Abstract
Chromosomal loci jiggle in place between segregation events in prokaryotic cells and during interphase in eukaryotic nuclei. This motion seems random and is often attributed to Brownian motion. However, we show here that locus dynamics in live bacteria and yeast are sensitive to metabolic activity. When ATP synthesis is inhibited, the apparent diffusion coefficient decreases, whereas the subdiffusive scaling exponent remains constant. Furthermore, the magnitude of locus motion increases more steeply with temperature in untreated cells than in ATP-depleted cells. This “superthermal” response suggests that untreated cells have an additional source of molecular agitation, beyond thermal motion, that increases sharply with temperature. Such ATP-dependent fluctuations are likely mechanical, because the heat dissipated from metabolic processes is insufficient to account for the difference in locus motion between untreated and ATP-depleted cells. Our data indicate that ATP-dependent enzymatic activity, in addition to thermal fluctuations, contributes to the molecular agitation driving random (sub)diffusive motion in the living cell.
Keywords: active diffusion, nonthermal fluctuations, intracellular transport, macromolecular motion
The cytoplasm is a crowded and dynamic medium, with molecules constantly jostling around and colliding with each other. This molecular motion is often attributed to Brownian motion, the random movement of suspended particles driven by thermal fluctuations of the solvent (1, 2). Classic Brownian motion theory assumes a system at thermal equilibrium. However, cells are far from equilibrium. They use the chemical energy of ATP (and GTP) to drive active biological processes, such as transport and metabolism.
Recent work in eukaryotic cells demonstrates that biological activity generates nonthermal fluctuations of greater magnitude than thermal fluctuations (3–8). These active fluctuations can drive diffusive-like motion of molecules inside the cell, a phenomenon known as “active” diffusion (9, 10). In vitro experiments and analytical theory suggest that these active fluctuations are generated by the cytoskeletal molecular motor myosin (11–13). Thus, random molecular motion in vivo, at least in eukaryotic cytoplasm, may be due to active motor-driven forces in addition to passive thermal forces.
Here we present evidence suggesting that ATP-dependent fluctuations contribute to the motion of chromosomal loci in bacterial and yeast cells. By modulating the temperature at which cells are observed, we were able to identify nonthermal forces that contribute to intracellular motion. Unlike active microrheology (7, 8, 11), temperature modulation presents a simple perturbation that can be applied to any experimental system to explore the physical processes underlying molecular motion in vivo. Our results suggest that “active” diffusion is not unique to systems containing eukaryotic cytoskeletal motors. This phenomenon may in fact be a general property of macromolecular motion in all living cells.
Results
Inhibition of ATP Synthesis Decreases Dapp of Chromosomal Loci.
To quantify macromolecular motion in vivo, we track the position of fluorescently labeled chromosomal loci in live Escherichia coli cells. The ensemble-averaged mean square displacement (MSD),
is calculated to determine the subdiffusive scaling exponent α and the apparent diffusion coefficient Dapp. We recently showed that the viscoelastic properties of the cytoplasm cause chromosomal loci to move subdiffusively with α = 0.39 ± 0.04 (14). Here we investigate the biological processes that determine the magnitude of Dapp.
If molecular transport in bacterial cells were a primarily passive process controlled by Brownian motion, then macromolecular motion should not require biological energy. To test this prediction, we examined the motion of chromosomal loci in cells that were treated with sodium azide and 2-deoxyglucose to inhibit ATP synthesis. Dapp of the 84′ locus decreased 49% ± 14% in ATP-depleted cells compared with untreated cells (Fig. 1 and Table 1) (14). Cells treated with sodium azide alone, which can still produce some ATP through glycolysis, exhibited an intermediate phenotype (Fig. 1 and Table 1). Furthermore, the subdiffusive scaling exponent α remained constant: αuntreated = 0.39 ± 0.04 and αtreated = 0.40 ± 0.04, indicating that the viscoelastic properties of the cytoplasm do not change significantly upon inhibition of ATP synthesis. Indeed, treatment with a different metabolic poison, 2,4-dinitrophenol, alone or in combination with 2-deoxyglucose, caused a 38% ± 12% reduction in Dapp but no change in α (Table 1). These observations raised the intriguing possibility that motion of chromosomal loci in bacteria is driven by active ATP-dependent fluctuations in addition to passive thermal fluctuations.
Fig. 1.
Ensemble-averaged MSD for a fluorescently tagged locus near the 84′ position on the E. coli chromosome in untreated cells and cells treated with sodium azide alone or in combination with 2-deoxyglucose at room temperature.
Table 1.
