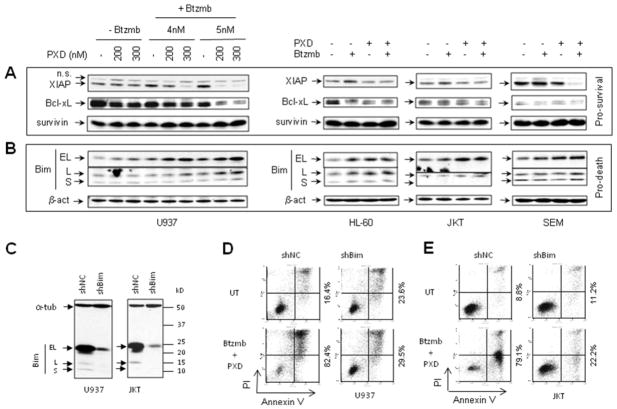
Previous studies have demonstrated that inhibition of HDACI-induced NF-κB activation (e.g., by pharmacologic IKK inhibitors) down-regulates multiple NF-κB-dependent antiapoptotic proteins such as Bcl-xL and XIAP in human leukemia cells exposed to HDACIs 33. To determine whether similar effects occurred when belinostat-induced NF-κB activation was inhibited by bortezomib in acute myeloid and lymphoid leukemia cells (Fig 2A and 2B), Western blot analysis was performed to monitor expression of established NF-κB-dependent anti-apoptotic proteins. As shown in Fig 3A, although results revealed variable changes in diverse cell types, co-administration of belinostat with bortezomib clearly resulted in down-regulation of XIAP, and to a lesser extent,, Bcl-xL in U937, HL-60, Jurkat, and SEM cells. In contrast, combined treatment did not down-regulate survivin, another anti-apoptotic protein, in any cell type.
Figure 3. Treatment with belinostat ± bortezomib down-regulates NF-κB-dependent pro-survival proteins, while up-regulates the pro-death protein Bim in AML and ALL cell lines.
(A-B) U937, HL-60, Jurkat, and SEM cells were exposed (24 hr) to belinostat ± bortezomib administered at the same concentrations as those described in Fig 2. Cells were then lysed and subjected to Western blot analysis to monitor expression of the anti-apoptotic proteins XIAP, Bcl-xL, and survivin (A), as well as the pro-apoptotic Bcl-2 family protein Bim, including three isoforms (BimEL, BimL, and BimS). Each lane was loaded with 30 μg of protein; blots were subsequently stripped and re-probed for expression of β-actin to ensure equivalent loading and transfer. Representative results are shown; two additional experiments yielded equivalent findings. (C) U937 and Jurkat cells were stably transfected with shRNA directed against Bim (shBim) or scrambled sequence controls (shNC). Western blot analysis demonstrates downregulation of the three Bim isoforms. Each lane was loaded with 30 μg of protein. In parallel, the same membrane was blotted for α-tubulin as a loading control. (D-E) U937 (D) and Jurkat (E) cells transfected with shBim or shNC were exposed (24 hr) to 300 nM belinostat + 5 nM bortezomib, after which cell death was monitored by annexin V/PI analysis by flow cytometry. Values shown represent the percentage of cells in the right quadrants (lower, annexin V+/PI−, and upper, annexin V+/PI+). Two additional experiments yielded equivalent results.
Co-administration of bortezomib and belinostat up-regulates the pro-apoptotic protein Bim, which plays a functional role in lethality of this regimen
It has been previously shown that HDACIs induce up-regulation of Bim, a BH3-only pro-apoptotic protein of the Bcl-2 family, in transformed cells 39, and that this phenomenon contributes to synergistic interactions between HDACIs and other targeted agents in human leukemia cells 29, 40. Studies were therefore undertaken to determine whether a similar mechanism might be involved in synergism between belinostat and bortezomib in human acute leukemia cells. As shown in Fig 3B, exposure of U937, HL-60, Jurkat, and SEM cells to belinostat, particularly in the presence of bortezomib, resulted in an increase in expression of Bim, including the BimEL isoform, and in less content, the BimL isoform in each cell type. To determine the functional role of Bim upregulation in belinostat/bortezomib lethality, U937 and Jurkat cells were stably transfected with a construct encoding human Bim shRNA. As shown in Fig 3C, these cells exhibited a marked decrease in expression of BimEL and BimL, compared to scrambled sequence controls. Notably, both U937 (Fig 3D) and Jurkat (Fig 3E) cells with shRNA knockdown of Bim displayed a pronounced reduction in apoptosis following bortezomib/belinostat co-treatment. These findings suggest that Bim up-regulation plays a significant functional role in the lethality of the bortezomib/belinostat regimen toward human acute myeloid and lymphoid leukemia cells.
