Abstract
During pregnancy, uterine quiescence is maintained by increased progesterone receptor (PR) activity, but labor is facilitated by a series of events that impair PR function. Previously, we discovered that miR-200 family members serve as progesterone (P4)-modulated activators of contraction-associated genes in the pregnant uterus. In this study, we identified a unique role for miR-200a to enhance the local metabolism of P4 in myometrium and, thus, decrease PR function during the progression toward labor. miR-200a exerts this action by direct repression of STAT5b, a transcriptional repressor of the P4-metabolizing enzyme 20α-hydroxysteroid dehydrogenase (20α-HSD). We observed that miR-200a expression increased and STAT5b expression coordinately decreased in myometrium of mice as they progressed to labor and in laboring myometrium from pregnant women. These changes were associated with a dramatic increase in expression and activity of 20α-HSD in laboring myometrium from mouse and human. Notably, overexpression of miR-200a in cultured human myometrial cells (hTERT-HM) suppressed STAT5b and increased 20α-HSD mRNA levels. In uterine tissues of ovariectomized mice injected with P4, miR-200 expression was significantly decreased, STAT5b expression was up-regulated, and 20α-HSD mRNA was decreased, but in 15 d postcoitum pregnant mice injected with the PR antagonist RU486, preterm labor was associated with increased miR-200a, decreased STAT5b, and enhanced 20α-HSD expression. Taken together, these findings implicate miR-200a as an important regulator of increased local P4 metabolism in the pregnant uterus near term and provide insight into the importance of miR-200s in the decline in PR function leading to labor.
It has long been appreciated that progesterone (P4) acting through progesterone receptor (PR) plays a critical role in maintaining uterine quiescence throughout most of pregnancy (see refs. 1 and 2 for review). The finding in rodents that circulating maternal P4 levels decline precipitously near term (3) has led to the concept that labor is associated with P4 withdrawal. On the other hand, in humans and guinea pigs, circulating P4 levels remain elevated throughout pregnancy and into labor, as do myometrial levels of PR (4, 5). Nonetheless, treatment with PR antagonists, mifepristone (RU486) or onapristone, can cause increased cervical ripening and spontaneous labor or enhanced sensitivity to labor induction by oxytocin or prostaglandins (6–10). It should be noted that even in mice maternal P4 levels at term remain well above the Kd for binding to PR. These collective findings have led to the concept that parturition in all species is initiated by a concerted series of biochemical mechanisms that antagonize the ability of the P4/PR to regulate target genes in the uterus and cervix that maintain myometrial quiescence. These mechanisms may include altered expression of PR coregulators (11–13), antagonistic interaction of PR with the inflammatory transcription factor NF-κB (14, 15) [which is activated in the myometrium near term (16)], increased expression of inhibitory PR isoforms (17), and enhanced local metabolism of P4 to inactive products (18, 19). Indeed, increased P4 metabolism by the pregnant uterus approaching term has been observed in a number of species (19–23). In myometrium of pregnant women at term, there is a dramatic increase in the ratio of 20α-dihydroprogesterone (20α-OHP) to P4 (23). 20α-OHP is an inactive metabolite of P4 generated by 20α-hydroxysteroid dehydrogenase (20α-HSD), a member of the aldo-ketoreductase (AKR) superfamily (24).
Recently, we have uncovered a role for microRNAs (miRNAs, miRs) in the regulation of genes that influence uterine quiescence/contractility during pregnancy and labor (25). We identified a conserved miRNA family, the miR-200 family, that is highly up-regulated at term in myometrium of mice and humans, as well as two coordinately down-regulated targets of miR-200, the zinc finger E-box binding homeobox proteins ZEB1 and ZEB2 (25). We further demonstrated that during pregnancy ZEB1 is directly up-regulated by P4/PR. Importantly, ZEB1 and ZEB2 inhibit expression of the contraction-associated genes, oxytocin receptor (OXTR) and connexin-43 (CX43) and block oxytocin-induced myometrial contractility. Near term, the decline in PR function results in decreased expression of ZEB1/2, an induction of miR-200 family expression, and increased transcription of contraction-associated genes leading to labor (25).
Interestingly, one of the members of the miR-200 family, miR-200a, is predicted by TargetScan analysis (http://www.targetscan.org/) to target the transcription factor, STAT5b, which serves as a P4-responsive transcriptional repressor of 20α-HSD in reproductive tissues (26, 27). Stat5b deficiency in mice resulted in pregnancy loss during midgestation. This finding was correlated with increased expression of ovarian 20α-HSD and decreased circulating P4 (27). Furthermore, the abortion rate in Stat5b-deficient mice was partially corrected by combined 20α-HSD deficiency (27). In the present study we have demonstrated that STAT5b is a bonafide target of miR-200a and observed that up-regulation of miR-200a within the myometrium of laboring mice and humans was associated with decreased STAT5b expression and increased expression and activity of 20α-HSD. P4 treatment significantly decreased miR-200a expression, up-regulated STAT5b mRNA and protein, and decreased 20α-HSD mRNA in mouse uterine tissues. Conversely, RU486 induction of preterm labor in mice was associated with increased miR-200a expression, decreased STAT5b, and induction of 20α-HSD expression. Taken together, these findings suggest that miR-200a plays a key role in the induction of local P4 metabolism in the pregnant uterus near term and in the decline in PR function leading to labor.
