Abstract
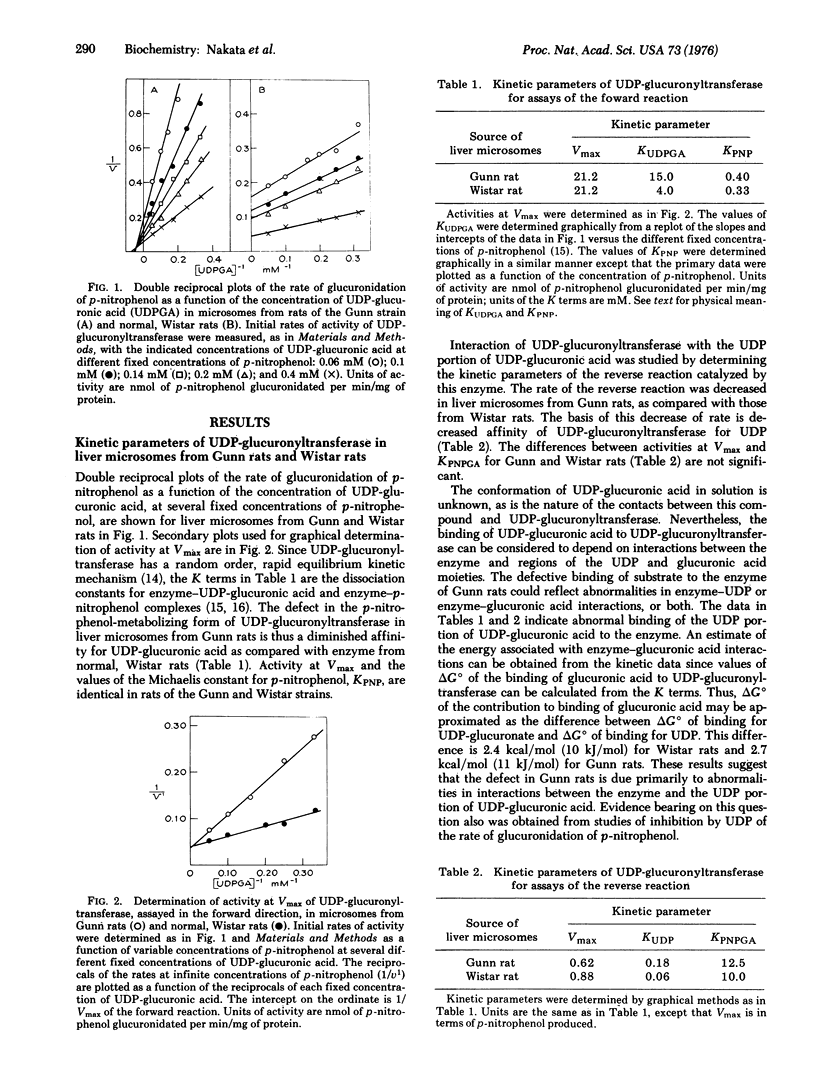
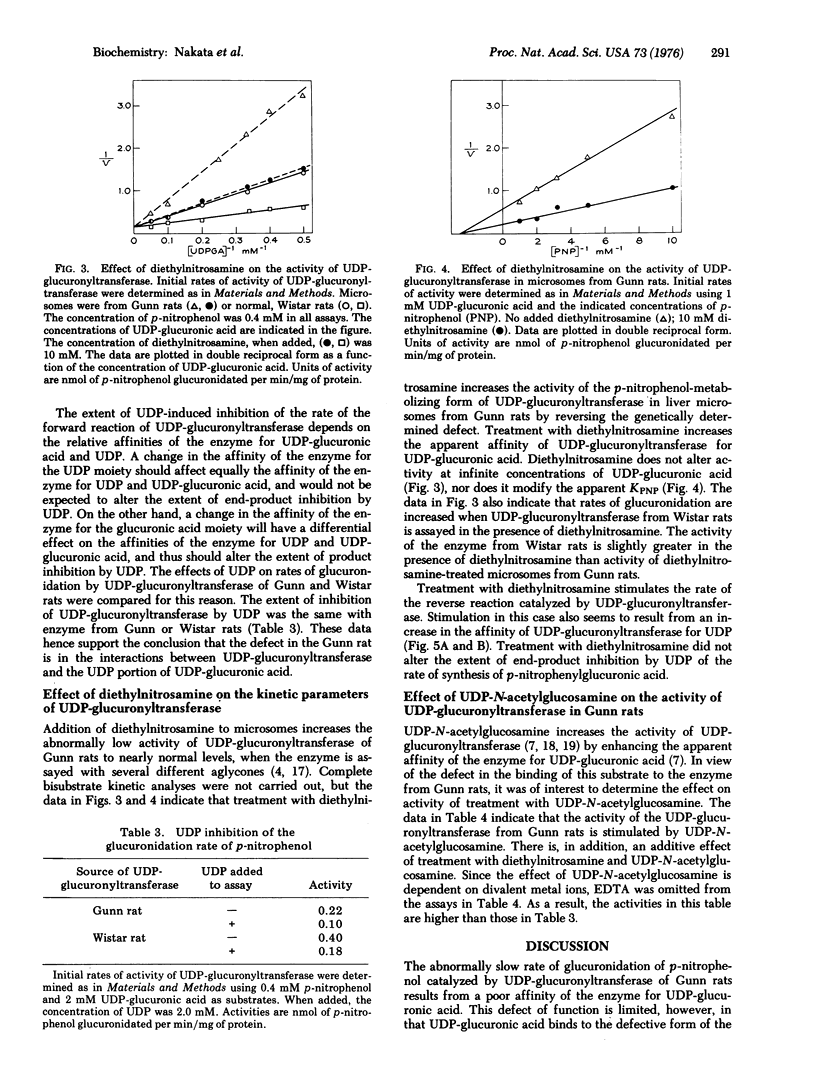
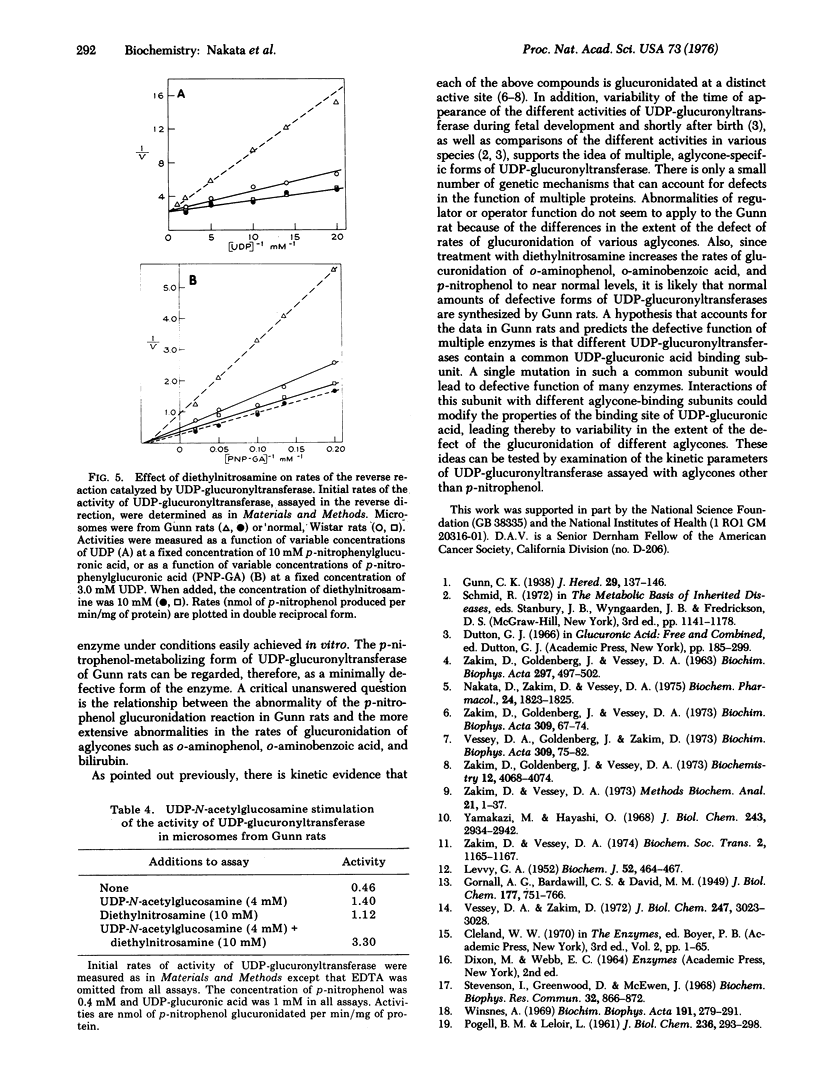
The kinetic parameters of the p-nitrophenol-metabolizing form of UDP-glucuronyltransferase [-UDPglucuronosyltransferase; UDPglucuronate beta-glucuronosyltransferase (acceptor-unspecific), EC 2.4.1.17] have been compared in liver microsomes from the Gunn strain of rat and from normal; Wistar rats. The abnormally low rate of glucuronidation of p-nitrophenol in the Gunn rats, as compared with Wistar rats, is due to decreased affinity of UDP-glucuronyltransferase for UDP-glucuronic acid. Activities at Vmax and the Michaelis constant for p-nitrophenol, KPNP, of UDP-glucuronyltransferase are the same for enzyme from either strain of rat. Studies of the kinetic parameters of the reverse reaction catalyzed by UDP-glucuronyltransferase indicate that the enzyme from Gunn rats also has decreased affinity for UDP. Calculated values of deltaG degrees for the binding of the UDP portion of UDP-glucuronic acid suggest that the defect of UDP-glucuronyltransferase of Gunn rats appears limited to abnormal interactions between the enzyme and the UDP portion of UDP-glucuronic acid. Studies of the extent of UDP-induced inhibition of the forward reaction support this idea. Diethylnitrosamine, added to microsomes in vitro, enhances the affinity of UDP-glucuronyltransferase for the UDP portion of UDP-glucuronic acid. Despite the defective conformation of the UDP-glucuronic acid binding site of UDP-glucuronyltransferase from Gunn rats this enzyme is activated in the normal way by UDP-N-acetylglucosamine, which is a K-type effector with regard to UDP-glucuronic acid.