Abstract
We describe a method for light-inducible and tissue-selective cell ablation using a genetically encoded photosensitizer, miniSOG (mini singlet oxygen generator). miniSOG is a newly engineered fluorescent protein of 106 amino acids that generates singlet oxygen in quantum yield upon blue-light illumination. We transgenically expressed mitochondrially targeted miniSOG (mito-miniSOG) in Caenorhabditis elegans neurons. Upon blue-light illumination, mito-miniSOG causes rapid and effective death of neurons in a cell-autonomous manner without detectable damages to surrounding tissues. Neuronal death induced by mito-miniSOG appears to be independent of the caspase CED-3, but the clearance of the damaged cells partially depends on the phagocytic receptor CED-1, a homolog of human CD91. We show that neurons can be killed at different developmental stages. We further use this method to investigate the role of the premotor interneurons in regulating the convulsive behavior caused by a gain-of-function mutation in the neuronal acetylcholine receptor acr-2. Our findings support an instructive role for the interneuron AVB in controlling motor neuron activity and reveal an inhibitory effect of the backward premotor interneurons on the forward interneurons. In summary, the simple inducible cell ablation method reported here allows temporal and spatial control and will prove to be a useful tool in studying the function of specific cells within complex cellular contexts.
Keywords: light inducible, locomotion, optogenetics, reactive oxygen species, pre-motor interneurons
Tools that allow for selective ablation of cells in a temporally and spatially precise manner have greatly facilitated our ability to dissect the function of a cell within a complex network such as the nervous system. Genetically encoded cell ablation reagents are highly desirable, as they can be used in combination with a variety of cellular manipulations. Key enzymes in programmed cell-death pathways, such as caspases (1), and cytotoxic molecules, such as gain-of-function mutant degenerins (2) or diphtheria toxin A (3, 4), are commonly used reagents to kill cells. However, these cell ablation methods rely on constitutive expression of such reagents, and the efficiency of cell ablation can vary depending on the cell type, physiological state, and possibly other factors. Several conditional cell ablation methods have also been reported and are primarily based on the combinatorial activity of exogenous immunotoxin or chemicals (5–8). For example, transgenic expression of Escherichia coli nitroreductase in zebrafish can ablate cells upon conversion of the prodrug metronidazole into a cytotoxic DNA cross-linking agent (6, 7). Targeted expression of the human interleukin 2 receptor α-subunit in mice can cause inducible cell killing upon binding of a recombinant immunotoxin (5). Developing additional inducible cell-killing methods will offer versatility to the toolbox for cellular manipulations.
Recent technological developments have identified exogenous photosensitizers that release reactive oxygen species (ROS) upon light excitation. Photo-inducible cell killing exploits the ability of ROS to potentially damage any macromolecules within the cell by over-oxidation; when the oxidative stress reaches a certain threshold, cell death ensues (9). Chemically based photosensitizing reagents have been used in photodynamic therapy, a clinically approved, minimally invasive procedure to kill malignant cells (10). Once administered, however, these chemical photosensitizers may accumulate in tissues other than cancerous cells, causing nonspecific toxicity. Genetically encoded photosensitizers enable more selective targeting and can offer a unique way to investigate cellular function in a site- and stage-specific manner (11–14). However, fully genetically encoded and functionally effective photosensitizers remain scarce. A promising photosensitizer recently reported is KillerRed, which generates ROS upon irradiation with green light and can ablate cells when targeted to mitochondria in cultured mammalian cells (12) or when targeted to plasma membrane in zebrafish (14). However, KillerRed is a relatively large protein and requires dimerization for its phototoxicity, which may impose limitations for efficient expression.
MiniSOG (mini singlet oxygen generator) is a green fluorescent flavoprotein engineered from Arabidopsis phototropin 2 (15). miniSOG consists of 106 amino acids and acts as a monomer. Upon blue-light illumination, miniSOG generates a sufficient quantity of singlet oxygen and has been shown to effectively catalyze local polymerization of diaminobenzidine into precipitates for imaging using electron microscopy (15). Here, we took advantage of the ROS-producing property of miniSOG and demonstrated that mitochondrially targeted miniSOG is a potent light-induced cell-ablation reagent in Caenorhabditis elegans. We also provide examples of the use of this method in studying neural function.
Results
Mitochondrially Targeted miniSOG Kills Cells After Blue-Light Illumination.