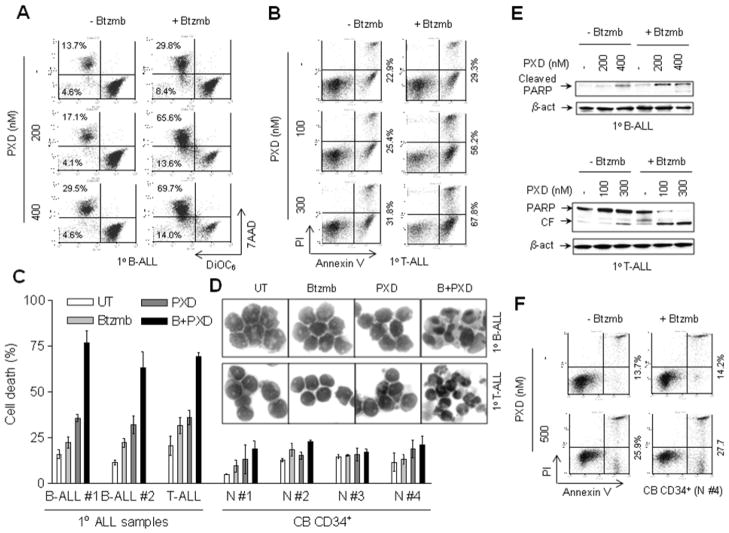
Bortezomib markedly promotes belinostat lethality in primary AML and ALL cells while sparing normal hematopoietic cells
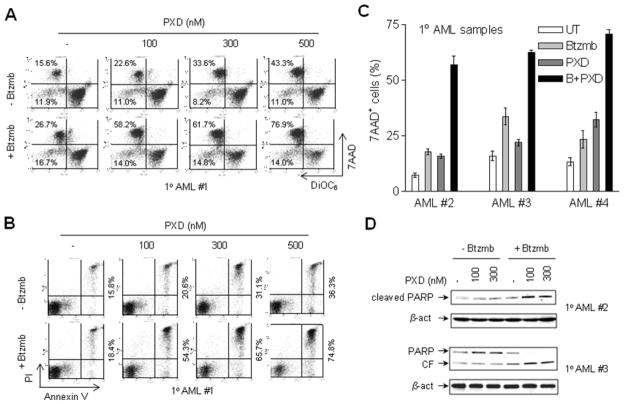
To determine whether the regimen combining bortezomib and belinostat is active against primary human acute myeloid and lymphoid leukemia cells, parallel studies were performed in multiple primary blast samples from patients with AML, B-cell ALL, T-cell ALL, as well as in normal cord blood (CB) CD34+ cells. First, blasts from patients with AML were exposed (24 hr) to a series of concentrations of belinostat (100–500 nM) in the presence or absence of bortezomib (range: 5–10 nM), and induction of apoptosis monitored by double staining with either 7-AAD/DiOC6 and/or annexin V/PI. Representative data illustrating flow cytometric analyses for one patient (patient #1) are shown in Fig 4A. While administration of bortezomib alone had only modest effects on mitochondrial integrity (manifested by low DiOC6 uptake/leftward shift in the histograms, reflecting loss of Δψm) or cell death (manifested by increased 7-AAD uptake, upward shift in the histograms), treatment with 100–500 nM belinostat alone exerted a modest and dose-dependent increase in cell death. Notably, belinostat lethality was sharply increased in the presence of 5 nM bortezomib (e.g., net increases in DiOC6−/7AAD+ cells over untreated control cells of 43 – 61% for combined treatment versus 7.0 – 27.7% for treatment with 100–500 nM belinostat alone). Assessment of apoptosis by annexin V/PI analysis revealed analogous results (e.g., net increases over controls of annexin V+/PI− and V+/PI+ cells of 39 – 59% for combination treatment versus 5 – 21% for 100–500 nM belinostat alone respectively, Fig 4B). Similar phenomena were observed in three additional primary AML blast samples, and representative quantitative cell death data (i.e., 7-AAD+ cells) are shown in Fig 4C. Moreover, these findings were accompanied by concordant increases in PARP cleavage in the two primary AML blast samples assayed (Fig 4D). In addition, the pronounced increase in lethality of the combination regimen toward primary AML cells was also confirmed by evaluating Wright Giemsa-stained cytospin slides under light microscopy, which revealed a marked increase in cells displaying classical apoptotic morphology following belinostat/bortezomib exposure (Supplemental Fig S1, upper panels).
Figure 4. Bortezomib increases belinostat lethality in primary AML blasts.
