Abstract
Most of the described and probably undescribed species on Earth are insects. Global models of species diversity rarely focus on insects and none attempt to address unknown, undescribed diversity. We assembled a database representing about 13,000 records for ant generic distribution from over 350 regions that cover much of the globe. Based on two models of diversity and endemicity, we identified regions where our knowledge of ant diversity is most limited, regions we have called “hotspots of discovery.” A priori, such regions might be expected to be remote and untouched. Instead, we found that the hotspots of discovery are also the regions in which biodiversity is the most threatened by habitat destruction. Our results not only highlight the immediate need for conservation of the remaining natural habitats in these regions, but also the extent to which, by focusing on well-known groups such as vertebrates, we may fail to conserve the far greater diversity of the smaller species yet to be found.
Keywords: biogeography, Formicidae
The global biodiversity crisis has made description and mapping of biological diversity a priority (1–4). Fortunately, distribution maps are available globally for vascular plants (5, 6) and terrestrial vertebrate animals (7, 8), helping target conservation plans for diversity hotspots (2, 9). Despite the fact that most species on Earth are insects (10) and that insects account for many ecosystem services (11) and disservices (12), no global maps of distribution or diversity exist for any major insect taxon. Understanding the patterns of distribution and diversity of even a single, diverse insect taxon would be disproportionately valuable.
The central challenge to modeling global diversity of insects is that even the “best-known” taxa and regions are known incompletely. A solution to this challenge is to model and map both what is known and what is unknown and, in doing so identify regions for both exploration and conservation. Here we describe the known diversity patterns of ants, model-predicted diversity patterns, and then use the difference between model predictions and empirical estimates of ant diversity as a measure of our ignorance. We model spatial patterns of diversity in two ways. First, we use an “interpolation” approach in which presences are conservatively interpolated. Second, we model climate–diversity relationships. Although the global diversity pattern of ants is similar to that of other taxa (e.g., vascular plants), many regions, which we designate “hotspots of discovery,” have much lower recorded diversity than expected given their climate and neighboring regions. Unfortunately, these hotspots of discovery are also the regions where deforestation is proceeding most quickly.
Results and Discussion
We accumulated a total of 13,072 presence records for the 300 described ant genera spanning 353 distinct regions (Table S1). Despite being very well known relative to most insect taxa, we estimated that a total of 2,400 genus*region occurrences remain to be documented. By comparing estimated and known patterns of diversity, we were able to map the spatial pattern in these undocumented occurrences.
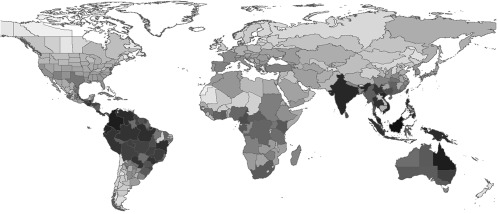
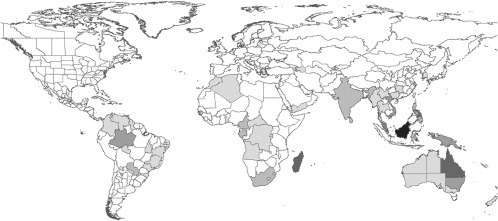
As for other taxa, richness decreased with latitude (e.g., refs. 13 and 14) (Fig. 1) and there were also strong regional effects on the magnitude of diversity. African regions were less diverse than would be expected given their latitude (or climate), the reverse of the pattern observed for termites (15, 16) and terrestrial mammals (17), although similar to that for vascular plants (5, 18). The 53 endemic genera were found almost exclusively in tropical regions that were diverse more generally, with four interesting exceptions in North Africa, Armenia, Azerbaijan, and South Korea (Fig. 2). Both overall generic diversity and endemic diversity showed a peak in the Oriental region, especially in Borneo, and were also high elsewhere in tropical Asia and Australia. Borneo was the most diverse region, which is also apparently the case for vascular plant species, suggesting concordance to some extent between ant and plant hotspots (18), perhaps because of causally linked diversification of ants and plants (19). South America includes important centers of diversity and endemicity in the Brazilian state of Amazonas and to a lesser extent in some of the surrounding regions. Just as for birds and flowering plants, the diversity of endemic genera (but not total generic diversity) is also high in Madagascar (five strictly endemic genera present), as has been noted elsewhere (20). The Congo basin seems to be another region of high endemicity. Not coincidentally, this list includes the regions where the greatest diversity of ant species also tends to have been recorded at local scales (21–26). It is noteworthy that the high endemicity observed for ants in Borneo, eastern Australia, regions of South East Asia, or the Amazonas province in Brazil contrasts with the lower levels of endemicity in terrestrial mammals (27) or birds (7) in those same regions.
Fig. 1.
Known generic diversity by political region. Grayscale corresponds to the diversity of genera in intervals of five genera, with 98 genera present in black regions and 0 genera present in white regions.
Fig. 2.
Map of observed richness of genera endemic to three or fewer political regions. Darker colors indicate the presence of more endemic genera. Endemic diversity ranges from zero (white) to eight (black) genera per region.
