Abstract
Metacaspases are distantly related caspase-family cysteine peptidases implicated in programmed cell death in plants and lower eukaryotes. They differ significantly from caspases because they are calcium-activated, arginine-specific peptidases that do not require processing or dimerization for activity. To elucidate the basis of these differences and to determine the impact they might have on the control of cell death pathways in lower eukaryotes, the previously undescribed crystal structure of a metacaspase, an inactive mutant of metacaspase 2 (MCA2) from Trypanosoma brucei, has been determined to a resolution of 1.4 Å. The structure comprises a core caspase fold, but with an unusual eight-stranded β-sheet that stabilizes the protein as a monomer. Essential aspartic acid residues, in the predicted S1 binding pocket, delineate the arginine-specific substrate specificity. In addition, MCA2 possesses an unusual N terminus, which encircles the protein and traverses the catalytic dyad, with Y31 acting as a gatekeeper residue. The calcium-binding site is defined by samarium coordinated by four aspartic acid residues, whereas calcium binding itself induces an allosteric conformational change that could stabilize the active site in a fashion analogous to subunit processing in caspases. Collectively, these data give insights into the mechanistic basis of substrate specificity and mode of activation of MCA2 and provide a detailed framework for understanding the role of metacaspases in cell death pathways of lower eukaryotes.
Keywords: apoptosis, clan CD, parasite, X-ray crystallography
Programmed cell death (PCD) is essential for animal development and the maintenance of adult tissues. PCD itself is a regulated process, and in animals, apoptosis is controlled through the action of caspases, aspartic acid-specific cysteine peptidases (1). PCD is also essential for plant development (2), and multiple markers for apoptosis have been described in yeast and a broad range of protozoan parasites (3), yet these organisms do not encode caspases in their genomes; thus, alternative pathways must have evolved for caspase-independent cell death to be regulated. In recent years, attention has focused on the metacaspases, which are a highly conserved group of caspase-like cysteine peptidases found in plants, fungi, and protozoa but not in the metazoa (4). In plants, metacaspases are essential for embryogenesis (5, 6) and have been shown to act in antagonistic relationships, functioning as both positive and negative regulators of PCD (7). In yeast and some protozoa, metacaspases have been implicated as mediators of cell death in response to oxidative stress and environmental change (3, 8–10).
Various attempts have been made to draw parallels between caspases and metacaspases in respect to structure, activation, and function (11). However, two striking differences between metacaspases and caspases are their substrate specificities and requirements for activation. Metacaspases have arginine/lysine specificity, do not necessarily require processing or dimerization for activity, and are activated by calcium (5, 12–15). There are few known natural targets of the proteolytic activity of metacaspases. One, Tudor staphylococcal nuclease, a substrate for mcII-Pa metacaspase in the Norway spruce, Picea abies, is also a substrate for human caspase-3, and this has been taken as indicating a level of evolutionary conservation between PCD pathways (16). It is now apparent, however, that metacaspases have a variety of cellular functions that are distinct from those of the caspases, which may reflect the distinct differences in substrate specificity and activation (11). For example, in yeast, the Yca1 metacaspase is required for clearance of cellular protein aggregates as well as regulation of the timing of the cell cycle, a function that has also been identified in the parasitic protozoon Leishmania (9, 17–19).
Metacaspases have been classified as members of the clan CD (20) structural superfamily, which is represented by six peptidase families: the caspases, legumains, separases, clostripains, gingipains, and the repeats in toxin (RTX) self-cleaving toxins (cysteine protease domain), all of which are predicted to share a core caspase/hemoglobinase fold (CHF) containing the catalytic His/Cys dyad. The core of the CHF domain is a simple α/β-fold (21) defined by a four-stranded, parallel β-sheet and three conserved α-helices. Currently, there are crystal structures available for the caspases (1) [including paracaspase (22)], gingipain (23), and an RTX toxin (24). Metacaspases are described as distant relatives of the caspases (4) and, despite low sequence identity with the caspases, have been grouped [along with the Arg-specific paracaspases (4, 22)] into a structural subfamily of the caspase-type peptidases (family C14B). In the caspase structures, the fundamental catalytic domain consists of a six-stranded β-sheet, which contains a large subunit (p20) and a small subunit (p10) (1) separated by an intersubunit linker between strands 4 and 5. Active caspases are functional dimers. The recently identified structure of the caspase domain of the mucosa-associated lymphoid tissue lymphoma translocation 1 (MALT1-C) paracaspase revealed an identical topology (22). Caspases are activated by processing of the intersubunit linker or by dimerization (1, 25). Metacaspases are divided into two structural types based on their primary amino acid sequence (4, 11). Both types have been predicted to exhibit a caspase-like domain composed of p20 and p10 subunits (26) but differ in that only type I has a predicted N-terminal prodomain, whereas type II contains an additional linker between the subunits. Plant genomes encode both types of metacaspases, whereas only type I has been reported in protozoa and fungi (11).