Fold change (mean ± SD) in α and Dapp for loci in treated cells compared with untreated cells at room temperature
| Species | Treatment | αtreated/αuntreated | Dtreated/Duntreated | No. of datasets |
| E. coli | Sodium azide | 0.95 ± 0.15 | 0.85 ± 0.25 | 5 |
| E. coli | Sodium azide + 2-deoxyglucose | 1.04 ± 0.13 | 0.51 ± 0.14 | 5 |
| E. coli | 2,4-Dinitrophenol ± 2-deoxyglucose | 1.10 ± 0.12 | 0.62 ± 0.12 | 3 |
| E. coli | Rifampin (1 min) | 1.02 ± 0.08 | 0.91 ± 0.01 | 4 |
| E. coli | Mecillinam | 1.03 ± 0.07 | 1.00 ± 0.11 | 5 |
| S. cerevisiae | Sodium azide + 2-deoxyglucose | 0.92 ± 0.13 | 0.12 ± 0.02 | 3 |
Superthermal Temperature Dependence of Locus Motion.
As another test of Brownian motion, we examined the temperature dependence of locus motion. If chromosomal loci move because of thermal fluctuations, then our fractional Langevin motion model (15) predicts a linear relationship between Dapp and the absolute temperature T:
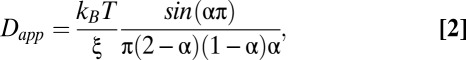 |
where ξ is the drag coefficient of a probe moving through a viscoelastic medium. This expression is equivalent to the generalized Stokes-Einstein relation (16, 17), 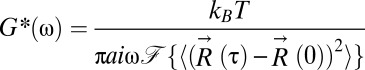 , where the frequency-dependent complex shear modulus G*(ω) is directly proportional to our fractional Langevin memory kernel K, such that
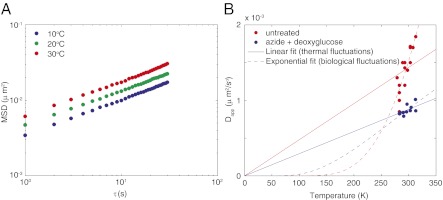
, where the frequency-dependent complex shear modulus G*(ω) is directly proportional to our fractional Langevin memory kernel K, such that  . To determine the temperature dependence of locus motion, cells were grown at 37 °C and shifted to a lower (or higher) temperature at least 5 min before imaging. As the shifted temperature was reduced from 30 °C to 10 °C, Dapp decreased almost twofold (Fig. 2A). Thus, lowering the temperature has a similar effect as depleting cells of ATP (compare Figs. 1 and 2A). This result is qualitatively consistent with Eq. 2, with a decrease in temperature leading to slower motion. However, the data are not fit well by a linear function that is constrained to pass through the point Dapp = 0 μm2/sα at 0 K (Fig. 2B). This failure to quantitatively fit Eq. 2 suggests that thermal fluctuations alone cannot account for the “superthermal” behavior observed for chromosomal loci.
. To determine the temperature dependence of locus motion, cells were grown at 37 °C and shifted to a lower (or higher) temperature at least 5 min before imaging. As the shifted temperature was reduced from 30 °C to 10 °C, Dapp decreased almost twofold (Fig. 2A). Thus, lowering the temperature has a similar effect as depleting cells of ATP (compare Figs. 1 and 2A). This result is qualitatively consistent with Eq. 2, with a decrease in temperature leading to slower motion. However, the data are not fit well by a linear function that is constrained to pass through the point Dapp = 0 μm2/sα at 0 K (Fig. 2B). This failure to quantitatively fit Eq. 2 suggests that thermal fluctuations alone cannot account for the “superthermal” behavior observed for chromosomal loci.
Fig. 2.
Temperature-dependence of locus motion in untreated and ATP-depleted cells. (A) Ensemble-averaged MSD for 84′ loci in untreated cells at 30 °C, 20 °C, and 10 °C. (B) Dapp at τ = 1 s for untreated cells or cells treated with sodium azide and 2-deoxyglucose as a function of temperature. Solid lines are fits to the linear Stokes-Einstein equation; dashed lines are fits to the exponential Arrhenius equation.
An alternative explanation for this superthermal behavior could be a temperature-dependent decrease in viscosity, which is indeed observed for many solvents. For example, the viscosity of water decreases from 1.308 centipoise (cP) at 10 °C to 0.8007 cP at 30 °C (18). This 38% decrease approaches the twofold change necessary to explain our observations. However, experimental measurements in E. coli and other cell types indicate that the temperature-dependence of cytoplasmic viscosity is substantially shallower than that of pure water (19). Moreover, because the cytoplasm is not purely viscous, it seems unlikely that a temperature-dependent change in viscosity would not also change α, which remains constant at all temperatures observed.