Results
miR-200a Is Up-Regulated During Late Gestation and Labor.
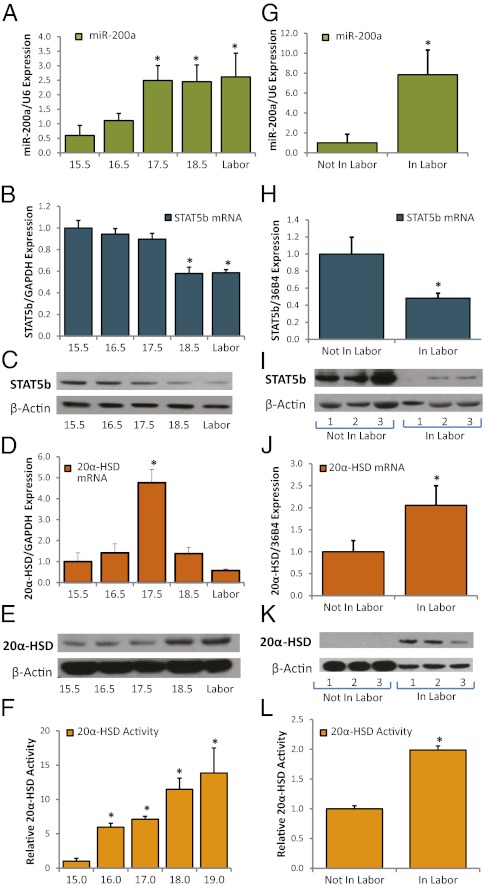
In previous microarray analysis of quiescent pregnant mouse myometrium at 15.5 d postcoitum (dpc) vs. contractile myometrium at 18.5 dpc, we observed that the miR-200 family was significantly up-regulated toward term but their targets, ZEB1 and ZEB2, were coordinately down-regulated (25). Quantitative RT-PCR (qRT-PCR) analysis of pregnant mouse myometrium during late gestation previously confirmed increased expression near term of the two miR-200 family members that were most significantly increased on the microarray, miR-200b and miR-429 (25). These miR-200 family members are clustered together with miR-200a on mouse chromosome 4 and on human chromosome 1. Notably, miR-200b/200a/429 are encoded within a 7.5-kb polycistronic primary miRNA transcript that was shown to be repressed by ZEB1 (28), suggesting their coordinate regulation. The 3′ UTR of ZEB1 contains binding sites for miR-200b/200a/429, which allow these miRNAs to collectively contribute to inhibition of ZEB1 expression in various tissues (25). Indeed, qRT-PCR of myometrial RNA from pregnant mice at 15.5 dpc to labor established that miR-200a expression increased in a pattern similar to that of miR-200b and miR-429 (Fig. 1A) and in a manner reciprocal to expression of ZEB1 (25).
Fig. 1.
miR-200a, STAT5b, and 20α-HSD are coordinately regulated during late gestation and labor in mouse and human myometrium. (A) qRT-PCR revealed that miR-200a expression was significantly increased in myometrium as mice progressed to labor in a pattern similar that previously observed for miR-200b and miR-429 (25). STAT5b mRNA (B) and protein (C) expression were coordinately decreased between 15.5 dpc and labor. 20α-HSD mRNA levels were markedly increased in pregnant mouse uterus between 15.5 and 17.5 dpc and subsequently declined (D), but 20α-HSD protein (E) and activity (F) progressively increased in mouse myometrium between 15.5 dpc and labor. qRT-PCR values in A, B, and D are the mean ± SEM of data from three independent gestational series of mice. Expression of each miR/mRNA was determined by qRT-PCR, normalized to U6/GAPDH, and expressed as arbitrary units. Immunoblots shown in C and E are representative of findings in three gestational series of mice. The 20α-HSD activity data shown in F are the mean ± SEM of values from three independent gestational series of mice. *Significantly different from values at 15.5 dpc (P < 0.05). (G) qRT-PCR revealed that miR-200a expression was increased in term myometrium of women in labor, compared with tissues from women not in labor. (H) STAT5b mRNA was decreased in myometrium from women in labor, compared with tissues from women not in labor. (I) Immunoblot analysis of STAT5b protein expression in myometrial samples from three women not in labor and three women in labor at term. Endogenous β-actin was analyzed as a loading/transfer control. (J) 20α-HSD mRNA was increased in term myometrium of women in labor, compared with women not in labor. (K) Immunoblot of 20α-HSD protein levels in myometrial samples from three women not in labor and three women in labor at term. Endogenous β-actin was analyzed as a loading/transfer control. (L) 20α-HSD activity was increased in homogenates of term myometrium from women in labor compared with homogenates from women not in labor. Data are the mean ± SEM of values from five women not in labor and five women in labor at term. Data shown in G, H, and J are the mean ± SEM of values from 12 myometrial samples from women not in labor and 11 myometrial samples from women in labor. *Significantly (P < 0.05) different from tissues of women not in labor.