The protozoan parasite Trypanosoma brucei is the causative agent of human African trypanosomiasis, and its genome encodes five metacaspases (MCA1–MCA5). MCA2, MCA3, and MCA5 have the conserved catalytic residues (histidine/cysteine dyad) (27) and are collectively required for the bloodstream form of the parasite (28). MCA2, which is an active peptidase, is located in RAB11-positive endosomes and has roles in precytokinesis cell cycle control (28). T. brucei mutants lacking MCA2 have no obvious defect, indicating that functional redundancy exists between MCA2, MCA3, and MCA5. MCA4 is an inactive pseudopeptidase because of a cysteine-to-serine substitution in the active site, yet it functions as a membrane-linked virulence factor (29). MCA4 is also bloodstream form-specific, released by the parasite and processed by MCA3 (29). This pseudopeptidase illustrates the diversity of metacaspase function and the challenges faced in attempting to assign functional roles based on sequence alone. This study aimed to understand the structural basis for the differences between metacaspases and caspases, including the substrate recognition properties and mechanisms for activation of the metacaspases. To this end, we undertook structural and biochemical analysis of MCA2 from T. brucei, determining the previously undescribed 3D structure of a metacaspase and providing incisive insights into how this family of enzymes functions.
Results and Discussion
Metacaspase Structure.
Attempts to crystallize active MCA2 (MCA2ac) were unsuccessful, most likely a result of the presence of mixed degradation products caused by in vitro autoprocessing of the full-length recombinant enzyme (14). Therefore, the crystal structures of two catalytically inactive forms of MCA2 [MCAC213G and MCAC213A (Table S1)] were determined to 1.4 and 1.6 Å, respectively (Tables S2 and S3). The MCAC213G and MCAC213A structures are essentially identical (SI Methods). The highest resolution structure of MCA2C213G is the most complete, and the term MCA2 is used to describe the overall structure, unless otherwise stated. The MCA2C213G structure consists of residues 3–347, with the exception of two disordered loop regions consisting of residues 166–170 and 269–275 (denoted the 280-loop). MCA2 is monomeric and comprises an eight-stranded β-sheet (β1–β8) consisting of six parallel and two antiparallel strands with 2↑1↑3↑4↑7↑8↓5↑6↓ topology (30) (Figs. 1 and 2D). The first four parallel strands lie in the same plane, exhibiting only a very slight right-handed twist, whereas the second half of the sheet is more twisted, with the C terminus of β7 rotating out of the plane of the sheet and crossing under the N terminus of β8 (Fig. 1A). The β-sheet is surrounded by five α-helices (α1–α5) that lie approximately parallel to it. Helices α1, α4, and α5 are found on one side of the sheet, with α2 and α3 on the opposite side. At the C terminus of β3, a small section of antiparallel β-sheet (βA-βC) is found with strands βB and βC, connected by a 10-residue linker containing a short section of α-helix (αA). This section of β-sheet is positioned at the N terminus of α2 and α3, capping the helices and the space between them (Fig. 1).
Fig. 1.
Structure and sequence of T. brucei MCA2C213G. General loop regions are shown in gray, the N-terminal region in cyan, α-helices in blue, and β-strands in red, apart from βA-βC, which is shown in purple. (A) Tertiary structure of the MCA2 monomer with the position of the catalytic dyad in yellow. (B) MCA2 sequence with the assigned secondary structure. The catalytic dyad is highlighted in red, and residues involved in the S1 pocket and samarium binding are shown in orange and cyan, respectively. Known cleavage sites (14) are highlighted (▼), and residues that are missing from the MCA2C213G electron density are shown in light gray and with a dashed line.
Fig. 2.
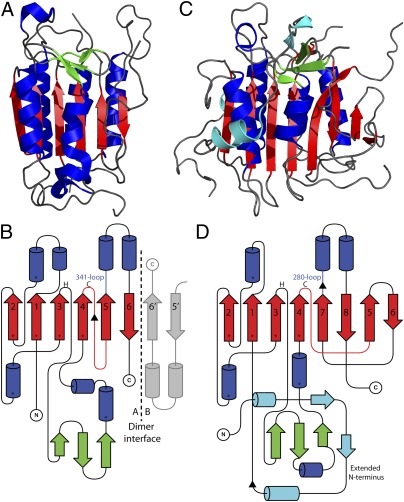
Comparison of MCA2 and caspase-7. Ribbon diagram of caspase-7 (A) and metacaspase (C). General loops are colored gray/black, the main β-sheet is colored red, surrounding α-helices are shown in blue, and a small section of the β-sheet at the C terminus of β3 is shown in green. In MCA2, secondary structural elements found on the N-terminal domain are colored cyan. (B) Topology diagram of caspase-7. The 341-loop and the position of the catalytic dyad are highlighted, and the β-strands are numbered from the N terminus. The intersubunit linker is drawn as a red line with the cleavage site highlighted (▲). (D) Topology diagram of MCA2, with the catalytic loop shown in red. The 280-loop affected during the Ca2+ activation of MCA2 and known autoprocessing sites (▲) (14) are shown. The CHF core secondary structural elements are highlighted (*) in B and D.