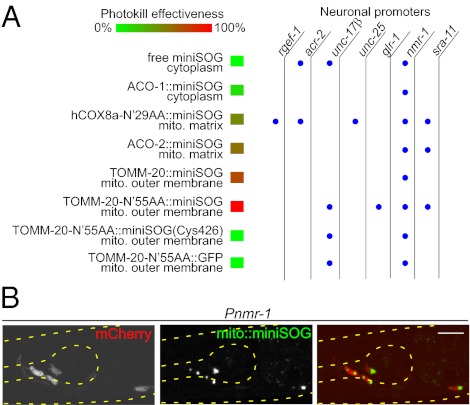
To explore the use of miniSOG in cell killing, we designed a series of transgenic expression constructs targeting miniSOG to subcellular locations (Fig. 1A; Table S1). Because mitochondria are prone to the damaging effects of oxygen radicals and play key roles in the regulation of cell death (9), we tagged miniSOG using a number of mitochondrial protein-targeting sequences (16). We previously reported the use of the N-terminal 29-amino-acid residues of human COX8a (cytochrome c oxidase subunit VIIIA) to target miniSOG to the mitochondrial matrix (15). To target miniSOG onto the outer mitochondrial membrane, we fused miniSOG to the C terminus of TOMM-20, the C. elegans ortholog of human TOM20, the major receptor for the import of polypeptides into mitochondria (17). We also used the N-terminal 55-amino-acid residues of TOMM-20 to target miniSOG, as this polypeptide was shown to localize GFP to mitochondria (18). Because an important mechanism underlying ROS toxicity appears to involve direct oxidation and inactivation of iron–sulfur proteins, such as mitochondrial aconitases (19), we also tagged miniSOG at the C terminus of ACO-2, the mitochondrial aconitase in C. elegans, as well as at the C terminus of ACO-1, the cytoplasmic aconitase (20). These miniSOG constructs were expressed using several neuron-type specific promoters (Fig. 1A; Tables S1 and S2). Under blue-light illumination, cells expressing free miniSOG showed diffuse green fluorescence that photobleached rapidly, whereas the various mitochondrially targeted miniSOG constructs showed green fluorescence primarily concentrated in cytoplasm of the neuronal cell body (Fig.1B), likely reflecting the relatively high concentration of mitochondria in the soma. When cultured under typical indoor illumination (∼0.2 mW/cm2), the transgenic animals expressing free miniSOG or mitochondrially targeted miniSOG in various types of neurons did not display any obvious behavioral defects (Table S1 and Movie S1), and neuronal morphology was grossly normal (Figs. 2A and 3A and B), indicating that miniSOG does not cause discernible toxicity under standard C. elegans culture conditions.
Fig. 1.
Specific neuronal ablation by light-activated mitochondrially targeted miniSOG. (A) miniSOG constructs and the effectiveness of cell ablation. The neuronal promoters are described in Table S2. rgef-1, pan-neurons; acr-2, A- and B-type cholinergic motor neurons; unc-17β, A- and B-type cholinergic motor neurons; unc-25, D-type GABAergic motor neurons; glr-1, premotor interneurons, a subset of head motor neurons and interneurons; nmr-1, a subset of premotor interneurons and RIM; sra-11, premotor interneurons AVB and interneurons AIA and AIY. Blue dots indicate that the transgene of miniSOG constructs driven by the corresponding neuronal promoters was tested. (B) Green fluorescence of mito-miniSOG is visible in soma when expressed in Pnmr-1-cells, which are colabeled by mCherry. (Scale bar, 10 μm.)
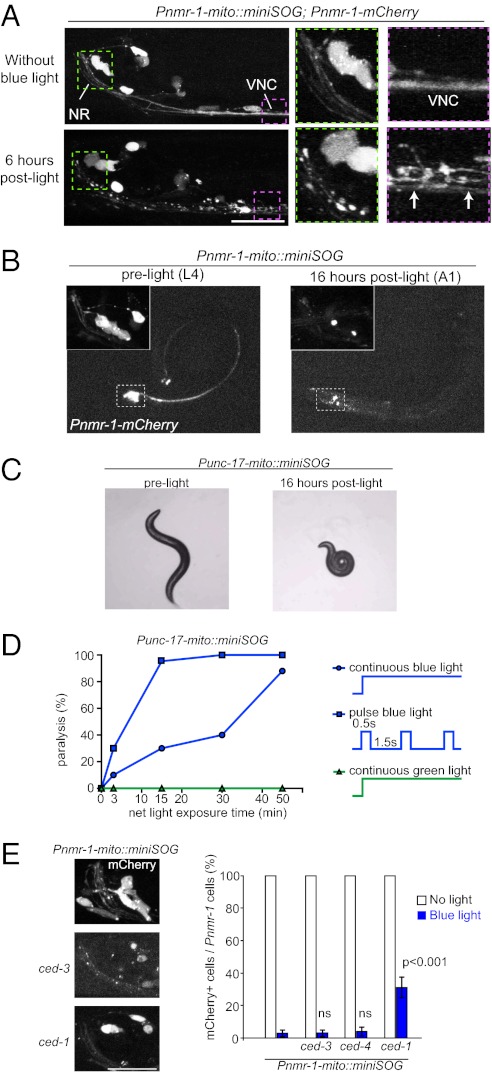
Fig. 2.