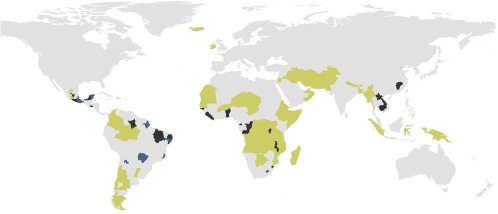
The patterns in modeled diversity reflect the diversity expected but, as the ant biologist Kusnezov wrote in 1957: “different local (ant) faunae have not been investigated equally thoroughly” (13), a truth that persists both for ants and for other insect taxa as well as plants (28–30). We remain ignorant, but now know enough to begin to map our ignorance and to more systematically make discoveries. Many regions had far fewer ant genera than would be predicted based on climate or their neighboring political regions (Fig. 3). A subset of these regions was identified as hotspots of discovery independent of which model we used. Our interpolation model identified 34 regions as relatively unknown (with a maximum of 57 new generic records predicted in Laos), whereas the environmental model predicted 75 regions to be relatively unknown (with a maximum of 66 new generic records predicted in New Guinea), over 20% of the regions we considered in our study. The differences between these two models could represent the influence of climatic and other historical factors (e.g., ref. 31) and potentially the influence of variables we did not consider, such as soils (32) or isolation. Nonetheless, many regions were identified as poorly known in both models (Table 1). Among those, regions of West Africa, southeast Africa, North East Brazil, southern Mexico, and to a lesser extent southeastern Asia are among the least explored (Fig. 3), similar to the unknown regions identified for vascular plants (17). A priori we predict that many of these same regions will be just as unknown, most likely more so, for other diverse taxa, such as flies and beetles, or specific clades, such as the mosquitoes or the tiger beetles. The great difficulty, however, is that with essentially no exceptions, maps are not available for other diverse groups, even at the level of genera.
Fig. 3.
Global hotspots of discovery as measured by the difference between the predicted and known diversity of ant genera. Regions where a minimum of 20 new records of genera are expected in both models appear in black. Regions with at least 20 new records of genera are predicted in the interpolation model (but not the environmental model) appear in blue. Regions with at least 20 new records of genera are predicted in the environmental model (but not the interpolation model) appear yellow-green.
Table 1.
Regions with the greatest difference between observed ant genus diversity and ant genus diversity predicted by our environmental model, interpolation model and the average of the two models
| Undersampled regions, environmental model | Undersampled regions, interpolation model | Undersampled regions, the mean of the two models | ||||||
| Region | Ĝ | Δ | Region | Ĝ | Δ | Region | Ĝ | Δ |
| New Guinea | 151 | 66 | Laos | 78 | 57 | Piaui, Brazil | 70 | 55 |
| Piaui, Brazil | 69 | 54 | Piaui, Brazil | 72 | 57 | Laos | 72 | 51 |
| Burundi | 66 | 53 | Cambodia | 78 | 52 | Cambodia | 76 | 51 |
| Sumatra | 133 | 51 | Sergipe, Brazil | 72 | 47 | Burundi | 58 | 45 |
| Cambodia | 75 | 49 | El Salvador | 75 | 47 | Sergipe, Brazil | 67 | 42 |
| Liberia | 68 | 46 | Paraiba, Brazil | 71 | 45 | R. Grande do Norte, Brazil | 65 | 40 |
| Laos | 64 | 45 | Rio Grande do Norte, Brazil | 70 | 45 | Equatorial Guinea | 66 | 40 |
| Pakistan | 67 | 44 | Campeche, Mexico | 63 | 41 | Liberia | 61 | 39 |
| Republic of the Congo | 86 | 44 | Burundi | 51 | 38 | Paraiba, Brazil | 65 | 39 |
| Sierra Leone | 67 | 43 | Equatorial Guinea | 64 | 38 | Campeche, Mexico | 58 | 36 |
| Colombia | 134 | 42 | Swaziland | 50 | 35 | Swaziland | 51 | 36 |
| Equatorial Guinea | 67 | 41 | Togo | 59 | 35 | El Salvador | 64 | 35 |
| Guinea Bissau | 46 | 40 | Lesotho | 51 | 33 | Sierra Leone | 59 | 35 |
| Dem. Rep. of Congo | 98 | 40 | Benin | 59 | 32 | Togo | 58 | 34 |
| Burkina Faso | 52 | 38 | Liberia | 54 | 32 | New Guinea | 118 | 33 |
| Mozambique | 70 | 38 | Yucatan, Mexico | 62 | 31 | Rwanda | 56 | 33 |
| Mauritania | 44 | 37 | Rwanda | 53 | 30 | Republic of the Congo | 74 | 32 |
| Western Sahara | 39 | 37 | Guanajuato, Mexico | 40 | 28 | Guinea-Bissau | 38 | 32 |
| Swaziland | 51 | 36 | Sierra Leone | 52 | 28 | Benin | 57 | 30 |
| Sergipe, Brazil | 61 | 36 | Roraima, Brazil | 80 | 26 | Sumatra | 110 | 28 |
| Eritrea | 65 | 36 | Alagoas, Brazil | 72 | 25 | Malawi | 54 | 28 |
| Rwanda | 59 | 36 | Espírito Santo, Brazil | 75 | 25 | Pakistan | 51 | 28 |
| Iraq | 54 | 36 | Quintana Roo, Mexico | 65 | 25 | Mozambique | 58 | 26 |
| Malawi | 61 | 35 | Mato Grosso do Sul, Brazil | 77 | 24 | Lesotho | 44 | 26 |
| Rio Grande do Norte, Brazil | 59 | 34 | Malawi | 47 | 24 | Guanajuato, Mexico | 38 | 26 |
The 25 regions in which we predict the greatest number of as yet unknown records of ant genera based on our environmental and interpolation models. Ĝ is the expected genus diversity and Δ is observed genus diversity minus Ĝ.
Poorly sampled regions are likely to represent regions where discoveries of new taxa in general are very likely. One might hope that these tend to be regions that are difficult to access geographically and, hence, relatively well preserved. Unfortunately, our results indicate otherwise. Poorly known regions are, on average, suffering higher rates of deforestation over the previous 20 y (−11.4% ± 3.1%) than well-known regions (+2.3% ± 2.1%) (Wilcoxon test; z = 3.7; P = 0.0002). For example, Togo and Burundi suffered the loss of 58% and 41% of their forests between 1990 and 2010 (33) and are predicted, by our models, to contain 39 and 38 undetected genera, respectively. As such, these regions should receive the highest conservation priority and investment in biological exploration.
