Abstract
Hydrophobic free energy for protein folding is currently measured by liquid-liquid transfer, based on an analogy between the folding process and the transfer of a nonpolar solute from water into a reference solvent. The second part of the analogy (transfer into a nonaqueous solvent) is dubious and has been justified by arguing that transfer out of water probably contributes the major part of the free energy change. This assumption is wrong: transfer out of water contributes no more than half the total, often less. Liquid-liquid transfer of the solute from water to liquid alkane is written here as the sum of 2 gas-liquid transfers: (i) out of water into vapor, and (ii) from vapor into liquid alkane. Both gas-liquid transfers have known free energy values for several alkane solutes. The comparable values of the two different transfer reactions are explained by the values, determined in 1991 for three alkane solutes, of the cavity work and the solute-solvent interaction energy. The transfer free energy is the difference between the positive cavity work and the negative solute-solvent interaction energy. The interaction energy has similar values in water and liquid alkane that are intermediate in magnitude between the cavity work in water and in liquid alkane. These properties explain why the transfer free energy has comparable values (with opposite signs) in the two transfers. The current hydrophobic free energy is puzzling and poorly defined and needs a new definition and method of measurement.
Keywords: solvation free energy, hydrophobicity, protein energetics, Ostwald coefficient, Pratt-Chandler analysis
Ever since Kauzmann’s revolutionary proposal (1) in 1959, the hydrophobic factor has been widely acknowledged as a major factor in protein folding energetics (1–3). In the widely used Kauzmann-Tanford approach to measuring it, hydrophobic free energy is synonymous with hydrophobic hydration and is measured by the preference of a nonpolar solute for a reference solvent over water, based on the solute’s relative solubility in the two solvents. This approach is still the standard one, although it has been criticized. Much of the current research into the nature of the hydrophobic factor is based on computer simulations (4). The basic criticism of the liquid-liquid transfer approach is that gas-liquid transfer (which is better understood) is used to determine the solvation free energy of the nonpolar solute. Moreover, the choice of a reference solvent ought not to change the apparent hydrophobic free energy, but it does (5). The purpose here is to investigate these criticisms with the aid of gas-liquid transfer results and their interpretation.
The 1971 paper by Nozaki and Tanford (2) is often cited as the starting point of quantitative work on measuring hydrophobic hydration. However, as ref. 2 emphasizes, it was based on the protocol developed in the Cohn and Edsall laboratory at Harvard by McMeekin, et al. (6) who used it to investigate the water solubility of polar as well as nonpolar protein side chains. The latter authors found that nonpolar groups have additive ΔGhyd values and consequently that model compound results can be used to interpret this aspect of protein folding energetics.
The protocol chosen by Nozaki and Tanford (2) contains three steps: first, measure the solubility (S) (pH 7) of the solute (chosen to model a side chain) in water (w) and in a reference solvent (r). Crystalline amino acids were used and results with r = ethanol or dioxane were compared (2). Second, compute KD = (Sr)/(Sw) and ΔGLL = -RT ln KD. Third, focus on the side chain properties by setting ΔGLL = ΔGaa - ΔGgly (where aa = amino acid and gly = glycine); subtracting the ΔGLL value for Gly was intended to remove the effects of the  and α-COO- groups. Nozaki and Tanford gave hydrophobicity values for twelve nonpolar protein side chains and they did not study polar side chains.
and α-COO- groups. Nozaki and Tanford gave hydrophobicity values for twelve nonpolar protein side chains and they did not study polar side chains.
The Polar Group Effect
Problems arise if the solute being transferred contains both polar and nonpolar groups. The perturbing polar group effect was discovered in 1935 when McMeekin, et al. (6) found that the characteristic hydrophobic contribution of the -CH2- group is missing when the side chains of aspartic acid, asparagine, glutamic acid, and glutamine are analyzed, apparently because there are nearby polar groups. The polar group effect can be understood by the iceberg model of Frank and Evans (7) and Kauzmann (1, 8). In the iceberg model, the hydrophobic free energy arises chiefly from the perturbed H-bonded structure of water that develops around nonpolar groups (1, 7, 8). Using the iceberg model, the polar group effect is explained as the result of strong interactions between water and polar groups that affect the perturbed water structure around nearby nonpolar groups.