(A-B) Primary blasts from a patient (#1) with AML (sub-type M2) were exposed (24 hr) to 100–500 nM belinostat in the presence or absence of 5 nM bortezomib, after which uptake of 7-AAD/DiOC6 (A) or annexin V/PI (B) were monitored by flow cytometry. For panel 4A, values refer to the percentage of cells in the upper (high 7-AAD uptake) or left (low DiOC6 uptake, reflecting loss of mitochondrial membrane potential or Δψm) quadrants. For panel 4B, values refer to the percentage of annexin V-positive (right quadrant) or PI-positive (upper quadrant) cells. (C) Blast samples from three additional AML patients were exposed to 300 nM belinostat ± bortezomib (#2 and #4, 5 nM; #3, 8 nM), after which 7-AAD uptake was monitored as described in panel 4A. Values represent the means ± S.D. for triplicate determinations. (D) Following treatment as described in panel C, blasts from two patients (#2 and #3) were lysed, and Western blot analysis performed to monitor PARP cleavage. CF = cleaved fragment. Each lane was loaded with 30 μg of protein; blots were subsequently stripped and re-probed for expression of β-actin to ensure equivalent loading and transfer.
Parallel studies were performed in primary blasts from patients with B-cell or T-cell ALL. As shown in Fig 5A and 5B, exposure of primary B-ALL and T-ALL cells to the combination of belinostat and bortezomib resulted in a sharp increase in DiOC6−/7AAD+ cells (B-ALL, Fig 5A; T-ALL, Supplemental Fig S2) and/or in annexin V+/PI− and/PI+ cells (T-ALL, Fig 5B) respectively. Quantitation of cell death (7-AAD+ cells) in primary blasts of B-cell (n = 2) and T-cell (n = 1) ALL demonstrated a striking increase in lethality with combined belinostat/bortezomib treatment, compared to the effects of each agent administrated alone (Fig 5C). Notably, Wright-Giemsa staining revealed classical features of apoptosis in both B-ALL and T-ALL blasts co-exposed to belinostat/bortezomib (Fig 5D, 60x magnification; Supplemental Fig S1, middle panels, 40x magnification), consistent with the marked increase in apoptosis detected by flow cytometry. Moreover, these results were further confirmed by a clear increase in PARP cleavage in primary B-ALL and T-ALL blasts (Fig 5E).
Figure 5. Combined treatment with bortezomib and belinostat markedly induces apoptosis in primary T- and B-cell ALL cells.
(A-B) Blasts from patients with B-cell ALL (A) or T-cell ALL (B) were exposed (24 hr) to the indicated concentrations of belinostat ± 5 nM bortezomib, after which uptake of 7-AAD/DiOC6 (A) or annexin V/PI (B) were determined by flow cytometry as described in Fig 4. (C) Three primary ALL (B-cell ALL = 2, and T-cell ALL = 1) samples and four cord blood (CB) CD34+ cell samples were exposed (24 hr) to belinostat (B-ALL, 400 nM; T-ALL, 300 nM; CB CD34+, 500 nM) ± 5 nM bortezomib, after which cell death (7AAD+ cells) was determined by flow cytometry as described in above. Values represent the means ± S.D. for triplicate determinations. (D) Representative photomicrographs of Wright-Giemsa stained cytospin slides for primary B- and one T-cell ALL specimens viewed under oil at 60x magnification. (E) Following treatment as described in panel 5A and 5B, blasts from B-cell and T-cell ALL patients were lysed, and Western blot analysis performed to monitor PARP cleavage. Each lane was loaded with 30 μg of protein; blots were subsequently stripped and re-probed for expression of β-actin to ensure equivalent loading and transfer. (F) Normal cord blood (CB) CD34+ cells were exposed to 500 nM belinostat ± 5 nM bortezomib, after which annexin V/PI uptake was monitored as in panel 5B.
Finally, to assess the selectivity of the bortezomib/belinostat combination regimen, toxicity toward normal cord blood (CB) CD34+ cells was examined following exposure to 500 nM belinostat, the highest concentration employed in the present studies. Interestingly, in contrast to the pronounced potentiation in cell killing in primary AML and ALL blasts by the combined treatment, co-administration of bortezomib (5 nM) failed to increase the lethality of belinostat in normal CD34+ cells, reflected by a minimal increase in annexin V+/PI− and annexin V+/PI+ cells (Fig 5C). Quantitation of cell death (7-AAD+ cells) demonstrated little or no toxicity of identical treatments toward 4 separate normal CB CD34+ cell samples (Fig 5C). In addition, there were no apoptotic feature observed in Wright-Giemsa stained CB CD34+ cells (Supplemental Fig S1, lower panels). Together, these findings argue that a strategy combining low concentrations of bortezomib and belinostat is highly active against primary human acute leukemia blasts, including AML, B-cell ALL, and T-cell ALL, while relatively sparing toward normal CD34+ cells.