Time course and light dose of cell ablation by activated mito-miniSOG. (A) Dying cells following photo-activation of mito-miniSOG. Shown are confocal images of the head region (NR, nerve ring) and anterior ventral nerve cord (VNC) of the animals 6 h after blue-light exposure. In green and pink dotted frames are the blow-up views of the same worm. Dying cells viewed by mCherry show swelling of soma and fragmentation of nerve bundles of VNC (arrows). (Scale bar, 10 μm.) (B) Light-induced cell ablation in transgenic Pnmr-1-mito-miniSOG animals 16 h post light illumination. (Insets) Enlarged views of mCherry in Pnmr-1 neurons before light illumination and disappearance of mCherry-labeled cells. (C) Ablation of Punc-17β–expressing cells (DA, DB, VA, and VB motor neurons) by mito-miniSOG causes paralysis. (D) The graph shows that light dosage correlates with the percentage of paralysis induced by mito-miniSOG upon illumination under continuous or pulsed light. A total of 20–30 animals were analyzed for each data point. (E) Ablation of Pnmr-1 cells by mito-miniSOG is independent of ced-3 or ced-4; the clearance of damaged cell bodies and processes is partially blocked by ced-1. A total of 20–40 animals of each genotype were analyzed. (Scale bar, 10 μm.) Statistics used two-tailed Student’s t test. Error bar shows SEM. Data for ced-3, ced-4, and ced-1 are compared with the data for the same transgene in wild-type background.
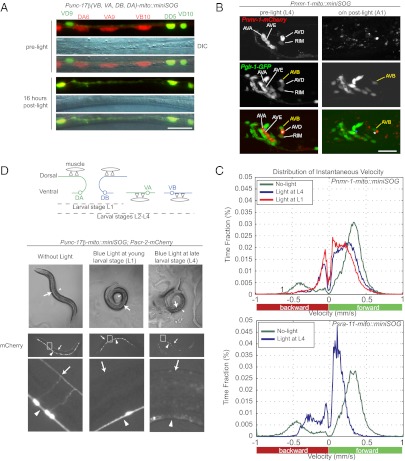
Fig. 3.
Activation of mito-miniSOG confers no detectable nonautonomous cytotoxicity and can be induced temporally. (A) Photokilling of Punc-17β neurons (labeled by mCherry) does not affect neighboring d-type motor neurons labeled by Punc-25-GFP. DIC images show the same segment of ventral nerve cord as appeared in fluorescence images. (Scale bar, 10 μm.) (B) Photokilling of Pnmr-1 neurons (labeled by mCherry) does not affect neighboring cells labeled by Pglr-1-GFP. AVB and other neurons labeled by Pglr-1 but not by Pnmr-1 are intact after light illumination. The positions of AVA (asterisk in cyan) and AVD (asterisk in white) are marked on images showing the animal before and after light illumination. (Scale bar, 10 μm.) (C) Plots of distribution of instantaneous locomotion speed when animals were transferred from food plate to food-free plate. Positive speed values reflect the forward motion, whereas negative speed values reflect the backward motion. When Pnmr-1 cells were photokilled (Upper), the backward movement pattern was severely affected (Movie S6) and the speed was dramatically reduced (the curve shifts toward zero on the velocity axis). Ablation of Pnmr-1 cells at young larval stage (L1) and late larval stage (L4) resulted in similar locomotion behaviors. When Psra-11 cells were photokilled (Lower), the animals exhibit severely disrupted forward locomotion (Movie S8). At least 15 animals of each illumination condition were tracked and analyzed. (D) Temporally controlled motor neuron ablation by Punc-17β-mito-miniSOG. Schematic drawings show that Punc-17β labels DA and DB at L1. At late L1, VA and VB are born, such that Punc-17β labels DA, DB, VA, and VB from then on (Top). The DIC images show young adult transgenic animals that had been illuminated under blue light at young larval stage (L1) and late larval stage (L4), respectively (Middle). When illuminated at L1, the animals are severely uncoordinated and coil invariably toward the ventral side (arrowheads). When illuminated at L4, the worms are paralyzed and tend to coil toward the dorsal side (arrows). (Bottom) mCherry fluorescence images of animals having received light illumination. When illuminated at L1, DA and DB are ablated, and fluorescence disappears only in the dorsal cord (arrows). When illuminated at L4, animals lose not only DA and DB, but also VA and VB (arrowheads).