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LEVVY G. A. The preparation and properties of beta-glucuronidase. IV. Inhibition by sugar acids and their lactones. Biochem J. 1952 Nov;52(3):464–472. doi: 10.1042/bj0520464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata D., Zakim D., Vessey D. A. Modification of protein-lipid interactions in the Gunn rat by treatment of microsomal UDP-glucuronyltransferase with diethylnitrosamine. Biochem Pharmacol. 1975 Oct 1;24(19):1823–1825. doi: 10.1016/0006-2952(75)90466-9. [DOI] [PubMed] [Google Scholar]
- POGELL B. M., LELOIR L. F. Nucleotide activation of liver microsomal glucuronidation. J Biol Chem. 1961 Feb;236:293–298. [PubMed] [Google Scholar]
- Stevenson I., Greenwood D., McEwen J. Hepatic UDP-glucuronyltransferase in Wistar and Gunn rats--in vitro activation by diethylnitrosamine. Biochem Biophys Res Commun. 1968 Sep 6;32(5):866–872. doi: 10.1016/0006-291x(68)90321-5. [DOI] [PubMed] [Google Scholar]
- Vessey D. A., Goldenberg J., Zakim D. Differentiation of homologous forms of hepatic microsomal UDP-glucuronyltransferase. II. Characterization of the bilirubin conjugating form. Biochim Biophys Acta. 1973 May 5;309(1):75–82. doi: 10.1016/0005-2744(73)90319-7. [DOI] [PubMed] [Google Scholar]
