Abstract
Given worldwide increases in the incidence of obesity and type 2 diabetes, new strategies for preventing and treating metabolic diseases are needed. The nuclear receptor PPARγ (peroxisome proliferator-activated receptor gamma) plays a central role in lipid and glucose metabolism; however, current PPARγ-targeting drugs are characterized by undesirable side effects. Natural products from edible biomaterial provide a structurally diverse resource to alleviate complex disorders via tailored nutritional intervention. We identified a family of natural products, the amorfrutins, from edible parts of two legumes, Glycyrrhiza foetida and Amorpha fruticosa, as structurally new and powerful antidiabetics with unprecedented effects for a dietary molecule. Amorfrutins bind to and activate PPARγ, which results in selective gene expression and physiological profiles markedly different from activation by current synthetic PPARγ drugs. In diet-induced obese and db/db mice, amorfrutin treatment strongly improves insulin resistance and other metabolic and inflammatory parameters without concomitant increase of fat storage or other unwanted side effects such as hepatoxicity. These results show that selective PPARγ-activation by diet-derived ligands may constitute a promising approach to combat metabolic disease.
Keywords: nuclear receptors, nutrition, compound screening, organic synthesis, x-ray structure
Over the last few decades metabolic diseases such as type 2 diabetes have evolved into a global epidemic (1). Exercise and dietary regimes can counteract the development of obesity and type 2 diabetes, but complementation of such strategies with safe preventive drugs or tailored food supplements may be needed to combat the epidemic of insulin resistance, a hallmark of metabolic disease (2). The nuclear receptor PPARγ (peroxisome proliferator-activated receptor gamma) is a key regulator of gene expression of metabolism, inflammation, and other pathways in many cell types, especially adipocytes (3). Following food intake, this nuclear receptor is activated by binding of lipid-derived ligands such as unsaturated fatty acids, which induces expression of a large number of genes involved in metabolism. Several structurally unrelated natural products, including flavonoids, polyphenols (e.g., resveratrol) or organic acids including punicic acid or abscisic acid, have been described to interact with PPARs in micromolar concentrations (4–6). However, these molecules do not show clear beneficial molecular or physiological in vivo effects, in part due to interaction with a number of other proteins, making further development of these compounds problematic. The antidiabetic thiazolidinediones (TZDs), including the widely applied drug rosiglitazone (Avandia), strongly activate PPARγ. Recently, these PPARγ activators have come under scrutiny because of undesirable clinical side effects (7) such as weight gain and other disorders (8). However, more subtle modulation of PPARs may promote specific gene expression profiles that result in more favorable outcomes. The use of selective PPARγ modulators (SPPARγMs) (9–11) as well as inhibition of phosphorylation of serine 273 of PPARγ by small molecules are two recently proposed approaches for improving insulin sensitivity while minimizing aforementioned side effects (12, 13).
A large proportion of drugs are based on natural products or their synthetic analogues (14), and purified natural products or extracts derived from edible biomaterials have recently become a major focus of nutrition research aiming to develop functional food and nutraceuticals with demonstrable health benefits (15).
Results and Discussion
Amorfrutins Are Dietary SPPARγMs with Potent Binding Affinity.
To identify new dietary molecules that could act as potent antidiabetic SPPARγMs, we screened a structurally diverse natural products library consisting of approximately 8,000 pure compounds derived from edible biomaterials, using mass spectrometry detection (SI Appendix, Fig. S1A). The screen revealed 90 potential PPARγ ligands (SI Appendix, Fig. S1B), which were characterized in additional assays to confirm PPARγ binding and activation. We identified the amorfrutins, a family of isoprenoid-substituted benzoic acid derivatives without any stereocentres, as structurally new PPARγ agonists with high binding affinity (Fig. 1A). The amorfrutins were isolated from the edible roots of licorice, Glycyrrhiza foetida, which are used in traditional medicine and are widely available. We also isolated amorfrutins from fruits of another legume, Amorpha fruticosa, an ingredient of some condiments.
Fig. 1.
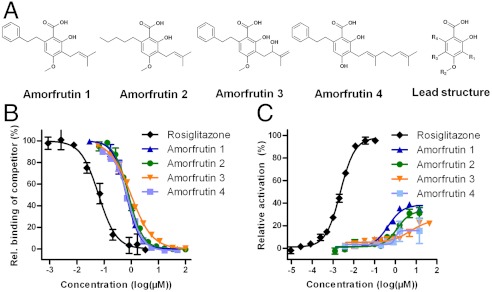
Amorfrutins are potent PPARγ modulators. (A) Structures of four amorfrutins described in this study and lead structure. R1: Isoprenoyl residues; R2: H or Me; R3: H or isoprenoyl residues; R4: H, aliphatic or aromatic residues or a combination thereof. (B) Binding of compounds on PPARγ-LBD in a competitive TR-FRET assay. (C) Cellular activation of PPARγ determined in a reporter gene assay in HEK 293H cells.