To date, when conservation targets have been chosen at the global scale, the assumption has been that regions that are diverse for birds, mammals, or plants will also be diverse for the rest of life. However, in many cases the diversity patterns of plant or vertebrate taxa do not correlate spatially (34–41), which is to say they are not congruent. An alternate approach to comparing patterns in the large-scale distribution of taxa is to consider the congruence of their complementarity (8). In other words, do they differ across the same gradients or among the same biogeographic regions? However, the ability of complementarity approaches to capture poorly known taxa, such has insects, has been questioned (39), in part because the same taxa that make regions complimentary tend to be the last ones to be discovered. In the long-term, comparisons of the congruence of complementarity of taxa, including insects, at the global scale would be valuable, but global maps or global data on the presence and absence of all taxa within diverse insect taxa remain scarce, and for most insect taxa may remain impossible long into the future (42). Having at least one diverse insect taxon, such as ants, for which we can begin to understand diversity and ignorance patterns and, in the long-term (once the hotspots of discovery have actually been explored), complementarity globally seems like a step forward. Although it would be naive to imagine that ants perfectly predict the patterns of diversity or complementarity of all other insect taxa, they appear to do a better job than do vertebrate taxa or even plants, at least in terms of their ability to predict diversity (43). In addition, if the same barriers that have prevented the study of ants in the regions we have identified as hotspots of discovery are also barriers to taxonomists working in other fields, hotspots of discovery for ants seem likely to be similar to those of other taxa.
Perhaps the biggest question is whether or not existing conservation programs tend to capture the regions of which we are most ignorant and, hence, in which discovery is most likely. We have shown that hotspots of discovery tend to be at an increased risk of deforestation. However, comparison of the least unexplored regions and the priority areas for conservation as established by Conservation International, one prominent model for global conservation (44), reveals substantial overlap and, as such, our results support the importance of conservation in those same regions already identified by Conservation International (Fig. S1). On the other hand, several regions of Africa (Benin, Equatorial Guinea, Republic of the Congo Togo), South America (e.g., Roraima), and Asia (Guizhou) are not included in Conservation International’s hotspot priority areas. These regions seem like important ones to target for immediate new discoveries. We hope that our study will encourage a new burst of scientific exploration in and conservation of the most poorly known regions of the Earth.
Materials and Methods
Ants are a useful starting point for understanding insect life more generally for several reasons. First, the systematics of the Formicidae is probably among the best resolved for any large group of terrestrial arthropods, especially at the taxonomic level of the genus (45, 46), where global and several regional taxonomical keys are available to local scientists (45, 47–49). Ants are one of the most abundant groups of insects in many ecosystems (50–53), play a considerable role in shaping ecosystems (51, 54–57), and participate in numerous and diverse interactions with other organisms (58–61). Most importantly, in the context of our goals, ants are well enough known to allow a global study to even be possible, which cannot be said for most other insect groups. In addition, to the extent that any group of organisms is predictive of the patterns of any others, ants appear to do relatively well (2, 40, 62, 63). A recent study, for example, found ants to be the best predictor of the diversity of twelve different groups of non-ant invertebrates, three groups of vertebrates, and vascular plants across Europe (43). For these reasons, we believe ants represent a reasonable group to begin with in terms of understanding patterns of global diversity and ignorance.
Taxonomic Scale.
Genera vs. species.
Ideally, one would want to know the species present in every region of Earth (or at least in a well-distributed subset of regions) to predict patterns of species diversity. The obvious problem is that species distributions of insect taxa, even relatively well-studied taxa such as ants, remain poorly documented in many regions and even in entire biomes (e.g., tropical forests). Over 12,500 species of ants have been described (64), but perhaps twice as many await description (51), whether through the discovery of unknown species or the recognition of cryptic species in species complexes (65). Even once such new species are named, they are typically first known in just a small part of their true geographic range (66). Finally, misidentification of specimens is more likely to occur at the species than generic level. For the above reasons, taxonomic levels higher than species may represent the most useful taxonomic level at which to consider patterns of global diversity and endemicity at large spatial grains. For ants in particular, many generic revisions have been completed in the last 10 y (67) and identification to genus level is relatively easy (68), if not fail proof. It is inevitable that some genera will be split into several genera in the future (46) and new genera (and even higher taxa) (69–77) have been recently described. More taxa will probably be discovered. However, the generic level is relatively stable in ant taxonomy (46, 78). Generic diversity patterns are useful in their own right for understanding process, areas to conserve, and areas in need of study, but they also tend to be indicative of species diversity and distribution patterns. In several studies, generic diversity of ants has been shown to be correlated with species richness and so can be used as a good predictor of species richness for large (79) and local scales (80–85), with some interesting exceptions (86). Other advantages and disadvantages of using higher taxonomic rank have been summarized by Balmford et al. (87) and Gaston (88), but all such summaries highlight the value of higher taxonomic level analyses.
Exotic genera.
For our analysis, we excluded records of genera in their nonnative ranges to focus on historical patterns of ant diversity before human-introduced genera. Inevitably, some genera now thought to be native in some regions will be shown in the future to be nonnative and vice versa, but such changes are likely to be relatively infrequent and few.
Sampling Grain.