The residues that constitute the catalytic dyad (H158 and C213G/A) are found on loops situated at the C-terminal end of strands β3 and β4, respectively (Figs. 1 and 2D). The loop connecting β4 and β5 comprising the catalytic cysteine (denoted the catalytic loop) is 11 residues long (Figs. 1B and 2D) and shows a degree of flexibility (as defined by poorer electron density and higher B-factors in this region). One of the most striking features of the MCA2 structure is the 70-residue N terminus preceding β1. This domain is an ordered loop containing only a small region of 310-helix (α0′), two short β-strands (β0′ and β0), and a seven-residue α-helix (α0) (Figs. 1 and 3A). It encircles the main body of the enzyme, traversing the top in the region of the catalytic dyad and interacting with itself as it crosses underneath (Fig. 3A). In MCA2C213A, there are 129 residues contributing to the interface between the N-terminal domain and the main body of the protein, creating 32 hydrogen bonds and eight salt bridges. The interfacing residues include the catalytic dyad of H158 and C213A, which are 100% and 50% buried by the N terminus, respectively.
Fig. 3.
N-terminal domain of MCA2 crosses over the S1 binding pocket. (A) N-terminal domain (blue) encircles the main body of the enzyme (gray). (B) Electrostatic surface potential of MCA2C213A (rotated ∼90° from A), calculated without the N-terminal domain, where blue and red denote positively and negatively charged surface areas, respectively. The N-terminal domain is shown crossing the catalytic dyad as sticks with Y31 labeled. The water molecules that line the active site are shown as cyan spheres. (C) Close-up view of the S1 binding pocket. (D) Y31 interacts with residues in the S1 binding pocket. (E) SDS/PAGE analysis of in vitro autoprocessing exhibited by MCA2 and mutants purified by Ni2+-affinity chromatography. (F) Activity of MCA2ac and mutants was compared by measuring the hydrolysis of the fluorogenic substrate Z-GGR-AMC (average of 3 experiments is shown ± SD).
Comparison with Caspase-7.
The overall fold of MCA2 is comparable to that of the mammalian caspases (SI Methods). It is most structurally similar to caspase-7, with 65% of its secondary structural elements identified in the caspase-7 structure. However, the structure of MCA2 reveals some striking architectural differences from the caspases, most notably in the length and arrangement of its β-sheet and in its extended N terminus (Fig. 2). The β-sheet in MCA2 is two strands longer than in caspase-7, which exhibits a six-stranded β-sheet with 2↑1↑3↑4↑5↑6↓ topology. These differences result in the monomeric structure of MCA2 being much less compact than that of a caspase monomer (Fig. 2). MCA2 exists as an enzymatically active monomer (Fig. S1), and the loop regions on its surface create an unfavorable environment for the type of β-sheet/β-sheet dimerization found in the caspases (Fig. 2). In addition, a structural overlay with the caspase-7 dimer reveals that β5 from MCA2 overlays with β6 from the second caspase monomer (β6′), and thus occupies the space used in the caspase-dimer interface and renders it unavailable (Fig. 2 B and D). Hence, the larger eight-stranded β-sheet in MCA2 prevents it from forming a dimer along the same interface as the caspases and stabilizes the protein as a monomer.
Recombinant MCA2ac undergoes autocatalytic processing at K55 in the N-terminal region and at K268 in the 280-loop (14) (Figs. 1B and 2D). However, cleavage does not generate separate subunits because the C-terminal region is embedded in the β-sheet and the N-terminal region remains associated with the main body of the protein (Fig. S1). This processing has not been observed in vivo (28) and is not required for the enzyme to be proteolytically active (14). Consequently, the autoproteolytic processing of recombinant MCA2ac in vitro may simply reflect the high concentrations of the enzyme and the position of basic residues on solvent-exposed loops.
Effector caspases, such as caspase-7, are activated by cleavage in the intersubunit linker generating the p10 and p20 subunits (Fig. 2B). This cleavage allows part of the intersubunit linker from one caspase monomer to interact with the neighboring monomer and helps to order the active site (31). MCA2 is very unusual in this respect; the topology of the β-sheet is different, with β4 and β5 not being adjacent but, instead, separated by two antiparallel strands (β7 and β8) (Fig. 2D), and the catalytic loop that connects these strands does not harbor any basic residues as potential cleavage sites (Fig. 1B), and, indeed, none have been identified (14). In addition, this loop is much shorter than the intersubunit linker in the caspases, suggesting that it does not have the same functional relevance. Thus, the eight-stranded β-sheet, the length and topology of which preclude caspase-like dimerization and explain its monomeric peptidase activity, clearly distinguishes MCA2 from other caspase family members. This is further supported by the lack of an intersubunit linker preventing the formation of two distinct subunits or activation by cleavage in this region. Consequently, the activation mechanism of MCA2 is quite distinct from that of the caspases.