The PPARγ binding affinity constants of amorfrutins 1–4 ranged from 236 to 354 nM (Fig. 1B, Table 1), indicating that these compounds bind about twice as strongly to PPARγ as the synthetic drug pioglitazone (Actos, Ki = 584 nM). The amorfrutins showed weaker binding to other PPAR subtypes with a selectivity factor for PPARγ of approximately 20 to 200 (SI Appendix, Fig. S2 A and B, Table 1). For example, amorfrutin 1 has binding affinities of 236 nM for PPARγ, which are more than 100-fold higher than for PPARα and PPARβ/δ (each have a binding constant of 27 μM). However, the amorfrutins also exhibited low-micromolar activity on PPARα (which is mainly expressed in the liver) and on ubiquitously expressed PPARβ/δ, suggesting that these compounds can potentially contribute to treatment of diabetes-associated disorders such as dyslipidemia and hypercholesterolemia (16).
Table 1.
Affinity constants (Ki), effective concentrations (EC50) and efficacy of investigated compounds binding to various PPAR subtypes
| Compound | PPARα | PPARβ/δ | PPARγ | ||
| Ki [μM] | Ki [μM] | Ki [μM] | EC50 [μM] | Efficacy [%] | |
| Amorfrutin 1 | 27 | 27 | 0.236 | 0.458 | 39 |
| Amorfrutin 2 | 25 | 17 | 0.287 | 1.200 | 30 |
| Amorfrutin 3 | 115 | 68 | 0.352 | 4.500 | 22 |
| Amorfrutin 4 | 8 | 6 | 0.278 | 0.979 | 15 |
| Rosiglitazone | n.d. | n.d. | 0.007 | 0.002 | 100 |
| Pioglitazone | n.d. | n.d. | 0.584 | n.d. | n.d. |
| nTZDpa | n.d. | n.d. | 0.029 | n.d. | n.d. |
| Telmisartan | n.d. | n.d. | 1.700 | n.d. | n.d. |
| GW0742 | n.d. | 0.0004 | n.d. | n.d. | n.d. |
| GW7647 | 0.001 | n.d. | 0.180 | n.d. | n.d. |
Ki values were obtained by using a competitive TR-FRET assay, EC50 and efficacy values were determined from a reporter gene assay.
Efficacy is the maximum activation relative to the rosiglitazone-induced activation of PPARs. n.d., not determined
In contrast to the full PPARγ agonist rosiglitazone, amorfrutins induced only partial recruitment of several transcriptional cofactors including CBP, PGC1a, TRAP220/DRIP, and PRIP/RAP250. Strikingly, amorfrutin 1 abolished recruitment of the corepressor NCoR showing IC50 values similar to those of rosiglitazone (51 nM for amorfrutin 1 vs. 64 nM for rosiglitazone, SI Appendix, Fig. S2 C–G, Table S1). As reported recently, NCoR deletion results in PPARγ activation and increased insulin sensitivity (17). We confirmed partial PPARγ activation by amorfrutins using a reporter gene assay and detected activation of 15 to 39% relative to full PPARγ activation (Fig. 1C, Table 1). Using cellular reporter gene or coactivator recruitment assays, we also tested for potential interaction with other nuclear receptors involved in adipocyte differentiation, metabolism, or xenobiotic sensing such as the estrogen receptors alpha and beta, the liver x receptor alpha, the constitutive androstane receptor, and the pregnane X receptor but did not detect any activation (SI Appendix, Fig. S3 A–E).
Crystal Structure of PPARγ-Binding Amorfrutin 1.
To gain further insight in the interaction of amorfrutins with PPARγ, we examined the structure of the complex of the PPARγ-ligand binding domain (LBD) and amorfrutin 1 by X-ray crystallography (2.0 Å resolution). In the resulting dimeric structure, polypeptide chain ‘B’ of PPARγ-LBD was distorted due to crystal contacts, consistent with previously published PPARγ structures (18–20). The other chain ‘A’ contained an amorfrutin 1 molecule bound between helix H3 and the β-sheet (Fig. 2A). The PPARγ-LBD recognized natural amorfrutin 1 in a similar way as the synthetic partial agonists nTZDpa, MRL-24, and BVT.13. All of these ligands stabilized helix H3 and the β-sheet and were linked to Ser342 and Arg288 of the LBD via hydrogen bonds and salt bridges (20) (Fig. 2B, SI Appendix, Fig. S4A). Disruption of these interactions by methylating the carboxyl group in amorfrutin weakened the binding to PPARγ by a factor of 40 (SI Appendix, Fig. S4B). The structure also revealed that the ortho-phenyl and meta-isoprenyl residues of amorfrutin 1 have extensive van der Waals contacts with the LBD.
Fig. 2.
Amorfrutins selectively regulate gene expression in adipocytes. (A) Structure of the PPARγ:amorfrutin 1 complex. PPARγ binds to amorfrutin 1 between helix H3 (red) and the β-sheet (green). The C-backbone of amorfrutin 1 is drawn in yellow and oxygens in red. (B) Atomic details of ligand recognition. Hydrogen bonds stabilizing the complex are shown as dashed lines, experimental amorfrutin 1 electron density is shown in gray. (C) Gene distance matrix of gene expression profiles in human adipocytes treated with amorfrutin 1 or 2 (30 μM), rosiglitazone, pioglitazone, nTZDpa (10 μM each) or telmisartan (30 μM). Squares show the distance of two compounds in Euclidean space, ranging from exactly the same profile (black) to completely different (red). (D) Venn diagram of differentially expressed genes after treatment of human adipocytes with different compounds. Numbers in circles indicate up- and down-regulated genes, numbers in parentheses represent a total of regulated genes for that compound. (E) Enriched pathways after treatment of human primary adipocytes using GSEA. Ten most highly significant pathways for amorfrutin 1 and corresponding normalized enrichment scores (NES) are shown. #, x, $, P ≤ 0.05 for amorfrutin 1, amorfrutin 2 or rosiglitazone.
