Abstract
Congenitally hypomyelinated shiverer mice fail to generate compact myelin, and die by 18–21 weeks of age. Using multifocal anterior and posterior fossa delivery of sorted fetal human glial progenitor cells into neonatal shiverer x rag2−/− immunodeficient and myelin-deficient mice, we achieved whole neuraxis myelination of the engrafted hosts, which in a significant fraction of cases rescued this otherwise lethal phenotype. The transplanted mice exhibited greatly prolonged survival, some surviving beyond a year, with progressive resolution of their neurological deficits. Accordingly, they exhibited substantial myelination of the brain, brainstem and cerebellum, as well as the spinal cord, optic nerves, and cranial ganglia. This was accompanied by the acquisition of normal nodes of Ranvier and transcallosal conduction velocities, ultrastructurally normal and complete myelination of most axons, and a restoration of a substantially normal neurological phenotype. Notably, the resultant mice were cerebral chimeras, with murine gray matter but a predominantly human white matter glial composition. These data demonstrate that the neonatal transplantation of human glial progenitor cells can effectively treat disorders of congenital and perinatal hypomyelination.
Keywords: oligodendrocyte progenitor, neural stem cell, transplantation, myelin, remyelination, leukodystrophy
Glial progenitor cells and the neural stem cells from which they derive may be isolated and transplanted into myelin-deficient hosts, as a means of introducing new oligodendrocytes able to myelinate host axons (Archer et al., 1997; Eftekharpour et al., 2007; Learish et al., 1999; Mitome et al., 2001; Yandava et al., 1999). We have previously noted that enriched preparations of human glial progenitor cells, when engrafted into the neonatal shiverer mouse, a mutant that lacks full-length myelin basic protein (Popko et al., 1987; Readhead et al., 1987; Roach et al., 1985), generate substantial myelin in these otherwise unmyelinated recipients (Windrem et al., 2004) However, the potential utility of this approach to the development of clinical remyelination strategies has been unclear, since previous studies have failed to note significant brainstem, cerebellar or spinal engraftment from intracerebral grafts, and no effect on disease phenotype or survival has yet been reported in hypomyelinated mice as a consequence of progenitor cell transplantation (reviewed in (Goldman, 2005; Keyoung and Goldman, 2007)). Indeed, our initial study of the efficacy of isolated human glial progenitors revealed no overt effect of cell transplantation on either the condition or fate of the engrafted recipients; despite widespread forebrain myelination, the transplanted mice typically died between 18 and 21 weeks of age, just as did unengrafted shiverers.
In the present study, using a newly developed set of approaches to both cell acquisition and transplantation, we have attempted a far more extensive and higher density cell engraftment than any previously noted. Our aim in doing so was to achieve sufficiently widespread central myelination so as to influence the phenotype and survival of the recipient animals. To avoid rejection as a complicating variable in these experiments, we crossed shiverers with rag2 null immunodeficient mice (Shinkai et al., 1992), and thereby generated an immunodeficient line of congenitally hypomyelinated mice in which to assess graft efficacy and effect. Using these double homozygous rag2−/− × shiverershi/shi mice, we established a multi-site injection protocol, with concurrent bilateral hemispheric and cerebellar cell injections delivered at birth. This procedure resulted in widespread donor cell engraftment throughout the neuraxis, with infiltration of the forebrain, brainstem and cerebellum, and ultimately the spinal cord and roots. The engrafted human glial progenitor cells exhibited robust, efficient and functional myelination, with progressive ensheathment of host axons and restoration of normal nodes of Ranvier and attendant conduction velocities. This ultimately led to the high efficiency myelination of the major intracerebral, ascending and descending tracts, the cranial nerves and intracranial ganglia, and the spinal cord to the thoracolumbar level. Most notably, the implanted animals exhibited a substantial recovery of normal neurological phenotype, such that a fraction were frankly rescued by perinatal transplantation, surviving well over a year until sacrificed for histology, which revealed both a remyelinated – and essentially humanized – central white matter. The neurological recovery and sustained survival of these transplanted mice was in sharp contrast to the fate of their untreated controls, which uniformly died by 5 months of age. To our knowledge, these data represent the first outright rescue of a congenital hypomyelinating disorder, by means of a stem or progenitor cell transplantation, and indicate that neonatal glial progenitor cell transplantation may prove an effective means of treating disorders of both hereditary and perinatal hypomyelination.
RESULTS
Engrafted shiverer mice exhibited substantially prolonged survival
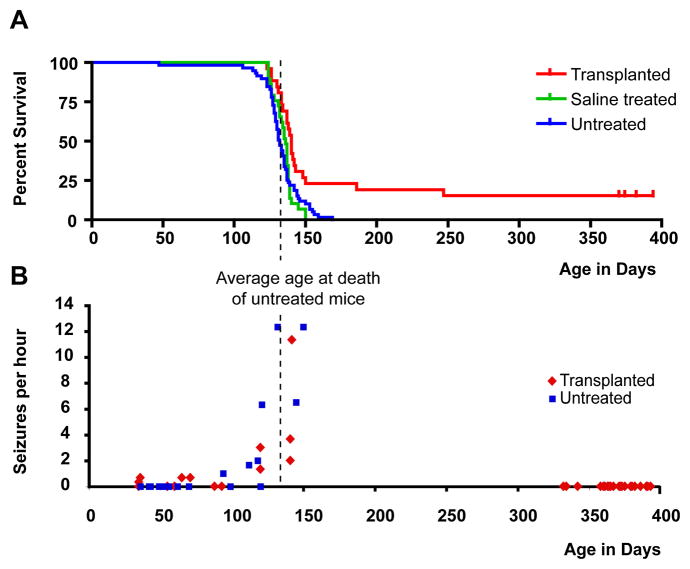
Newborn double-homozygous shiverer (shi/shi) × rag2−/− immunodeficient mice were implanted with either 300,000 human glial progenitor cells (GPCs) (n=26), with PBS vehicle control (n=29), or with nothing (n=59). Cells were delivered at 5 sites, including the anterior and posterior corpus callosa bilaterally, and the presumptive cerebellar peduncle as a single midline injection; PBS controls received equal volume injections at each site, while the no-injection controls were not injected. The mice were then returned to their mothers, and allowed to develop normally, with weaning at 21 days and small group housing thereafter. All mice were observed to undergo progressive neurological deterioration, typically first manifest by a progressive truncal instability worse upon ambulation, followed with marked hindlimb weakness by 14–16 weeks of age, and seizures beginning at 4–6 weeks but rapidly increasing in frequency by 18–19 weeks. Thus, by 18 weeks, all mice exhibited markedly impaired forward ambulation, and frequent episodes of sustained seizures. Over a range of 130–150 days postnatally, all of the 29 PBS-treated and 53 untreated control shiverer mice died, with median and mean (± SE) survivals of 135.0 ± 1.4 and 132.4 ± 2.1 days, respectively.
In sharp contrast, of the 26 implanted mice, 20 died during this period, but 6 (23.1%) survived. Whereas the average survival of the untreated controls approximated 130 days, and none of the 82 total control mice survived to 150 days, these 6 implanted mice survived over 160 days, and 4 appeared to have been frankly rescued, surviving over a year before being sacrificed for analysis. Remarkably, these mice exhibited overtly improved neurological function, with decreased seizure incidence and improved mobility and self-care. Indeed, transplanted mice surviving beyond 190 days exhibited apparent treatment-dependent cure, with sustained survival over a year, accompanied by a virtually complete recovery of normal neurological phenotype. As a result, the engrafted mice as a group exhibited significantly prolonged survival: Kaplan-Meier analysis(Hosmer and Lemeshow, 1999) confirmed that the treatment-associated improvement in survival was statistically significant, and profoundly so (p=0.0003; hazard ratio = 0.4718 (95% CI=0.30–0.70) (Figure 1A).
Figure 1. Engrafted shiverer mice exhibited substantially prolonged survival.