N-Terminal Domain and Active Site.
The catalytic dyad of MCA2 is buried beneath the N-terminal domain, which crosses the active site in the direction of the N terminus to the C terminus (Fig. 3B). This is the same direction as the peptide inhibitors bound to the other clan CD peptidases (23, 32, 33) and the predicted direction for an MCA2 substrate. A 3D alignment of the structures of MCA2 and caspase-7 revealed that the position of the catalytic dyad is conserved between the two enzymes (Fig. 2 B and D), and this is also the case for clan CD members from other families: gingipain R (23) and the cysteine peptidase domain of the Vibrio cholerae RTX (24). However, in all the structures apart from MCA2, the dyad is found on the surface of the protein. In contrast, the N-terminal domain of MCA2 appears to block substrate access to the active site sterically, with the nature of the residues at the crossover preventing intramolecular autocleavage.
Occlusion of the active site by an N-terminal domain is also observed in the proforms of the lysosomal cathepsins [clan CA, family C1 (20)], where proteolytic cleavage and removal of the prodomain are required for activation (34). However, in cathepsin B (35), the prodomain is only weakly associated with the enzyme, through five hydrogen bonds, and it crosses the active site in the opposite orientation to that observed in MCA2. Structural elucidation of a caspase prodomain is yet to be achieved, but it has been reported to be flexible, and hence disordered, in the crystal structures (36). In contrast, the N-terminal domain of MCA2 was both visible and highly ordered in our crystal structures, with its position clearly showing that it would be required to undergo a conformational shift to allow substrates access into the active site.
Examining the structure of MCA2 without its N-terminal region reveals that the catalytic dyad forms part of a large acidic pocket (Fig. 3B), consistent with a binding site for the basic P1 amino acid of an MCA2 substrate. The functional groups of C92, D95, S156, and D211 all point toward the surface of the S1 pocket, and the bottom is lined with three well-ordered water molecules (Fig. 3C), the locations of which were found to be conserved in all the MCA2 crystal structures. The N terminus of MCA2 crosses the S1 pocket and the catalytic dyad at residues 30–36, with Y31 forming hydrogen bonds to D95 and S156, along with a water molecule, which is, in turn, coordinated to a second water molecule and D211 (Fig. 3D). There are no other hydrogen bonds between the S1 pocket and the N-terminal domain. Residues C92, D95, S156, and D211 are conserved within the metacaspase family, whereas Y31 is conserved in the N-terminal domain of T. brucei metacaspases (Fig. S2). In addition to their conservation, their location and orientation in the S1 pocket suggest that these residues play a part in substrate recognition. To investigate their significance, single alanine point mutations in MCA2ac were constructed and their proteolytic activity was assessed using a fluorogenic peptide substrate (Z-GGR-AMC; Fig. S3).
D95 is positioned at the back of the S1 pocket in close proximity to D211. Alanine mutations of these residues resulted in enzymes that exhibited no autoprocessing in vitro or any noteworthy activity toward Z-GGR-AMC compared with MCA2ac (0% for MCA2D95A, <5% for MCAD211A) (Fig. 3 E and F). Consequently, these aspartic acid residues are aptly positioned to recognize and coordinate the basic side chain of an arginine or lysine residue and are involved in the enzyme’s substrate specificity in P1. MCA2C92A showed approximately half of the activity toward the substrate compared with MCA2ac, and the equivalent of C92 in Arabidopsis thaliana metacaspase 9 (C29) has similarly been shown to be important for activity (37). Thus, C92 of MCA2 contributes to correct formation of the substrate binding pocket. MCA2Y31A exhibited a much higher degree of autoprocessing in vitro than MCA2ac, or any of the other mutants (Fig. 3E), suggesting that Y31 controls access to the active site. MCA2Y31A had only 60% of MCA2ac activity, probably attributable to the extensive autoprocessing degrading the enzyme. In contrast MCA2S156A activity was threefold higher than MCA2ac. We propose a model whereby Y31 acts as a gatekeeper by using S156 as a latch; when the latch is released, substrate can access the active site. These data are consistent with the N-terminal domain acting to regulate the activity of the enzyme by protecting the active site until it is activated by calcium (see below).