Furthermore, the temperature-dependence of the motion of chromosomal loci in untreated cells is much steeper than in cells treated with sodium azide and 2-deoxyglucose (Fig. 2B). In untreated cells, Dapp increases approximately twofold as the temperature is raised from 10 to 40 °C, whereas it increases only 1.2-fold for ATP-depleted cells. In contrast to untreated cells, Dapp of loci in ATP-depleted cells can be reasonably well fit by a line that extrapolates to 0 μm2/sα at 0 K. This indicates that thermal fluctuations are sufficient to explain DNA motion in these cells, as predicted by Eq. 2. This fit is also consistent with the suggestion that cytoplasmic viscosity changes relatively little over this temperature range (19). Therefore, untreated cells must have another source of molecular agitation, in addition to thermal motion.
ATP-dependent enzymatic activity could be a major source for generating excess agitation in the cytoplasm. Unlike thermal motion, which is by definition directly proportional to temperature, the reaction rates of enzymes are often described by the Arrhenius equation,  which has an exponential dependence on temperature. Interestingly, the diffusion coefficients of atoms in solids also exhibit Arrhenius behavior (20). When fit to the exponential function
which has an exponential dependence on temperature. Interestingly, the diffusion coefficients of atoms in solids also exhibit Arrhenius behavior (20). When fit to the exponential function
chromosomal loci give a value for the activation energy, Ea = 12 kJ/mol, that is approximately one quarter the energy released by ATP hydrolysis. This Arrhenius fit demonstrates that active biological fluctuations can account for the superthermal behavior of chromosomal loci in untreated cells (Fig. 2B). We note that Dapp of loci in untreated and ATP-depleted cells become comparable near 4 °C, where most biological enzymes become inactive. Thus, at this temperature extreme, thermal fluctuations are likely the dominant driving force of intracellular motion.
ATP-dependent fluctuations might arise because of the energy dissipated from active biological processes. Because enzymes are not perfectly efficient, not all of the chemical energy in ATP is converted into useful work. The excess is dissipated as heat into the cellular environment. For example, respiration in the mitochondria of eukaryotic cells produces heat, which can be detected by calorimetry (21, 22). Furthermore, cancer tissue produces more heat than nontumor tissue, perhaps owing to an enhanced metabolic activity (23). Thus, cells with greater metabolic capacity may generate more heat and consequently drive faster thermal motion of their intracellular components. To test the plausibility of this thermal model, we calculated the increase in intracellular temperature due to the heat dissipated by glycolysis and oxidative phosphorylation. In E. coli, the breakdown of a glucose molecule is ≈60% efficient, with 1,150 kJ of heat per mole of glucose released into the cytoplasm (24). This heat is quickly dissipated to the environment, and the intracellular temperature increases only fractions of a degree (from 310 K to 310.0001 K). Such a small rise in temperature is not sufficient to account for the difference in Dapp between loci in untreated and ATP-depleted cells. Indeed, the difference in Dapp at physiological temperature (310 K) predicts an intracellular temperature of ≈613 K, rendering the heat dissipation model unreasonable.
Alternatively, ATP-dependent fluctuations might arise from mechanical agitation. For example, conformational changes of DNA-bound proteins, the binding/unbinding of proteins to/from DNA, or the active translocation of protein machines along DNA, could cause chromosomal loci to move more than they would in the absence of ATP. Such a mechanism would be analogous to myosin contractions in a cross-linked actin network, which mechanically stress the network (11, 13). Furthermore, conformational changes of transmembrane proteins, driven by light (25) or ATP (26), have been shown to enhance the magnitude of fluctuations of a membrane. In bacteria, these nonequilibrium fluctuations driving random motion of chromosomal loci are likely due to the combination of many different enzymes undergoing conformational changes and binding reactions, as opposed to a single dominant motor.
Molecular Candidates Driving Intracellular Motion.
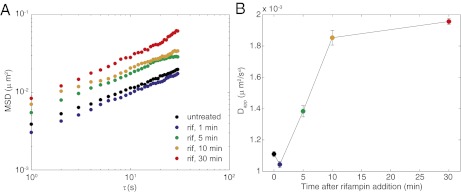
One molecular candidate for an energy-dependent enzyme that mechanically moves DNA is RNA polymerase, which can exert forces up to ≈25 pN (27). To test whether RNA polymerase activity contributes to locus motion, we treated cells with rifampin to inhibit transcription. After 1 min of drug treatment, Dapp does decrease (Fig. 3), slightly but significantly and consistently. However, after longer times of drug treatment, Dapp increases, plateauing to approximately twofold greater magnitude between 10 and 30 min (Fig. 3 and Table 1) (14). This nonmonotonic response is likely due to opposing effects on different timescales. Rifampin binds to the β subunit of the polymerase and prevents initiation (28), so transcriptional activity will stop in ≈30 s, the time required to finish the current transcript. The short-time decrease in Dapp is consistent with polymerase activity producing fluctuations that increase locus motion in untreated cells. At longer times, the cellular pool of mRNA will decay. The median lifetime of mRNA in E. coli is 2–4 min (29). Loss of mRNA will decrease the effective viscosity of the cytoplasm, which may result in faster motion. Furthermore, dissociation of RNA polymerase and nascent transcripts from the chromosome will reduce the effective size of a locus, as well as remove entanglements, enabling loci to move more quickly.