Early collections of insect specimens often simply note localities, regions, or even countries (e.g., Ghana, 1943) and many publications that provide checklists of species focus on specific political regions [e.g., Morocco (89); Paraguay (90); Tabasco (91)]. In light of these problems, many studies of the distribution patterns of diverse taxa focus on the political region as the grain of analysis (6, 10). We follow this approach, but recognize that its key disadvantage is that political regions vary greatly in their geographic area. As such, where possible, we have divided large political regions (e.g., Argentina, Australia, Brazil, China, Columbia, Japan, Mexico, and the United States) into smaller political regions, such as states. In one case, a state—Western Australia—was further divided into northern and southern domains. Russia was divided into five main entities according to the division used in the database Fauna Europaea (92). Where political regions included multiple geographic regions, those regions were separated, even if small. For example, many islands were considered separately than the countries they belong to (e.g., Sardinia and Sicily for Italy). Some islands were considered as single unit even if shared by several countries (e.g., Borneo, New Guinea). Oceanic islands and islands with an area less than 20,000 km2 were not included in our analysis, so as to minimize effects in our overall model associated with the influence of small islands (93, 94). The complete list of entities considered appears in Table S1.
Data Compilation.
Data were compiled from literature resources, Web sites that collate the work of ant biologists, museum collections, and direct contact with local or taxon specific experts. We have included publications from the 19th century until May 2011, independent of the language in which they were published. Where data were derived from species records (e.g., we know the genus Pachycondyla to be present in North Carolina because the species Pachycondyla chinensis is present), the species name was checked in Bolton et al. (67) to verify its taxonomic status. In addition to direct contacts with study authors for poorly studied regions, we searched Google Scholar, country by country (e.g., country + Formicidae), genus by genus (for papers that discuss the distribution of a particular genus or its species), and finally we searched on every pair-wise combination of genus and country (e.g., Camponotus + Chile).
Predicting Regions for New Discoveries.
On the basis of genus records in our database, we mapped known occurrences of all ant genera. These maps, when overlaid, represent the known generic diversity of ants. We then took two approaches to identify those political regions where more discoveries are likely.
Our first approach was based on interpolation. We began by identifying known presences and absences within the different regions for each genus. In those regions where a genus has not yet been recorded, the genus is either truly absent or it has simply not yet been detected. To account for those cases where genera are likely to be present but not yet collected, we interpolated across gaps. We considered a “gap” to be a geographical entity or a series of entities for which a genus was absent but surrounded by areas where the genus is present. Gaps were approached conservatively and “interpolated” only if they were surrounded by presences and if the interpretation was ecologically sensible (95). For example, tropical arboreal ants are highly unlikely in deserts or savannahs, so even if a gap exists in a tropical desert, a tropical arboreal genus would not be interpolated into it. However, any interpolation made by the authors during revision of a specific taxonomical group was integrated into the genus distribution as interpolation. Since we developed our first version of the maps in April 2009 we have been able to find records for 620 initially interpolated genus*region occurrences, which represent 20% of our total initial interpolations. Finally, we summed the different interpolations obtained for each region to get an estimate of unknown generic richness by political region.
Our second approach was to statistically model the expected number of genera in those regions where the number of genera is fewer than would be expected given their climate (temperature and precipitation), geographic area, and topographic range (absolute latitude, longitude and hemisphere). To estimate regional climate, we calculated the spatial minimum, maximum, mean, median, and SD (based on all points within a region) for annual values of temperature and precipitation (96). Data for the number of ecoregions and biomes per political region were extracted from Olson et al. (27). A list of the 26 variables considered is presented in Table S2. In these models, we included data only from political regions thought to be well sampled. To select those regions, we first ran a generalized linear model with the entire set of regions, including the 26 variables related to geographic and environmental conditions of each region. We assumed that some regions had been undersampled relative to others. This process yielded a general equation that underestimated the generic diversity for each region (because it included poorly known regions). We then estimated the difference between the known diversity and the expected diversity based on this first model. All of the regions for which the expected values were inferior to the known value were rejected and considered as poorly known. This method gave us a subset of 170 regions considered as suitable for the second-step model. We ran a similar generalized linear model with only the suitable regions. We used the set of significant variables to produce the equation that predicted the generic diversity for all of the regions considered in our study (Tables S3).
Both approaches are complementary and have both strengths and weaknesses when attempting to identify regions where new discoveries are likely. The main advantage of our interpolation approach is that it predicts where individual genera are likely to occur. In other words, it predicts not just that a genus will be discovered in a particular region, but which genus. Implicitly, the interpolation model also accounts, to some extent, for regional differences in fauna because of factors, such as history and geography, beyond those captured by climate. However, the weakness of interpolation is that it is conservative and is, for example, unlikely to predict a genus will occur far beyond where it is already known. Conversely, the environmental model has the advantage of representing a more general and replicable approach for estimating diversity. However, this model has the disadvantage that is contingent on including the “right” environmental variables in the first place and ignores regional influences of geography, history, and other factors on diversity.
Evaluation of the Quality of Data Collected.
Ultimately, we obtained three values for the generic richness of each political region: (A) the known generic diversity, (B) the known generic diversity plus the number of interpolations, and (C) the generic diversity predicted by the environmental model. It was then possible to calculate the difference between A and B and A and C to infer the number of unknown generic records within each region. We use these metrics as estimates of the degree of our ignorance of ant generic diversity in each region. We considered five different levels of “generic ant knowledge” based on the number of new generic records predicted for each model: good (0 generic records predicted), acceptable (1–5 generic records predicted), mediocre (6–10 generic records predicted), poor (11–20 generic records predicted), and very poor (more than 21 generic records predicted).
Endemism.
Ideally, one wants to conserve areas in such a way as to maximize the complementarity of conserved regions and, in a similar vein, make discoveries of the most unique genera and generic records first. Understanding the complementarity of regions, however, depends on knowing not just how many known and predicted genera are in a region, but which genera they are. Although our interpolation model, in theory, estimates which genera are missing from regions, using such data to estimate complementarity puts undue weight on the most uncertain aspect of our models (which genera are present). In the absence of perfect knowledge about the identity of missing genera, one approach to identifying regions with unique genera is to focus on endemic lineages. This is the approach we took. Because of their limited distribution, endemic taxa are more prone to extinction and as such should be prioritized within conservation plans (8). Here, genera present in three or fewer political regions were considered endemic. We combined the number of endemic genera per region to obtain the total number of endemic genera within each region. Only four endemic genera could be interpolated into new regions and these interpolations were not included in the figure. The genus Poecilomyrma, known only from the Fiji Islands, was not included in our analysis.