To test if activation of miniSOG could result in cell ablation, we exposed freely moving transgenic animals under wide-field blue light (475 ± 20 nm) at an intensity of 57 mW/cm2 for 30 min using an epi-fluorescence compound microscope (Materials and Methods and SI Materials and Methods). Under the tested conditions, free miniSOG did not induce detectable effects, whereas all mitochondrially targeted miniSOG caused animals to exhibit behavioral deficits to variable degrees within 30 min after illumination (Fig.1A; Table S1). To visualize the morphological effects on the targeting neurons, we coexpressed mCherry under the corresponding promoters for mito-miniSOG. Within a few hours post light illumination, mCherry-labeled cells began to show shape changes, including rounding up, shrinkage, vacuolation, and swellings in the soma and neuronal processes (Fig. 2A). By 16 h, the mCherry fluorescence in cell bodies had disappeared, and neuronal processes appeared to have disintegrated (Fig. 2B). Using Nomarski differential interference contrast (DIC) microscopy, we inspected the nuclei of the targeting neurons and confirmed that disappearance of mCherry was indeed correlated with absence of the cells (Fig. 3A). It is worth noting that the mCherry marker provided further measure that the ablation resulted in a complete disintegration of the dying cells. Under parallel blue-light illumination, transgenic animals expressing mitochondrially targeted GFP or mCherry alone did not show any behavioral or cellular changes (Fig. 1A). The phototoxicity of miniSOG was specifically induced by blue light, as green light (560 ± 20 nm) caused no effects (Fig. 2D), consistent with the absorption spectrum of miniSOG. Moreover, expression of a miniSOG variant containing Cys426, which cannot divert light energy into 1O2 generation (15), did not produce any fluorescence and was not able to kill cells (Fig. 1A; Table S1). miniSOG fused in-frame to the ACO-1 cytoplasmic aconitase produced limited damage that was not sufficient to kill cells (Fig. 1A; Table S1), consistent with the very short range of 1O2 (15) and the key role of mitochondria in triggering cell death. Among the different mitochondrial tags, targeting to the mitochondrial outer membrane using the N-terminal 55-amino-acid residues of TOMM-20 provided the most potent phototoxic effect (Fig. 1A; Table S1). We therefore focused on TOMM-20(N′55aa)-miniSOG and hereafter refer to this as mito-miniSOG.
Cell Ablation by Mitochondrially Targeted miniSOG Is Light-Dose-Dependent and Enhanced by Pulse Illumination.
We empirically determined that the effectiveness of cell ablation with mitochondrially targeted miniSOG (mito-miniSOG) correlated with whether green fluorescence of miniSOG was visually detectable under a standard epi-fluorescence compound microscope (SI Materials and Methods). We also tested how the light dosage might affect the effectiveness of cell ablation. mito-miniSOG–induced ablation of the ventral cord A- and B-type motor neurons resulted in paralysis (Fig. 2C; Movies S1 and S2). We analyzed the percentage of paralyzed animals expressing Punc-17β-mito-miniSOG following various time periods of continuous blue-light treatment and found that the paralysis increased with longer durations of light exposure (Fig. 2D). We also devised a repetitive pulsed blue lighting procedure (0.5 s light; 1.5 s dark) (Materials and Methods) and found that it resulted in three times more effective cell ablation, given the same intensity and total dose of blue-light illumination (Fig. 2D). Under pulsed blue-light exposure for a total of 15–30 min, the animals remained healthy and reproduced normally. Thus, photo-ablation effects by activated mito-miniSOG appear to be graded, and pulsed lighting can be advantageous. Further experimentation would be necessary to investigate why different regimens for delivering a given photon dose vary in effectiveness and whether the efficacy of cell killing can be further optimized.
Caspase ced-3 Is Not Required for Cell Death Induced by mito-miniSOG.
We wanted to gain clues as to whether mito-miniSOG–mediated cell killing involves known cell-death pathways. Two genes essential for apoptotic programmed cell death in C. elegans are the caspase ced-3 and its activator ced-4 (21). We found that null mutations of ced-3 or ced-4 did not block the photoablation of neurons expressing Pnmr-1– or Punc-17β–mito-miniSOG, as monitored by the disappearance of mCherry fluorescence or the defective locomotion behavior (Fig. 2E; Table S1). The phagocytic receptor ced-1, a homolog of the human CD91 and LRP proteins, acts in phagocytosis of both apoptotic cells and nonapoptotic debris (21, 22) and is also shown to up-regulate the unfolded protein response in C. elegans innate immunity (23). In a strong loss-of-function ced-1 mutant, roughly 30–40% of Pnmr-1-mito-miniSOG–expressing cells retained normal expression of mCherry, with little morphological evidence of disintegration 16 h after light exposure (Fig. 2E). However, analysis of the locomotion speed of such animals showed that the ced-1 mutation did not significantly alleviate the locomotion deficit resulting from ablation of Pnmr-1 neurons by mito-miniSOG (Fig. S1). These data imply that clearance of the damaged cells caused by mito-miniSOG involves dedicated cellular pathways including ced-1. The above observations thus suggest that the mechanism of mito-miniSOG–induced cell death does not require the core programmed cell-death pathway and that phagocytosis of the damaged cells contributes to their elimination. It will be of future interest to investigate the mechanisms underlying miniSOG toxicity.
Activated mito-miniSOG Does Not Cause Cell-Nonautonomous Cytotoxicity.
As dying cells may send damaging signals to other cells and cause nonautonomous consequences, we addressed whether miniSOG–induced cell death could affect the neighboring cells. Punc-17β–expressing cholinergic motor neurons in the ventral nerve cord are interdigitated with the GABAergic motor neurons, which express Punc-25-GFP (24, 25). Killing Punc-17β–expressing motor neurons by mito-miniSOG did not cause detectable abnormality of the neighboring GABAergic neurons (Fig. 3A). To test the cell-autonomous ablation in a more complex environment, we examined the interneurons that reside in the densely packed head ganglia. Pnmr-1–expressing neurons include four pairs of interneurons: AVA, AVE, AVD, and RIM (26). Pglr-1-GFP labels all of the Pnmr-1–expressing cells and additional adjacent neurons (26, 27). We generated a strain containing both Pnmr-1-mito-miniSOG and Pglr-1-GFP. When Pnmr-1–expressing neurons (marked by mCherry) were photokilled, the neighboring GFP-labeled cells showed normal morphology (Fig. 3B).