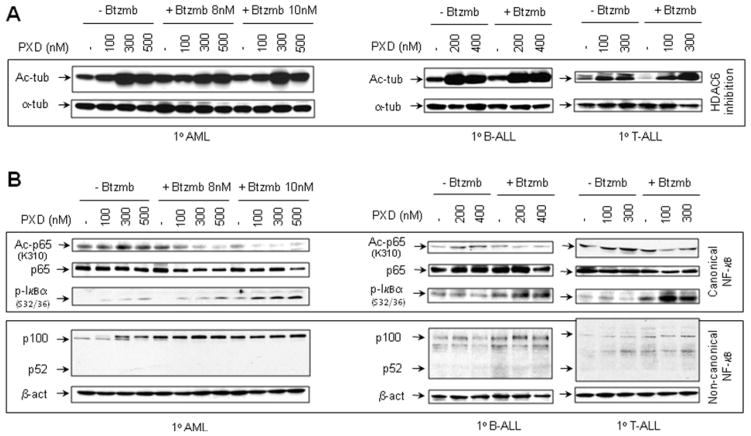
Effects of bortezomib and belinostat on α-tubulin acetylation and NF-κB signaling proteins and in primary acute leukemia cells
To determine whether the preceding effects observed in leukemia cell lines could be extended to primary acute leukemia cells, blasts from patients with AML, B-cell ALL, and T-cells ALL were exposed (24 hr) to 100–500 nM belinostat in the presence or absence of bortezomib. As observed in leukemia cell lines, exposure of primary leukemia blasts to belinostat with or without bortezomib resulted in a pronounced increase in α-tubulin K40 acetylation (Fig 6A). Notably, in primary leukemia samples, bortezomib also diminished RelA/p65 K310 acetylation, associated with increased accumulation of the S32/36 phosphorylated form of IκBα in belinostat-treated cells (Fig 6B; upper panels). Moreover, coadministration of bortezomib with belinostat resulted in accumulation of the precursor p100 in primary AML blasts, and to a lesser extent, B-cell and T-cell ALL, although expression of the active form p52 was not detectable in these primary leukemia samples (Fig 6B; lower panels). Finally, similar results were obtained in another primary AML blast sample (Supplemental Fig S3A and B),
Figure 6. Co-exposure to belinostat and bortezomib induces α-tubulin hyperacetylation, and interrupts both canonical and non-canonical NF-κB signaling in primary AML and ALL blasts.
(A-B) Blasts from patients with AML, B-cell ALL, or T-cell ALL were exposed (24 hr) to belinostat (AML, 100, 300 and 500 nM; B-ALL, 200 and 400 nM; T-ALL, 100 and 300 nM) ± bortezomib (AML, 8 and 10nM; B-ALL and T-ALL, 5 nM), after which cells were lysed and subjected to Western blot analysis to assess acetylation of α-tubulin (lysine 40, A) and RelA/p65 (lysine 310), phosphorylation of IκBα (serine 32/36), and processing of p100 to p52 (B). Levels of total p65 and α-tubulin are shown as controls. Each lane was loaded with 30 μg of protein; blots were subsequently stripped and reprobed for β-actin to ensure equivalent loading and transfer of protein. Duplicate experiments yielded equivalent results.
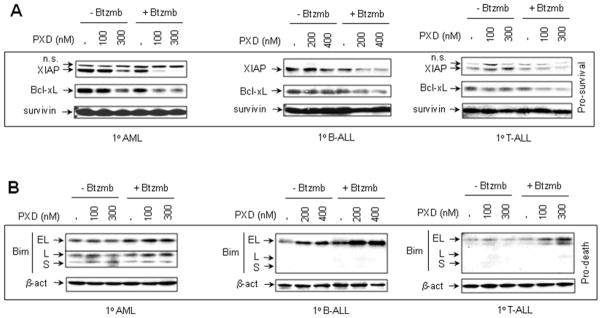
Effects of bortezomib and belinostat on expression of NF-κB-dependent anti-apoptotic proteins and the pro-apoptotic protein Bim in primary acute leukemia cells
To determine whether effects of belinostat and bortezomib on expression of the NF-κB-dependent anti-apoptotic proteins and the BH3-only pro-apoptotic protein Bim, observed in human leukemia cell lines, would be recapitulated in primary AML and ALL cells, Western blot analysis was performed to detect XIAP, Bcl-xL, survivin, and Bim. As shown in Fig 7A, co-exposure (24 hr) to 100–400 nM belinostat in combination with bortezomib (AML, 8 nM; B-cell and T-cell ALL, 5 nM) also resulted in down-regulation of XIAP and Bcl-xL in these primary leukemia samples. Similar changes were observed in another AML specimen (Supplemental Fig 3C). Analogous to results obtained in leukemia cell lines, co-exposure of primary AML and (B-cell and T-cell) ALL cells to belinostat and bortezomib resulted in a clear increase in expression of Bim, particulary the BimEL isoform (Fig 7B). As in the case of cell lines, changes in expression of survivin were not apparent. Together, these findings indicate that as in the case of continuously cultured cell lines, co-administration of belinostat and bortezomib in primary AML and ALL cells results in a shift from pro-survival to pro-death signaling pathways.