Deforestation Rate and Our Knowledge of Life.
We compared the deforestation rates for the past 20 y [based on data available by the Food and Agriculture Organization (FAO) (33)] for the 109 regions that have been particularly well sampled according to the interpolation model (i.e., fewer than 10 genera predicted to be newly recorded) to the 34 regions considered to be the most poorly known according to the interpolation model (i.e., more than 10 genera predicted to be recorded) (Fig. S2 and Table S4). These 143 regions correspond to the regions for which data were available at the same spatial grain in both the FAO database and our ant generic distribution database. As the scale of our dataset and the one available from the FAO have different grain sizes for some regions of the world (e.g., Mexico, United States, China), we only used regions of the world with a country level resolution. The data provided by the FAO have been discussed elsewhere and, like all data on human impacts have limits (97, 98). One of their limits is the coarseness of their resolution [remotely sensed data are available at a finer resolution (99), although only for small patches of many of the regions in which we were interested]. However, in our case the FAO data are gathered at a spatial grain similar to that at which we consider the distribution of ant genera. We compared both rate of deforestation for well-known and poorly known regions with a nonparametric Wilcoxon test. All statistical analyses were performed with the statistical software JMP (100).
All data are available in Dataset S1.
Supplementary Material
Acknowledgments
We thank L. Alonso, G. Alpert, I. Ambretch, F. Azorsa, A. Bernadou, R. Brandao, F. Cuezzo, S. Dash, S. De Greef, F. Esteves, R. Feitosa, J. Fellowes, F. Fernandez, B. Fisher, S. Groc, N. Gunawardene, B. Jahyny, R. Johnson, M. Karaman, J. Lattke, I. Leal, J. Longino, J. Mac Gown, C. Moreau, O. Paknia, M. Philippi, S. Philpott, S. Powell, Y. Quinet, C. Rabeling, E. Rodriguez, S. Shattuck, A. Suarez, G. Tohme, W. Tschinkel, J. Wagenknecht, P. Ward, J. Wetterer, and A. Wild. This work was supported by Department of Energy Program for Ecosystem Research Grant DE-FG02-08ER64510, National Aeronautic and Space Administration Award NNX09AK22G, and National Science Foundation Faculty Early Career Development Grant 09533390 (to R.R.D., M.D.W., and B.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper are available in the Supporting Information as Dataset S1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113867109/-/DCSupplemental.
References
- 1.Reid WV. Biodiversity hotspots. Trends Ecol Evol. 1998;13:275–280. doi: 10.1016/s0169-5347(98)01363-9. [DOI] [PubMed] [Google Scholar]
- 2.Alonso LE. In: Ants: Standard Methods for Measuring and Monitoring Biodiversity. Agosti D, Majer JD, Alonso LE, Schultz TR, editors. Washington, DC: Smithsonian Institution; 2000. pp. 80–88. [Google Scholar]
- 3.Rands MRW, et al. Biodiversity conservation: Challenges beyond 2010. Science. 2010;329:1298–1303. doi: 10.1126/science.1189138. [DOI] [PubMed] [Google Scholar]
- 4.Walpole M, et al. Ecology. Tracking progress toward the 2010 biodiversity target and beyond. Science. 2009;325:1503–1504. doi: 10.1126/science.1175466. [DOI] [PubMed] [Google Scholar]
- 5.Francis AP, Currie DJ. A globally consistent richness-climate relationship for angiosperms. Am Nat. 2003;161:523–536. doi: 10.1086/368223. [DOI] [PubMed] [Google Scholar]
- 6.Mutke J, Barthlott W. Patterns of vascular plant diversity at continental to global scales. Biol Skr. 2005;55:521–531. [Google Scholar]
- 7.Orme CDL, et al. Global hotspots of species richness are not congruent with endemism or threat. Nature. 2005;436:1016–1019. doi: 10.1038/nature03850. [DOI] [PubMed] [Google Scholar]
- 8.Lamoreux JF, et al. Global tests of biodiversity concordance and the importance of endemism. Nature. 2006;440:212–214. doi: 10.1038/nature04291. [DOI] [PubMed] [Google Scholar]
- 9.Fazey I, Fischer J, Lindenmayer DB. What do conservation biologists publish? Biol Conserv. 2005;124(1):63–73. [Google Scholar]
- 10.Pearson DL, Cassola F. World-wide species richness patterns of tiger beetles (Coleoptera: Cicindelidae): Indicator taxon for biodiversity and conservation studies. Conserv Biol. 1992;6:376–391. [Google Scholar]
- 11.Kremen C, et al. Terrestrial arthropods assemblages: Their use in conservation planning. Conserv Biol. 1993;7:796–808. [Google Scholar]
- 12.Dunn RR. Global mapping of ecosystem disservices: The unspoken reality that nature sometimes kills us. Biotropica. 2010;42:555–557. [Google Scholar]
- 13.Kusnezov N. Numbers of species of ants in faunae of different latitudes. Evolution. 1957;11:298–299. [Google Scholar]
- 14.Brown WL., Jr . In: Tropical Forest Ecosystems in Africa and South America: A Comparative Review. Meggers BJ, Ayensu ES, Duckworth WD, editors. Washington, DC: Smithsonian Institution; 1973. pp. 161–185. [Google Scholar]