Amorfrutins Selectively Modulate PPARγ Gene Expression Networks in Adipocytes.
Consistent with partial activation of PPARγ in vitro and the observation of amorfrutin-LBD binding in the X-ray structure, we confirmed activation of expression of known PPARγ target genes by the amorfrutins. Classical PPARγ target genes such as Fabp4, Slc2a4, and Nr1h3 were upregulated in differentiated adipocytes but to a much lower degree compared to rosiglitazone (SI Appendix, Fig. S5A). Knockdown of PPARγ reduced significantly or abolished amorfrutin-induced gene expression, suggesting specific activation of PPARγ-dependent gene expression networks by these natural products (SI Appendix, Fig. S5B). Upregulation of PPARγ target genes by amorfrutins was in general weaker than observed for the full agonist rosiglitazone, in concomitant with markedly less pronounced adipocyte differentiation. We further compared gene expression profiles of human primary adipocytes treated with amorfrutins, the full PPARγ agonists rosiglitazone and pioglitazone, and the selective PPARγ modulators nTZDpa (21) and telmisartan (22). Gene Ontology and Gene Set Enrichment Analysis (GSEA) revealed molecular networks of PPARγ modulation by amorfrutins. The most enriched pathway for amorfrutin 1 and 2 was the PPAR signaling pathway. Gene distance matrix comparison (Fig. 2C), hierarchical clustering (SI Appendix, Fig. S5C), and principal component analyses (SI Appendix, Fig. S5 D and E) strongly support classification of the amorfrutins as natural SPPARγMs, showing characteristically different expression patterns compared to known synthetic PPARγ agonists. Notably, gene expression profiles of amorfrutins 1 and 2 were partially distinct, indicating that small changes in ligand structure may contribute to fine tuning of transcriptional regulation (Fig. 2D). Cholesterol biosynthesis, fatty acid elongation, and fatty acid oxidation genes were efficiently upregulated by amorfrutin treatment. In contrast, inflammation pathways were downregulated (Fig. 2E, SI Appendix, Fig. S5 F and G). As for many approved drugs and natural products (23, 24), we can of course not completely rule out the possibility of off-target effects of the amorfrutins; for example the inhibition of NF-κB pathways in some cells (25). Nevertheless, our in vitro results and detailed analyses of gene expression data, including application of the Connectivity Map approach (26) for drug discovery, strongly suggested that the amorfrutins act mainly as insulin sensitizers (SI Appendix, Table S2).
Amorfrutins Act as Antidiabetics in Mouse Models for Type 2 Diabetes.
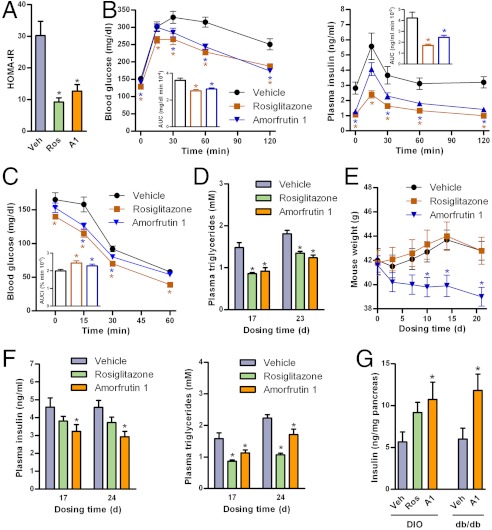
Next, we evaluated the in vivo effects of amorfrutin 1 on insulin resistance in high-fat diet-induced obesity (DIO) C57BL/6 mice. For this purpose, we developed a chemical synthesis that provided multigram quantities of amorfrutin 1 of greater than 99% purity (see SI Appendix, Methods). A panel of ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) studies did not reveal any adverse effects of amorfrutin 1 application (SI Appendix, Fig. S6A, Table S3). Furthermore, using an in vitro micronucleus assay, we observed no genotoxicity of amorfrutin 1 at physiologically relevant doses (SI Appendix, Fig. S6B). After feeding a high-fat diet (HFD) for 12 w, the DIO mice were treated for 23 d with 100 mg/kg/d amorfrutin 1, a dosage for which we anticipated antidiabetic and nontoxic effects based on the affinity to PPARγ and the ADMET properties observed. In the mouse studies, liver toxicity indicating plasma alanine transaminase (ALT) assays showed reduced ALT levels in mice treated with amorfrutin 1 compared to mice treated with vehicle control or rosiglitazone (SI Appendix, Fig. S6C). Similarly, whole-genome expression analysis of mice livers suggested no toxic effects after amorfrutin treatment (SI Appendix, Fig. S6D). Amorfrutin 1 and rosiglitazone both showed equal reduction of insulin resistance in DIO mice as assessed by homeostatic modelling (Fig. 3A). Amorfrutin 1 considerably enhanced glucose tolerance [19% decrease in glucose area under the curve (AUC), 42% decrease in insulin AUC vs. vehicle] and insulin sensitivity (14% increase in glucose AUCi vs. vehicle) during oral glucose tolerance tests (OGTT, Fig. 3B) and intraperitoneal insulin sensitivity tests (IPIST, Fig. 3C). Moreover, amorfrutin 1 strongly decreased plasma triglycerides, free fatty acids, insulin, and glucose comparable to rosiglitazone (Fig. 3D, SI Appendix, Fig. S7A).