The participation of D95, D211, and Y31 in S1 binding of MCA2 is further supported by analysis of substrate binding in caspase-7 and MALT1-C. In caspase-7, P1 substrate recognition is dependent on the three highly conserved residues, R87, Q184, and R233 (1), and in MALT1-C, three negatively charged residues, D365, D462, and E500, were found to coordinate the P1 Arg of the inhibitor (22). The structural overlay revealed that the secondary structure of MCA2 is conserved with caspase-7 residues R87 and Q184, overlaying with residues L89 and D211, respectively. L89 would not be important in recognizing a basic metacaspase substrate; however, D95, which sits nearby on the N-terminal end of α1 and overlays with D365 in MALT1-C, was shown to be essential for MCA2 activity. R233 of caspase-7, which lies on a loop (termed the 341-loop; ref. 1, Fig. 2B) at the C terminus of β5 and undergoes significant conformational changes during activation (33), has no ordered equivalent in MCA2. In the structure of MALT1-C, this loop contains the P1 binding residue E500. In the MCA2 structure, this corresponds to the disordered loop region at the C terminus of β7 (denoted the 280-loop; Figs. 1B and 2D), suggesting that this loop could play a role in substrate binding of MCA2. In addition, Y31 from the MCA2 N-terminal domain occupies the same position as R233 in caspase-7 (and E500 in MALT1-C), implying that Y31 would be involved in binding a substrate at the P1 position.
Despite the distinct structural and functional differences between the caspases and metacaspases, MCA2 uses residues at similar structural positions in its S1 binding pocket to other caspase family members to determine its substrate specificity. Although two of the three proposed MCA2 P1 binding residues do not share conserved secondary structure with the caspases, the position of the residues in the S1 binding pocket remains preserved. This suggests a common substrate recognition mechanism for caspases, paracaspases, and metacaspases.
Calcium Binding/Activation.
MCA2 and other metacaspases are dependent on calcium for activation (5, 12–15). Furthermore, the peptide-based inhibitor Z-VRPR-FMK inhibits the autoprocessing activity of MCA2ac, and calcium is required for inhibitor binding (Fig. 4A and SI Methods). Varying concentrations of CaCl2 were added to crystallization trials in an attempt to identify calcium-binding sites in the protein. However, even concentrations as low as 10 μM inhibited crystal formation. Lanthanides are often used to define calcium-binding sites in proteins (38); consequently, samarium was tested as a heavy atom derivative for the structure solution. A samarium-derivatized crystal (MCA2C213G-Sm) was obtained that revealed a single, fully occupied Sm3+-binding site on the surface of the molecule (Fig. 4B). The samarium ion exhibits a coordination number of 7 from two water molecules and four highly conserved aspartic acid residues: D173, D189, D190, and D220. These residues are well conserved in metacaspases from a diverse range of organisms (Fig. S2) but are not found in the caspases or paracaspases, which correlates with their lack of calcium-dependent activation. The structures of MCA2C213G and MCA2C213G-Sm are essentially identical, with the exception that MCA2C213G-Sm lacks electron density for several additional residues in the 280-loop (266–279 compared with 269–275 for MCA2C213G; Table S3), showing an increased degree of flexibility in this region.
Fig. 4.
Allosteric binding of calcium induces a conformational shift in MCA2C213A. (A) Inhibitor Z-VRPR-FMK binds to MCA2ac on the addition of Ca2+, causing a small size shift on SDS/PAGE, and protects MCA2ac from autoproteolysis in the presence of Ca2+. (B) Samarium ion (Sm3+) binds to the surface of MCA2C213G via four aspartic acid residues and two water molecules (cyan spheres), defining the Ca2+-binding site. (C) SDS/PAGE analysis of in vitro autoprocessing exhibited by MCA2ac and MCA2D189A/D190A. (D) Composite diagram shows the structure of MCA2C213A-Ca (gray) and its 280-loop (+Ca2+, red) with the catalytic dyad highlighted in orange and the N-terminal domain shown in green. Addition of the 280-loop from the MCA2C213A structure (APO, blue) and the bound Sm3+ ion from the MCA2C213G-Sm structure shows the 280-loop movement with respect to the position of the calcium-binding site. Missing residues from the 280-loop regions are represented by dotted lines. (Inset) Structure of MCA2C213A-Ca shows an approximate 5.6-Å, 110° shift in the position of the MCA2C213A 280-loop. (E) In the structure of MCA2C213A-Ca, the 280-loop (red) forms a section of the β-sheet with the N-terminal domain (green) that sits above the catalytic dyad (orange).