- 15.Eggleton P, Williams PH, Gaston KJ. Explaining global termite diversity: Productivity or history? Biodivers Conserv. 1994;3:318–330. [Google Scholar]
- 16.Eggleton P. In: Termites: Evolution, Sociality, Symbioses, Ecology. Abe T, Bignell DE, Higashi M, editors. Dordrecht: Kluwer; 2000. pp. 25–51. [Google Scholar]
- 17.Schipper J, et al. The status of the world’s land and marine mammals: Diversity, threat, and knowledge. Science. 2008;322:225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- 18.Kier G, et al. Global patterns of plant diversity and floristic knowledge. J Biogeogr. 2005;32:1107–1116. [Google Scholar]
- 19.Moreau CS, Bell CD, Vila R, Archibald SB, Pierce NE. Phylogeny of the ants: Diversification in the age of angiosperms. Science. 2006;312:101–104. doi: 10.1126/science.1124891. [DOI] [PubMed] [Google Scholar]
- 20.Fisher BL. In: Biogeography of Madagascar (in French) Lourenco WR, Goodman SM, editors. Paris: Orstom; 1996. pp. 457–465. [Google Scholar]
- 21.Bruhl CA, Gunsalam G, Linsenmair KE. Stratification of ants (Hymenoptera: Formicidae) in a primary rain forest in Sabah, Borneo. J Trop Ecol. 1998;14:285–297. [Google Scholar]
- 22.Longino JT, Coddington J, Colwell RK. The ant fauna of a tropical rain forest: Estimating species richness three different ways. Ecology. 2002;83:689–702. [Google Scholar]
- 23.Malsch AKF, Rosciszewki K, Maschwitz U. In: Pasoh: Ecology of a Lowland Rain Forest in Southeast Asia. Okuda T, et al., editors. Tokyo: Springer; 2003. pp. 347–373. [Google Scholar]
- 24.Malsch AKF, et al. An analysis of declining ant species richness with increasing elevation at Mount Kinabalu, Sabah, Borneo. Asian Myrmecology. 2008;2:33–49. [Google Scholar]
- 25.Verhaagh M. The Formicidae of the rain forest in Panguana, Peru: The most diverse local ant fauna ever recorded. In: Veeresh GK, Mallik B, Viraktamath CA, editors. Proceedings of the 11th International Congress of the International Union for the Study of Social Insects. The Netherlands: EJ Brill Publishing Company, Leiden; 1990. pp. 217–218. [Google Scholar]
- 26.Ryder Wilkie KT, Mertl AL, Traniello JFA. Species diversity and distribution patterns of the ants of Amazonian Ecuador. PLoS ONE. 2010;5:e13146. doi: 10.1371/journal.pone.0013146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olson DM, et al. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience. 2001;51:933–938. [Google Scholar]
- 28.Joppa LN, Roberts DL, Myers N, Pimm SL. Biodiversity hotspots house most undiscovered plant species. Proc Natl Acad Sci USA. 2011;108:13171–13176. doi: 10.1073/pnas.1109389108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahrends A, et al. Conservation and the botanist effect. Biol Conserv. 2010;144(1):131–140. [Google Scholar]
- 30.Ahrends A, et al. Funding begets biodiversity. Divers Distrib. 2011;17:191–200. [Google Scholar]
- 31.Dunn RR, et al. Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol Lett. 2009;12:324–333. doi: 10.1111/j.1461-0248.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 32.Bestelmeyer BT, Wiens JA. Ant biodiversity in semiarid landscape mosaics: The consequences of grazing vs natural heterogeneity. Ecol Appl. 2001;11:1123–1140. [Google Scholar]
- 33.Food and Agriculture Organization of the United Nations 2010. Global forest resources assessment 2010. Main report. ( http://www.fao.org/forestry/fra/fra2010/en/). Accessed May 10, 2011.
- 34.Wilcox BA, Murphy DD, Ehrlich PR, Austin GT. Insular biogeography of the montane butterfly faunas in the Great Basin: Comparison with birds and mammals. Oecologia. 1986;69(2):188–194. doi: 10.1007/BF00377620. [DOI] [PubMed] [Google Scholar]
- 35.Kremen C. Assessing the indicator properties of species assemblages for natural area monitoring. Ecol Appl. 1992;2:203–217. doi: 10.2307/1941776. [DOI] [PubMed] [Google Scholar]
- 36.Prendergast JR, Quinn RM, Lawton JH, Eversham BC, Gibbons DW. Rare species, the coincidence of diversity hotspots and conservation strategies. Nature. 1993;365:335–337. [Google Scholar]
- 37.Flather CH, Wilson KR, Dean DJ, McComb WC. Identifying gaps in conservation networks: Of indicators and uncertainty in geographic-based analyses. Ecol Appl. 1997;7:531–542. [Google Scholar]
- 38.Howard PC, et al. Complementarity and the use of indicator groups for reserve selection in Uganda. Nature. 1998;394:472–475. [Google Scholar]
- 39.Freitag S, et al. van Jaarsveld AS Biodiversity assessment and conservation strategies. Science. 1998;279:2106–2108. doi: 10.1126/science.279.5359.2106. [DOI] [PubMed] [Google Scholar]
- 40.Allen CR, Pearlstine LG, Wojcik DP, Kitchens WM. The spatial distribution of diversity between disparate taxa: Spatial correspondence between mammals and ants across South Florida, USA. Landscape Ecol. 2001;46:453–464. [Google Scholar]
- 41.Palitzsch Lund M, Rahbek C. Cross-taxon congruence in complementarity and conservation of temperate biodiversity. Anim Conserv. 2002;5(2):163–172. [Google Scholar]