Fig. 3.
Amorfrutins have potent antidiabetic effects in mouse models of type 2 diabetes. (A) Effect of treatment over 17 d on insulin resistance determined by homeostatic model assessment of insulin resistance (HOMA-IR) in DIO mice (N = 13). (B) Glucose and insulin concentrations during oral glucose tolerance test (OGTT) after 17 d of treatment with 100 mg/kg/d amorfrutin 1 in DIO mice (N = 13). Inlet, AUC. (C) Glucose levels during intraperitoneal insulin sensitivity test (IPIST) of the same DIO mice after 23 d of treatment (N = 13). Inlet, inverse area under the curve (AUCi). (D) Effect of treatment on fasted plasma triglycerides in these DIO mice (N = 13). (E) Effect of treatment on body weight of DIO mice (N = 13). (F) Effect of treatment with vehicle control, 4 mg/kg/d rosiglitazone or 100 mg/kg/d amorfrutin 1 on fasting plasma insulin and triglyceride level of genetically diabetic db/db mice (N = 7–12). (G) Pancreatic insulin content in DIO mice (N = 13) or db/db mice (N = 7) after treatment over 3 w. Data are expressed as mean ± SEM. *, P ≤ 0.05 vs. vehicle.
Both rosiglitazone and amorfrutin 1 increased food intake (SI Appendix, Fig. S7B) as previously described for PPARγ ligands (27). But in contrast to rosiglitazone, amorfrutin 1 treatment over three weeks reduced significantly body weight gain in DIO mice by approximately 10% compared to DIO mice treated with vehicle control (Fig. 3E). Such a surprising effect has also been reported for the pan-PPAR agonist bezafibrate (28), while for many synthetic SPPARγMs reduced food consumption and concomitantly decreased weight gain have been observed (29). The reduced weight gain in our DIO mice was associated with slightly elevated plasma concentration of thyroxine (T4), a marker for increased energy expenditure (SI Appendix, Fig. S7C). Because the complex effects of PPARγ agonism on various endocrine systems and downstream physiological changes (e.g., change in thermogenesis, fatty acid oxidation, or activity) are not fully understood, it is difficult to probe all potential mechanisms by which the amorfrutins may affect weight regulation. For example, recent studies suggest that complex interaction of brain PPARγ-signaling with peripheral organs may contribute to the physiological regulation of energy balance (30, 31). Presumably, the amorfrutins as partial agonists may act on neuronal PPARγ by antagonising diet-derived endogenous agonists such as fatty acids, thereby leading to relative weight loss. Notably, in our study an increase in food intake became apparent not until day 10 of the treatment with amorfrutin, whereas beneficial reduction of weight gain already started during the first days. Orexigenic effects may therefore be secondary to weight gain reduction.
We also investigated the antidiabetic effects of amorfrutin 1 in leptin receptor-deficient db/db mice, a genetic model of severe diabetes. In this model, rosiglitazone strongly increased body weight by approximately 30% within 3 w, whereas amorfrutin 1 treatment had no significant effects on mouse body weight (SI Appendix, Fig. S7D). Strikingly, amorfrutin 1 reduced plasma insulin concentrations more strongly than rosiglitazone (36% vs. 19% decrease after 24 d) (Fig. 3F). Amorfrutin 1 treatment also decreased plasma concentrations of glucose, triglycerides, and free fatty acids (Fig. 3F, SI Appendix, Fig. S7E). Possibly as a result of enhanced insulin sensitivity, amorfrutin 1 also appeared to prevent deterioration of pancreatic function in insulin-resistant mice, as pancreatic insulin levels improved compared to nontreated control mice (Fig. 3G).
Amorfrutins Inhibit HFD-Induced PPARγ Ser273 Phosphorylation in Mouse Adipocytes.