Transcriptional Repressor STAT5b and Its Predicted Target 20α-HSD Are Reciprocally Regulated with miR-200a in Late Gestation and Labor.
Using TargetScan prediction software, we identified two putative binding sites for miR-200a in the 3′ UTR of STAT5b, a P4-responsive transcription factor that inhibits transcription of 20α-HSD (27, 29). In mice, STAT5b deficiency caused pregnancy loss during midgestation; this was correlated with increased expression of ovarian 20α-HSD and decreased circulating levels of P4 (27). In contrast, 20α-HSD−/− mice manifest delayed parturition and increased fetal demise at birth (27, 30). In one of these studies, circulating P4 levels declined at term in a manner similar to wild-type (30), suggesting that local P4 metabolism in PR target tissues, such as the uterus, may be critical. In the present study, qRT-PCR and immunoblotting revealed a decline in STAT5b mRNA and protein expression in the mouse myometrium between 15.5 dpc and labor (Fig. 1 B and C). This gestational decline in STAT5b expression during late gestation was associated with a dramatic, but transient increase in 20α-HSD mRNA (Fig. 1D) that resulted in a sustained increase in both 20α-HSD protein (Fig. 1E) and enzymatic activity (Fig. 1F).
To determine whether this temporal pattern of regulation is conserved from mouse to human, we analyzed expression of miR-200a and STAT5b, as well as 20α-HSD expression and activity in myometrial biopsies from women at term who were either in labor or not in labor. Similar to our findings in the mouse, myometrial miR-200a was increased (Fig. 1G), but STAT5b mRNA (Fig. 1H) and protein (Fig. 1I) expression were decreased in myometrium from women in labor compared with those not in labor. In contrast, 20α-HSD expression (Fig. 1 J and K) and activity (Fig. 1L) were increased in myometrium from women in labor, compared with women not in labor. These findings corroborate previous observations of increased levels of the 20α-HSD metabolite, 20α-OHP, in the myometrium from pregnant women at term (23).
STAT5b Negatively Regulates 20α-HSD in Myometrium and Is a Direct Target of miR-200a.
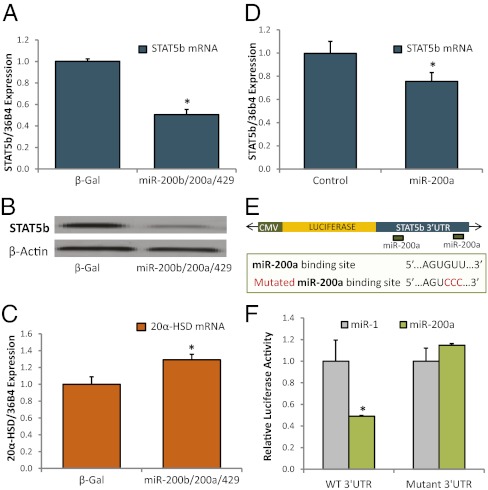
Although previous studies indicate that STAT5b negatively regulates 20α-HSD expression in the ovary (27), this relationship has not been established in the myometrium. To investigate whether STAT5b regulates 20α-HSD expression in the myometrium, we used an immortalized human myometrial cell line (hTERT-HM) (31). Transfection of hTERT-HM cells with a CMV-driven expression plasmid for STAT5b augmented STAT5b expression at the mRNA and protein levels and caused a decrease in 20α-HSD mRNA levels (Fig. 2 A–C). Conversely, 20α-HSD mRNA expression was increased as a consequence of siRNA-mediated knockdown of STAT5b in hTERT-HM (Fig. 2 D–F).
Fig. 2.
STAT5b negatively regulates 20α-HSD in human myometrial cells. Binding of endogenous STAT5b to response elements in the 20α-HSD/AKR1C18 promoter declines in pregnant mouse myometrium at term. (A–C) hTERT-HM cells were transfected with a pCMV–STAT5b expression plasmid or with an empty vector (control). Levels of STAT5b mRNA (A) and protein (B) were increased after 24 h. Up-regulation of STAT5b was associated with a significant suppression of 20α-HSD mRNA levels (C). (D–F) hTERT-HM cells were transfected with STAT5b siRNA or with a scrambled siRNA (control). Levels of STAT5b mRNA (D) and protein (E) were significantly reduced after 72 h. (F) The decline in STAT5b expression was associated with a significant induction of 20α-HSD mRNA levels. Data shown in A, C, D, and F are the mean ± SD of values from three independent experiments, each conducted in triplicate. *Significantly (P < 0.05) different from control. (G) Binding of endogenous STAT5b to the AKR1C18 promoter is significantly decreased in pregnant mouse myometrium between 15.5 dpc and just before labor. ChIP using antibodies to STAT5b was used to assess binding of endogenous STAT5b to the region of the AKR1C18 promoter that contains putative STAT5b response elements. STAT5b binding was quantified by PCR, normalized to input and expressed as fold-increase over binding using nonimmune IgG. Data are the mean ± SEM (*P < 0.05; n = 5 mice per group).