To investigate the location of the calcium-binding site further, a double mutant, MCA2D189A/D190A, was made. In the presence of calcium, this mutant had no detectable activity against Z-GGR-AMC (specific activity <0.14 mU/mg), nor did it autoprocess (Fig. 4C). Furthermore, CD spectra of MCA2D189A/D190A confirmed that the mutation had not adversely affected the overall structure of the protein (Fig. S4). This confirms the role of these aspartic acid residues in the putative calcium-binding site and shows they are essential for MCA2 activity. To gain additional information on how calcium influences MCA2 activity, various CaCl2 soaks were carried out on MCA2C213A crystals (MCA2C213A-Ca). Two datasets were collected from each crystal soak at wavelengths of 0.97 Å and 1.7 Å, but Ca2+ could not be detected in any of these datasets (SI Methods). However, structures determined from calcium-soaked crystals revealed a large Ca2+-induced conformational change in the position of the 280-loop. This loop changes direction at the Cα position of A280, resulting in a 5.6-Å, 110° shift in the positions of the Cα positions of G279 between MCA2C213A-Ca and MCA2C213A (Fig. 4, inset). This movement also resulted in the disorder of the first 15 residues of the N-terminal domain of MCA2C213A-Ca, which does not appear to be functional but, instead, is a direct result of the 280-loop movement in the crystals.
In both MCA2C213A and MCA2C213A-Ca, residues 280 and 281 form a section of β-sheet with N-terminal residues 32 and 31, respectively. In MCA2C213A, this loop then moves away from the N-terminal domain and crosses over a short loop between α5 and β8. In MCA2C213A-Ca, the 280-loop continues to run parallel, forming a section of β-sheet with the N-terminal domain (Fig. 4E). In contrast to MCA2C213A, the 280-loop in MCA2C213A-Ca is in line with the wall of the S1 binding pocket, sitting directly above the catalytic dyad (Fig. 4E). Because the 280-loop is structurally homologous to the 341-loop in the caspases (Fig. 2 B and D), it appears that in MCA2, the 280-loop moves to stabilize substrates in the active site in response to calcium binding. We also found that calcium binding alone does not induce a conformational shift in the N-terminal region crossing the active site suggesting that the presence of a substrate is also required to trigger the release of the N-terminal latch.
Conclusions.
The 1.4-Å structure of T. brucei MCA2 has given insights into the substrate specificity and mode of activation of MCA2. It has revealed that MCA2 possesses an unusual N-terminal domain, which likely serves to regulate substrate access to the active site until the enzyme is activated by calcium in the presence of a substrate. In MCA2, calcium is required for both activation of the enzyme and binding of the peptide inhibitor Z-VRPR-FMK, and it was found to induce an allosteric conformational change in the 280-loop, bringing it closer to the active site. This suggests that MCA2 requires this calcium-induced loop movement to stabilize substrate binding, and hence to regulate activity. Little is known about Ca2+ concentrations and/or Ca2+ signaling in African trypanosomes, and nothing is known about Ca2+ in the RAB11-positive recycling endosome compartment in which MCA2 primarily resides. However, endoplasmic reticulum stress-induced cell death in Leishmania is Ca2+-dependent (39); thus, it will be interesting to investigate whether there is any relationship between such cell death and the Ca2+ activation central to metacaspase function.
The structural similarity of MCA2 to the caspases suggests that they evolved from a common ancestor. However, the length and construction of their β-sheets, and the differences identified in the intersubunit linker and domain structure, show that they are architecturally very distinct. Furthermore, the lack of structurally conserved autocatalytic cleavage sites and dimerization interfaces in MCA2 is consistent with MCA2 having a mode of activation different from that of the caspases. These findings, together with metacaspase’s occluded active site, distinct substrate specificity, and dependence on calcium for activation (14), show that MCA2 activity is regulated very differently from that of the caspases. Thus, caspases and metacaspases may have evolved independently from an ancestral metacaspase-like peptidase, with each family of enzymes evolving distinct activation mechanisms to regulate cell death pathways. Overall, this work provides a context for the design of metacaspase-specific inhibitors that can potentially be used for the development of novel antiparasite drugs (40).
Methods
The construction of plasmids, purification of protein, and enzyme assays were carried out as described previously (14) and in SI Methods. Initial crystals of MCA2C213G or MCA2C213A were grown at 4 °C using vapor diffusion techniques in a 96-well sitting drop plate (Innovadyne) containing 500 nL of protein and 500 nL of reservoir against a reservoir of 50 mM Hepes (pH 7.0), 0.1% tryptone, and 20% (wt/vol) PEG3350 (PEG/Ion screen; Hampton). These crystals were composed of multiple plates and were optimized in 24-well sitting drop plates (Cryschem; Hampton) using microseeding techniques. Crystals were flash-frozen in liquid nitrogen using the reservoir solution plus 20% (vol/vol) 2-methyl-2,4-pentanediol (MPD) as a cryoprotectant before data collection. A samarium-derivatized crystal (MCA2C213G-Sm) was prepared by soaking an MCA2C213G crystal in artificial mother liquor [AML; 50 mM Hepes (pH 7.0), 0.1% tryptone, 20% PEG3350] containing 60 mM samarium acetate for 2 min. In addition, an MCA2C213A crystal soaked in 5 mM CaCl2 for 1 h (MCA2C213A-Ca) was prepared and cryoprotected in AML containing 20% MPD and 1 mM CaCl2. All native and multiwavelength anomalous dispersion diffraction data were collected at Diamond Light Source (DLS; Didcot, United Kingdom) on beamlines I03 and/or I04 and processed with Fast_dp at DLS. Details of data collection, structure determination, and structural analyses are described in SI Methods.