Fig. 3.
Locus motion in response to rifampin treatment. (A) Ensemble-averaged MSD for 84′ loci at 0, 1, 5, 10, and 30 min after addition of rifampin. (B) Mean Dapp ± SD as a function of time after rifampin addition for four independent data sets.
The modest decrease in Dapp at short times after rifampin treatment indicates that RNA polymerase cannot be the only source of ATP-dependent fluctuations. Cell wall synthesis presents another candidate process that could produce ATP-dependent fluctuations in bacteria. The peptidoglycan wall is constantly under construction by penicillin-binding proteins (PBPs), which catalyze the glycosyl transfer and transpeptidation reactions necessary to polymerize new cell wall (30, 31). Although PBPs do not hydrolyze ATP directly, synthesis of their substrate, lipid II, involves ATP-dependent reactions (32). Recent experiments suggest that the activity of these enzymes generates mechanical forces that drive motion of the actin homolog MreB in the cytoplasm (33–35). We saw no change in locus dynamics upon treatment with mecillinam, an antibiotic that inhibits PBP2. At concentrations under which mecillinam blocked motion of MreB in E. coli (35), the fold change in Dapp of chromosomal loci in mecillinam-treated cells compared with loci in untreated cells was 1.03 ± 0.07 (Table 1). Thus, mechanical fluctuations generated by cell wall synthesis do not penetrate into the nucleoid.
Previously, we showed that neither inhibition of DNA gyrase nor depolymerization of MreB significantly affect Dapp, ruling out these enzymes as potential sources of motion (14). Thus, our candidate approach failed to identify one single enzyme or molecular process that can account for the enhanced motion of chromosomal loci in untreated cells, but did raise RNA polymerase as a candidate that is partially responsible. These results suggest that ATP-dependent fluctuations in bacteria are caused not by a single dominant motor, as has been suggested for eukaryotes (36), but rather by the combined effect of all enzymatic activity in the cell.
Superthermal Motion of Chromosomal Loci in Yeast.
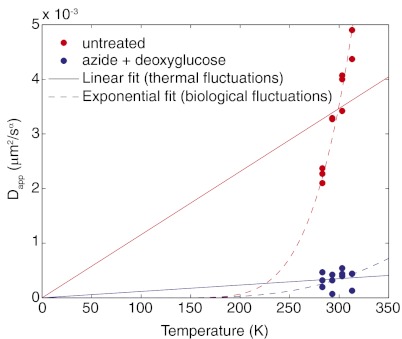
Finally, we performed similar experiments in the budding yeast Saccharomyces cerevisiae. A haploid strain carrying a lacO array inserted at the LEU2 locus and expressing GFP-LacI was used to quantify locus motion in the eukaryotic nucleus. Cells were grown at 30 °C and transferred to the appropriate temperature at least 5 min before imaging. As observed in E. coli, Dapp of LEU2 loci increased with temperature more steeply than expected from Eq. 2 (Fig. 4). Furthermore, treatment with sodium azide and 2-deoxyglucose caused a 10-fold decrease in mobility, an even stronger effect than that observed in E. coli (Table 1). Interestingly, even at 10 °C, where Dapp for bacterial loci in untreated and ATP-depleted cells began to converge, a significant difference between these two conditions remained for yeast loci. This observation suggests that active fluctuations persist in yeast at much colder temperatures than in E. coli. Yeast are adapted to cooler temperatures than E. coli, so perhaps their enzymes remain active across a lower temperature range. Indeed, wild-type S. cerevisiae can grow at 4–5 °C (37), and some strains even produce wine at −2 °C (38).
Fig. 4.
Dapp for chromosomal loci in S. cerevisiae as a function of temperature for untreated cells and cells treated with sodium azide and 2-deoxyglucose. Solid lines are fits to the linear Stokes-Einstein equation; dashed lines are fits to the exponential Arrhenius equation.