A, Shiverer/rag2−/− mice, either engrafted with human glial progenitor cells (GPCs) at birth (n=26, red), injected with saline (n=29, green), or untreated (n=59, blue) were maintained in small group housing and monitored daily until death. The Kaplan-Meier survival graph, plotting the percentage of each group alive as a function of age in weeks, shows that most mice die between 18 and 20 weeks. However, a fraction of engrafted mice (n=6, or 23.1%) lived substantially longer than any control mouse; 4 survived more than one year, at which point the experiment was terminated. B, Shiverer mice uniformly manifested a seizure disorder that was typically apparent by 5 weeks of age, and then worsened between 16–18 weeks. When seizure frequency was scored by video with blinded post hoc assessment, both transplanted and control shiverers were noted to seize frequently during weeks 18–20, corresponding to the time span during which most mice died. However, the seizure incidence among the transplanted shiverers fell thereafter, such that by 47 weeks of age, all surviving mice were seizure-free.
Transplantation was associated with neurological improvement and diminished seizures
The rescued mice exhibited substantial resolution of their neurological deficits. Shiverer mice typically exhibit truncal instability and marked intention tremor, evident within weeks of birth, which becomes complicated by a progressive hindlimb weakness, and multimodal sensory and perceptual deficits that include blindness, such that by 18–19 weeks of age they are severely impaired (Supplemental video 1). In addition, they manifest a progressively worsening seizure disorder (Supplemental video 2), often succumbing to status epilepticus. Given the substantially longer survivals noted in a fraction of the transplanted shiverers, we asked what the behavioral concomitants were to transplantation, as a function of time after cell delivery. We were especially interested in any discernible differences in the behavior or neurological status of shiverer mice that were rescued by neonatal GPC transplant, relative to transplanted littermates that nonetheless died. We noted that all of the shiverers, both transplanted and controls, deteriorated identically over the first several months after birth and transplantation. Indeed, up to 130 days, the point at which mice typically began to die in number, little difference was observed in the behaviors of transplanted relative to untransplanted shiverer mice. However, those mice that survived the period spanning 130–150 days postnatally, exhibited noticeable improvement in their neurological exams thereafter, frankly manifest by 7–8 months of age as diminished frequency of seizures and improved ambulation, with more forward motion and less retropulsion or freezing. Over the several months thereafter, the transplanted mice incrementally improved, regaining normal fluidity in ambulation, normal voluntary explorative behavior, and less truncal intention tremor. All 5 mice surviving to at least 35 weeks of age were substantially normal by that point and thereafter in terms of their grossly assessable neurological function, save for a coarse axial intention tremor, manifesting as a wobble on forward ambulation (Supplemental video 3).
Since the death of most mice at 130–150 days of age was coincident with the period of sharply increasing seizure activity, we next assessed the frequency and duration of spontaneous seizures in both untreated and transplanted shi/shi x rag2 nulls, as a function of age; we paid special attention to the incidence of seizures in transplanted mice that were rescued by transplant, compared to their treated counterparts that nonetheless succumbed. We found that the first seizures of shiverer mice typically characterized by absence-like episodes of tonic akinesia, followed by a rapid evolution to brief tonic-clonic events - appeared by 35–42 days of age (Fig. 1B). At approximately 120 days, the incidence of seizures was noted to substantially increase, in both treated and untreated animals alike. Over the period spanning 120–140 days, the seizure incidence of each group increased, yielding frequent seizures every hour (see Supplemental fig. 1 for a representative EEG recording); these ictal events progressed to sustained periods of status epilepticus, often associated with death. However, in those animals that survived this period to enjoy long-term survival, seizure incidence fell dramatically, such that no seizure activity whatsoever was observed at 12 months (p<0.0001 by 1 way ANOVA, separately comparing 12 month transplanted animal seizure incidence to that of 4 month transplanted and control shiverers). Thus, perinatal GPC transplantation was associated with markedly diminished seizure activity in those shi/shi × rag2 null mice that were rescued by perinatal transplantation, such that by a year, none manifested any residual spontaneous seizure activity, while otherwise exhibiting virtually complete neurological recovery.
Besides spontaneous seizures, we noted that shiverers exhibited stimulus-evoked seizure activity that increased in both frequency and duration as a function of age. To quantify this pathological response to handling, we established a brief screening test by which mice were briefly and abruptly suspended by the tail, and their behavioral responses observed. We found that such tail suspension was sufficient to induce seizures in a large proportion of shiverer mice, whether treated or not, by 3 months of age. The induction of seizure activity within 30 seconds of tail suspension was thus chosen as a metric by which to assess the effect of cell transplant on seizure incidence and duration. We observed that among the transplanted shiverers, the percentage of individual tail suspension challenges resulting in clinically-overt seizures rose to 75 ± 2.4% by 3 to 4 months. Among the transplanted mice that survived at least 5 months, 47 ± 2.8% of tail suspension challenges resulted in seizures. In contrast, by 8 months, none of the 4 surviving mice could be induced to seize by tail suspension. Linear regression of the percentage of mice induced to seize, plotted against their age in days, revealed a best-fit of y=−0.324x + 116.7. Regression analysis confirmed that the negative correlation between seizure incidence and age (r=0.826; r2=0.68) was significant (p<0.0001; F=53.68 [1, 25 d.f.]) (see Supplemental fig. 2).
Although these data do not yet allow us to causally attribute the sustained survival of these transplanted shiverers to the diminution of their seizure activity, the increased incidence of both spontaneous and stimulus-evoked seizures coincident with the period during which most shiverers die, coupled with the diminished seizure incidence of those transplanted survivors that go on to survive (compare Fig. 1B to 1A), suggests that improved seizure control contributes to the sustained viability of the long-surviving transplant recipients.
Perinatal grafts of human glial progenitors yield widespread and dense host myelination
To assess the terminal distribution of donor cells and robustness of myelination in the transplanted animals, and to compare the extent of donor cell dispersal and myelination between short- and long-term survivors, the latter were ultimately sacrificed at 13 months of age, after assessment of their transcallosal conduction velocities and seizure frequency. The brains and spinal cords of these mice were then analyzed in terms of donor cell distribution and density, myelin production and the proportion of myelinated axons, nodal architecture and reconstitution, and ultrastructural metrics including myelinated axons, and myelin G-ratios. Each of these metrics was then compared to those obtained from transplanted mice that had died earlier, as well as to unimplanted shiverer controls, as well as to wild-type, normally myelinated rag2 null mice.
These histological data supported the compelling nature of the survival data. Human donor cell engraftment was extraordinarily extensive, with essentially whole neuraxis penetration and colonization by the human donor OPCs (Fig. 2A). High donor cell densities were observed throughout the forebrain, cerebellum, brainstem and cervical spinal cord, diminishing only at the level of the thoracolumbar cord, yet increasing again in the sacral cord and conus medullaris. The pattern of myelination, as indicated by MBP expression, reflected this widespread engraftment, with equally widespread and dense myelination (Figs. 2B–F), including not only all major central white matter tracts, but also structures as distant and diverse as the cranial ganglia, optic chiasm and conus medullaris (e.g., Fig. 2G). These long-term survivors, whose neurological exams had largely normalized by 9 months of age, exhibited essentially complete myelination of the brain, brainstem and cerebellum, with substantial myelination of the optic nerves (Fig. 2B), spinal cord (Figs. 2E), and spinal roots (Fig. 2G), as well as of the cranial roots and ganglia (Figs. 3A–C). In regards to the latter, the cessation of donor GPC migration at the border of CNS and PNS was striking, such that donor-derived myelination occurred up to, but not beyond, the transition points demarcating central ganglia and roots from peripheral nerve (Figs. 3A–E). The resultant densities and patterns of donor cell dispersal resulted in the virtually complete chimerization of the murine hosts’ central nervous systems, which thereby acquired a largely humanized white matter. Three-dimensional reconstructions confirmed that both the pattern and density of donor-derived myelination in the brains of transplanted shiverers approximated that of wild-type, normal mice.
Figure 2. Perinatal grafts of human glial progenitors yield widespread and dense host myelination.
A–B. Serial sagittal images of an engrafted shi/shi x rag2−/− brain, sacrificed at 1 year of age. Each image in a and b represents a montage of 50–100 images at 10×. Each series begins 750 μm lateral to the midline, and continues at 600 μm intervals. A. human donor cells, immunolabeled in 14 μm cryosections using an anti-human nuclear antibody (hN; red). B. Alexa 488-labeled myelin basic protein (MBP; green) in sections adjacent or nearly so to their matched sections in A. All major white matter tracts, including those of the corpus callosum, capsules, striatum, fimbria, cerebellum and brainstem heavily express MBP.