Criticisms by Hildebrand (1968) and Ben-Naim (1979)
In 1968 Hildebrand (9) criticized the concept of “hydrophobic bonds” [Kauzmann’s term (1)] because hydrocarbons interact favorably with water. An oil drop added to a beaker of water does not remain a droplet, but instead spreads out to form a surface film in order to interact with as much water as possible. Tanford pondered his reply for eleven years. In 1979 he agreed (10) that hydrocarbons make favorable van der Waals (dispersion force) interactions with water. He also used data for the interfacial energies of water and hexane to argue, however, that the hydrophobicity of water is caused by its high cohesive energy density, which tends to squeeze out all solutes. Polar solutes resist being squeezed out by making strong interactions with water. Other workers had a different view of the role of the solute-solvent interaction: Sharp, Nicholls, Friedman and Honig said in 1991: “For a nonpolar solute in water, the solute-solvent interaction is the origin of the hydrophobic effect.” (11).
Today there are mainly two opposing views (12) on the hydrophobicity of water: either it is caused chiefly by the small size of the water molecule (13) or by the perturbed H-bonded water structure around nonpolar groups (the iceberg model) (1, 7, 8). The analysis given here is not based on either view, but rather on the solute insertion model for the gas-liquid solution process (14, 15).
In 1979 Ben-Naim (16) criticized the Nozaki-Tanford approach to determining hydrophobic free energy (2), saying it should be measured by gas-liquid, not liquid-liquid, transfer experiments because the solvation free energy of a solute is defined by gas-liquid transfer experiments (17). Ben-Naim’s criticism is accepted here and gas-liquid transfer results are used to investigate the free energy values found by the Kauzmann-Tanford approach (liquid-liquid transfer), which are the standard values used in protein folding studies. The free energy values depend on the concentration scale: the ones given here for liquid-liquid transfer are based on gas-liquid transfers with the solute concentration being molar (or number density) in both the gas and liquid phases (16, 17).
Results and Discussion
Two Factors Determine Solubility in Gas-Liquid Transfer.
In the solute insertion model used by Lee (14) and Pollack (15), there are two factors that determine the gas-liquid transfer free energy and the solubility of the solute: the cavity work ΔGc and the solute-solvent interaction energy Ea. Dissolving a solute is divided into two steps: In the first step, thermal fluctuations in the solvent make a cavity for the solute, and in the second step, the solute is inserted into the cavity where it interacts with the solvent. Thus, the cavity is made by solvent reorganization. In principle, Ea also includes a work of solvent reorganization when the solute and solvent interact, but this term is minor and can be dropped (14). Ea is chiefly the van der Waals interaction energy between solute and solvent. Lee used the potential-distribution theory of Widom (18) to calculate Ea by appropriate ensemble averaging and he found that the minor term mentioned above can be dropped (14). Lee’s two-step analysis of hydrophobic hydration (14) is conceptually similar to the 1977 Pratt-Chandler (19) analysis of the hydrophobic interaction between a pair of nonpolar molecules in water. The small size of the water molecule (13) helps to account for the large cavity work in water (14) because several water molecules must move aside to form a cavity for the nonpolar solute.
The transfer free energy ΔGGL of the solute between gas and liquid is based on the chemical potential μ of the solute being the same in the gas and liquid phases at equilibrium. Importantly, ΔGGL is the sum of ΔGc and Ea (14, 15), but the two terms have opposite signs so that ΔGGL is a difference between them.
| [1] |
As mentioned above, a minor term has been dropped from Eq. 1 and this omission makes Eq. 1 approximate. The Ostwald coefficient L (20) is used to measure ΔGGL provided the solute has a measurable tendency to escape into the gas phase (15, 17). L is the equilibrium ratio of the molar concentrations of the solute in the liquid phase over the gas phase; L is related to ΔGGL by (15, 17)
| [1a] |
For gas-liquid transfer to a liquid alkane, L is found from the molar volume and the vapor pressure. For gas-liquid transfer to water, L is found from the solubility of the solute and its partial pressure.