Previous studies suggest that STAT5b inhibits 20α-HSD expression by binding to a putative STAT5b response elements within the proximal 20α-HSD promoter. In those studies, cotransfection of reporter constructs containing −2,500 bp of the 20α-HSD 5′-flanking region fused to luciferase together with an expression vector for constitutively active Stat5b caused substantial inhibition of 20α-HSD promoter activity (32). This genomic region contains putative STAT5b response elements at −155 and −547 bp. To assess gestational changes in binding of endogenous STAT5b to the proximal region of the 20α-HSD promoter that contains these putative STAT5b response elements, we performed ChIP assays comparing myometrial tissues from pregnant mice at 15.5 dpc to myometrium collected just before labor at 18.5 dpc. Our findings revealed that STAT5b binding to the 20α-HSD promoter was relatively high at 15.5 dpc and declined markedly just before labor (Fig. 2G). Taken together, these data suggest that during most of pregnancy, STAT5b-mediated inhibition of 20α-HSD expression within the myometrium helps to maintain high local P4 levels and sustains uterine quiescence. However, during the transition to labor, STAT5b levels decline within the myometrium allowing for increased expression of 20α-HSD and enhanced local P4 metabolism.
As mentioned, the 3′ UTR of STAT5b contains two putative binding sites for miR-200a; however, direct binding of miR-200a to the STAT5b 3′ UTR and miR-200 inhibition of STAT5b expression have not previously been tested. To determine whether miR-200a inhibits endogenous STAT5b expression in human myometrial cells, hTERT-HM cells were infected with recombinant adenovirus expressing miR-200b/200a/429. Following transduction of miR-200 family members, STAT5b expression was repressed at the mRNA (Fig. 3A) and protein (Fig. 3B) levels, but 20α-HSD mRNA expression was up-regulated (Fig. 3C). Moreover, transfection of the hTERT-HM cells with miR-200a mimics also significantly decreased STAT5b mRNA expression (Fig. 3D). To assess whether miR-200a directly targets STAT5b, we transfected COS7 cells with miR-200a mimics and a luciferase reporter plasmid comprised of a portion of the STAT5b 3′ UTR, containing two putative miR-200a binding sites subcloned downstream of the luciferase gene (Fig. 3E). A miR-1 mimic was cotransfected as a control in these experiments, because miR-1 is not predicted to target STAT5b by TargetScan analysis (http://www.targetscan.org/). Enhanced expression of miR-200a significantly repressed luciferase reporter activity, but transfection of miR-1 had no effect (Fig. 3F). In cells transfected with a luciferase reporter construct in which both miR-200a binding sites were mutated, this repression was lost (Fig. 3F).
Fig. 3.
miR-200s directly target STAT5b and inhibit 20α-HSD expression in human myometrial cells. (A–C) hTERT-HM cells were infected overnight with recombinant adenoviruses expressing the miR-200b-200a-429 cluster. After 72 h, miR-200 family overexpression inhibited STAT5b mRNA (A) and protein (B) and increased 20α-HSD mRNA (C) levels. (D) Transfection of hTERTs with miR-200a mimics also caused a significant reduction in STAT5b mRNA expression. Data shown in A, C, and D are the mean ± SD of values from three independent experiments, each conducted in triplicate. *Significantly (P < 0.05) different from control or β-Gal. (E) Schematic diagram of luciferase reporter containing the subcloned 3′ UTR of STAT5b with miR-200a binding sites. Wild-type and mutated binding site sequences are also shown. (F) Luciferase assays were conducted in COS7 cells cotransfected with either miR-200a or miR-1 (as control) mimics and the luciferase:STAT5b-3′ UTR reporter containing WT or mutant miR-200 binding sites. Luciferase activity in cells cotransfected with miR-200a are plotted relative to miR-1, which had no effect on reporter activity and was assigned an arbitrary value of “1.” Values are the mean ± SD of three independent experiments, each conducted in triplicate. *Significantly (P < 0.05) different from cells cotransfected with miR-1. Cotransfection data indicate that miR-200a directly targets the STAT5b 3′ UTR. Repression was lost when the putative miR-200a binding sites were mutated.
Progesterone Regulates miR-200a, STAT5b, and 20α-HSD in the Uterus.