C–G. Black-and-white images of MBP-immunoreactive fibers in a number of sites reveal high efficiency axonal myelination; all images of transplanted shi/shi x rag2−/− mice at > 1 year post-transplant. C, The rostral striatum, corpus callosum, and neocortical layers 5 and 6 are shown in sagittal section. D. Higher magnification of c shows the MBP-defined myelination of individual fibers within the striatum, as well as the larger bundles of corticostriatal and striopallidal fibers. E. Donor-myelinated MBP+ fibers in a longitudinal section of the cervical spinal cord; dorsal column to the left, central gray to the right. F. Interwoven donor-myelinated fibers of the brainstem, in the pontine base. G. Donor-derived MBP in the conus medullaris; exiting myelinated roots of the cauda equina to the left.
Scale: A, B = 2.5 mm; C = 200 μm; D = 40 μm; E = 50 μm; F = 60 μm; G = 125 μm
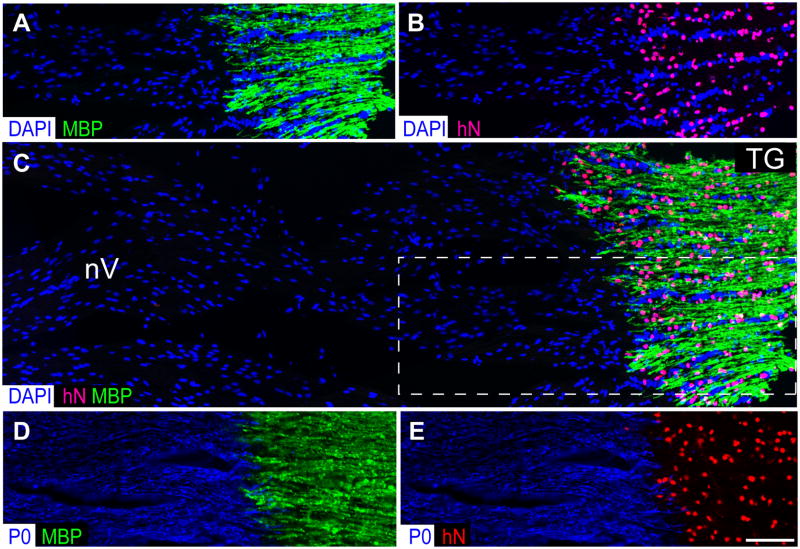
Figure 3. Transplanted GPCs invade the cranial and spinal roots but obey the CNS-PNS border.
Transplanted mice exhibited robust myelination throughout the entire CNS neuraxis by 9 months of age, that included not only the brain, brainstem, cerebellum and spinal cord, but also the cranial nerves, ganglia, and both cranial and spinal roots (see Figs. 2 and 4). Of note, the invasion of human glial progenitor cells, all derived from the fetal forebrain, was sharply delimited to the CNS, with no invasion whatsoever of the peripheral nerves beyond the root take-offs. A–B show both the dense concentration of human donor cells (anti-human nuclear antigen, red) in the trigeminal ganglion (TG), and the concurrent prohibition of donor cell infiltration into the trigeminal nerve (nV), a peripheral nerve. Accordingly, donor-derived myelin (MBP, green) was limited to the ganglion and trigeminal nerve take-off, and did not extend into nV proper. C shows a wider field color composite of A–B (A–B correspond to the boxed area of C), further demonstrating that the transplanted GPCs strictly respect the CNS-PNS border. In contrast, D–E show an adjacent section stained for the peripheral myelin protein P0 (blue), and for either human nuclear antigen (red) or central myelin basic protein (green). The human cells are seen to have stopped at the P0 protein-defined threshold to the PNS.
Scale=50 μm.
Xenografted shiverer brains exhibit restored nodes of Ranvier
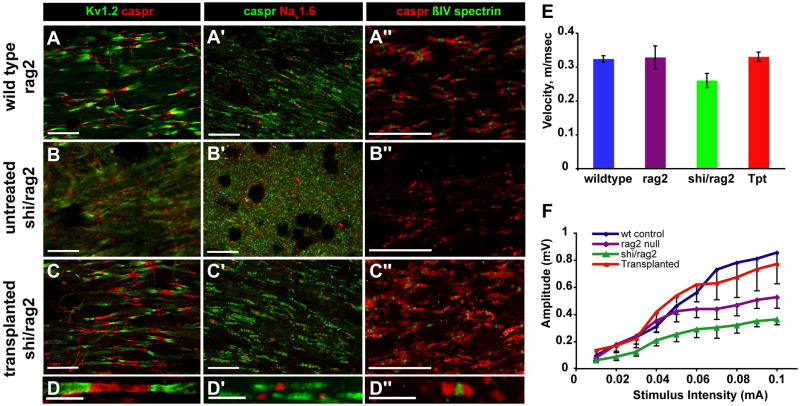
We next asked if donor cell-derived myelination of shiverer axons was accompanied by the acquisition of normal nodes of Ranvier and paranodal structure (Fig. 4A–D). Using high-resolution confocal imaging of the corpus callosa, cervical spinal cords, and optic nerves of implanted shiverers killed at 35 or 52 weeks of age, we assessed the distribution pattern of the paranodal and juxtaparanodal proteins Caspr and the KV1.2 voltage-gated potassium channel, respectively, the contiguous interaction of which characterizes the normal node of Ranvier (Rasband and Trimmer, 2001; Schafer and Rasband, 2006). These potassium channels are assembled at - and functionally define - the juxtaparanodes in myelinated axons, but they are broadly and nonspecifically expressed in unmyelinated fibers (Rasband and Trimmer, 2001). In addition, we determined the axonal expression and compartmentalization of NaV1.6 fast sodium channels, which are typically sequestered at nodes of Ranvier in intact myelinated axons, but dispersed broadly along unmyelinated or dysmyelinated fibers. Similarly, we immunostained for βIV-spectrin, which couples to ankyrin to organize fast sodium channels at the node of Ranvier, and hence typically coincides with nodal Nav1.6 expression (Schafer and Rasband, 2006; Sherman and Brophy, 2005; Yang et al., 2007).
Figure 4. Engrafted shi/shi brains exhibit restored nodes of Ranvier and callosal conduction velocities.
The expression patterns of several antigens characteristic of nodes of Ranvier, including nodal (NaV1.6, βIV-spectrin), paranodal (Caspr) and juxtaparanodal (KV1.2) proteins, were investigated in the spinal cord (A–D and A″–D″) and optic nerves (A″–D″) of normally-myelinated wild-type (rag2−/−) mice (A–A″), and compared to the corresponding expression patterns in the optic nerves of both untreated (B–B″), and transplanted (C–C″ and D–D″) shiverer × rag2−/− mice. The nodal architecture of the transplanted shiverers was indistinguishable from that of wild-type controls for every antigen tested; both exhibited the sodium channel and spectrin clustering, flanked by the paranodal Caspr and juxtaparanodal Kv1.2, of mature nodes. The nodal integrity of the transplanted shiverers (C–C″ and D–D″) contrasted sharply to the disorganized and indistinct antigen expression patterns of the untreated mice (B–B″), in which neither nodal channel clustering nor paranodal Caspr sequestration was noted.
E–F. Transcallosal responses were evoked by electrical stimulation in mice in vivo. E, plots the transcallosal conduction velocities obtained from wild-type, rag2−/−, shiverer x rag2−/−, and transplanted shiverer x rag2−/− mice, all assessed between 12–13 months after neonatal transplant. F, graphs the telationship between stimulus intensity and signal amplitude in C3H wild-type mice, rag2−/− mice, shiverer x rag2−/− mice, and transplanted shiverer x rag2−/− mice, respectively.