- Vessey D. A., Zakim D. Regulation of microsomal enzymes by phospholipids. V. Kinetic studies of hepatic uridine diphosphate-glucuronyltransferase. J Biol Chem. 1972 May 25;247(10):3023–3028. [PubMed] [Google Scholar]
- Winsnes A. Studies on the activation in vitro of glucuronyltransferase. Biochim Biophys Acta. 1969 Nov 4;191(2):279–291. doi: 10.1016/0005-2744(69)90247-2. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Hayaishi O. Allosteric properties of nucleoside diphosphatase and its identity with thiamine pyrophosphatase. J Biol Chem. 1968 Jun 10;243(11):2934–2942. [PubMed] [Google Scholar]
- Zakim D., Goldenberg J., Vessey D. A. Differentiation of homologous forms of hepatic microsomal UDP-glucuronyltransferase. I. Evidence for the glucuronidation of o-aminophenol and p-nitrophenol by separate enzymes. Biochim Biophys Acta. 1973 May 5;309(1):67–74. doi: 10.1016/0005-2744(73)90318-5. [DOI] [PubMed] [Google Scholar]
- Zakim D., Goldenberg J., Vessey D. A. Effects of metals on the properties of hepatic microsomal uridine diphosphate glucuronyltransferase. Biochemistry. 1973 Oct 9;12(21):4068–4074. doi: 10.1021/bi00745a007. [DOI] [PubMed] [Google Scholar]
- Zakim D., Goldenberg J., Vessey D. A. Regulation of microsomal enzymes by phospholipids. VI. Abnormal enzyme-lipid interactions in liver microsomes from Gunn rats. Biochim Biophys Acta. 1973 Feb 28;297(2):497–502. doi: 10.1016/0304-4165(73)90097-4. [DOI] [PubMed] [Google Scholar]