Figure 7. Co-treatment with belinostat and bortezomib leads to down-regulation of NF-κB-dependent proteins XIAP and Bcl-xL, accompanied by up-regulation of Bim in primary AML and ALL blasts.
(A-B) Primary AML, B-ALL, and T-ALL blasts were treated (24 hr) with the indicated concentrations of belinostat ± bortezomib (AML, 8 nM; B-ALL and T-ALL, 5 nM) as described in Fig 6. Following treatment, Western blot analysis was performed to monitor expression levels of the pro-apoptotic proteins XIAP, Bcl-xL, and survivin, as well as the proapoptotic protein Bim (BimEL, BimL, and BimS). Each lane was loaded with 30 μg of protein; blots were subsequently stripped and re-probed for expression of β-actin to ensure equivalent loading and transfer. Duplicate experiments yielded equivalent results.
DISCUSSION
The results of the present study indicate that low, sub-micromolar concentrations of the pan-HDAC inhibitor belinostat interact in a highly synergistic manner with nanomolar concentrations of bortezomib to induce apoptosis in human acute myeloid and lymphoid leukemia cells. Several groups, including our own, have described synergistic interactions between proteasome and HDAC inhibitors, in indolent lymphoid malignancies such as multiple myeloma 21, 22, CLL 23, and non-Hodgkin’s lymphoma cells 24, 25. On the other hand, analogous interactions have been much less extensively explored in the setting of acute myeloid or lymphoid leukemias. In this context, synergistic interactions between bortezomib and vorinostat have been observed in Bcr/abl+ leukemia cells 26 and between the non-peptide proteasome inhibitor NPI-0052 and the HDACI MS-275 or valproic acid in AML and ALL cells 41. In the case of combinations of belinostat and bortezomib, synergistic lethality has been observed in mantle cell lymphoma 24 and multiple myeloma cells 20. However, to the best of our knowledge, interactions between belinostat and bortezomib have not previously been investigated in acute leukemia cells. The present study now demonstrates that the belinostat/bortezomib regimen is highly effective in inducing cell death in acute myeloid and lymphoid leukemia cells, particularly in primary blast specimens.
In contrast to the pronounced lethality of the belinostat/bortezomib combination regimen in primary AML and ALL cells, toxicity toward normal CD34+ hematopoietic cells was significantly less. Notably, proteasome inhibitors have been shown to exert preferential toxicity toward transformed versus normal cells 14. Analogously, HDACIs selectively target neoplastic cells, possibly representing a consequence of up-regulation of antioxidant proteins in normal cells 2, or alternatively, down-regulation of DNA repair proteins in transformed cells 42. The selectivity of the bortezomib/belinostat regimen suggests that similar mechanisms might also be operative when these two classes of agents are combined.
The present findings also suggest that some of the mechanisms invoked to explain interactions between HDAC and proteasome inhibitors (e.g., inhibition of NF-κB signaling pathways, up-regulation of the pro-apoptotic protein Bim) in B-cell malignancies 23, 25 may also be operative in human acute leukemia cell types. There is accumulating evidence that HDACIs activate the NF-κB pathway in transformed cells, and that interruption of this process leads to potentiation of lethality. HDACI-induced activation of NF-κB, at least in human leukemia cells, is likely initiated from HDACI-mediated DNA damage (e.g., double-strand break/DSB) associated with oxidative stress (e.g., ROS generation) through the atypical ATM/NEMO [IKKγ]/SUMOylation pathway 43. This event leads to phosphorylation and nuclear export of NEMO, which forms the IKK complex with other components (e.g., IKKα, IKKβ, and ELKS) in the cytoplasm, resulting in activation of IKKs and then the canonical NF-κB pathway via IKK-mediated IκBα S32/36 phosphorylation, ubiquitination, and proteasomal degradation 44. On the other hand, exposure to HDACIs also results in hyperacetylation of RelA/p65, most likely through prevention of the deacetylation reaction via inhibition of nuclear class I HDACs (e.g., HDAC3). Because RelA/p65 acetylation at several lysine sites (e.g., (e.g., K310, K221) prevents its binding to de novo synthesized IκBα and nuclear export which collectively silence NF-κB signaling (e.g., in the case of TNFα) 45, prevention of RelA/p65 deacetylation by HDACIs leads to enhanced and relatively sustained NF-κB activation. Furthermore, interruption of this process e.g., by pharmacologic IKK inhibitors (e.g., Bay 11-7082 or parthenolide), blocks IκBα S32/36 phosphorylation, ubiquitination, and proteasomal degradation, thereby trapping RelA/p65 in the cytoplasm and prevents HDACI-mediated RelA/p65 acetylation in the nucleus 11. Moreover, analogous phenomenon are observed when leukemia cells are transfected with IκBα superrepressor (mutations of S32/36 that prevents IκBα phosphorylation and degradation). Notably, both approaches result in a significant increase of HDACI lethality, associated with down-regulation of NF-κB-dependent anti-apoptotic proteins (e.g., XIAP, Bcl-xL, SOD2) 11, as well as NF-κB inhibition-related activation of the stress-related SAPK/JNK pathway 11, 46. In this context, proteasome inhibitors such as bortezomib, by directly blocking IκBα degradation 23, may act through a similar mechanism. In the present study, co-administration of bortezomib attenuated belinostat-mediated RelA/p65 K310 acetylation, an event associated with accumulation of the S32/36 phosphorylated form of IκBα, in both continuously cultured cell lines and primary acute leukemia blasts. Moreover, these events were accompanied by inhibition of RelA/p65 binding activity and NF-κB luciferase reporter activity, as well as diminished expression of multiple NF-κB-dependent anti-apoptotic proteins (e.g., XIAP and Bcl-xL). Together, these results support the notion that interruption of the canonical NF-κB pathway may contribute to potentiation of belinostat lethality by bortezomib in acute myeloid and lymphoid leukemia cells.