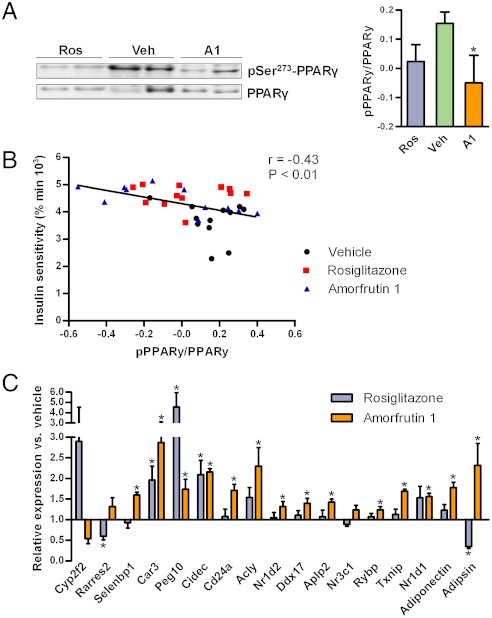
Phosphorylation by protein kinase Cdk5 at serine 273 of PPARγ in adipocytes leads to dysregulation of a large number of genes whose expression is altered in obesity (12). Inhibition of the Ser273-phosphorylation was thus proposed as a new strategy to increase insulin sensitivity specifically, without activating the full range of PPARγ targets, and thereby avoiding known side effects such as weight gain. Phosphorylation of PPARγ in viscerale white adipose tissue of DIO mice was blocked by amorfrutin 1 (Fig. 4A). This effect was significantly correlated with improved insulin sensitivity (Fig. 4B). As shown above, compared to rosiglitazone the amorfrutins do not induce expression of large gene sets (Fig. 2D), leading for example to reduced expression of genes for fat storage such as Fabp4. We further observed in vivo that amorfrutin 1 more efficiently than rosiglitazone counterregulated a set of 17 genes (Fig. 4C) that had recently been reported (12) to be altered by HFD-induced activation of the kinase Cdk5 in white adipose tissue, which is consistent with decreased PPARγ-Ser273 phosphorylation. Thus, the amorfrutins were more efficient than rosiglitazone in reversing the gene expression changes induced by high-fat diet. The striking inhibition of NCoR recruitment by amorfrutin 1, as revealed by cofactor recruitment analysis (SI Appendix, Fig. S2G), may play an important role in this mechanism as NCoR interacts with Cdk5 (17).
Fig. 4.
Phosphorylation of PPARγ Ser273 in visceral white adipose tissue (vWAT) of insulin-resistant mice treated with indicated compounds. (A) Exemplary Western blots, and densitometric analyses (N = 11–12 each). (B) Correlation between insulin sensitivity measured in the insulin sensitivity test (inverse area under the curve) and PPARγ Ser273 phosphorylation. Pearson correlation coefficient and P value (two-tailed) are shown (N = 35). (C) Expression of genes regulated by PPARγ phosphorylation on Ser273 (N = 8). Data are expressed as mean ± SEM. *, P ≤ 0.05 vs. vehicle.
Amorfrutins Prevent Formation of Insulin Resistence, Dyslipidemia, and Liver Steatosis Induced by HFD.
To investigate the potential of the amorfrutins to prevent development of insulin resistance, C57BL/6 mice were fed for 15 w either a low-fat diet (LFD) or a HFD in the absence or the presence of rosiglitazone (HFD+R), or low-dose amorfrutin 1 (37 mg/kg/d) from the beginning of HFD feeding (HFD+A1), respectively. Amorfrutin 1 reduced the HFD-induced weight gain by 22% without affecting food intake (SI Appendix, Fig. S8 A and B), which indicates that early intervention by low-dose natural PPARγ agonists can reduce diet-induced weight gain and development of concomitant disorders such as insulin resistance. In the corresponding control experiment, synthetic rosiglitazone reduced HFD-induced weight gain even more strongly (SI Appendix, Fig. S8B), indicating that in general early intervention with PPARγ-modulating molecules may have different effects than late-stage treatment.
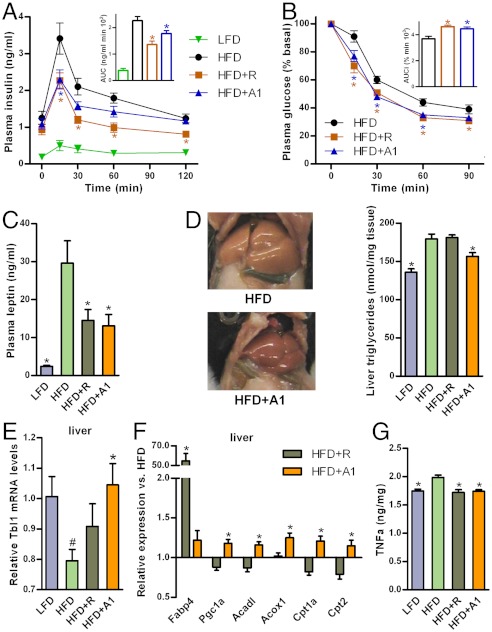
Consistently, presumably as an effect of both reduced weight gain and increased insulin sensitivity, preventive administration of amorfrutin 1 significantly improved glucose tolerance (22% decrease in insulin AUC) (Fig. 5A) and insulin sensitivity (21% increase in glucose AUCi) (Fig. 5B). Additionally, this natural product substantially diminished the rise of plasma free fatty acids and triglycerides (SI Appendix, Fig. S8C). Preventive administration of amorfrutin 1 also maintained the integrity of the pancreas, as indicated by the plasma level of proinsulin that did not increase during 15 w of HFD-feeding (SI Appendix, Fig. S8D). Furthermore, amorfrutin 1 significantly reduced the increase of plasma concentrations of the adipose derived hormone leptin (Fig. 5C), which could have in part contributed to the improved metabolic profile.