The cavity work was calculated directly from ΔGGL and Ea in Lee’s study (14) and his values of Ea are believed to be reliable. Lee used the heat of vaporization to calculate Ea for liquid alkanes and he used the Monte Carlo simulations of Jorgensen, et al. (21, 22) to find Ea for the aqueous solutions. The simulation results for liquid hydrocarbons were tested against experiment (21, 23) by calculating the heats of vaporization and densities of the liquid hydrocarbons; the estimated and experimental values agreed within 2% for alkanes with six or fewer carbon atoms (23). Lee’s goal (14) in 1991 was to test how well scaled particle theory calculates the cavity work, as his 1985 model for the hydrophobicity of water [based on the small size of the water molecule (13)] relied on calculations made with scaled particle theory, as did much of the earlier work on the hydrophobicity of water (24, 25). In contrast to scaled particle theory, the solute insertion model (14, 15) is not based on the hard sphere assumption.
Taking Apart Liquid-Liquid Transfer into Two Gas-Liquid Transfers.
Table 1 analyzes the liquid-liquid transfers of three alkane solutes from water to liquid alkane by treating each liquid-liquid transfer as the sum of two gas-liquid transfers. The gas-liquid transfers are characterized by their values of the cavity work (ΔGc) and the solute-solvent interaction energy (Ea) (14). Surprisingly, Table 1 shows that ΔGLL is divided almost equally between the two values of ΔGGL for transfer out of water and transfer into liquid alkane. Both Kauzmann (1) and Nozaki and Tanford (2) expected transfer out of water to account for the major part of ΔGLL. Nozaki and Tanford say: “Hydrophobic moieties have a strongly unfavorable interaction with water, which is abolished when the group in question is removed from contact with water and placed inside the native (protein) structure, where such groups are not generally involved in any strong favorable or unfavorable interactions…”. (2).
Table 1.
Transfer energetics for gas-liquid transfer of three alkane solutes to water and to liquid alkane
| Water | Liquid alkane | |||||
| Solute | ΔGc | Ea | ΔGGL | ΔGc | Ea | ΔGGL |
| Propane | 8.6 | −6.6 | 2.0 | 4.3 | −6.4 | −2.1 |
| Isobutane | 10.1 | −7.8 | 2.3 | 5.8 | −8.4 | −2.6 |
| Neopentane | 11.1 | −8.6 | 2.5 | 6.6 | −9.5 | −2.9 |
Values from (14) are given in kcal/mol at 25 °C. ΔGc is the work of making a cavity for the solute in the solvent; Ea is the solute-solvent interaction energy; ΔGGL is the algebraic sum of ΔGc and Ea.
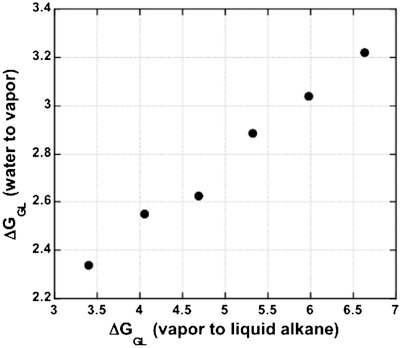
In order to examine further the surprisingly large values of ΔGGL for transfer to liquid alkane, the two types of transfer are compared (Fig. 1) for linear alkanes with n (the number of carbon atoms) between 5 and 10; those with n between 1 and 4 are gases, not liquids, at 25 °C and atmospheric pressure. Values of ΔGGL for the two types of transfer were taken from Ben-Naim and Marcus (17). The linear alkane data confirm that ΔGGL for transfer out of water is substantially smaller than ΔGGL for transfer into liquid alkane (Fig. 1).