- 42.Hamilton AJ, et al. Quantifying uncertainty in estimation of tropical arthropod species richness. Am Nat. 2010;176:90–95. doi: 10.1086/652998. [DOI] [PubMed] [Google Scholar]
- 43.Schuldt A, Assmann T. Invertebrate diversity and national responsibility of species conservation across Europe—A multi-taxon approach. Biol Conserv. 2010;143:2747–2756. [Google Scholar]
- 44.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 45.Ward PS. Phylogeny, classification, and species-level taxonomy of ants (Hymenoptera: Formicidae) Zootaxa. 2007;1668:549–563. [Google Scholar]
- 46.Bolton B. Identification Guide to the Ant Genera of the World. Cambridge, MA: Harvard Univ Press; 1994. [Google Scholar]
- 47.Shattuck SO. Australian Ants: Their Biology and Identification, Monographs on Invertebrate Taxonomy. Collingwood, Australia: CSIRO; 1999. [Google Scholar]
- 48.Fernandez F. Introduction to the Ants of the Neotropical Region. 2003. (in Spanish) (Instituto de Investigación de Recursos Biológicos Alexander von Humboldt, Bogotá) [Google Scholar]
- 49.Fisher BL, Cover SP. Ants of North America: A Guide to the Genera. Berkeley: Univ of California Press; 2007. [Google Scholar]
- 50.Fittkau EJ, Klinge H. On biomass and trophic structure of the Central Amazonian rain forest ecosystem. Biotropica. 1973;5(1):2–14. [Google Scholar]
- 51.Holldobler B, Wilson EO. The Ants. Cambridge, MA: Belknap, Harvard Univ Press; 1990. [Google Scholar]
- 52.Tobin JE. In: Forest Canopies. Lowman MD, Nadkarni NM, editors. New York: Academic; 1995. pp. 129–147. [Google Scholar]
- 53.Davidson DW. The role of resource, imbalances in the evolutionary ecology of tropical arboreal ants. Biol J Linn Soc Lond. 1997;61(2):153–181. [Google Scholar]
- 54.Lal R. Effects of macrofauna on soil properties in tropical ecosystems. Agric Ecosyst Environ. 1988;24(1–3):101–116. [Google Scholar]
- 55.Folgarait PJ. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers Conserv. 1998;7:1221–1244. [Google Scholar]
- 56.Petal J, et al. Biological and chemical properties of fen soils affected by anthills of Myrmica spp. Pol J Ecol. 2003;51(1):67–78. [Google Scholar]
- 57.Jouquet P, Dauber J, Lagerlof J, Lavelle P, Lepage M. Soil invertebrates as ecosystem engineers: Intended and accidental effects on soil and feedback loops. Appl Soil Ecol. 2006;32(2):153–164. [Google Scholar]
- 58.Way MJ. Mutualism between ants and honeydew-producing Homoptera. Annu Rev Entomol. 1963;8:307–344. [Google Scholar]
- 59.Beattie AJ. The Evolutionary Ecology of Ant-Plant Mutualisms. London: Cambridge Univ Press; 1985. [Google Scholar]
- 60.Rico Gray V, Oliveira PS. The Ecology and Evolution of Ant-Plant Interactions. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 61.Stadler B, Dixon T. Mutualism: Ants and Their Insect Partners. Cambridge: Cambridge Univ Press; 2008. [Google Scholar]
- 62.Majer JD, Orabi G, Bisevac L. Ants (Hymenoptera: Formicidae) pass the bioindicator scorecard. Myrmecol News. 2007;10:69–76. [Google Scholar]
- 63.Leal IR, Bieber AGD, Tabarelli M, Andersen AN. Biodiversity surrogacy: Indicator taxa as predictors of total species richness in Brazilian Atlantic forest and Caatinga. Biodivers Conserv. 2010;19:3347–3360. [Google Scholar]
- 64.Agosti D, Johnson NF. 2005. Antbase, World Wide Web electronic publication. antbase.org, version (05/2005). Accessed April 20, 2011.
- 65.Seifert B. Cryptic species in ants (Hymenoptera: Formicidae) revisited: We need a change in the alpha-taxonomic approach. Myrmecol News. 2009;12:149–166. [Google Scholar]
- 66.Ryder Wilkie KT, Mertl AL, Traniello JFA. Biodiversity below ground: Probing the subterranean ant fauna of Amazonia. Naturwissenschaften. 2007;94:725–731. doi: 10.1007/s00114-007-0250-2. [DOI] [PubMed] [Google Scholar]
- 67.Bolton B, Alpert G, Ward PS, Naskrecki P. Bolton's Catalogue of the Ants of the World: 1758–2005. 2007. CD version (Harvard Univ Press, Cambridge, MA) [Google Scholar]
- 68.Vasconcelos HL. In: Soil Biodiversity in Amazonian and Other Brazilian Ecosystems. Moreira FMS, Siqueira JO, Brussaard L, editors. Trowbridge, UK: CABI; 2006. pp. 129–141. [Google Scholar]
- 69.Eguchi K, Bui TV. Parvimyrma gen. nov. belonging to the Solenopsis genus group from Vietnam (Hymenoptera: Formicidae: Myrmicinae: Solenopsidini) Zootaxa. 2007;1461:39–47. [Google Scholar]
- 70.Bolton B, Fisher BL. Afrotropical ants of the ponerine genera Centromyrmex Mayr, Promyopias Santschi gen. rev. and Feroponera gen. n., with a revised key to genera of African Ponerinae (Hymenoptera: Formicidae) Zootaxa. 2008;1929:1–37. [Google Scholar]
- 71.Rabeling C, Brown JM, Verhaagh M. Newly discovered sister lineage sheds light on early ant evolution. Proc Natl Acad Sci USA. 2008;105:14913–14917. doi: 10.1073/pnas.0806187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamane S, Bui TV, Eguchi K. Opamyrma hungvuong, a new genus and species of ant related to Apomyrma (Hymenoptera: Formicidae: Amblyoponinae) Zootaxa. 2008;1767:55–63. [Google Scholar]