Using these complementary nodal markers, we identified an essentially normal organization of the nodes of Ranvier in transplanted mice, which was indistinguishable from that of wild-type mice. Caspr and KV1.2 were expressed in organized paranodal and juxtaparanodal apposition, with an expression pattern that contrasted sharply with the grossly uncoordinated pattern of diffuse Caspr and KV1.2 immunolabeling that was evident in the untransplanted controls (Figs. 4A–D). Similarly, both NaV1.6 (Figs. 4A′-D′) and βIV-spectrin (Figs. 4A″–D″) clearly identified nodes of Ranvier in the transplanted shi/shi mice, flanked by Caspr defining the paranodes, whereas their untransplanted controls showed no such sequestration of either NaV1.6 or βIV-spectrin expression. Together, these observations suggest that despite interspecies chimerization, the glio-axonal interactions of human GPC-derived oligodendrocytes with host mouse axons were functionally appropriate. More broadly, they indicate that GPC-derived oligodendrocytes are able to communicate effectively with host axons, organizing structurally appropriate nodes of Ranvier while sequestering fast sodium channels within the nodes, and thereby myelinating their axonal substrates both effectively and appropriately.
Transcallosal conduction velocities are restored in xenografted shiverer brains
In light of the apparent histological reconstitution of normal myelin, we next asked if donor OPC-derived myelin was sufficient in both extent and functional competence to restore the conduction speed of newly myelinated central axons. To this end, we assessed the conduction velocity across the corpus callosum in a sample of 4 long-surviving transplanted shiverer mice, between 12 and 13 months after neonatal xenograft. The transcallosal nerve conduction velocities were determined by recording response amplitudes and times from depth electrodes placed at several sites in the corpus callosa of each of these mice, after contralateral stimulation at symmetric sites during open craniotomy. Equal numbers of age-matched wild-type (congenic C3h) mice and rag2-null controls were assessed identically, as was a necessarily younger (4 months-old) sample of untransplanted shiverer × rag2 null mice. As this was a terminal procedure, these animals - all of which had exhibited not only sustained survival but also a substantial restoration of normal neurological function - were sacrificed after measurement of their transcallosal conduction velocities, thus ending the survival study in which they were subjects.
We found that whereas both control Fvb wild-type (n=3) and rag2 null C3h mice (n=4) exhibited conduction velocities of 0.324 ± 0.01 and 0.328 ± 0.03 m/sec respectively, the shiverer x rag2 mice (n=4), also on the C3h background, exhibited substantially slower conduction, at 0.260 ± 0.02 m/sec (Fig. 4E). In contrast, transplanted shiverer × rag2 mice, tested just prior to sacrifice 12–13 months post-transplant (n=3), had an average conduction velocity of 0.330 ± 0.01 m/sec. Repeated measures ANOVA with post hoc Boneferroni t tests revealed a significant treatment effect (F=35.15 [3, 9 df]), such that callosal conduction by the transplanted mice was significantly faster than untransplanted shiverer x rag2−/− mice (p<0.001), and indistinguishable from that of normally myelinated Fvb wild-type and rag2 null mice. The more rapid transcallosal conduction exhibited by the transplanted mice was sustained across stimulus intensities, and thus appeared to represent improved conduction across a wide spectrum of fiber diameters (Fig. 4F). Thus, neonatal transplantation of human OPCs yielded sufficient myelin, in terms of both density and physiological competence, to restore normal inter-hemispheric conduction velocity to a major central tract, the corpus callosum.
Myelination and axonal ensheathment were progressive over time
We had previously established that the dispersal of donor cells following neonatal implantation of human GPCs was relatively rapid, with the terminal distributions of engrafted progenitors occurring within 4–8 weeks of neonatal administration, and myelination proceeding over the several months thereafter(Windrem et al., 2004). In the present study, given the extended survival of mice transplanted in both brain and brainstem, we were able to assess the later progression of myelination in the graft recipients. We asked whether myelination continued to progress even after extended survival had been achieved. To that end, we examined the brains of transplanted shiverers at 18–20 (n=10), 27 (n=1), 35 (n=1) and 52–56 (n=4) weeks of age, and assessed the distribution pattern and densities of human donor cells, as well as of donor-derived myelin, in these recipient brains. (The 20 week-olds had died natural deaths despite their extensive donor cell engraftment, while the 52–56 week-olds were long-survivors, which had been killed to allow histological analysis. The deaths of the 27 and 35 week-old mice – natural and accidental deaths, respectively - provided informative, if singular, intermediate time points.)
We found that while cerebral and cerebellar myelination, as followed by MBP expression, were both substantial and geographically widespread at 20 weeks, both the density and distribution of MBP expression in the brainstem and cervical spinal cord were more extensive at 35 weeks than 20, and much moreso at 52–56 weeks (Figs. 5A–C and D–F). In particular, the 52–56 week-old transplanted mice exhibited essentially complete myelination of the brainstem (Fig. 5C; also 2B), whereas the 20 week-olds still exhibited a number of regions of relative hypomyelination relative to wild-type controls (Fig. 5A). The areas of relatively delayed myelination included the ventral long tracts of the brainstem, as well as the brainstem tegmentum and intrinsic internuclear tracts, all of which were more extensively myelinated at 52 weeks of age than at earlier time-points. By scoring the proportion of ensheathed host axons in confocal optical sections immunostained for MBP and neurofilament, we found that by 52 weeks, 78.0 ± 4.8% of axons in the cervical corticospinal tract at the cervico-medullary junction were myelinated (Fig. 5G–H), only a marginally smaller proportion than that observed in wild-types (93.9 ± 0.9%). At that same timepoint, the proportion of myelinated axons in both the corpus callosum and corticospinal tract of the transplanted animals was indistinguishable from that of their wild-type controls; each exceeded 60% (Fig. 5G–H). We next used transmission electron microscopy (TEM), concentrating on the longitudinal and largely parallel fibers of the cervical spinal cord, to validate the criteria by which we defined myelin-ensheathed axons in our confocal analysis. TEM of the cervical corticospinal tract of 12–13 month-old transplanted shiverers established that the majority of axons manifested ultrastructurally normal myelin, with both major dense lines and multilayer lamination (Figs. 5I–K), thereby confirming that axons which appeared ensheathed in confocal optical sections were indeed so. Furthermore, the major dense lines of the observed myelin indicated its necessarily donor cell origin, since shiverer oligodendrocytes do not make major dense lines, as formation of the latter require myelin basic protein - in which shiverers are genetically deficient(Readhead et al., 1990). In addition, the calculated G-ratio, defined as the ratio of axonal diameter to total myelin-ensheathed fiber diameter, was significantly higher in the untreated shiverers than in either transplanted shiverers or wild-type rag2 nulls, while the latter groups did not differ from one another (Figs. 5L). This indicated that whereas untreated shiverers had little or no myelin ensheathment, their transplanted kindreds had myelin sheaths as thick, on average, as their normally-myelinated wild-type × rag2 null controls.
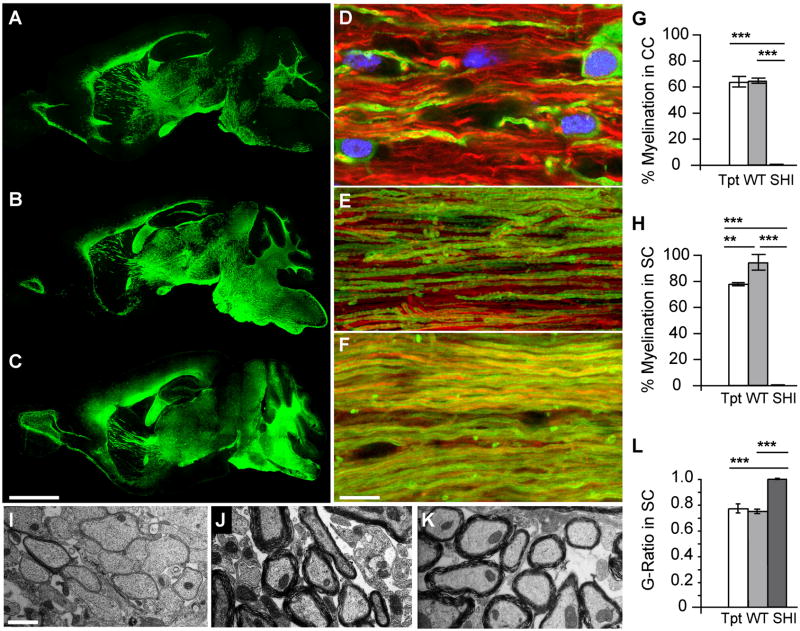
Figure 5. Myelination and axonal ensheathment were progressive over time.