These results demonstrate that superthermal motion of chromosomal loci is not unique to E. coli. Furthermore, although myosin I has been found in the nucleus of mammalian cells (39, 40), there is no evidence for nuclear localization of any myosin isoform in S. cerevisiae (41). Thus, the enhanced motion of loci in the yeast nucleus suggests that, even in eukaryotes, active fluctuations can arise from nonmyosin ATP-dependent activity.
Discussion
Here we present evidence for “active” diffusion in bacterial cells and the eukaryotic nucleus. By examining the energy- and temperature-dependence of chromosomal locus dynamics in E. coli and S. cerevisiae, we show that ATP-dependent fluctuations contribute to macromolecular motion in vivo. These fluctuations behave like thermal fluctuations but with a greater magnitude and steeper temperature-dependence. They likely arise from enzymatic activity that mechanically agitates the cytoplasm/nucleoplasm.
Our results have significant implications for understanding macromolecular motion in vivo. Chemical reactions in the cell are governed by molecular collisions. These collisions may be driven by the chaotic motion of ATP-dependent enzymatic activity rather than purely thermal forces. This suggests that diffusion-limited reactions may occur more quickly than expected in vivo because “diffusion” is accelerated by ATP-consuming processes. Furthermore, if thermal fluctuations are not the only driving force for intracellular motion, then the application of equilibrium theories, such as the Stokes-Einstein equation, to biological processes may not be valid. Indeed, several studies have demonstrated that the fluctuation-dissipation theorem is violated in vivo (4, 5, 7, 8, 42). These exciting findings may fundamentally change how we think about cellular processes and the nature of the cytoplasm (36).
Although “active” diffusion driven by ATP-dependent fluctuations is an appealing interpretation of our data, it is not the only one. An alternative explanation for the data presented in Fig. 1 is that treatment with sodium azide and 2-deoxyglucose increases the viscosity of the cytoplasm. We have not ruled out this possibility experimentally, but it seems unlikely for two reasons. First, if depletion of ATP caused a stiffening of the cytoplasm, such as that observed for actomyosin cytoskeletal networks at low ATP concentrations (11), then we would expect to see a change in the scaling exponent. However, α does not change upon this treatment, or many others (14), including 2,4-dinitrophenol (Table 1). Second, as shown in Fig. 2B, the temperature-dependence of locus motion in ATP-depleted cells differs from that in untreated cells. If sodium azide and 2-deoxyglucose increased the cytoplasmic viscosity, then one would expect the entire Dapp vs. T curve to shift down but not to change slope. It seems unlikely that sodium azide and 2-deoxyglucose would change the temperature-dependence of the cytoplasm's viscosity.
Although “active” diffusion was first observed in eukaryotic cytoplasm (3–8), our results suggest that ATP-dependent fluctuations can also drive macromolecular motion in bacterial cells and in the eukaryotic nucleus. However, there are some important differences between the motor-driven fluctuations measured in eukaryotic cytoplasm (11, 13) and the ATP-dependent fluctuations described here. In particular, the scaling of (nondirected) motor-driven motion in eukaryotic cytoplasm is diffusive-like, with the power spectrum of intracellular forces exhibiting an ω−2 frequency dependence. In contrast, the MSD scaling of ATP-dependent motion in prokaryotes is subdiffusive and does not change when ATP synthesis is inhibited (Fig. 1). This robust scaling suggests that the power spectrum of ATP-dependent fluctuations has a frequency dependence similar to that of thermal fluctuations in a viscoelastic medium, although with a larger magnitude. Development of physical models will give new insight into these distinct fluctuation spectra and may lead to the identification of additional molecular sources of ATP-dependent fluctuations in prokaryotes. Such a theoretical approach, combined with experimental data, has significantly advanced our understanding of eukaryotic cytoskeletal networks (36).
The central role of myosin in generating nonthermal fluctuations in eukaryotes raises the intriguing possibility of cellular control. Eukaryotic cells may be able to tune the rate of intracellular diffusion by regulating myosin activity. This regulation mechanism seems unlikely in prokaryotes, because there is currently no known primary tunable source of ATP-dependent fluctuations. Rather, it seems that such intracellular forces may be a byproduct of global enzymatic activity. Nevertheless, an in vitro study of LacI-mediated DNA looping suggests that nonequilibrium fluctuations could influence transcriptional regulation (43). Applied fluctuations of only 5% above thermal fluctuations caused a twofold increase in the rate of DNA loop formation. Importantly, the rate of unlooping was insensitive to the fluctuations. Because DNA looping is rate-limiting for repression of the lac operon in E. coli (44), the authors conclude that environmental fluctuations, which raise the “effective” temperature in the cell, may be used to mechanically regulate gene expression in vivo (43).