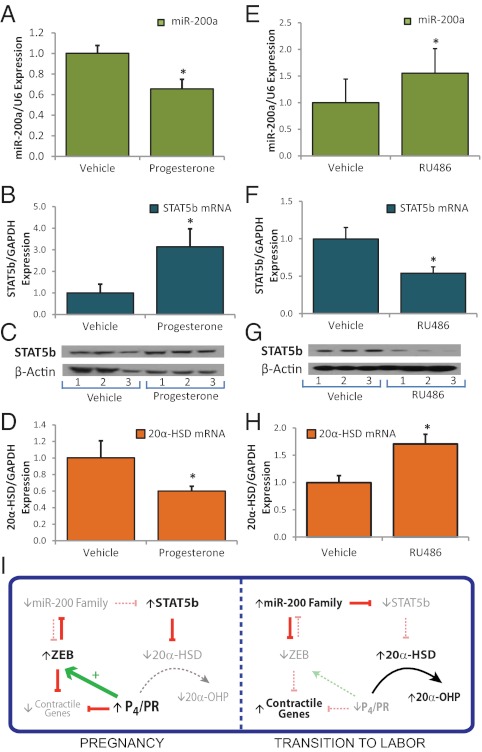
As mentioned, ZEBs and miR-200 family members exist in a double-negative feedback loop, whereby miR-200s suppress expression of ZEB1/2 posttranscriptionally and ZEBs inhibit miR-200 transcription (28, 33). Previously, we observed that P4 treatment of mice and cultured myometrial cells specifically induced ZEB1, resulting in inhibition of miR-200b/429 expression (25). P4 was previously reported to repress 20α-HSD expression in rat corpus luteum (34, 35) and to induce STAT5 expression in human breast cancer cells (26). To test the hypothesis that P4/PR-mediated repression of miR-200a results in enhanced expression of STAT5b and inhibition of 20α-HSD in myometrium, we analyzed effects of P4 injection in uterine tissues of ovariectomized mice after 24 h. We observed that P4 treatment inhibited miR-200a expression in myometrium (Fig. 4A). This inhibition was associated with a P4-mediated induction of STAT5b mRNA (Fig. 4B) and protein (Fig. 4C) and a coordinate repression of 20α-HSD mRNA (Fig. 4D). In contrast, treatment of 15.5 dpc mice with the PR antagonist RU486 to induce preterm labor significantly increased miR-200a expression (Fig. 4E), inhibited STAT5b mRNA (Fig. 4F) and protein (Fig. 4G), and increased 20α-HSD expression (Fig. 4H). These findings suggest that P4/PR-mediated repression of miR-200 expression caused by induction of ZEB1 maintains uterine quiescence, in part, via up-regulation of STAT5b and subsequent repression of 20α-HSD. Conversely, the increase in myometrial miR-200 expression caused by RU486 treatment inhibits STAT5b and induces 20α-HSD, resulting in enhanced local metabolism of P4 and a further decline in PR function leading to preterm labor.
Fig. 4.
P4/PR regulates 20α-HSD in the uterus via modulation of the miR-200-STAT5b axis. (A–D) Ovariectomized mice were injected with P4 (1 mg) or with vehicle; uterine tissues were harvested 24 h later. P4 treatment inhibited miR-200a expression (A) induced STAT5b mRNA (B) and protein (C) and inhibited 20α-HSD mRNA (D). (E–H) Timed pregnant 15.5 dpc mice were injected subcutaneously with the PR antagonist RU486 (200 μg) or with vehicle. RU486 injected mice were killed upon the preterm birth of one pup; a vehicle-injected control was killed immediately afterward and myometrial tissues were isolated. RU486 treatment increased miR-200a (E), inhibited STAT5b mRNA (F) and protein (G), and induced 20α HSD mRNA (H) expression. Expression of each miRNA/mRNA was determined by qRT-PCR, normalized to U6/GAPDH, and expressed as fold-change over vehicle-treated controls. Mean ± SEM values are shown. Student t test, *P < 0.05, n = 5 mice per treatment group. (I) Schematic diagram of the regulation of PR function during pregnancy and labor via the miR-200/STAT5b/20α-HSD axis.
Discussion
The molecular mechanisms that maintain quiescence of the myometrium throughout most of pregnancy and mediate its conversion into a synchronously contractile unit, culminating in parturition, remain incompletely understood. It is clear that P4 acting through PR is essential for maintaining myometrial quiescence, and an enhanced inflammatory response caused by signals from mother and fetus promotes the progression to labor (1). P4/PR mediates uterine quiescence, in part by suppressing NF-κB activation of contraction-associated genes (15). Our recent studies revealed that P4/PR also contributes to the maintenance of uterine quiescence during pregnancy via up-regulation of the transcription factor, ZEB1, which inhibits expression of the contraction-associated genes, OXTR and CX43, and suppresses the miR-200 family to promote further up-regulation of ZEB1 and ZEB2 (25).