Fig. 1.
The ΔGGL values for two gas-liquid transfers are compared for linear alkanes with 5, 6,… 10 carbon atoms. The signs of the two transfers refer to transfer: (1) from vapor into water, and (2) from liquid alkane into vapor. The plot shows that ΔGGL for transfer into water is considerably smaller than ΔGGL for transfer out of liquid alkane. The values of ΔGGL (kcal/mol at 25 °C) are given for both types of transfer by Ben-Naim and Marcus (17), and their sum (ΔGLL) is given by Sharp, et al. (11). For n = 9 and 10, the values of ΔGGL for transfer into water are not given in (17) and were found by using the ΔGLL values in (11).
The large fraction of ΔGLL produced by transfer into liquid alkane is explained by the values of ΔGc and Ea (Table 1) for the two types of transfer. In size ΔGGL is a difference between the positive cavity work (which opposes transfer) and the negative solute-solvent interaction energy (which favors transfer). The two types of transfer have free energies with opposite signs and the directions of transfer are also opposite, so that the two ΔGGL values add to give ΔGLL. The key point is that the values of Ea are nearly the same for the two types of transfer and the Ea values are midway between the ΔGc values for water and liquid alkane; this ensures that the magnitudes of the two ΔGGL values are comparable. When Kauzmann (1959) and Nozaki and Tanford (1971) wrote their papers (1, 2), the values of ΔGc and Ea [obtained by Lee (14) in 1991] were not yet available and it wasn’t possible to foresee this behavior. The basic result today is that the hydrophobic factor defined by Kauzmann (1) and Nozaki and Tanford (2) is doubtful because it is based on an assumption that is no longer justified. It will be necessary in future work to reassess the hydrophobic free energy found by liquid-liquid transfer. For other current problems involving the hydrophobic factor, see review (26).
The hallmark of the hydrophobic factor in protein folding energetics is its unusual temperature dependence (8), expressed as a large value of ΔCp, the difference between the heat capacity values of the native and unfolded protein forms. The enthalpy change for unfolding increases as much as sixfold between 25 and 110 °C for some proteins (27) and this behavior is attributed entirely to the hydrophobic factor, since the much smaller dependence from the peptide groups has the opposite sign and the enthalpy change from breaking van der Waals contact interactions is considered temperature-independent (28). The unusual temperature dependence of the hydrophobic factor is observed in both model compound results and protein unfolding data (29, 30).
Other Approaches to Measuring Hydrophobic Free Energy.
The results reported here reinforce the need to find other approaches to measuring hydrophobic free energy and enthalpy in protein folding. A promising alternative approach has been reported recently: the hydrophobic factor in polymer collapse has been studied with collapsed hydrophobic polymers in aqueous solution by pulling out the polymer using atomic force microscopy and analyzing the force required (31). By studying polymers of different monomer types, Li and Walker analyzed how hydrophobic free energy contributes to the pulling energetics. The results showed strong correlations with the Kauzmann-Tanford approach to hydrophobic free energy but also showed quantitative differences (31). The pulling free energy values were smaller than expected from solvent transfer studies; the pulling experiments may well contain contributions from pairwise hydrophobic interactions as well as from hydrophobic hydration.
There is a clear analogy between transferring a nonpolar solute out of water into vapor and removing protein nonpolar side chains from water via folding. Lazaridis, et al. (32) examined this factor in the folding energetics by comparing protein unfolding enthalpies converted to vacuum conditions with force-field simulations of protein unfolding in a vacuum. Lazaridis, et al. (32) took calorimetric data for the enthalpy of protein unfolding from Makhatadze and Privalov (33), who converted it to unfolding in vacuum by using empirical calibrations of the heats of desolvating exposed polar and nonpolar groups. This work uncovered a major practical problem in converting experimental unfolding enthalpies to conditions in vacuum: the heats of desolvating the exposed nonpolar and polar groups are large and not known with the accuracy needed. The combined heats of desolvating the exposed polar and nonpolar groups are about 20 times larger than the enthalpy of protein unfolding in water at 25 °C (32).