Chromosomal loci are not the only example of energy-dependent molecular motion in bacteria. Plasmids lacking a partition system are highly mobile in E. coli cells growing on agarose pads, but slow down or stop in nongrowing cells imaged on glass slides, perhaps because of nutrient and energy depletion (45). Furthermore, Winther et al. (46) used optical tweezers to track the motion of single molecules of LamB, an E. coli outer membrane protein that serves as the receptor for bacteriophage λ. LamB motility decreased an order of magnitude when cells were depleted of energy by treatment with azide and arsenate. A similar decrease in motion was seen upon treatment with ampicillin, an antibiotic that inhibits cross-linking of the peptidoglycan, indicating that PBP activity is the dominant source of fluctuations driving LamB motion. Together with our experiments on chromosomal loci, these data support the hypothesis that ATP-dependent enzymatic activity produces mechanical fluctuations that actively drive the motion of molecules in the bacterial cell.
In conclusion, we have used a simple approach—temperature modulation—to study the physical basis of molecular motion in vivo. Our results demonstrate that ATP-dependent fluctuations significantly increase the mobility of macromolecules in the bacterial cytoplasm and the eukaryotic nucleus at physiological temperatures. These results have important implications for reaction rates inside the cell and suggest that “active” diffusion is a fundamental feature of cytoplasm that is common to both prokaryotes and eukaryotes.
Materials and Methods
Strains.
Stuart Austin (National Institutes of Health, Bethesda, MD) provided an E. coli strain with P1 parS inserted at the 84.3′ position on the chromosome and carrying plasmid pALA2705, which encodes GFP-Δ30ParB (47). Aaron Straight (Stanford University, Stanford, CA) provided AFS479, an S. cerevisiae strain with a lacO array inserted at the LEU2 locus and GFP-LacI integrated at the HIS3 locus (48).
Growth Conditions.
E. coli were grown overnight at 37 °C in LB medium with 100 μg/mL ampicillin. Cultures were diluted 1:100 into M9 minimal medium containing ampicillin and grown to an OD600 of ≈0.3–0.5. No isopropyl β-D-1-thiogalactopyranoside induction was required to see chromosomal loci, as described by Nielsen et al. (47). S. cerevisiae were grown overnight at 30 °C in synthetic dropout medium lacking histidine and leucine, and supplemented with adenine. Cultures were diluted 1:50 in the same medium and grown to midlog phase. No induction was required to see fluorescent labeling of chromosomal loci.
Metabolic inhibitors were added immediately before imaging at the following concentrations: sodium azide (0.1% for E. coli, 0.02% for yeast), 2-deoxyglucose (1 mM), and 2,4-dinitrophenol (10 mM). Rifampin (100 μg/mL) was added to liquid cultures at 1, 5, 10, and 30 min before imaging, or directly to an agarose pad. Mecillinam (100 μg/mL) was added to liquid cultures 30 min before imaging.
Microscopy.
Two microliters of media containing cells were placed on a 1% agarose pad made with the appropriate minimal media. For drug treatments at room temperature, cells were imaged on a Zeiss Axioplan 2 upright microscope. Temperature shifts were done with a water bath-controlled stage adapter on a Nikon Diaphot 300 inverted microscope. Both species were viewed with a 60× objective lens. Images were collected on a cooled CCD camera (Princeton Instruments) using MetaMorph software (Molecular Devices). Time-lapse movies were taken for 100 frames at 1-s intervals with a 200-ms exposure time.
Data Analysis.
Movies were analyzed with custom software in MATLAB (Mathworks). The positions of chromosomal loci were determined by nonlinear least squares fitting to a 2D Gaussian function and used to calculate the ensemble-averaged MSD, 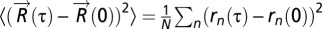 , where N is the number of loci. Alpha and Dapp were determined by fitting MSD data to Eq. 1 for τ = 1–30 s.
, where N is the number of loci. Alpha and Dapp were determined by fitting MSD data to Eq. 1 for τ = 1–30 s.
Acknowledgments
We thank Stuart Austin and Aaron Straight for generously providing bacterial and yeast strains. This work was supported by the National Science Foundation (NSF) Graduate Research Fellowship Program, an NSF Faculty Early Career Development award, the Howard Hughes Medical Institute, and by National Institute of Allergy and Infectious Diseases (NIAID) Grant AI-67712.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7138.