It remains possible that cell-nonautonomous damages might occur independently of morphological changes. To address this, we next examined the behavioral consequences of selective ablation of the premotor interneurons. Pnmr-1–expressing interneurons regulate backward locomotion (26, 28) and reside in the near neighborhood of the interneurons AVB, which regulate forward locomotion and express Psra-11 (29). When worms are transferred from a plate with food to a plate without food, they display a dramatically changed locomotion pattern, characterized by high velocity and infrequent short reversals (26, 30). We tracked the locomotion speed and pattern of the animals before and after photoablation (Materials and Methods). The adult animals with Pnmr-1 cells photo-ablated at L4 showed impaired backward locomotion (Fig. 3C; Movies S3, S4, S5, and S6) in a pattern similar to that of animals in which Pnmr-1 cells were killed by either overexpression of the proapoptotic factor ICE (26) or laser ablation in L1 larvae (28). The sinusoidal pattern of the forward motion in these animals was largely normal, supporting that the AVB neurons were unharmed. We note that, despite the normal sinusoidal forward pattern, the animals with Pnmr-1 cells ablated showed a modest and significant decrease in the locomotion speed (Fig. 3C; Fig. S1B), the underlying cause of which would be of future investigation. Conversely, when AVB neurons were ablated by Psra-11-mito-miniSOG, the forward sinusoidal locomotion pattern was severely disrupted (Fig. 3C; Movies S7 and S8). Nonetheless, these animals could drag along in a forward motion, suggesting that the pulling force may likely come from the head-and-neck motor system. The backward locomotion of the animals with Psra-11 cells ablated was largely normal, indicating that AVA/AVD/AVE neurons were unharmed. Together, these data support that the mito-miniSOG–mediated cell ablation occurs in a cell-specific and autonomous manner.
Temporally Activated mito-miniSOG Selectively Ablates Motor Neurons Arising from Specific Stages.
The light-inducible cell killing by mito-miniSOG offers an advantage for temporally controlled cell ablation. To explore such use, we illuminated the Punc-17β-mito-miniSOG animals at young (L1) and older (L4) larval stages. The Punc-17β–expressing DA and DB neurons are embryonically born and present in young L1, whereas the Punc-17β–expressing VA and VB neurons are born in late L1 and early L2 stages (Fig. 3D) (31). We observed that, following light illumination at the young L1 stage, the animals lacked mCherry-labeled processes from DA and DB in the dorsal nerve cord (Fig. 3D). Behaviorally, these animals displayed uncoordinated movement as they reached adult stages and coiled toward the ventral side (Fig. 3D), consistent with a lack of excitatory inputs from the DA and DB neurons to the dorsal body muscles. In contrast, when Punc-17β-mito-miniSOG animals were light-illuminated at L4 larval stage, all mCherry-labeled processes in the dorsal and ventral nerve cords disintegrated, and the animals displayed severe paralysis (Fig. 3D), consistent with the elimination of excitatory inputs to both ventral and dorsal body muscles. We also performed stage-specific ablation on the Pnmr-1–expressing interneurons, which are born during embryogenesis and persist through adulthood. We observed that killing Pnmr-1 neurons in L1 and L4 animals resulted in a similar defective locomotion pattern (Fig. 3C, Upper). The latter analysis indicates that the Pnmr-1 interneurons are required continuously throughout development to regulate locomotion speed. In all cases, mCherry did not reappear in the L1-illuminated worms when they reached adult stages, indicating that cell ablation is permanent.
Ablation of Premotor Interneurons Reveals a Role of the AVB Interneurons in the Convulsive Behavior of acr-2(gf) Mutants.