- 73.Fisher BL. Two new dolichoderine ant genera from Madagascar: Aptinoma gen. n. and Ravavy gen. n. (Hymenoptera: Formicidae) Zootaxa. 2009;2118:37–52. [Google Scholar]
- 74.Klingerberg C, Brandao CRF. Revision of the fungus-growing ant genera Mycetophylax Emery and Paramycetophylax Kusnezov rev. stat., and description of Kalathomyrmex n. gen. (Formicidae: Myrmicinae: Attini) Zootaxa. 2009;2052:1–31. [Google Scholar]
- 75.Silva RR, Feitosa RM, Brandao CRF, Diniz JLM. Tropidomyrmex elianae, a new myrmicine ant genus and species from Brazil, tentatively assigned to Solenopsidini (Hymenoptera, Formicidae) Zootaxa. 2009;2052:32–48. [Google Scholar]
- 76.Terayama M. A synopsis of the family Formicidae of Taiwan (Insecta; Hymenoptera) The Research Bulletin of Kanto Gakuen University. 2009;17:81–266. [Google Scholar]
- 77.LaPolla JS, Brady SB, Shattuck SO. Phylogeny and taxonomy of the Prenolepis genus-group of ants (Hymenoptera: Formicidae) Syst Entomol. 2010;35(1):118–131. [Google Scholar]
- 78.Ward PS. In: Ant Ecology. Lach L, Parr CL, Abbott K, editors. New York: Oxford Univ Press; 2010. pp. 3–17. [Google Scholar]
- 79.Groc S, et al. A new method based on taxonomic sufficiency to simplify studies on Neotropical ant assemblages. Biol Conserv. 2010;143:2832–2839. [Google Scholar]
- 80.Andersen AN. In: Conservation Outside Nature Reserves. Hale P, Lamb D, editors. Brisbane, Australia: Centre For Conservation Biology, University of Queensland; 1997. pp. 319–325. [Google Scholar]
- 81.Neville PJ, New TR. In: The Other 99%. The Conservation and Biodiversity of Invertebrates. Ponder W, Lunney D, editors. Mosman: Transactions of the Royal Zoological Society of New South Wales; 1999. pp. 133–137. [Google Scholar]
- 82.Pik AJ, Oliver I, Beattie AJ. Taxonomic sufficiency in ecological studies of terrestrial invertebrates. Aust J Ecol. 1999;24:555–562. [Google Scholar]
- 83.Negi HR, Gadgil M. Cross-taxon surrogacy of biodiversity in the Indian Garhwal Himalaya. Biol Conserv. 2002;105(2):143–155. [Google Scholar]
- 84.Baldi A. Using higher taxa as surrogates of species richness: A study based on 3700 Coleoptera, Diptera, and Acari species in Central-Hungarian reserves. Basic Appl Ecol. 2003;4:589–593. [Google Scholar]
- 85.Schnell MR, Pik AJ, Dangerfield JM. Ant community succession within eucalypt plantations on used pasture and implications for taxonomic sufficiency in biomonitoring. Austral Ecol. 2003;28:553–565. [Google Scholar]
- 86.Andersen AN. Measuring more of biodiversity: Genus richness as a surrogate for species richness in Australian ant faunas. Biol Conserv. 1995;73(1):39–43. [Google Scholar]
- 87.Balmford A, Jayasuriya AHM, Green MJB. Using higher-taxon richness as a surrogate for species richness. II. Local applications. P Roy Soc Lond B Bio. 1996;263:1571–1575. [Google Scholar]
- 88.Gaston KJ. Biodiversity: Higher taxon richness. Prog Phys Geogr. 2000;24(1):117–127. [Google Scholar]
- 89.Cagniant H. Actualized list of ants of Maroc (Hymenoptera: Formicidae) (in French) Myrmecol News. 2006;8:193–200. [Google Scholar]
- 90.Wild AL. A catalogue of the ants of Paraguay. Zootaxa. 2007;1622:1–55. [Google Scholar]
- 91.Del Toro I, Vazquez M, MacKay WP, Rojas P, Zapata-Mata R. Ants Hormigas (Hymenoptera: Formicidae) of Tabasco: Exploration of the myrmecofauna diversity in tropical forests of lower altitude. Dugesiana. 2009;16(1):1–14. [Google Scholar]
- 92.de Jong YSDM. 2010. Fauna Europaea version 2.3. Web Service available online at http://www.faunaeur.org. Accessed December 5, 2010.
- 93.MacArthur RH. Patterns of species diversity. Biol Rev Camb Philos Soc. 1965;40:510–533. [Google Scholar]
- 94.Frankham R. Do island populations have less genetic variation than mainland populations? Heredity (Edinb) 1997;78:311–327. doi: 10.1038/hdy.1997.46. [DOI] [PubMed] [Google Scholar]
- 95.Brown WL. In: Ants: Standard Methods for Measuring and Monitoring Biodiversity. Agosit D, Majer JD, Alonso LE, Schultz TR, editors. Washington, DC: Smithosian Institution; 2000. pp. 45–79. [Google Scholar]
- 96.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 97.Stokstad E. Ecology. U.N. report suggests slowed forest losses. Science. 2001;291:2294. doi: 10.1126/science.291.5512.2294. [DOI] [PubMed] [Google Scholar]
- 98.Grainger A. Difficulties in tracking the long-term global trend in tropical forest area. Proc Natl Acad Sci USA. 2008;105:818–823. doi: 10.1073/pnas.0703015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hansen MC, et al. Global percent tree cover at a spatial resolution of 500 meters: First results of the MODIS vegetation continuous field algorithm. Earth Interact. 2003;7(10):1–15. [Google Scholar]
- 100.SAS, editor. JMP 8.0.2– Statistical discovery software. Cary, NC: SAS Institute; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.