In rodents, labor is heralded by a pronounced decline in P4 production by the corpus luteum, whereas in humans, circulating P4 remains elevated throughout pregnancy and into labor because of increased placental P4 synthesis (4, 5). As mentioned above, even in rodents, levels of circulating P4 at term remain higher than the Kd for its binding to PR. This finding suggests that a decline in PR function and increased local metabolism of P4 near term are of paramount importance for the initiation of parturition in all mammals. Notably, in the present study we discovered that the P4-metabolizing enzyme 20α-HSD is markedly up-regulated in pregnant mouse and human myometrium near term and elucidated a unique role for the miR-200 family member, miR-200a, in its regulation. Our findings indicate that within myometrium, miR-200a directly targets STAT5b (Fig. 3), a transcription factor known to suppress 20α-HSD expression (27). As noted above, Stat5b-deficient mice manifest midgestation pregnancy loss, correlated with increased ovarian 20α-HSD expression and decreased circulating P4 levels (27). Although the midgestation abortions in the STAT5b null mice were presumed to be caused by enhanced ovarian 20α-HSD expression, circulating P4 levels in these animals remained higher than the Kd for binding to PR (36). This finding suggests the potential importance of STAT5b and 20α-HSD in the control of local P4 metabolism in tissues such as myometrium before labor. Notably, STAT5b expression declined in mouse and human myometrium during the progression to labor in concert with the up-regulation of miR-200a and induction of 20α-HSD expression and activity (Fig. 1). Identical findings were obtained upon induction of preterm labor in mice using RU486 (Fig. 4 E–H) and in an inflammatory preterm labor model using intra-amniotic injection of LPS (Fig. S1). Because the seed sequence of miR-200a is nearly identical to that of miR-200b and miR-429, which are encoded within the same transcript, it is likely that other members of the miR-200 family similarly target myometrial STAT5b and regulate 20α-HSD. Furthermore, miR-200a also directly targets ZEB1 and ZEB2 (37), suggesting that near term, increasing levels of miR-200a act cooperatively with miR-200b/429 to inhibit ZEB expression, allowing for derepression of contraction-associated genes.
Increased P4 metabolism by the pregnant uterus approaching term has been observed in a number of species, including humans. In rats (20), mice (19), sheep (21), and guinea pigs (22), there is a significant decline in local P4 levels in uterine tissues near term. In myometrium of pregnant women at term, there is a dramatic decrease in the ratio of P4 to 20α-OHP, an inactive metabolite of P4 generated by 20α-HSD, compared with women in early pregnancy (23). Indeed, we observed a significant increase in 20α-HSD mRNA and protein expression and activity in myometrium from women in labor, compared with those not in labor (Fig. 1 J–L). In pregnant mice, we found that 20α-HSD mRNA expression increased dramatically in the myometrium at 17.5 dpc and then declined (Fig. 1D). On the other hand, 20α-HSD protein expression and enzyme activity continued to increase to term and into labor (Fig. 1 E and F), suggesting that this enzyme serves an important role in local P4 metabolism during the transition to labor.
As mentioned, 20α-HSD is a member of the AKR superfamily of NADPH-dependent oxidoreductases, comprising >100 proteins, which catalyze the reduction of a wide variety of substrates, including steroid hormones, prostaglandins, carbohydrates, and xenobiotics (38) (http://www.med.upenn.edu/akr/index.html). 20α-HSD belongs to the steroid-metabolizing HSD subgroup of the AKR1 family. In humans, there are four clustered HSD genes on chromosome 10, corresponding to 20α-HSD (AKR1C1), 3α-HSD type III (AKR1C2), 17β-HSD type V (AKR1C3), and 3α-HSD type I (AKR1C4). In mice, there are four distinct HSD genes that correspond to their human gene counterparts in the order listed above (AKR1C18, AKR1C6, AKR1C12, and AKR1C13), as well as four novel HSDs, all clustered on chromosome 13 (39). The AKR family members in human and mouse that metabolize P4 with the highest activity are AKR1C1 and AKR1C18, respectively (40).
In rats and mice, 20α-HSD is expressed at relatively high levels in the corpus luteum of the ovary, and increases in 20α-HSD expression during the estrous cycle and at the end of pregnancy have been associated with functional luteolysis (30, 41). The correlation of the increase in corpus luteum 20α-HSD expression and the fall in circulating P4 levels at the end of pregnancy in rodents has led to the view that this enzyme plays a crucial role in the decline in ovarian P4 secretion leading to parturition. Consequently, mice carrying targeted deletions in the 20α-HSD gene manifest a delay in parturition of several days (27, 30). Although in one of these studies, circulating P4 levels in the 20α-HSD null mice were sustained at levels three- to four-times higher than those of wild-type mice at term (27), in the other, P4 levels were only marginally elevated in the knockouts, compared with wild-type (30). These findings suggest that the decline in ovarian production of P4 at term may not be sufficient and that actions of 20α-HSD to catalyze local metabolism of P4 also may be critical for the decline in PR function leading to labor. This paradigm is exemplified in women, where circulating P4 levels remain elevated into labor. Importantly, mice deficient in the P4 metabolizing enzyme 5α-reductase type I fail to deliver because of a lack of cervical ripening (19). This parturition defect, which is manifest despite a decline in circulating P4, is caused by impaired local metabolism of P4 within the cervix. Based on these collective findings, we suggest that increased local metabolism of P4 in the myometrium near term by 20α-HSD may contribute to the loss of PR function leading to labor.