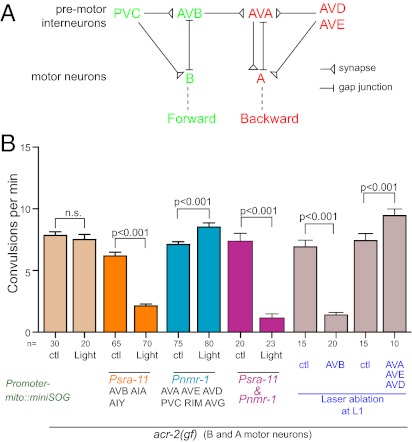
As an application of the mito-miniSOG–induced cell ablation method in analyzing neural circuit function, we investigated the neural control of the convulsive behavior caused by a gain-of-function mutation in acr-2. ACR-2 is a nicotinic acetylcholine receptor that is expressed in the ventral cord cholinergic B and A classes of motor neurons and functions in maintaining the excitability of these neurons (32). The acr-2(gf) mutation causes increased cholinergic transmission, and the mutant animals exhibit a severe disruption of undulatory locomotion accompanied with periodic seizure-like whole-body contraction (Fig. 4B). It is unclear how the convulsive events are initiated and regulated and whether the movement defect is the result of convulsion. The major neural inputs to the motor neurons are from the premotor interneurons AVB and PVC, which regulate the B class of motor neurons in forward locomotion, and from the premotor interneurons AVA, AVD, and AVE, which regulate the A class of motor neurons in backward locomotion (Fig. 4A) (28, 33). We therefore selectively ablated the premotor interneurons to address their roles in the convulsive behavior of acr-2(gf) mutants (Tables S1 and S2). When AVB neurons were ablated by Psra-11-mito-miniSOG in acr-2(gf) adult animals, the convulsion rates significantly decreased (Fig. 4B; Movies S9 and S10). In contrast, when the premotor Pnmr-1–expressing interneurons were killed by light illumination, the convulsion frequency of acr-2(gf) animals instead significantly increased (Fig. 4B; Movies S11 and S12). For comparison, we performed traditional laser ablation of AVB or AVA/AVD/AVE in L1 worms of acr-2(gf) and observed a similar suppression of the convulsion by killing AVB and an enhancement by killing AVA/AVD/AVE (Fig. 4B). These data indicate that AVB neurons positively regulate the convulsion events and suggest that the premotor interneurons AVA/AVD/AVE may impose a negative regulation on shrinking events through inhibiting AVB activity. Indeed, when we simultaneously ablated both Pnmr-1 and Psra-11 neurons, the convulsion frequency of acr-2(gf) dropped to the same level as ablating Psra-11 neurons alone. It is worth noting that ablation of the interneurons affected only the onset of convulsion events and did not alleviate the overall locomotion pattern defect of acr-2(gf) animals (Movies S9, S10, S11, S12, S13, and S14). Thus, it is the abnormal motor neuron activities caused by the ACR-2(gf) channel that account for the uncoordinated movement pattern in the mutants.
Fig. 4.
Ablation of the premotor interneurons reveals their roles in the regulation of convulsions in acr-2(gf) animals. (A) Shown is the connectivity among premotor interneurons and ventral cord motor neurons that regulates forward and backward movement (based on refs. 25, 28, 33). (B) acr-2(gf) animals exhibit periodic convulsions; frequency of convulsions (min−1) in young adult animals are quantified in the graph. Ablation of specific subsets of premotor interneurons in young adult animals by activation of mito-miniSOG causes differential effects on convulsion. Ablating AVB results in a suppression of convulsive events, whereas ablating AVA and other backward premotor interneurons enhances the convulsive behavior. Control (ctl) groups are the same genotype without light illumination. Ablation of cells using both Psra-11 and Pnmr-1 together resulted in the decrease of convulsions frequency. Similar results are observed when AVB (labeled by Psra-11-GFP) or AVA, AVE, and AVD (labeled by Pnmr-1-mCherry) are killed by laser ablation at L1 stage. Control (ctl) groups are the animals of same genotype with laser aimed at the epidermis instead of mCherry-labeled neurons. Statistics used two-tailed Student’s t test. Error bar shows SEM.
Discussion
We have reported a simple inducible cell ablation method in C. elegans using the genetically encoded singlet oxygen generator protein, miniSOG. We provide both morphological and behavioral evidence that photokilling of cells by targeting miniSOG to mitochondria is specific to the targeted cells and does not impose discernible damages on neighboring cells. We also show that photoablation by mito-miniSOG is effective in larval and adult stages and that the use of stable transgenic mito-miniSOG ensures the feasibility of comparing a large number of samples with a uniform standard. In comparison with the traditional laser-killing methodology (35), photoablation by mito-miniSOG requires only a standard fluorescent light source in setup and does not need extensive anatomical expertise. Moreover, laser killing of neurons is usually performed only in young L1 larvae because the body depth of older larvae and adults increases considerably, precluding precise focusing of a laser beam on targeting cells. It is also known that neuronal processes may remain intact even when the nucleus is eliminated by laser and could provide unknown function (35). We have shown that ablation by mito-miniSOG causes a complete disintegration, followed by clearance, of the dying cells. Current transgenic cell ablation methodologies in C. elegans predominantly rely on the constitutive expression of toxic proteins or caspases (36). Depending on the expression timing and strength of the promoters, these methods may often result in ablation of precursor cells or incomplete killing. Thus, mito-miniSOG–mediated cell ablation offers better temporal and spatial control in desired developmental and adult stages. We envision a particular advantage of mito-miniSOG in its application to our understanding of neuronal functions in aged adult animals.