A–C, Sagittal sections of hGPC-implanted mice immunolabeled for MBP (green) at 20 weeks (A), 35 weeks (B) and 52 weeks (C). A, Major white matter regions of the brain, including the corpus callosum, fimbria, optic tract, and both the cerebral and cerebellar peduncles, are already myelinated at 20 weeks. B. At 35 weeks, the area of dense myelination has expanded into the midbrain and hindbrain. C. By a year, myelin was well-distributed, and myelination appeared complete, throughout the forebrain and hindbrain, and includes the lowers layers of neocortex, the colliculi, the pons and medulla, as well as the major corticopontine and corticospinal tracts.
D–F, corresponding confocal optical sections of transplanted shiverer mouse corpus callosum taken at 20 (D), 35 (E) and 52 (F) weeks, immunolabeled for neurofilament (red) and MBP (green), reveal the progressive increase in axonal ensheathment with time.
G–H, plot the proportion of MBP-ensheathed axons, as determined by confocal analysis, in the corpus callosa (CC) (G) and cervical corticospinal tracts of the spinal cord (SC) (H) in 1 year-old transplanted shi/shi × rag2−/− mice compared to both untreated and wild-type rag2−/− controls. At both sites, most axons in the transplant recipients were ensheathed by MBP-defined myelin. In contrast, no ensheathment was noted in their untreated counterparts.
I–K, electron micrographs of spinal cord cross-sections in untreated shi/shi × rag2−/− mice (I), wild-type rag2−/− controls (J), and 1-year old transplanted shi/shi × rag2−/− (K). L plots the G-ratios calculated in 1 year-old implanted shiverers, compared to their wild-type and untreated shiverer controls. The correlation between myelin sheath thickness and axonal diameter in implanted mice is indistinguishable from that of wt/rag2 null mice, while myelin sheaths are virtually undetectable in untreated shiverers. Scale: A–C, 2.5 mm; D–F, 10 μm; I–K, 1 μm
The progressive myelination of transplanted shiverers did not appear to be a function of the rate or kinetics of donor cell dispersal, in that the topography of donor cells at 35 weeks did not differ substantially from that observed at 52 weeks. Nonetheless, the local densities of donor-derived cells did appear to rise over time; this rise was asymptotic (Fig. 6A), which appeared to reflect the fall in mitotic competence of the donor cell pool following their initial expansion in the first half-year or so after transplantation (Fig. 6B). These data suggest that long after human donor cells achieve their destinations, myelinogenesis and axonal ensheathment continue to progress slowly, ultimately achieving the myelination of the recipient neuraxis only after a protracted period of postnatal maturation; this may reflect the incremental engagement of local axons by single oligodendrocytes, as the latter mature and expand their individual domains of myelin ensheathment, adding axons to their ensheathed cohort one at a time over a period of many months.
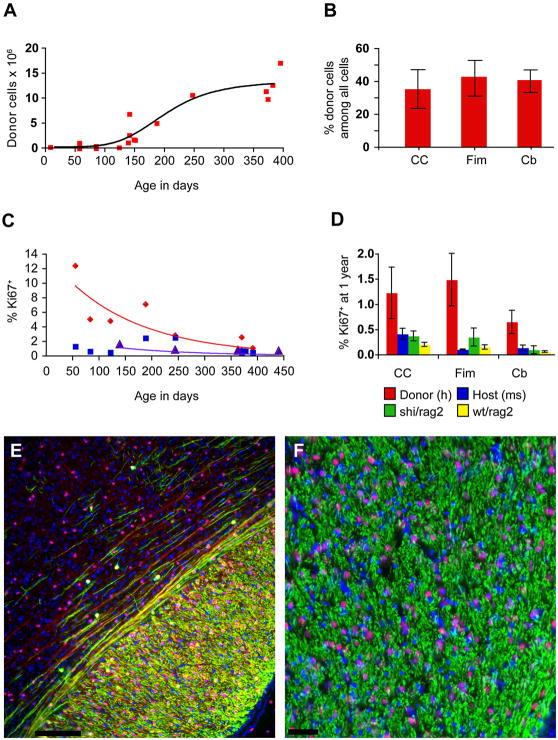
Figure 6. Long-term survival was associated with humanization of the recipient white matter.
A. Perinatally transplanted human GPCs increase in number asymptotically over the course of a year. From an initial dose of 300,000 on postnatal day 1, the cells increase to an average of 12 million/brain by one year post transplantation (y = −9,898,000 + 733,632×+ (−5709)2; r2 = 0.83).
B. By one year, donor cells comprised over 40% of all cells in the fimbria and cerebellar white matter, and over a third in the corpus callosum. Since the total cell count includes host vascular cells and microglia, the human donor-derived cells appeared to comprise a net majority of all glial cells by that stage. C. Over the year after implantation, the rate of human GPC proliferation in white matter declined exponentially (red; y = 14.013e−0.0475×; r2 = 0.79). At 8 weeks, 12.35% of human GPCs in the mouse corpus callosum are Ki67 positive, but by one year, an average of 1.22% are Ki67%. From 5 to 12 months, the percentage of Ki67+ mouse cells in the corpus callosum of untreated rag2 null mice also declines exponentially, but beginning at a lower rate (purple; y = 2.9154e−0.0497×; r2 = 0.83). The Ki67+ percentages of endogenous mouse cells in the same sections of transplanted mice from which the hGPC percentages were obtained, however, do not follow a pattern of exponential decline (blue; y = 1.3684e-0.02× r2 = 0.1855). D. At one year, the percentage of hGPCs in white matter that are Ki67+ (red) exceeds that of the endogenous mouse cells in the same mice (blue), as well as that of untreated rag2 null mice (yellow), and that of 4 month old untreated shiverer/rag2 homozygotes (green) in corpus callosum, fimbria and cerebellum.
E–F. Progressive myelination (MBP, green) of mouse axons (neurofilament, in red) was attended by chimerization of the recipient white matter, such that by 20 weeks, host cells (DAPI, blue) are exceeded by human donor cells (human nuclear antigen, hNA, purple, as blue co-labeled with hNA, red). E. Parasagittal section including dorsal callosum and overlying cortex of a transplanted shiverer at 20 weeks, showing human donor-derived myelination of callosum, and admixture of host (blue, DAPI) and donor cells (purple, as blue co-labeled with hNA, red). Both myelinated (MBP, green) and unmyelinated (NF, far red) fibers are evident traversing lower cortical layers. F. Higher magnification section through fimbria of hippocampus, showing myelinated fibers viewed en face, with admixed mouse (blue) and human (purple, representing blue co-labeled with hNA, red) cells. Scale: E, 100 μm; F, 50 μm.
Long-term survival was associated with humanization of the recipient white matter
The selective expansion of the human glial population in the shiverer mouse white matter appears to be at least in part a product of the more sustained proliferation of the transplanted human GPCs (Figs. 6B and 6D), which as derived from the late second trimester fetal SVZ, would be expected to have continued actively dividing for at least another 9–12 months, assuming cell-autonomous regulation of expansion potential. Accordingly, when we plotted the number of all human cells in the recipient mouse brains, as a function of time, we found that the initial dose of 300,000 cells/recipient had expanded to an average of 12 million human donor glia by 12–14 months in the long-term survivors (Fig. 6A). When the incidence of Ki67+ cells was assessed in three sample regions - the corpus callosum, fimbria and cerebellar white matter - the fraction of mitotic human donor cells was found to be much higher than that of the local host cells, both perinatally and for many months thereafter; only at a year after engraftment was the Ki67+ fraction of human donor cells observed to fall below 2% (Fig. 6B). Even then, the fraction of Ki67+ human glia remained higher than the corresponding proportion of Ki67+ mouse cells, in both the transplanted hosts, and in the rag2 wild-type or shi/shi x rag2−/− mouse controls (F=12.42 [3, 2 df] by 2-way ANOVA permuting cell type and region; p<0.05 for each comparison, by Boneferroni post hoc t tests) (Fig. 6D). Ultimately though, despite the preferential expansion of the human donor cell pool, its relative mitotic quiescence was achieved by a year after transplantation, according to the approximate time course by which normal human GPCs attenuate their expansion in situ. Importantly in this regard, no evidence of heterotopic foci, anaplasia or neoplastic transformation was ever noted in over 100 transplanted mice serially examined.