Supplementary Material
Acknowledgments
We thank E. Brown for help with cloning, A. Scott for protein purification, T. Z. Murray for inhibitor assays, S. M. Kelly for CD analysis, M. Gabrielsen for helpful discussion, and DLS for access (Proposal Mx6683). This work was supported by Wellcome Trust Grant 091790 and Medical Research Council Grant 0700127. The Wellcome Trust Centre for Molecular Parasitology is supported by core funding from Wellcome Trust Grant 085349.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors reported in this paper have been deposited in the Protein Data Bank in Europe, www.ebi.ac.uk/pdbe/ (PDB ID 4AF8, 4AFP, 4AFR, and 4AFV).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200885109/-/DCSupplemental.
References
- 1.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozhkov PV, Filonova LH, Suarez MF. Programmed cell death in plant embryogenesis. Curr Top Dev Biol. 2005;67:135–179. doi: 10.1016/S0070-2153(05)67004-4. [DOI] [PubMed] [Google Scholar]
- 3.Reece SE, Pollitt LC, Colegrave N, Gardner A. The meaning of death: Evolution and ecology of apoptosis in protozoan parasites. PLoS Pathog. 2011;7:e1002320. doi: 10.1371/journal.ppat.1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uren AG, et al. Identification of paracaspases and metacaspases: Two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell. 2000;6:961–967. doi: 10.1016/s1097-2765(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 5.Bozhkov PV, et al. Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc Natl Acad Sci USA. 2005;102:14463–14468. doi: 10.1073/pnas.0506948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suarez MF, et al. Metacaspase-dependent programmed cell death is essential for plant embryogenesis. Curr Biol. 2004;14:R339–R340. doi: 10.1016/j.cub.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Coll NS, et al. Arabidopsis type I metacaspases control cell death. Science. 2010;330:1393–1397. doi: 10.1126/science.1194980. [DOI] [PubMed] [Google Scholar]
- 8.Herker E, et al. Chronological aging leads to apoptosis in yeast. J Cell Biol. 2004;164:501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zalila H, et al. Processing of metacaspase into a cytoplasmic catalytic domain mediating cell death in Leishmania major. Mol Microbiol. 2011;79:222–239. doi: 10.1111/j.1365-2958.2010.07443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan MA, Chock PB, Stadtman ER. Knockout of caspase-like gene, YCA1, abrogates apoptosis and elevates oxidized proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2005;102:17326–17331. doi: 10.1073/pnas.0508120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsiatsiani L, et al. Metacaspases. Cell Death Differ. 2011;18:1279–1288. doi: 10.1038/cdd.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vercammen D, et al. Type II metacaspases Atmc4 and Atmc9 of Arabidopsis thaliana cleave substrates after arginine and lysine. J Biol Chem. 2004;279:45329–45336. doi: 10.1074/jbc.M406329200. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe N, Lam E. Two Arabidopsis metacaspases AtMCP1b and AtMCP2b are arginine/lysine-specific cysteine proteases and activate apoptosis-like cell death in yeast. J Biol Chem. 2005;280:14691–14699. doi: 10.1074/jbc.M413527200. [DOI] [PubMed] [Google Scholar]
- 14.Moss CX, Westrop GD, Juliano L, Coombs GH, Mottram JC. Metacaspase 2 of Trypanosoma brucei is a calcium-dependent cysteine peptidase active without processing. FEBS Lett. 2007;581:5635–5639. doi: 10.1016/j.febslet.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe N, Lam E. Calcium-dependent activation and autolysis of Arabidopsis metacaspase 2d. J Biol Chem. 2011;286:10027–10040. doi: 10.1074/jbc.M110.194340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundström JF, et al. Tudor staphylococcal nuclease is an evolutionarily conserved component of the programmed cell death degradome. Nat Cell Biol. 2009;11:1347–1354. doi: 10.1038/ncb1979. [DOI] [PubMed] [Google Scholar]
- 17.Lee RE, Puente LG, Kaern M, Megeney LA. A non-death role of the yeast metacaspase: Yca1p alters cell cycle dynamics. PLoS ONE. 2008;3:e2956. doi: 10.1371/journal.pone.0002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RE, Brunette S, Puente LG, Megeney LA. Metacaspase Yca1 is required for clearance of insoluble protein aggregates. Proc Natl Acad Sci USA. 2010;107:13348–13353. doi: 10.1073/pnas.1006610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambit A, Fasel N, Coombs GH, Mottram JC. An essential role for the Leishmania major metacaspase in cell cycle progression. Cell Death Differ. 2008;15:113–122. doi: 10.1038/sj.cdd.4402232. [DOI] [PubMed] [Google Scholar]