In the course of developing the method of selective cell ablation using mito-miniSOG, we have also analyzed the roles of the premotor interneurons in regulating locomotion behaviors. The functions of these neurons have long been inferred from the wiring diagram (33) and were partly supported by early laser ablation experiments (28). Numerous investigators have recently revisited the roles of these neurons in locomotion by using a combination of laser ablation, genetic mutations, and optogenetic manipulations (30, 34, 37, 40). The laser ablations were performed in L1 larvae, and the animal behaviors were examined in adults, leaving the possibility that loss of the neurons might be compensated during animal growth. Although we cannot make precise parallel comparisons for the locomotion speed and pattern with those reported by other investigators, our observations that ablation of AVB leads to a severe disruption of the forward locomotion and that ablation of AVA/AVD/AVE causes a loss of the backward movement are consistent with the conclusions drawn in the recently published studies (34, 37, 40). Because we ablated these interneurons in adult animals, our studies provide evidence that the behavior deficits are direct consequences of the loss of these neurons. Furthermore, from our understanding of the convulsion behaviors of acr-2(gf) animals, we have identified an essential connection between the onset of convulsion and the forward locomotor motor system controlled by the premotor interneurons AVB. Unexpectedly, we found that the removal of AVA/AVD/AVE by mito-miniSOG or by laser ablation can enhance the convulsion frequency. We infer from this observation a negative feed-forward pathway from these neurons onto the AVB neurons. This is supported by our data that indicate simultaneous loss of both AVB and AVA/AVD/AVE results in a similar effect as the ablation of AVB alone. Our interpretation is also consistent with the recent report showing that in the gap junction mutants, elevated AVA activity is associated with decreased activity in AVB (37). Because there are no extensive synaptic or gap junctions from the backward- to the forward-regulating premotor interneurons (33), these results raise important questions for the ongoing understanding of motor circuit modulation. Increasing studies have elegantly shown the power of optogenetic manipulations and optic imaging in dissecting the neural basis of behaviors in living animals (34, 37, 40). In conjuncture with these tools, the inducible and selective cell ablation by miniSOG will prove to be valuable in understanding neuronal function.
Various constitutive and inducible cell-ablation methods have been used in many cellular manipulations (1–8, 12). It may be difficult to accurately compare the efficiency of various genetically encoded cell-killing reagents as it requires knowledge of the protein concentration in vivo. The reconstituted caspase-mediated cell ablation method offers selective killing when the two halves of caspases are coexpressed in the same cell (36). However, variations in the expression levels driven by different promoters can often cause inconsistent cell ablations. Use of diphtheria toxin A for cell killing in C. elegans can also be problematic due to its high toxicity. The phototoxic protein KillerRed, developed from a red chromoprotein homolog of GFP, has been used to kill cultured cells when targeted to mitochondria, membranes, or histones and illuminated with green light (12, 38). KillerRed works through generation of radicals or hydrogen peroxide rather than through singlet oxygen (15, 39). miniSOG has an additional advantage for tagging functional proteins because it is a monomer less than one quarter the size of the obligate dimer of KillerRed (12, 15). In any case, the availability of two nonhomologous phototoxic proteins using different wavelengths and generating different reactive intermediates will add versatility to the optogenetic toolbox. We have previously suggested that miniSOG may also be targeted to other cellular compartments to allow mechanistic dissection of oxidative injury (15). The application of miniSOG presented here and its extended use in the future should greatly benefit functional studies in biology and clinical therapy.
Materials and Methods
Genetics and Molecular Biology and Transgenes.
C. elegans strains were grown following standard procedure and animals were handled under ambient light unless noted. Strains and genotypes are shown in Table S1. All expression constructs are described in Table S2.
Photo Illumination.
We used an upright Zeiss Axioplan 2 microscope equipped with an X-Cite 120 series fluorescence illumination lamp. Plates containing worms were placed, without any covers, on the stage with the blue light passing through without an objective lens. For light illumination, we restricted worms by placing a ring of filter paper soaked with 100 mM CuCl2 with a hole of 15-mm diameter, about the size of the light illumination spot. The opening was spread with OP50 paste, and animals were placed on the food. Animals were exposed to either continuous blue light or pulse blue light (0.5 s on, 1.5 s off). The blue light (475 ± 20 nm) intensity that worms received was measured as 57 mW/cm2. We used a Uniblitz unit (VMM-D1) to control the shutter (30 mm W/HS, LUDL Electronics).
Behavioral Analysis and Quantification of Convulsion.
We used Worm Tracker 2.0 to track locomotion (see SI Materials and Methods). Quantification of convulsion was as described (32).
Supplementary Material
Acknowledgments
We thank Suk-Ryool Kang for help in modifying tracker software; Claudiu Giurumescu for help in light control; and S. Cherra, N. Liu, A. D. Chisholm, and our laboratory members for comments. We thank Oliver Hobert, Villu Maricq, and Kenneth Miller for promoter constructs. This work was supported by National Institutes of Health Grants R01 NS035546 (to Y.J.) and R01 GM086197 (to R.Y.T.). Y.J. and R.Y.T. are Investigators of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204096109/-/DCSupplemental.