These data indicate that donor human GPCs exhibit more robust and sustained mitotic expansion than their host murine counterparts after transplantation, and that over time, this results in the relative humanization of the recipient white matter. Indeed, quantification of the human donor cell complement revealed that in 4 long-surviving transplanted mice sacrificed at 12–14 months, at least a third of all cells in the corpus callosum, fimbria and cerebellar white matter were of human origin (35.3 ± 11.8%, 42.9 ± 10.9%, and 40.8 ± 6.9%, respectively) (Fig. 6C). In 3 of the 4, over 40% of all cells in each of these white matter regions were human, and in the densest engraftment among these, that of a mouse sacrificed at 13 months, 54.6% of all cells in its callosum were human. Since just under a third of all cells in the shiverer white matter are non-glial (unpublished data) these include microglia, endothelial cells, and pericytes - we estimate that at least 80% of all callosal glial cells in this “best-case” mouse were of human origin by a year after engraftment; more broadly, over half of all callosal glia were human in each of the long-surviving recipients assessed.
DISCUSSION
In these experiments, we delivered highly-enriched isolates of human glial progenitor cells into neonatal shiverer × rag2 null (shi/shi x rag2−/−) immunodeficient and myelin-deficient mice, using a multifocal delivery strategy that achieved both widespread and dense donor cell engraftment throughout the recipient CNS. Our injection sites were chosen so as to permit contiguous infiltration of migrating donor cells into all major brain, brainstem and spinal white matter tracts, without hindrance from intervening gray matter structures, which may delimit the migration and sustained multilineage competence of engrafted glial progenitor cells(Windrem et al., 2002). By this strategy, we achieved the complete, whole neuraxis myelination of the engrafted hosts; in a fraction of these animals, this resulted in the effective rescue of this otherwise lethal phenotype. These mice exhibited essentially complete myelination of the brain, brainstem and cerebellum, with substantial myelination of the optic nerves, cranial roots and ganglia, spinal cord, and spinal roots, that was associated with clinical rescue, as reflected by both sustained survival and substantially restored functional competence, the latter as manifested both electrophysiologically and behaviorally.
This transplant-associated reduction in both morbidity and mortality was accompanied by the acquisition of normal nodes of Ranvier and paranodal structure, a restitution of transcallosal conduction velocity, ultrastructurally normal and complete myelination of the overwhelming majority of axons, and a restoration of a substantially normal neurological phenotype. These transplants were also attended by a virtually complete chimerization of the recipient central nervous systems, such that the surviving recipients, and in particular the long-term survivors, developed a largely humanized white matter. Donor cell expansion occurred in an asymptotic fashion, such that chimerization evolved over the 8–9 months after transplantation, with progressive myelination reflecting ongoing axonal ensheathment as much as persistent cell expansion.
Interestingly, the progressive chimerization of the host white matter with admixed human donor cells was attended by a significant loss of both host glial progenitor cells and oligodendroglia (data not shown). Whether the competitive strength of the human donor cells was the result of more effective axonal interactions by myelinogenic donor cells relative to non-myelinogenic host progenitors, or rather reflected the selective mitotic expansion of the human donor cells in the postnatal murine environment, or even the selective geographic displacement of the host cells by their donor-derived counterparts, is unclear; it seems likely that each of these factors contributed to the marked competitive advantage of the human donor cells. Yet setting aside the nature of this donor-host competition, the degree of the resultant humanization was remarkable: By 13 months of age, over a third of all cells, and the majority of the glial cells within the recipient callosa, fimbria, cerebellar white matter, and cervical spinal cords indeed, in every region quantified - were human. Moreover, of the recipient axons in these regions, >60% were successfully ensheathed by donor oligodendrocytes; the nodal architecture of the resultant chimerized brains was thus established by human myelin-associated proteins. As a result, multifocal neonatal delivery of human glial progenitor cells to myelin-deficient immunodeficient mice - intended initially as a proof-of-principle of a promising therapeutic approach - has also provided us mice with largely humanized white matter (Figs. 6E–F), in which the responses of human glial oligodendrocytes and astrocytes to injury and disease may now be observed in real-time, in vivo. This may prove an invaluable experimental model going forward, quite apart from the value of somatic chimerization via progenitor cell allografts as a potential therapeutic approach in the dysmyelinating disorders.
Several reports have noted the potential utility of neural stem or progenitor cell grafts in alleviating pathology in congenital leukodystrophies associated with lysosomal storage disorders; these efforts have capitalized upon the provision of wild-type enzyme by engrafted donor cells to the otherwise deficient host cells with which they structurally integrate (Lee et al., 2007; Pellegatta et al., 2006; Snyder et al., 1995). Yet no cell-based treatment of any congenital hypomyelination has ever proven sufficient to rescue the underlying phenotype. A number of previous studies demonstrated that transplants of fetal tissue (Lachapelle et al., 1983), tissue dissociates (Gumpel et al., 1989; Gumpel et al., 1987), neural stem cells (Mitome et al., 2001; Yandava et al., 1999), and embryonic stem cell-derived glia (Nistor et al., 2005) could give rise to myelinogenic oligodendrocytes in the shiverer mouse, as well as in other animal models of hypomyelination, including both the myelin-deficient rat (Archer et al., 1994; Hammang et al., 1997) and shaking pup (Archer et al., 1997). However, none of these efforts yielded sufficiently abundant or extensive myelination to provide significant survival or long-term functional benefit to the treated recipients. In contrast, our present results suggest the feasibility of an outright rescue of the disease phenotype, using highly enriched preparations of human glial progenitor cells delivered to multiple sites of the neonatal neuraxis, at relatively high cell dose levels.
It is important to note that only a minority of the transplanted shiverers were rescued by neonatal transplantation. A major challenge going forward will be to better define the critical sites that need to be rapidly myelinated to avoid death, and hence allow progressive myelination to ultimately assuage disease progression. Our results suggest that early myelination of the brainstem, and an early transplant-associated diminution in seizure activity, are both associated with clinical rescue; as such, future studies may combine antiepileptic treatment with more posteriorly biased progenitor grafts, as a potential strategy to improve the likelihood of clinical rescue. These caveats aside, our results indicate that multifocal perinatal transplantation of human glial progenitor cells can be sufficient to rescue a congenital lethal hypomyelination. The sustained viability and restored functional competence of these animals augers well for the potential utility of this approach in the treatment of precociously apparent leukodystrophies, in particular those hypomyelinating disorders manifesting neonatally, such as Pelizaeus-Merzbacher disease (PMD) (Garbern and Hobson, 2002; Nave and Griffiths, 2004; Powers, 2004). Indeed, the pathological similarity of PMD, an X-linked misexpression of proteolipid protein, to the periventricular leukomalacia of cerebral palsy (Deguchi et al., 1997; Rezaie and Dean, 2002; Volpe, 2001), suggests the potential applicability of this glial progenitor cell-based treatment strategy to a wide range of both hereditary and ischemic (Back et al., 2001) childhood disorders of myelin.
METHODS
Cells
Fetal OPCs were extracted from second trimester human fetuses (19 to 22 weeks gestational age, g.a.), obtained at abortion as described (Windrem et al., 2004). The forebrain ventricular/subventricular zones were dissected from the remaining brain parenchyma, the samples chilled on ice, and the minced samples then dissociated using papain/DNAse as described (Keyoung et al., 2001), always within 3 hours of extraction. The dissociates were maintained overnight in minimal media of DMEM/F12/N1 with 20 ng/ml FGF. A total of 5 tissue samples (1 at 19 wks g.a., 1 at 20 wks, 3 at 22 wks) were used for this study, all from chromosomally normal fetal donors. All samples were obtained with consent under approved protocols of the University of Rochester, Cornell/New York Presbyterian Hospital, and Albert Einstein College of Medicine and Jacoby Hospital Institutional Review Boards.