Fig. 5.
Effects of LFD or HFD without or with 4 mg/kg/d rosiglitazone (HFD+R) or 37 mg/kg/d amorfrutin 1 (HFD+A1) on glucose tolerance and insulin sensitivity in C57BL/6 mice. (A) Insulin concentrations during an oral glucose tolerance test (OGTT) after 10 w of dosing. Inlet, AUC (N = 8–12). (B) Glucose levels during an intraperitoneal insulin sensitivity test (IPIST) after 13 w of dosing. Inlet, inverse area under the curve (AUCi) (N = 8–12). (C) Plasma leptin levels after 15 w of treatment (N = 9–12). (D) Effect of treatment over 15 w on liver triglycerides (N = 6–7). (E) Change in PPAR-cofactor Tbl1 gene expression in liver of these mice (N = 12). (F) Expression of genes involved in lipogenesis and fatty acid catabolism in liver of these mice (N = 12). (G) TNFα protein concentrations in liver of C57BL/6 mice (N = 6). Data are expressed as mean ± SEM. #, P ≤ 0.05 vs. LFD. *, P ≤ 0.05 vs. HFD.
Heavily overweight mice usually develop liver steatosis due to storage of fat in central organs (32). In stark contrast to rosiglitazone, amorfrutin 1 reduced HFD-induced accumulation of liver triglycerides by approximately 50% (Fig. 5D). To shed light on the potentially underlying mechanism of amorfrutin-based prevention of liver disorders in HFD mice, we determined gene expression profiles in liver tissue. As reported recently, accumulation of triglycerides in the liver is—although the exact molecular mechanism is still unclear—causally linked to decreased expression of transducin beta-like 1 (Tbl1), a transcriptional cofactor of PPARα, which is the master regulator of fatty acid oxidation (33). Consistent with previous results, Tbl1 expression negatively correlated with liver steatosis (SI Appendix, Fig. S9A), and HFD feeding of mice led to significant reduction in Tbl1 expression compared to LFD-fed animals (Fig. 5E). Treatment with amorfrutin 1, but not rosiglitazone, increased the gene expression of Tbl1 significantly (Fig. 5E). Rosiglitazone further hyper-activated for example Fabp4 expression by a factor of 55, accounting potentially in part for the increased lipid storage in the mouse liver (34) (Fig. 5F). In contrast, amorfrutin 1 rather induced the expression of genes responsible for fatty acid oxidation (Fig. 5F), likely at least in part via regulation of Tbl1. Furthermore direct interaction of amorfrutin 1 with the liver specific nuclear receptor PPARα and potentially additional modulation of PPARβ/δ pathways (Table 1, SI Appendix, Fig. S2A) may have contributed to the observed reduction of liver steatosis (Fig. 5D) (35).
Obesity is further characterized by the expression of inflammatory mediators and macrophage recruitment to different tissues (2, 36). In HFD-fed mice amorfrutin 1 decreased inflammation and macrophage accumulation in liver and viscerale white adipose tissue (SI Appendix, Fig. S9 B–E). This anti-inflammatory effect was also reflected in reduced tumor necrosis factor α (TNFα) protein concentration in liver (Fig. 5G). Thus, amorfrutin treatment led to additional liver protective effects, including higher liver glycogen content, likely as a result of reduced insulin resistance in HFD-mice (SI Appendix, Fig. S9F) (37).
Potential Applications of the Amorfrutins.
In summary, our results suggest that the plant-derived amorfrutins function as selective PPARγ modulators that induce beneficial changes in glucose metabolism and lipid profiles. In our mouse models, we further observed a reduction of inflammatory responses to metabolic stress. In contrast to many synthetic PPARγ agonists including the thiazolidinediones, amorfrutin treatment additionally had significant liver protective effects.
Much debate in the diabetes field has focused on the various side effects of the thiazolidinediones. For example, the widely applied strong PPARγ activator rosiglitazone did not only cause weight gain but also led to increased rates of cardiovascular disease in humans after long-term treatment, at least in part as a result of fluid or water retention. Consistently, in DIO mice rosiglitazone significantly decreased plasma protein concentration, suggesting increased fluid retention, whereas the selective PPARγ agonist amorfrutin 1 did not change this physiological parameter compared to vehicle control (SI Appendix, Fig. S9G).
The fact that the amorfrutins are derived from edible plants may encourage more detailed study of their mode of action, as eventual regulatory approval for use in humans will be easier to obtain. PPARγ also plays central roles in inflammation (38) and aging processes (39). Thus it is possible that amorfrutin treatment could benefit other ageing-associated or inflammatory disorders, and cancer. Clearly, as for all potentially health-beneficial molecules, further in-depth studies including human studies will be required to assess the therapeutic potential of the amorfrutins. In general, further mechanistic studies on the PPARs will help to better describe the effects of structually new PPAR-modulating compounds.
Our discovery of the highly antidiabetic legume-derived amorfrutins highlights the fascinating structural and biological properties of natural products, and suggests that dietary small molecules represent a largely unexplored resource for pharmaceutical and nutraceutical development. As ingredients of functional food or plant-based medicine for inhibiting insulin resistance and liver steatosis, dietary molecules such as the amorfrutins may have a great potential to be accepted by the consumers and patients as emerging alternatives to conventional treatment with synthetic drugs.