References
- 1.Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75:653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- 2.Hong K, Driscoll M. A transmembrane domain of the putative channel subunit MEC-4 influences mechanotransduction and neurodegeneration in C. elegans. Nature. 1994;367:470–473. doi: 10.1038/367470a0. [DOI] [PubMed] [Google Scholar]
- 3.Palmiter RD, et al. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- 4.Breitman ML, et al. Genetic ablation: Targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238:1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K, et al. Immunotoxin-mediated conditional disruption of specific neurons in transgenic mice. Proc Natl Acad Sci USA. 1995;92:1132–1136. doi: 10.1073/pnas.92.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curado S, et al. Conditional targeted cell ablation in zebrafish: A new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 7.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev. 2007;124:218–229. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito M, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 9.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: Implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 10.Agostinis P, et al. Photodynamic therapy of cancer: An update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tour O, Meijer RM, Zacharias DA, Adams SR, Tsien RY. Genetically targeted chromophore-assisted light inactivation. Nat Biotechnol. 2003;21:1505–1508. doi: 10.1038/nbt914. [DOI] [PubMed] [Google Scholar]
- 12.Bulina ME, et al. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 13.Marek KW, Davis GW. Transgenically encoded protein photoinactivation (FlAsH-FALI): Acute inactivation of synaptotagmin I. Neuron. 2002;36:805–813. doi: 10.1016/s0896-6273(02)01068-1. [DOI] [PubMed] [Google Scholar]
- 14.Teh C, et al. Optogenetic in vivo cell manipulation in KillerRed-expressing zebrafish transgenics. BMC Dev Biol. 2010;10:110. doi: 10.1186/1471-213X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shu X, et al. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 17.Abe Y, et al. Structural basis of presequence recognition by the mitochondrial protein import receptor Tom20. Cell. 2000;100:551–560. doi: 10.1016/s0092-8674(00)80691-1. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S, et al. Protein localization in electron micrographs using fluorescence nanoscopy. Nat Methods. 2011;8:80–84. doi: 10.1038/nmeth.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 20.Gourley BL, Parker SB, Jones BJ, Zumbrennen KB, Leibold EA. Cytosolic aconitase and ferritin are regulated by iron in Caenorhabditis elegans. J Biol Chem. 2003;278:3227–3234. doi: 10.1074/jbc.M210333200. [DOI] [PubMed] [Google Scholar]
- 21.Conradt B, Xue D. Programmed cell death. WormBook. 2005:1–13. doi: 10.1895/wormbook.1.32.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangahas PM, Zhou Z. Clearance of apoptotic cells in Caenorhabditis elegans. Semin Cell Dev Biol. 2005;16:295–306. doi: 10.1016/j.semcdb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Haskins KA, Russell JF, Gaddis N, Dressman HK, Aballay A. Unfolded protein response genes regulated by CED-1 are required for Caenorhabditis elegans innate immunity. Dev Cell. 2008;15:87–97. doi: 10.1016/j.devcel.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin Y, Jorgensen E, Hartwieg E, Horvitz HR. The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J Neurosci. 1999;19:539–548. doi: 10.1523/JNEUROSCI.19-02-00539.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White JG, Southgate E, Thomson JN, Brenner S. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 1999;24:347–361. doi: 10.1016/s0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 27.Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- 28.Chalfie M, et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenick AS, Hobert O. Genomic cis-regulatory architecture and trans-acting regulators of a single interneuron-specific gene battery in C. elegans. Dev Cell. 2004;6:757–770. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2005;102:3184–3191. doi: 10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulston JE. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:287–297. doi: 10.1098/rstb.1976.0084. [DOI] [PubMed] [Google Scholar]
- 32.Jospin M, et al. A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biol. 2009;7:e1000265. doi: 10.1371/journal.pbio.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 34.Piggott BJ, Liu J, Feng Z, Wescott SA, Xu XZ. The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell. 2011;147:922–933. doi: 10.1016/j.cell.2011.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang-Yen C, Gabel CV, Samuel AD, Bargmann CI, Avery L. Laser microsurgery in Caenorhabditis elegans. Methods Cell Biol. 2012;107:177–206. doi: 10.1016/B978-0-12-394620-1.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chelur DS, Chalfie M. Targeted cell killing by reconstituted caspases. Proc Natl Acad Sci USA. 2007;104:2283–2288. doi: 10.1073/pnas.0610877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawano T, et al. An imbalancing act: Gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron. 2011;72:572–586. doi: 10.1016/j.neuron.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Waldeck W, et al. Autofluorescent proteins as photosensitizer in eukaryontes. Int J Med Sci. 2009;6:365–373. doi: 10.7150/ijms.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vegh RB, et al. Reactive oxygen species in photochemistry of the red fluorescent protein “Killer Red”. Chem Commun (Camb) 2011;47:4887–4889. doi: 10.1039/c0cc05713d. [DOI] [PubMed] [Google Scholar]
- 40.Faumont S, et al. An image-free opto-mechanical system for creating virtual environments and imaging neuronal activity in freely moving Caenorhabditis elegans. PLoS One. 2011;6(9):e24666. doi: 10.1371/journal.pone.0024666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.