References
- 1.Brown R. A brief account of microscopical observations on the particles contained in the pollen of plants and on the general existence of active molecules in organic and inorganic bodies. Philos Mag. 1828;4:161–173. [Google Scholar]
- 2.Einstein A. Investigations on the theory of brownian movement. Annalen der Physik. 1905;17:549–560. [Google Scholar]
- 3.Caspi A, Granek R, Elbaum M. Enhanced diffusion in active intracellular transport. Phys Rev Lett. 2000;85:5655–5658. doi: 10.1103/PhysRevLett.85.5655. [DOI] [PubMed] [Google Scholar]
- 4.Lau AWC, Hoffman BD, Davies A, Crocker JC, Lubensky TC. Microrheology, stress fluctuations, and active behavior of living cells. Phys Rev Lett. 2003;91:198101. doi: 10.1103/PhysRevLett.91.198101. [DOI] [PubMed] [Google Scholar]
- 5.Bursac P, et al. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4:557–561. doi: 10.1038/nmat1404. [DOI] [PubMed] [Google Scholar]
- 6.Brangwynne CP, MacKintosh FC, Weitz DA. Force fluctuations and polymerization dynamics of intracellular microtubules. Proc Natl Acad Sci USA. 2007;104:16128–16133. doi: 10.1073/pnas.0703094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelm C. Out-of-equilibrium microrheology inside living cells. Phys Rev Lett. 2008;101:028101. doi: 10.1103/PhysRevLett.101.028101. [DOI] [PubMed] [Google Scholar]
- 8.Gallet F, Arcizet D, Bohec P, Richert A. Power spectrum of out-of-equilibrium forces in living cells: amplitude and frequency dependence. Soft Matter. 2009;5:2947–2953. [Google Scholar]
- 9.Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA. Cytoplasmic diffusion: Molecular motors mix it up. J Cell Biol. 2008;183:583–587. doi: 10.1083/jcb.200806149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA. Intracellular transport by active diffusion. Trends Cell Biol. 2009;19:423–427. doi: 10.1016/j.tcb.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno D, Tardin C, Schmidt CF, Mackintosh FC. Nonequilibrium mechanics of active cytoskeletal networks. Science. 2007;315:370–373. doi: 10.1126/science.1134404. [DOI] [PubMed] [Google Scholar]
- 12.Brangwynne CP, Koenderink GH, Mackintosh FC, Weitz DA. Nonequilibrium microtubule fluctuations in a model cytoskeleton. Phys Rev Lett. 2008;100:118104. doi: 10.1103/PhysRevLett.100.118104. [DOI] [PubMed] [Google Scholar]
- 13.MacKintosh FC, Levine AJ. Nonequilibrium mechanics and dynamics of motor-activated gels. Phys Rev Lett. 2008;100:018104. doi: 10.1103/PhysRevLett.100.018104. [DOI] [PubMed] [Google Scholar]
- 14.Weber SC, Spakowitz AJ, Theriot JA. Bacterial chromosomal loci move subdiffusively through a viscoelastic cytoplasm. Phys Rev Lett. 2010;104:238102. doi: 10.1103/PhysRevLett.104.238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber SC, Theriot JA, Spakowitz AJ. Subdiffusive motion of a polymer composed of subdiffusive monomers. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;82:011913. doi: 10.1103/PhysRevE.82.011913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mason TG, Weitz DA. Optical measurements of frequency-dependent linear viscoelastic moduli of complex fluids. Phys Rev Lett. 1995;74:1250–1253. doi: 10.1103/PhysRevLett.74.1250. [DOI] [PubMed] [Google Scholar]
- 17.Mason TG. Estimating the viscoelastic moduli of complex fluids using the generalized stokes-einstein equation. Rheologica Acta. 2000;39:371–378. [Google Scholar]
- 18.Segur JB, Oberstar HE. Viscosity of glycerol and its aqueous solutions. Ind Eng Chem. 1951;43:2117–2120. [Google Scholar]
- 19.Keith AD, Snipes W. Viscosity of cellular protoplasm. Science. 1974;183:666–668. doi: 10.1126/science.183.4125.666. [DOI] [PubMed] [Google Scholar]
- 20.Dill KA, Bromberg S. Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology. New York: Garland Science; 2003. [Google Scholar]
- 21.Prusiner S, Poe M. Thermodynamic cosiderations of mammalian thermogenesis. Nature. 1968;220:235–237. doi: 10.1038/220235a0. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Matsuoka I. Calorimetric studies of heat of respiration of mitochondria. J Biochem. 1978;84:39–46. doi: 10.1093/oxfordjournals.jbchem.a132117. [DOI] [PubMed] [Google Scholar]
- 23.Karnebogen M, Singer D, Kallerhoff M, Ringert RH. Microcalorimetric investigations on isolated tumorous and non-tumorous tissue samples. Thermochim Acta. 1993;229:147–155. [Google Scholar]
- 24.Bennett BD, et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manneville JB, Bassereau P, Levy D, Prost J. Activity of transmembrane proteins induces magnification of shape fluctuations of lipid membranes. Phys Rev Lett. 1999;84:4356–4359. [Google Scholar]