Materials and Methods
Compounds were purchased from the following sources: rosiglitazone (Cayman, Biozol), nTZDpa (Tocris, Biozol), pioglitazone (Sigma Aldrich), telmisartan, troglitazone, GW0742, GW7647 (all from Sigma-Aldrich), amorfrutin 1 (NP-003520), amorfrutin 2 (NP-003521), amorfrutin 3 (NP-006430), amorfrutin 4 (NP-009525), natural product library (all available from Analyticon Discovery) (SI Appendix, SI Methods). For screening of ligands we established a mass spectrometry based heterogeneous binding assay that is particularly useful for rapid screening of natural product libraries containing many autofluorescent compounds and new target proteins for which no specific assay is available (SI Appendix, SI Methods). For in vivo testing, we developed a method for the synthesis of multigram quantities of pure amorfrutin 1 (SI Appendix, SI Methods). PPARγ ligands were further characterized using competitive binding assays (Lanthascreen, Invitrogen), coactivator recruitment assays (Lanthascreen, Invitrogen and Cerep Inc.) and reporter gene assays (GeneBLAzer, Invitrogen) (SI Appendix, SI Methods).
Effects of PPARγ ligands were investigated in murine 3T3-L1 cells (ATCC, LGC Promochem) and human primary adipocytes (Zen-Bio, BioCat). Gene expression was measured with quantitative PCR (Applied Biosystems) and Expression BeadChips (Illumina) (SI Appendix, SI Methods). A panel of ADMET assays were performed to assess pharmacokinetic properties of the amorfrutins (SI Appendix, SI Methods).
Animal studies have been validated and approved by the State Office of Health and Social Affairs Berlin and were carried out according internationally approved guidelines. For the therapy study we subjected DIO mice to a short-term-medium-dose treatment. Male C57BL/6 mice at age of 6 w were fed with HFD for 12 w to induce obesity and insulin resistance. The mice were then weighed and distributed equally to three groups (N = 13 each). Mice were fed over 3 w with HFD without compound (vehicle), HFD with 4 mg/kg/d rosiglitazone or HFD with 100 mg/kg/d amorfrutin 1. To test the compounds in a diabetic model, leptin receptor deficient db/db mice (Charles River Laboratories) at age of 9 w were fed with standard diet without compound (vehicle), with 4 mg/kg/d rosiglitazone or 100 mg/kg/d amorfrutin 1 over 3 w. For the prevention study we designed a long-term low-dose study in C57BL/6 mice. Therefore, male C57BL/6 mice at age of 9 w were weighed and distributed equally to four groups (N = 12). Mice were fed over 15 weeks with either LFD (10 kcal% fat), HFD (60 kcal% fat) or HFD with 4 mg/kg/d HFD+R or 37 mg/kg/d amorfrutin 1 (HFD+A1) (SI Appendix, SI Methods). Further experimental details can be found online (SI Appendix, SI Methods).
Supplementary Material
Acknowledgments.
We thank A. Schoop and M. Ristow for fruitful discussions, suggestions, and material provided. We are deeply grateful to K. Hansen, U. Schröder, and L. Hartmann for technical assistance during the animal studies. We thank R. Feldmann for critical reading of the manuscript. This work is part of the Ph.D. theses of C.W. and J.C.d.G. Our work is supported by the German Ministry for Education and Research (BMBF, grant number 0315082), the National Genome Research Net (NGFN, grant number 01 GS 0828), the European Union [FP7/2007-2013, under grant agreement number [HEALTH-F4-2008-201418], entitled READNA, and [FP7/2007-2011], under grant agreement n° 262055 (ESGI)], the TRIAD foundation (to F.C.S.), and the Max Planck Society.
Footnotes
Conflict of interest statement: The authors declare no conflict of interest, with the exception of K. S. and L. M.-K. from Analyticon Discovery, a company that sells natural products.
This article is a PNAS Direct Submission.
Data deposition: The X-ray crystallography data have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2yfe). The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE28384).
See Commentary on page 7136.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116971109/-/DCSupplemental.