As depicted in Fig. 4I, our collective findings suggest that miR-200a serves a critical role in the regulation of P4 metabolism in the myometrium during pregnancy and labor. During pregnancy, high levels of P4/PR function increase expression of transcription factor ZEB1, which inhibits expression of miR-200a and other members of the miR-200 family (25), as described above. The decreased levels of miR-200 enhance expression of STAT5b, which represses 20α-HSD to sustain elevated P4 levels within the myometrium. During the transition to term and preterm labor, an increased inflammatory response promotes a decline in PR function in myometrium (11, 14, 17, 42), resulting in decreased levels of ZEB, which releases repression of the miR-200 family. The increased levels of miR-200a inhibit STAT5b expression, releasing repression of 20α-HSD. The increase in 20α-HSD, in turn, catalyzes metabolism of P4 to reduce local P4 levels in the myometrium and further promote the progression of labor. The decline in PR function may act in a positive feed-forward manner to further increase miR-200 expression, suppress STAT5b, and induce 20α-HSD. Taken together, these findings highlight another important function for the miR-200 family in the timing of parturition and emphasize its potential importance as a therapeutic target for prevention of preterm labor.
Materials and Methods
Mouse and Human Myometrial Preparations.
Myometrial tissues from timed-pregnant mice and pregnant women undergoing cesarean section before and during the onset of active labor were collected and prepared as described previously (25) and in SI Materials and Methods.
Mouse Model of Preterm Labor Induced by Pharmacological P4/PR Withdrawal.
Induction of preterm labor using RU486 was implemented as described previously (10, 25) and in SI Materials and Methods.
P4 Treatment Studies.
Ovariectomized mice were injected with P4 or with sesame oil (vehicle) and uterine tissues collected, as described in SI Materials and Methods.
Transfection and Transduction of Human Myometrial Cells.
Immortalized human myometrial cells (hTERT-HM) (31) were cultured, transduced with recombinant adenoviruses, or transfected, as described in SI Materials and Methods.
qRT-PCR.
RNA was DNase treated (Invitrogen) and reverse-transcribed using the SuperScript III-RT kit (Invitrogen) or TaqMan microRNA reverse transcription kit (Applied Biosystems). Gene expression analysis was conducted using SYBR Green (Applied Biosystems) or TaqMan Universal PCR Master Mix (Applied Biosystems). Relative gene expression was calculated using the ΔΔCt method. Primer sets are listed in SI Materials and Methods.
Immunoblot Analysis.
Cytoplasmic and nuclear proteins were isolated and analyzed for STAT5b and 20α-HSD proteins by immunoblotting using specific antibodies as described in SI Materials and Methods.
Luciferase Reporter Assays.
The pMIR-REPORT system (Ambion) was used to validate miR-200a binding sites in STAT5b 3′-UTR, as described in SI Materials and Methods.
20α-HSD Enzyme Activity.
Uterine tissue homogenates from pregnant Institute for Cancer Research mice from 15.0 to 19.0 dpc and from human myometrial biopsies were analyzed for 20α-HSD enzymatic activity using [14C]P4 and TLC, as described in SI Materials and Methods.
ChIP.
Quantitative ChIP was performed using a ChIP Assay Kit (catalog no. 17–295; Millipore) to assess binding of endogenous STAT5b to the 20α-HSD/AKR1C18 promoter in myometrial tissues from timed-pregnant mice at 15.5 and 18.5 dpc, as described in SI Materials and Methods.
Statistical Analysis.
Excel (Microsoft) was used to perform statistical analyses. The two-tailed Student t test was used to calculate statistical significance. We considered P > 0.05 to be significant.
Supplementary Material
Acknowledgments
We thank Dr. Geula Gibori (University of Illinois at Chicago) for the generous provision of polyclonal antibody for 20α-hydroxysteroid dehydrogenase. This research was supported by National Institutes of Health Grant 5-P01-HD11149 (to C.R.M.) and March of Dimes Birth Defects Foundation Grant 21FY11-30 (to C.R.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200650109/-/DCSupplemental.
References
- 1.Mendelson CR. Minireview: Fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23:947–954. doi: 10.1210/me.2009-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 3.Virgo BB, Bellward GD. Serum progesterone levels in the pregnant and postpartum laboratory mouse. Endocrinology. 1974;95:1486–1490. doi: 10.1210/endo-95-5-1486. [DOI] [PubMed] [Google Scholar]
- 4.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 5.Smith R, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94:2066–2074. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- 6.Frydman R, et al. Labor induction in women at term with mifepristone (RU 486): A double-blind, randomized, placebo-controlled study. Obstet Gynecol. 1992;80:972–975. [PubMed] [Google Scholar]
- 7.Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol. 1998;92:804–809. doi: 10.1016/s0029-7844(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 8.Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone—A randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78:793–798. [PubMed] [Google Scholar]
- 9.Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9(Suppl 1):131–161. doi: 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- 10.Dudley DJ, Branch DW, Edwin SS, Mitchell MD. Induction of preterm birth in mice by RU486. Biol Reprod. 1996;55:992–995. doi: 10.1095/biolreprod55.5.992. [DOI] [PubMed] [Google Scholar]