Sorting
Glial progenitor cells were isolated from dissociated tissue using a dual immunomagnetic sorting strategy. On the day after dissociation the cells were incubated with mouse anti-PSA-NCAM (Chemicon) at 1:100. then washed and labeled with microbead-tagged rat anti-mouse IgM (Miltenyi Biotech), and removed by MACS depletion. The remaining PSA-NCAM− cells were next incubated 1:1 with MAb A2B5 supernatant (clone 105; ATCC, Manassas, VA), for 20 minutes, then washed and labeled with microbead-tagged rat anti-mouse IgM (Miltenyi Biotech). All incubations were done on ice(Keyoung et al., 2001; Roy et al., 2000). Magnetic separation of A2B5+ cells (MACS; Miltenyi) was then performed, as described(Nunes et al., 2003). The bound cells were then eluted, yielding a highly enriched population of A2B5+/PSA-NCAM− cells. After sorting, the cells were maintained in vitro for 1–2 days in DMEM/F12/N1 with 20 ng/ml bFGF, then frozen and stored in liquid nitrogen, at 2 × 106 cells/ml in 7%DMSO/93% FBS.
Transplantation and husbandry
Homozygous shiverers were crossed to homozygous rag2 null immunodeficient mice (Shinkai et al., 1992), to generate a line of shi/shi × rag2−/− myelin-deficient, immunodeficient mice. Newborn pups of this line were transplanted within a day of birth, using a total of 300,000 donor cells dispersed over 5 injection sites. The pups were first cryoanesthetized for cell delivery. 5 × 104 donor cells in 0.5 μl HBSS were then injected at each of 4 locations in the forebrain subcortex, specifically into the anterior and posterior anlagen of the corpus callosum bilaterally. These injections were delivered to a depth of 1.0 to 1.2 mm ventrally, depending on the weight/size of the pup (which varied from 1–1.5 g). A fifth injection of 105 cells in 1 μl was delivered into the cerebellar peduncle dorsally, to gain access to the major cerebellar and dorsal brainstem tracts. All cells were injected through pulled glass pipettes, inserted directly through the skull into the presumptive target sites. The pups were then returned to their mother, until weaning at 21 days; at that point, each litter was moved to separate enriched housing. After weaning, mice were checked at least twice daily. Mice that died from immediate surgical complications or before weaning (2 saline-injected and 1 GPC-transplanted) were excluded from the experiment. Typically as of 130 days of age, mice in all groups began to die. They were checked several times daily, and if found so moribund as to be unable to right themselves upon being moved, they were given barbiturate anesthesia, then perfusion-fixed with HBSS followed by 4% paraformaldehyde. When mice were instead found dead, their brains were removed and post-fixed for 2 hrs in cold paraformaldehyde.
Survival analysis and statistics
Kaplan-Meier analysis was used to assess the different survivals of transplanted and control mice, as described (Hosmer and Lemeshow, 1999). No difference in survival was observed between saline-injected and untreated mice, so the two populations were combined as a single control population for the Kaplan-Meier comparison with GPC-implanted mice.
Analyses of variance (ANOVA) were performed using GraphPad Prism (v4.0c for Macintosh; GraphPad Software, San Diego, CA).
Immunolabeling
Human cells were identified with mouse anti-human nuclei, clone 235-1 at 1:100 (MAB1281, Millipore, Billerica, MA). Myelin basic protein was labeled with rat anti-MBP at 1:25 (Ab7349, Abcam, Cambridge MA), and axons with mouse anti-neurofilament cocktail at 1:1000 (SMI-311 and -312, Covance, Princeton, NJ). Monoclonal antibodies against Caspr, Nav1.6 and Kv1.2 were used at 1:600, 1:200 and 1:200, respectively, and were obtained from NeuroMab (Davis, CA). Rabbit anti-Caspr and anti-βIV spectrin were generated as described (Rasband and Trimmer, 2001; Yang et al., 2007), while rabbit anti-Caspr2 was obtained from Millipore. Rabbit anti-olig2 was obtained from Abcam (Ab33427) and used at 1:1,500. Alexa Fluor secondary antibodies, goat anti-mouse, rat, and rabbit 488, 568, 594 and 647 were used at 1:400 (Invitrogen, Carlsbad, CA).
Myelinated axon counts
Uniform random sagittal sections of the cervical spinal cord, and coronal sections of the corpus callosum, were both selected for neurofilament and MBP staining; in the spinal cord samples, the most medial sections were analyzed with respect to the percentage of myelinated host axons. A 1 μm stack of 10 superimposed optical slices taken at 0.1 μm intervals (Olympus FluoView 300) was made for each of 3 fields of view in the dorsal columns, beginning rostrally and progressing caudally. Three parallel, equidistant lines were laid over the images perpendicular to the axons. Axons were scored at intersections with the lines as either myelinated (closely apposed to MBP on both sides) or unmyelinated. This procedure was then repeated for the coronally-cut samples of corpus callosum.
Proportionate representation of donor cells
The percentage of human cells in the recipient white matter was assessed as a function of time after transplantation. Randomly initiated, uniformly sampled sagittal sections of the brains were labeled for human nuclei and DAPI (Vector Labs). 4–6 sections (depending on the persistence of the structure in the selected range of sections) of the corpus callosum, fimbria, and cerebellar white matter were counted, with data entry and reconstruction using BioQuant. All human nuclei and DAPI-labeled cells in the white matter regions of these 14 μm sections were counted.
Electron Microscopy
The four mice that survived over a year were perfused transcardially with HBSS, followed by 4% paraformaldehyde with 0.25% glutaraldehyde and 6% sucrose in phosphate buffer (sucrose-PB). One hemisphere of each brain and longitudinal half of each spinal cord were post-fixed in 2% paraformaldehyde, 2.5% glutaraldehyde in sucrose-PB for electron microscopy; the other half of each brain and spinal cord were post-fixed in 4% paraformaldehyde in sucrose-PB for immunohistochemistry. Tissue samples used for electron microscopy were osmicated, dehydrated in ethanol, and embedded in Epon. Ultrathin sectioning was performed using a PowerTome X Ultramicrotome (RMC products by Boeckeler, Tucson, AZ). The ultrathin sections were collected on formvar-coated copper one-hole grids and contrasted with lead citrate and uranyl acetate, then examined in a JEOL 100CX transmission electron microscope.
Seizure counts
Mice were placed in a sterilized Plexiglass cage with a camera embedded in the ceiling (PhenoTyper, Noldus, Wageningen, the Netherlands) and left undisturbed overnight while their movements were recorded by infra-red light. Six non-overlapping half-hour video segments were randomly selected from each 8 hour videotape, excluding the first 3 hour segment so as to diminish any effects of the novel environment. Two segments for each mouse scored were assigned to each of 3 observers, blinded as to the mouse’s age and treatment. The observers recorded and timed each mouse’s seizures, which were defined as such when the mouse fell to its side and assumed a rigid, stereotypically tonic posture, typically complicated by clonic flexion-extension of the trunk and limbs. A seizure was timed as ending when the mouse first moved to right itself. The number of seizures per hour, and the total ictal time per hour, were thereby scored.