Calculating ΔGhyd on the Molar (Not Mole Fraction) Concentration Scale.
When calculating ΔGLL via two gas-liquid transfers, it is necessary to express the solute concentration on the molar (or number density) scale. Kauzmann (1) recommended calculating ΔGLL on the mole fraction scale to avoid including in ΔGLL a contribution from the entropy of mixing. There is, however, a basic reason (15–17) for using the molar (or number density) scale when gas-liquid transfer is involved, namely that the large contribution from translational entropy vanishes on this scale. It is intriguing that Ostwald knew this fact (15, 17, 20). Using the mole fraction scale results in including a significant and unwanted contribution from translational entropy (15, 17). Pollack (15) gives the contribution of motional entropy to the chemical potential of a monatomic ideal gas as -20.4 kT, where kT is thermal energy, about -0.59 kcal per mole at 25 °C. The ΔGLL values for liquid-liquid transfer of a solute differ significantly between the molar and mole fraction scales (11) and the ΔGGL values for gas-liquid transfer differ hugely between the two scales.
Acknowledgments.
I thank Henry Ashbaugh, Ken Dill, William Jorgensen, B-K Lee, George Rose, and Catherine Royer for discussion. I am much indebted to B-K Lee for answering my questions about his 1991 paper (14).
Footnotes
The author declares no conflict of interest.
References
- 1.Kauzmann W. Factors in interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 2.Nozaki Y, Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions: establishment of a hydrophobicity scale. J Biol Chem. 1971;246:2211–2217. [PubMed] [Google Scholar]
- 3.Dill KA. Dominant forces in protein folding. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 4.Ashbaugh HS, Pratt LR. Colloquium: scaled particle theory and the length scales of hydrophobicity. Rev Mod Phys. 2006;78:159–178. [Google Scholar]
- 5.Radzicka A, Wolfenden R. Comparing the polarities of the amino acids: side-chain distribution coefficients between the vapor phase, cyclohexane, 1-octanol and neutral aqueous solution. Biochemistry. 1988;27:1644–1670. [Google Scholar]
- 6.McMeekin TL, Cohn EJ, Weare JH. Studies in the physical chemistry of amino acids, peptides and related substances III. The solubility of derivatives of the amino acids in alcohol-water mixtures. J Am Chem Soc. 1935;57:626–633. [Google Scholar]
- 7.Frank HS, Evans MW. Free volume and entropy in condensed systems III. Entropy in binary liquid mixtures; partial molal entropy in dilute solutions; structure and thermodynamics in aqueous electrolytes. J Chem Phys. 1945;13:507–532. [Google Scholar]
- 8.Kauzmann W. Thermodynamics of unfolding. Nature. 1987;325:763–764. [Google Scholar]
- 9.Hildebrand JH. A criticism of the term “hydrophobic bond. J Phys Chem. 1968:1841–1842. [Google Scholar]
- 10.Tanford C. Interfacial free energy and the hydrophobic effect. Proc Natl Acad Sci USA. 1979;76:4175–4176. doi: 10.1073/pnas.76.9.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp KA, Nicholls A, Friedman R, Honig B. Extracting hydrophobic free energies from experimental data: relationship to protein folding and theoretical models. Biochemistry. 1991;30:9686–9697. doi: 10.1021/bi00104a017. [DOI] [PubMed] [Google Scholar]
- 12.Lazaridis T. Solvent size vs cohesive energy as the origin of hydrophobicity. Acc Chem Res. 2001;34:931–937. doi: 10.1021/ar010058y. [DOI] [PubMed] [Google Scholar]