- 11.Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100:9518–9523. doi: 10.1073/pnas.1633616100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong X, Shylnova O, Challis JR, Lye SJ. Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J Biol Chem. 2005;280:13329–13340. doi: 10.1074/jbc.M409187200. [DOI] [PubMed] [Google Scholar]
- 13.Dong X, et al. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1) Mol Endocrinol. 2009;23:1147–1160. doi: 10.1210/me.2008-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271:6217–6224. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- 15.Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor (PR) plays a major anti-inflammatory role in human myometrial cells by antagonism of NF-κB activation of cyclooxygenase 2 (COX-2) expression. Mol Endocrinol. 2006;20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 16.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA. 2004;101:4978–4983. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20:764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- 18.Mahendroo MS, Cala KM, Russell DW. 5 α-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 19.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5α-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 20.Puri CP, Garfield RE. Changes in hormone levels and gap junctions in the rat uterus during pregnancy and parturition. Biol Reprod. 1982;27:967–975. doi: 10.1095/biolreprod27.4.967. [DOI] [PubMed] [Google Scholar]
- 21.Power SG, Challis JR. The effects of gestational age and intrafetal ACTH administration on the concentration of progesterone in the fetal membranes, endometrium, and myometrium of pregnant sheep. Can J Physiol Pharmacol. 1987;65:136–140. doi: 10.1139/y87-027. [DOI] [PubMed] [Google Scholar]
- 22.Csapo AI, Eskola J, Tarro S. Gestational changes in the progesterone and prostaglandin F levels of the guinea-pig. Prostaglandins. 1981;21:53–64. doi: 10.1016/0090-6980(81)90196-9. [DOI] [PubMed] [Google Scholar]
- 23.Runnebaum B, Zander J. Progesterone and 20 alpha-dihydroprogesterone in human myometrium during pregnancy. Acta Endocrinol Suppl (Copenh) 1971;150:3–45. [PubMed] [Google Scholar]
- 24.Penning TM, Drury JE. Human aldo-keto reductases: Function, gene regulation, and single nucleotide polymorphisms. Arch Biochem Biophys. 2007;464:241–250. doi: 10.1016/j.abb.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renthal NE, et al. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA. 2010;107:20828–20833. doi: 10.1073/pnas.1008301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richer JK, et al. Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem. 1998;273:31317–31326. doi: 10.1074/jbc.273.47.31317. [DOI] [PubMed] [Google Scholar]
- 27.Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20α-hydroxysteroid dehydrogenase. Mol Endocrinol. 2005;19:431–440. doi: 10.1210/me.2004-0302. [DOI] [PubMed] [Google Scholar]
- 28.Bracken CP, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 29.Lin VC, et al. Progesterone induces cellular differentiation in MDA-MB-231 breast cancer cells transfected with progesterone receptor complementary DNA. Am J Pathol. 2003;162:1781–1787. doi: 10.1016/S0002-9440(10)64313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishida M, et al. Reproductive phenotypes in mice with targeted disruption of the 20alpha-hydroxysteroid dehydrogenase gene. J Reprod Dev. 2007;53:499–508. doi: 10.1262/jrd.18125. [DOI] [PubMed] [Google Scholar]
- 31.Condon J, et al. Telomerase immortalization of human myometrial cells. Biol Reprod. 2002;67:506–514. doi: 10.1095/biolreprod67.2.506. [DOI] [PubMed] [Google Scholar]
- 32.Bao L, et al. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;148:2326–2334. doi: 10.1210/en.2006-1643. [DOI] [PubMed] [Google Scholar]
- 33.Wellner U, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 34.Sugino N, Telleria CM, Gibori G. Progesterone inhibits 20α-hydroxysteroid dehydrogenase expression in the rat corpus luteum through the glucocorticoid receptor. Endocrinology. 1997;138:4497–4500. doi: 10.1210/endo.138.10.5572. [DOI] [PubMed] [Google Scholar]
- 35.Stocco CO, Chedrese J, Deis RP. Luteal expression of cytochrome P450 side-chain cleavage, steroidogenic acute regulatory protein, 3β-hydroxysteroid dehydrogenase, and 20α-hydroxysteroid dehydrogenase genes in late pregnant rats: Effect of luteinizing hormone and RU486. Biol Reprod. 2001;65:1114–1119. doi: 10.1095/biolreprod65.4.1114. [DOI] [PubMed] [Google Scholar]
- 36.Udy GB, et al. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregory PA, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 38.Penning TM, Byrns MC. Steroid hormone transforming aldo-keto reductases and cancer. Ann N Y Acad Sci. 2009;1155:33–42. doi: 10.1111/j.1749-6632.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vergnes L, Phan J, Stolz A, Reue K. A cluster of eight hydroxysteroid dehydrogenase genes belonging to the aldo-keto reductase supergene family on mouse chromosome 13. J Lipid Res. 2003;44:503–511. doi: 10.1194/jlr.M200399-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Veliça P, et al. Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: Implications for modelling human cancers. Mol Cancer. 2009;8:121. doi: 10.1186/1476-4598-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiest WG, Kidwell WR, Balogh K., Jr Progesterone catabolism in the rat ovary: A regulatory mechanism for progestational potency during pregnancy. Endocrinology. 1968;82:844–859. doi: 10.1210/endo-82-4-844. [DOI] [PubMed] [Google Scholar]
- 42.Merlino AA, et al. Nuclear progesterone receptors in the human pregnancy myometrium: Evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.