In addition to contributions to the regulation of the canonical NF-κB pathway, proteasome-mediated mechanisms may also play an important regulatory role in the non-canonical NF-κB pathway via processing of the precursor p100 (NF-κB2) into its active form p52 37. It is noteworthy that co-administration of bortezomib, particularly in the presence of belinostat, resulted in accumulation of p100 and reduced expression of p52, indicating that the bortezomib/belinostat regimen interrupts the non-canonical NF-κB pathway in acute myeloid and lymphoid leukemia cells. The non-canonical pathway has been implicated in the survival of certain malignant B-cells 47, and disorders such as multiple myeloma are characterized by frequent aberrations in genes related to this pathway 48. However, although NF-κB activation via the canonical pathway has impacts on the survival of AML cells 49, including AML-initiating cells 16, as well as certain ALL sub-types (e.g. T-cell leukemia) 50, the functional role of disrupting the non-canonical NF-κB pathway in AML or ALL cells by the belinostat/bortezomib regimen remains to be more clearly defined.
Exposure to belinostat resulted in K40 acetylation of α-tubulin in continuously cultured cell lines of both AML and ALL, as well as in primary leukemic blasts. Lysine hyperacetylation (e.g., K40) of α-tubulin occurs in response to agents that inhibit the class IIb HDAC6, including both HDAC6-specific inhibitors (e.g., tubacin) 3 and pan-HDACIs 3. By inhibiting HDAC6, such agents, when combined with proteasome inhibitors, also disrupt aggresome formation in response to misfolded protein-induced stress by preventing recruitment of misfolded cargo proteins to dynein motors for transport to aggresomes. This leads to amplification of proteotoxic stress, potentially contributing to enhanced lethality of concomitant HDAC/proteasome inhibition in transformed cells e.g., myeloma or leukemia cells 21, 27. Consequently, although co-administration of bortezomib did not enhance belinostat-mediated α-tubulin K40 acetylation (reflecting HDAC6 inhibition), the possibility that this mechanism contributes to belinostat/bortezomib synergism in AML or ALL cells cannot be excluded.
Previous studies have shown that HDACIs induce expression of the BH3-only pro-apoptotic protein Bim, likely through an E2F1-dependent mechanism 39, which has been known to play a key role in activation of apoptotic signaling pathways in response to numerous anti-cancer agents 51. Moreover, this phenomenon contributes to anti-leukemic synergism between HDACIs and other targeted agents e.g., the Bcl-2/Bcl-xL antagonist ABT-737 29, or the dual Bcr/abl and aurora kinase inhibitor MK-0457 52. Furthermore, proteasome inhibition can also lead to accumulation of Bim in malignant cells 53. Thus, up-regulation of Bim by HDACIs may cooperate with bortezomib-mediated inhibition of proteasomal degradation to up-regulate Bim expression. In this context, exposure to belinostat, particularly when combined with bortezomib, resulted in clear up-regulation of Bim in both continuously cultured or primary cells of AML and ALL. Importantly, the observation that shRNA knockdown of Bim substantially blocked the lethality of co-administered belinostat and bortezomib in both AML and ALL cells argues that Bim up-regulation plays a significant functional role in the anti-leukemia activity of this regimen.