- 13.Lee B. The physical origin of the low solubility of nonpolar solutes in water. Biopolymers. 1985;24:813–823. doi: 10.1002/bip.360240507. [DOI] [PubMed] [Google Scholar]
- 14.Lee B-K. Solvent reorganization contribution to the transfer thermodynamics of small nonpolar molecules. Biopolymers. 1991;31:993–1008. doi: 10.1002/bip.360310809. [DOI] [PubMed] [Google Scholar]
- 15.Pollack GL. Why gases dissolve in liquids. Science. 1991;251:1323–1330. doi: 10.1126/science.251.4999.1323. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Naim A. Standard thermodynamics of transfer. Uses and misuses. J Phys Chem. 1979;82:792–803. [Google Scholar]
- 17.Ben-Naim A, Marcus Y. Solvation thermodynamics of nonionic solutes. J Chem Phys. 1984;81:2016–2027. [Google Scholar]
- 18.Widom B. Potential distribution theory and the statistical mechanics of fluids. J Phys Chem. 1982;86:869–887. [Google Scholar]
- 19.Pratt LR, Chandler D. Theory of hydrophobic effect. J Chem Phys. 1977;67:3683–3704. [Google Scholar]
- 20.Battino R. The Ostwald coefficient of gas solubility. Fluid Phase Equilbr. 1984;15:231–240. [Google Scholar]
- 21.Jorgensen WL, Madura JD, Swenson CJ. Optimized intermolecular potential functions for liquid hydrocarbons. J Am Chem Soc. 1984;106:6638–6646. [Google Scholar]
- 22.Jorgensen WL, Gao J, Ravimohan C. Monte Carlo simulations of alkanes in water: hydration numbers and the hydrophobic effect. J Phys Chem. 1985;89:3470–3473. [Google Scholar]
- 23.Thomas LL, Christakis TJ, Jorgensen WL. Conformation of alkanes in the gas phase and pure liquids. J Phys Chem B. 2006;110:21198–21204. doi: 10.1021/jp064811m. [DOI] [PubMed] [Google Scholar]
- 24.Pierotti RA. Aqueous solutions of nonpolar gases. J Phys Chem. 1965;69:281–288. [Google Scholar]
- 25.Stillinger FH. Structure in aqueous solutions of nonpolar solutes from the standpoint of scaled-particle theory. J Solution Chem. 1973;2:141–158. [Google Scholar]
- 26.Ball P. More than a bystander. Nature. 2011;478:467–468. doi: 10.1038/478467a. [DOI] [PubMed] [Google Scholar]
- 27.Privalov PL, Gill SJ. Stability of protein structure and hydrophobic interaction. Adv Protein Chem. 1988;39:191–234. doi: 10.1016/s0065-3233(08)60377-0. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin RL. Desolvation penalty for burying hydrogen-bonded peptide groups in protein folding. J Phys Chem B. 2010;114:16223–16227. doi: 10.1021/jp107111f. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin RL. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci USA. 1986;83:8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livingstone JR, Spolar RS, Record MT. Contribution to the thermodynamics of protein folding from the reduction in water-accessible nonpolar surface area. Biochemistry. 1991;30:4237–4244. doi: 10.1021/bi00231a019. [DOI] [PubMed] [Google Scholar]
- 31.Li ITS, Walker GC. Signature of hydrophobic hydration in a single polymer. Proc Natl Acad Sci USA. 2011;108:16527–16532. doi: 10.1073/pnas.1105450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazaridis T, Archontis G, Karplus M. Enthalpic contribution to protein stability: insights from atom-based calculations and statistical mechanics. Adv Protein Chem. 1995;47:231–306. doi: 10.1016/s0065-3233(08)60547-1. [DOI] [PubMed] [Google Scholar]
- 33.Makhatadze GI, Privalov PL. Energetics of protein structure. Adv Protein Chem. 1995;47:307–425. doi: 10.1016/s0065-3233(08)60548-3. [DOI] [PubMed] [Google Scholar]