- 20.Rawlings ND, Barrett AJ, Bateman A. MEROPS: The peptidase database. Nucleic Acids Res. 2010;38(Database issue):D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aravind L, Koonin EV. Classification of the caspase-hemoglobinase fold: Detection of new families and implications for the origin of the eukaryotic separins. Proteins. 2002;46:355–367. doi: 10.1002/prot.10060. [DOI] [PubMed] [Google Scholar]
- 22.Yu JW, Jeffrey PD, Ha JY, Yang X, Shi Y. Crystal structure of the mucosa-associated lymphoid tissue lymphoma translocation 1 (MALT1) paracaspase region. Proc Natl Acad Sci USA. 2011;108:21004–21009. doi: 10.1073/pnas.1111708108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichinger A, et al. Crystal structure of gingipain R: An Arg-specific bacterial cysteine proteinase with a caspase-like fold. EMBO J. 1999;18:5453–5462. doi: 10.1093/emboj/18.20.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lupardus PJ, Shen A, Bogyo M, Garcia KC. Small molecule-induced allosteric activation of the Vibrio cholerae RTX cysteine protease domain. Science. 2008;322:265–268. doi: 10.1126/science.1162403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Vercammen D, Declercq W, Vandenabeele P, Van Breusegem F. Are metacaspases caspases? J Cell Biol. 2007;179:375–380. doi: 10.1083/jcb.200705193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mottram JC, Helms MJ, Coombs GH, Sajid M. Clan CD cysteine peptidases of parasitic protozoa. Trends Parasitol. 2003;19(4):182–187. doi: 10.1016/s1471-4922(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 28.Helms MJ, et al. Bloodstream form Trypanosoma brucei depend upon multiple metacaspases associated with RAB11-positive endosomes. J Cell Sci. 2006;119:1105–1117. doi: 10.1242/jcs.02809. [DOI] [PubMed] [Google Scholar]
- 29.Proto WR, et al. Trypanosoma brucei metacaspase 4 is a pseudopeptidase and a virulence factor. J Biol Chem. 2011;286:39914–39925. doi: 10.1074/jbc.M111.292334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Kim SH. The anatomy of protein beta-sheet topology. J Mol Biol. 2000;299:1075–1089. doi: 10.1006/jmbi.2000.3678. [DOI] [PubMed] [Google Scholar]
- 31.Shi YG. Caspase activation: Revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Shen A, et al. Mechanistic and structural insights into the proteolytic activation of Vibrio cholerae MARTX toxin. Nat Chem Biol. 2009;5:469–478. doi: 10.1038/nchembio.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei Y, et al. The structures of caspases-1, -3, -7 and -8 reveal the basis for substrate and inhibitor selectivity. Chem Biol. 2000;7:423–432. doi: 10.1016/s1074-5521(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 34.Coulombe R, et al. Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 1996;15:5492–5503. [PMC free article] [PubMed] [Google Scholar]
- 35.Podobnik M, Kuhelj R, Turk V, Turk D. Crystal structure of the wild-type human procathepsin B at 2.5 A resolution reveals the native active site of a papain-like cysteine protease zymogen. J Mol Biol. 1997;271:774–788. doi: 10.1006/jmbi.1997.1218. [DOI] [PubMed] [Google Scholar]
- 36.Vaidya S, Velázquez-Delgado EM, Abbruzzese G, Hardy JA. Substrate-induced conformational changes occur in all cleaved forms of caspase-6. J Mol Biol. 2011;406:75–91. doi: 10.1016/j.jmb.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belenghi B, et al. Metacaspase activity of Arabidopsis thaliana is regulated by S-nitrosylation of a critical cysteine residue. J Biol Chem. 2007;282:1352–1358. doi: 10.1074/jbc.M608931200. [DOI] [PubMed] [Google Scholar]
- 38.Martin RB, Richardson FS. Lanthanides as probes for calcium in biological systems. Q Rev Biophys. 1979;12(2):181–209. doi: 10.1017/s0033583500002754. [DOI] [PubMed] [Google Scholar]
- 39.Dolai S, Pal S, Yadav RK, Adak S. Endoplasmic reticulum stress-induced apoptosis in Leishmania through Ca2+-dependent and caspase-independent mechanism. J Biol Chem. 2011;286:13638–13646. doi: 10.1074/jbc.M110.201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berg M, et al. Design and evaluation of Trypanosoma brucei metacaspase inhibitors. Bioorg Med Chem Lett. 2010;20:2001–2006. doi: 10.1016/j.bmcl.2010.01.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.