While monotherapy with HDACIs has shown some efficacy in acute leukemias 6, 7, the activity of bortezomib in this setting is less clear. Bortezomib has substantial single agent activity in multiple myeloma and mantle cell lymphoma, and has been approved for use in these diseases. Experience with bortezomib in acute leukemias is considerably more limited. In a Phase I trial of bortezomib in patients with refractory acute leukemia, lowering of blast counts was frequently observed, but objective responses were not obtained despite significant reductions in proteasome activity (e.g., > 50%) in several patients 19. More recently, attempts have been made to exploit the dependence of leukemia-initiating cells on NF-κB by combining bortezomib with conventional cytotoxic chemotherapy in patients with AML, and initial results appear promising 17. Analogously, bortezomib might act to enhance the activity of other targeted agents in this setting. In this context, a Phase I trial of the pan-HDACI vorinostat and bortezomib in patients with refractory multiple myeloma revealed significant activity for this regimen, including responses in several patients refractory to bortezomib alone 54. Based upon the present and earlier findings 26, a strategy combining HDACIs, and more specifically belinostat, with proteasome inhibitors warrants consideration in acute leukemia, including myeloid and lymphoid leukemias. Accordingly, a Phase I trial of belinostat administered in conjunction with bortezomib in patients with refractory acute myeloid and lymphoid leukemia has recently been initiated to determine the tolerability of such a regimen and to identify an MTD for this combination regimen. It will be of considerable interest to determine whether events observed in acute myeloid and lymphoid leukemia cells in vitro (e.g., inhibition of RelA/p65 acetylation and NF-κB activation, down-regulation of NF-κB-dependent proteins such as XIAP, up-regulation of Bim) are recapitulated in the blasts of patients receiving these agents in vivo. Plans for such correlative laboratory studies are underway.
Supplementary Material
Acknowledgments
This work was supported by awards CA63753, CA 93738, and CA100866, and 1 P50 CA130805-01 from the National Cancer Institute, award 6045-03 from the Leukemia and Lymphoma Society of America, and award from the V Foundation.
Footnotes
Contributions: Y.D. planned and performed experiments, and wrote the manuscript. S.C., L.W., X-Y.P., and L.B.K. performed experiments. P.D. helped plan experiments. S.G. planned experiments and wrote the manuscript.
Conflict-of-interest disclosure: There is no potential conflict of interest to disclose.
Reference List
- 1.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 2.Ungerstedt JS, Sowa Y, Xu WS, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102(3):673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100(8):4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 5.Grant S, Easley C, Kirkpatrick P. Vorinostat. Nature Review Drug Discovery. 2007;6:1–2. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111(3):1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Manero G, Assouline S, Cortes J, et al. Phase I study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112(4):981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23(17):3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- 9.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293(5535):1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Rahmani M, Dent P, Grant S. Blockade of Histone Deacetylase Inhibitor-Induced RelA/p65 Acetylation and NF-{kappa}B Activation Potentiates Apoptosis in Leukemia Cells through a Process Mediated by Oxidative Damage, XIAP Downregulation, and c-Jun N-Terminal Kinase 1 Activation. Mol Cell Biol. 2005;25(13):5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14(6):1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 13.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277(19):16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 14.An B, Goldfarb RH, Siman R, Dou QP. Novel dipeptidyl proteasome inhibitors overcome Bcl-2 protective function and selectively accumulate the cyclin-dependent kinase inhibitor p27 and induce apoptosis in transformed, but not normal, human fibroblasts. Cell Death Differ. 1998;5(12):1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 15.Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene. 2003;22(46):7108–7122. doi: 10.1038/sj.onc.1206863. [DOI] [PubMed] [Google Scholar]
- 16.Guzman ML, Swiderski CF, Howard DS, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci U S A. 2002;99(25):16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attar EC, De Angelo DJ, Supko JG, et al. Phase I and pharmacokinetic study of bortezomib in combination with idarubicin and cytarabine in patients with acute myelogenous leukemia. Clin Cancer Res. 2008;14(5):1446–1454. doi: 10.1158/1078-0432.CCR-07-4626. [DOI] [PubMed] [Google Scholar]
- 18.Horton TM, Pati D, Plon SE, et al. A phase 1 study of the proteasome inhibitor bortezomib in pediatric patients with refractory leukemia: a Children’s Oncology Group study. Clin Cancer Res. 2007;13(5):1516–1522. doi: 10.1158/1078-0432.CCR-06-2173. [DOI] [PubMed] [Google Scholar]
- 19.Cortes J, Thomas D, Koller C, et al. Phase I study of bortezomib in refractory or relapsed acute leukemias. Clin Cancer Res. 2004;10(10):3371–3376. doi: 10.1158/1078-0432.CCR-03-0508. [DOI] [PubMed] [Google Scholar]