References
- 1.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Huang TH, Kota BP, Razmovski V, Roufogalis BD. Herbal or natural medicines as modulators of peroxisome proliferator-activated receptors and related nuclear receptors for therapy of metabolic syndrome. Basic Clin Pharmacol. 2005;96:3–14. doi: 10.1111/j.1742-7843.2005.pto960102.x. [DOI] [PubMed] [Google Scholar]
- 5.Hontecillas R, O’Shea M, Einerhand A, Diguardo M, Bassaganya-Riera J. Activation of PPAR gamma and alpha by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J Am Coll Nutr. 2009;28:184–195. doi: 10.1080/07315724.2009.10719770. [DOI] [PubMed] [Google Scholar]
- 6.Guri AJ, Hontecillas R, Si H, Liu D, Bassaganya-Riera J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 2007;26:107–116. doi: 10.1016/j.clnu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. Regulators restrict Avandia in the US and suspend it in the EU. Nat Rev Drug Discov. 2010;9:828–828. [Google Scholar]
- 8.Rosen CJ. Revisiting the rosiglitazone story--lessons learned. N Engl J Med. 2010;363:803–806. doi: 10.1056/NEJMp1008233. [DOI] [PubMed] [Google Scholar]
- 9.Argmann CA, Cock TA, Auwerx J. Peroxisome proliferator-activated receptor gamma: the more the merrier? Eur J Clin Invest. 2005;35:82–92. doi: 10.1111/j.1365-2362.2005.01456.x. discussion 80. [DOI] [PubMed] [Google Scholar]
- 10.Cock TA, Houten SM, Auwerx J. Peroxisome proliferator-activated receptor-gamma: too much of a good thing causes harm. EMBO Rep. 2004;5:142–147. doi: 10.1038/sj.embor.7400082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 12.Choi JH, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones D. Potential remains for PPAR-targeted drugs. Nat Rev Drug Discov. 2010;9:668–669. doi: 10.1038/nrd3271. [DOI] [PubMed] [Google Scholar]
- 14.Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–165. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- 15.Muller M, Kersten S. Nutrigenomics: goals and strategies. Nat Rev Genet. 2003;4:315–322. doi: 10.1038/nrg1047. [DOI] [PubMed] [Google Scholar]
- 16.Cho N, Momose Y. Peroxisome proliferator-activated receptor gamma agonists as insulin sensitizers: from the discovery to recent progress. Curr Top Med Chem. 2008;8:1483–1507. doi: 10.2174/156802608786413474. [DOI] [PubMed] [Google Scholar]
- 17.Li P, et al. Adipocyte NCoR knockout decreases PPARgamma phosphorylation and enhances PPARgamma activity and insulin sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolte RT, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395:137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 19.Cronet P, et al. Structure of the PPARalpha and -gamma ligand binding domain in complex with AZ 242; ligand selectivity and agonist activation in the PPAR family. Structure. 2001;9:699–706. doi: 10.1016/s0969-2126(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 20.Bruning JB, et al. Partial agonists activate PPARgamma using a helix 12 independent mechanism. Structure. 2007;15:1258–1271. doi: 10.1016/j.str.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Berger JP, et al. Distinct properties and advantages of a novel peroxisome proliferator-activated protein [gamma] selective modulator. Mol Endocrinol. 2003;17:662–676. doi: 10.1210/me.2002-0217. [DOI] [PubMed] [Google Scholar]
- 22.Schupp M, et al. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–3452. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- 23.Fischer JJ, et al. Dasatinib, imatinib and staurosporine capture compounds—Complementary tools for the profiling of kinases by Capture Compound Mass Spectrometry (CCMS) J Proteomics. 2011;10:160–168. doi: 10.1016/j.jprot.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Ong SE, et al. Identifying the proteins to which small-molecule probes and drugs bind in cells. Proc Natl Acad Sci USA. 2009;106:4617–4622. doi: 10.1073/pnas.0900191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dat NT, et al. Phenolic constituents of Amorpha fruticosa that inhibit NF-kappaB activation and related gene expression. J Nat Prod. 2008;71:1696–1700. doi: 10.1021/np800383q. [DOI] [PubMed] [Google Scholar]
- 26.Lamb J, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 27.Larsen PJ, et al. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 2003;52:2249–2259. doi: 10.2337/diabetes.52.9.2249. [DOI] [PubMed] [Google Scholar]
- 28.Alegret M, et al. Relationship between plasma lipids and palmitoyl-CoA hydrolase and synthetase activities with peroxisomal proliferation in rats treated with fibrates. Br J Pharmacol. 1994;112:551–556. doi: 10.1111/j.1476-5381.1994.tb13109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doshi LS, Brahma MK, Bahirat UA, Dixit AV, Nemmani KV. Discovery and development of selective PPAR gamma modulators as safe and effective antidiabetic agents. Expert Opin Inv Drug. 2010;19:489–512. doi: 10.1517/13543781003640169. [DOI] [PubMed] [Google Scholar]
- 30.Ryan KK, et al. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu M, et al. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchesini G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 33.Kulozik P, et al. Hepatic deficiency in transcriptional cofactor TBL1 promotes liver steatosis and hypertriglyceridemia. Cell Metab. 2011;13:389–400. doi: 10.1016/j.cmet.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Spiegelman BM, Frank M, Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983;258:10083–10089. [PubMed] [Google Scholar]
- 35.Artis DR, et al. Scaffold-based discovery of indeglitazar, a PPAR pan-active anti-diabetic agent. Proc Natl Acad Sci USA. 2009;106:262–267. doi: 10.1073/pnas.0811325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 37.Kuda O, et al. Prominent role of liver in elevated plasma palmitoleate levels in response to rosiglitazone in mice fed high-fat diet. J Physiol Pharmacol. 2009;60:135–140. [PubMed] [Google Scholar]
- 38.Rizzo G, Fiorucci S. PPARs and other nuclear receptors in inflammation. Curr Opin Pharmacol. 2006;6:421–427. doi: 10.1016/j.coph.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Argmann C, et al. Ppargamma2 is a key driver of longevity in the mouse. PLoS Genet. 2009;5:e1000752. doi: 10.1371/journal.pgen.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.