- 26.Girard P, Prost J, Bassereau P. Passive or active fluctuations in membranes containing proteins. Phys Rev Lett. 2005;94:088102. doi: 10.1103/PhysRevLett.94.088102. [DOI] [PubMed] [Google Scholar]
- 27.Wang MD, et al. Force and velocity measured for single molecules of RNA polymerase. Science. 1998;282:902–907. doi: 10.1126/science.282.5390.902. [DOI] [PubMed] [Google Scholar]
- 28.Campbell EA, et al. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell. 2001;104:901–912. doi: 10.1016/s0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein JA, Lin PH, Cohen SN, Lin-Chao S. Global analysis of Escherichia coli RNA degradosome function using DNA microarrays. Proc Natl Acad Sci USA. 2004;101:2758–2763. doi: 10.1073/pnas.0308747101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 31.Matteï PJ, Neves D, Dessen A. Bridging cell wall biosynthesis and bacterial morphogenesis. Curr Opin Struct Biol. 2010;20:749–755. doi: 10.1016/j.sbi.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Barreteau H, et al. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:168–207. doi: 10.1111/j.1574-6976.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 33.Garner EC, et al. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science. 2011;333:222–225. doi: 10.1126/science.1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domínguez-Escobar J, et al. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science. 2011;333:225–228. doi: 10.1126/science.1203466. [DOI] [PubMed] [Google Scholar]
- 35.van Teeffelen S, et al. The bacterial actin MreB rotates and rotation depends on cell-wall assembly. Proc Natl Acad Sci USA. 2011;108:15822–15827. doi: 10.1073/pnas.1108999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackintosh FC, Schmidt CF. Active cellular materials. Curr Opin Cell Biol. 2010;22:29–35. doi: 10.1016/j.ceb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Singh A, Manney TR. Genetic analysis of mutations affecting growth of Saccharomyces cerevisiae at low temperature. Genetics. 1974;77:651–659. doi: 10.1093/genetics/77.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argiriou T, et al. Psychrotolerant Saccharomyces cerevisiae strains after an adaptation treatment for low temperature wine making. Process Biochem. 1996;31:639–643. [Google Scholar]
- 39.Nowak G, et al. Evidence for the presence of myosin I in the nucleus. J Biol Chem. 1997;272:17176–17181. doi: 10.1074/jbc.272.27.17176. [DOI] [PubMed] [Google Scholar]
- 40.Pestic-Dragovich L, et al. A myosin I isoform in the nucleus. Science. 2000;290:337–341. doi: 10.1126/science.290.5490.337. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann WA, Richards TA, de Lanerolle P. Ancient animal ancestry for nuclear myosin. J Cell Sci. 2009;122:636–643. doi: 10.1242/jcs.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robert D, Nguyen TH, Gallet F, Wilhelm C. In vivo determination of fluctuating forces during endosome trafficking using a combination of active and passive microrheology. PLoS ONE. 2010;5:e10046. doi: 10.1371/journal.pone.0010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YF, Milstein JN, Meiners JC. Protein-mediated DNA loop formation and breakdown in a fluctuating environment. Phys Rev Lett. 2010;104:258103. doi: 10.1103/PhysRevLett.104.258103. [DOI] [PubMed] [Google Scholar]
- 44.Müller J, Oehler S, Müller-Hill B. Repression of lac promoter as a function of distance, phase and quality of an auxiliary lac operator. J Mol Biol. 1996;257:21–29. doi: 10.1006/jmbi.1996.0143. [DOI] [PubMed] [Google Scholar]
- 45.Derman AI, Lim-Fong G, Pogliano J. Intracellular mobility of plasmid DNA is limited by the ParA family of partitioning systems. Mol Microbiol. 2008;67:935–946. doi: 10.1111/j.1365-2958.2007.06066.x. [DOI] [PubMed] [Google Scholar]
- 46.Winther T, Xu L, Berg-Sørensen K, Brown S, Oddershede LB. Effect of energy metabolism on protein motility in the bacterial outer membrane. Biophys J. 2009;97:1305–1312. doi: 10.1016/j.bpj.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen HJ, Li Y, Youngren B, Hansen FG, Austin S. Progressive segregation of the Escherichia coli chromosome. Mol Microbiol. 2006;61:383–393. doi: 10.1111/j.1365-2958.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 48.Straight AF, Belmont AS, Robinett CC, Murray AW. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–1608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]