- 20.Feng R, Oton A, Mapara MY, Anderson G, Belani C, Lentzsch S. The histone deacetylase inhibitor, PXD101, potentiates bortezomib-induced anti-multiple myeloma effect by induction of oxidative stress and DNA damage. Br J Haematol. 2007;139(3):385–397. doi: 10.1111/j.1365-2141.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 21.Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102(24):8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei XY, Dai Y, Grant S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin Cancer Res. 2004;10(11):3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- 23.Dai Y, Chen S, Kramer LB, Funk VL, Dent P, Grant S. Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res. 2008;14(2):549–558. doi: 10.1158/1078-0432.CCR-07-1934. [DOI] [PubMed] [Google Scholar]
- 24.Paoluzzi L, Scotto L, Marchi E, Zain J, Seshan VE, O’Connor OA. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin Cancer Res. 2010;16(2):554–565. doi: 10.1158/1078-0432.CCR-09-1937. [DOI] [PubMed] [Google Scholar]
- 25.Dasmahapatra G, Lembersky D, Kramer L, et al. The pan-HDAC inhibitor vorinostat potentiates the activity of the proteasome inhibitor carfilzomib in human DLBCL cells in vitro and in vivo. Blood. 2010;115(22):4478–4487. doi: 10.1182/blood-2009-12-257261. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood. 2003;102(10):3765–3774. doi: 10.1182/blood-2003-03-0737. [DOI] [PubMed] [Google Scholar]
- 27.Bali P, Pranpat M, Bradner J, et al. Inhibiition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis of antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005 doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 28.Nawrocki ST, Carew JS, Pino MS, et al. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006;66(7):3773–3781. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Dai Y, Pei XY, Grant S. Bim Up-regulation by Histone Deacetylase Inhibitors Mediates Interactions with the Bcl-2 Antagonist ABT-737: Evidence for Distinct Roles for Bcl-2, Bcl-xL and Mcl-1. Molecular and Cellular Biology. 2009:29. doi: 10.1128/MCB.01481-08. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahmani M, Anderson A, Habibi JR, et al. The BH3-only protein Bim plays a critical role in leukemia cell death triggered by concomitant inhibition of the PI3K/Akt and MEK/ERK1/2 pathways. Blood. 2009;114(20):4507–4516. doi: 10.1182/blood-2008-09-177881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai Y, Yu C, Singh V, et al. Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res. 2001;61(13):5106–5115. [PubMed] [Google Scholar]
- 32.Dai Y, Rahmani M, Pei XY, Dent P, Grant S. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104(2):509–518. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- 33.Dai Y, Rahmani M, Dent P, Grant S. Blockade of Histone Deacetylase Inhibitor-Induced RelA/p65 Acetylation and NF-{kappa}B Activation Potentiates Apoptosis in Leukemia Cells through a Process Mediated by Oxidative Damage, XIAP Downregulation, and c-Jun N-Terminal Kinase 1 Activation. Mol Cell Biol. 2005;25(13):5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp. 2004;259:208–217. [PubMed] [Google Scholar]
- 35.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Li N, Caron C, et al. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22(5):1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao G, Fong A, Sun SC. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J Biol Chem. 2004;279(29):30099–30105. doi: 10.1074/jbc.M401428200. [DOI] [PubMed] [Google Scholar]
- 38.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25(51):6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci U S A. 2005;102(44):16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai Y, Chen S, Venditti CA, et al. Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood. 2008 doi: 10.1182/blood-2007-10-116376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller CP, Ban K, Dujka ME, et al. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood. 2007;110(1):267–277. doi: 10.1182/blood-2006-03-013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosato RR, Kolla SS, Hock SK, et al. Histone deacetylase inhibitors activate NF-kappaB in human leukemia cells through an ATM/NEMO-related pathway. J Biol Chem. 2010;285(13):10064–10077. doi: 10.1074/jbc.M109.095208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311(5764):1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 45.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21(23):6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai Y, Guzman ML, Chen S, et al. The NF (Nuclear factor)-kappaB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol. 2010 doi: 10.1111/j.1365-2141.2010.08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parameswaran R, Muschen M, Kim YM, Groffen J, Heisterkamp N. A functional receptor for B-cell-activating factor is expressed on human acute lymphoblastic leukemias. Cancer Res. 2010;70(11):4346–4356. doi: 10.1158/0008-5472.CAN-10-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12(2):115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98(8):2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 50.Vilimas T, Mascarenhas J, Palomero T, et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat Med. 2007;13(1):70–77. doi: 10.1038/nm1524. [DOI] [PubMed] [Google Scholar]
- 51.Akiyama T, Dass CR, Choong PF. Bim-targeted cancer therapy: a link between drug action and underlying molecular changes. Mol Cancer Ther. 2009;8(12):3173–3180. doi: 10.1158/1535-7163.MCT-09-0685. [DOI] [PubMed] [Google Scholar]
- 52.Chen CC, Kennedy RD, Sidi S, Look AT, D’Andrea A. CHK1 inhibition as a strategy for targeting Fanconi Anemia (FA) DNA repair pathway deficient tumors. Mol Cancer. 2009;8:24. doi: 10.1186/1476-4598-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4(3):443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 54.Badros A, Burger AM, Philip S, et al. Phase I study of vorinostat in combination with bortezomib for relapsed and refractory multiple myeloma. Clin Cancer Res. 2009;15(16):5250–5257. doi: 10.1158/1078-0432.CCR-08-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.