Transcallosal transmission
Mice were anesthetized with katamine (60 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), intubated through a tracheotomy and ventilated with a ventilator (SAR-830, CWE, Inc., Ardmore, PA). A femoral artery was catheterized for monitoring mean artery blood pressure and blood gases, and body temperature was maintained at 37°C by a warming blanket (Harvard Apparatus, Holliston, MA). Mice were secured with a custom-made metal frame that was glued to the skull with acrylic cement. Two burr holes, each 3 mm in diameter, were made bilaterally, centered 1–2 mm posterior to bregma and 2–3 mm from the midline. The dura was removed and agarose (0.75% in saline) was poured into the craniotomy sites, which were then closed with a 0.17 mm thick glass coverslip. The head frame was then attached to a second frame that was coupled to the microscope stage. Glass micropipettes filled with 2M NaCl solution were then inserted to a depth of 200 μm into the right cortex, at 1.5 mm posterior to bregma and 2.5 mm from the midline, for recording the local field potentials (LFPs) generated by transcallosal electric stimulation. Electrical stimulation (100 μs at 10–1000 μA, via an ISO-Flex isolator controlled by a Master-8 programmer; AMPI, Israel) was applied using a bipolar electrode inserted at the same coordinates in the contralateral (left) hemisphere. Evoked LFPs were recorded by a multiClamp 700A amplifier, filtered at a cutoff frequency of 1 kHz, and sampled at an interval of 200 μs by a pCLAMP 9.2 program and DigiData 1332A interface (Axon Instruments Inc.). The same electrode was used to continuously monitor the electrocorticogram (ECoG). ECoG was recorded continuously by a multiClamp 700A Amplifier (Axon) with a low frequency filter at 1 Hz and high frequency filter at 100 Hz (51, 52), and a pCLAMP 9.2 program and DigiData 1332A interface (Axon) with an interval of 200 μs. The amplitude of stimulus-evoked transcallosal response was then calculated as the difference between the peak and baseline, whereby the baseline was defined as the average potential measured during the 20 ms before the stimulation was delivered. The velocity of transcallosal response was calculated, together with the latency of the response and the distance between the stimulating and recording electrodes. The response latency was defined as the difference between the stimulus start and the peak. Two recordings of the transcallosal responses to electric stimulation (0.10 ms, 0.01–0.10 mA) were obtained from each animal.
Supplementary Material
Acknowledgments
This work was supported by the Miriam and Sheldon Adelson Medical Research Foundation, the G. Harold and Leila Y. Mathers Charitable Foundation, the CNS Foundation and Ataxia-Telangiectasia Children’s Project, the National Multiple Sclerosis Society, and NINDS R01NS039559. We thank Devin Chandler-Militello for advice and assistance in cell preparation, and Carolyn Moyle for technical assistance. We are grateful to Dr. Brad Poulis of the Fetal Human Tissue Repository of the Albert Einstein College of Medicine for some of the fetal tissue samples used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer D, Cuddon P, Lipsitz D, Duncan I. Myelination of the canine central nervous sytem by glial cell transplantation: A model for repair of human myelin disease. Nature Medicine. 1997;3:54–59. doi: 10.1038/nm0197-54. [DOI] [PubMed] [Google Scholar]
- Archer DR, Leven S, Duncan ID. Myelination by cryopreserved xenografts and allografts in the myelin-deficient rat. Exp Neurol. 1994;125:268–277. doi: 10.1006/exnr.1994.1029. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C, Fehlings M. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J Neuroscience. 2007;27:3416–3428. doi: 10.1523/JNEUROSCI.0273-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern J, Hobson G. Prenatal diagnosis of Pelizaeus-Merzbacher disease. Prenat Diagn. 2002;22:1033–1035. doi: 10.1002/pd.465. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Stem and progenitor cell-based therapy of the human central nervous sytem. Nature Biotech. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- Gumpel M, Gout O, Lubetzki C, Gansmuller A, Baumann N. Myelination and remyelination in the central nervous system by transplanted oligodendrocytes using the shiverer model. Discussion on the remyelinating cell population in adult mammals. Dev Neurosci. 1989;11:132–139. doi: 10.1159/000111894. [DOI] [PubMed] [Google Scholar]
- Gumpel M, Lachapelle F, Gansmuller A, Baulac M, Baron van Evercooren A, Baumann N. Transplantation of human embryonic oligodendrocytes into shiverer brain. Ann N Y Acad Sci. 1987;495:71–85. doi: 10.1111/j.1749-6632.1987.tb23666.x. [DOI] [PubMed] [Google Scholar]
- Hammang JP, Archer DR, Duncan ID. Myelination following transplantation of EGF-responsive neural stem cells into a myelin-deficient environment. Exp Neurol. 1997;147:84–95. doi: 10.1006/exnr.1997.6592. [DOI] [PubMed] [Google Scholar]
- Hosmer D, Lemeshow S. Applied Survival Analysis. New York: John Wiley and Sons; 1999. [Google Scholar]
- Keyoung HM, Goldman SA. Glial progenitor-based repair of demyelinating neurological diseases. Neurosurg Clin NA. 2007;18:93–104. doi: 10.1016/j.nec.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Keyoung HM, Roy N, Louissant A, Benraiss A, Mori Y, Okano H, Goldman SA. Specific identification, selection and extraction of neural stem cells from the fetal human brain. Nature Biotechnology. 2001;19:843–850. doi: 10.1038/nbt0901-843. [DOI] [PubMed] [Google Scholar]
- Lachapelle F, Gumpel M, Baulac C, Jacque C. Transplantation of fragments of CNS into the brains of shiverer mutant mice: Extensive myelination by transplanted oligodendrocytes. Dev Neurosci. 1983;6:326–334. doi: 10.1159/000112359. [DOI] [PubMed] [Google Scholar]
- Learish RD, Brustle O, Zhang SC, Duncan ID. Intraventricular transplantation of oligodendrocyte progenitors into a fetal myelin mutant results in widespread formation of myelin. Ann Neurol. 1999;46:716–722. [PubMed] [Google Scholar]
- Lee JP, Jeyakumar M, Gonzalez R, Takahashi H, Lee P, park KI, Butters T, Dwek R, Schwartz P, Tong G, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nature Medicine. 2007;13:439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- Mitome M, Low HP, van Den Pol A, Nunnari JJ, Wolf MK, Billings-Gagliardi S, Schwartz WJ. Towards the reconstruction of central nervous system white matter using neural precursor cells. Brain. 2001;124:2147–2161. doi: 10.1093/brain/124.11.2147. [DOI] [PubMed] [Google Scholar]
- Nave K-A, Griffiths IR. Models of Pelizaeus-Merzbacher Disease. In: Lazzarini R, editor. The Myelin Biology and Disorders. San Diego: Elsevier Academic; 2004. pp. 1125–1143. [Google Scholar]
- Nistor G, Totoiu M, Haque NS, Carpenter M, Keirstead H. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature Medicine. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- Pellegatta S, Tunici P, Poliani P, Dolcetta D, Cajola L, Colombelli C, Ciusani E, DiDonato S, Finocchiaro G. The therapeutic potential of neural stem/progenitor cells in murine globoid cell leukodystrophy is conditioned by macrophage/microglial activation. Neurobiol Dis. 2006;21:314–323. doi: 10.1016/j.nbd.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Popko B, Puckett C, Lai E, Shine HD, Readhead C, Takahashi N, Hunt SW, 3rd, Sidman RL, Hood L. Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell. 1987;48:713–721. doi: 10.1016/0092-8674(87)90249-2. [DOI] [PubMed] [Google Scholar]
- Powers J. The Leukodystrophies: Overview and Classification. In: Lazzarini RA, editor. Myelin Biology and Disorders. San Diego: Elsevier Academic Press; 2004. pp. 663–690. [Google Scholar]
- Rasband M, Trimmer J. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Readhead C, Popko B, Takahashi N, Shine HD, Saavedra RA, Sidman RL, Hood L. Expression of a myelin basic protein gene in transgenic shiverer mice: correction of the dysmyelinating phenotype. Cell. 1987;48:703–712. doi: 10.1016/0092-8674(87)90248-0. [DOI] [PubMed] [Google Scholar]
- Readhead C, Takasashi N, Shine HD, Saavedra R, Sidman R, Hood L. Role of myelin basic protein in the formation of central nervous system myelin. Ann N Y Acad Sci. 1990;605:280–285. doi: 10.1111/j.1749-6632.1990.tb42401.x. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- Roach A, Takahashi N, Pravtcheva D, Ruddle F, Hood L. Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell. 1985;42:149–155. doi: 10.1016/s0092-8674(85)80110-0. [DOI] [PubMed] [Google Scholar]
- Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, Fraser RA, Couldwell WT, Kawaguchi A, Okano H, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- Schafer D, Rasband M. Glial regulation of the axonal membrane at nodes of Ranvier. Curr Opinion in Neurobiology. 2006;16:508–514. doi: 10.1016/j.conb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Sherman D, Brophy P. Mechanisms of axon ensheathment and myelin growth. Nature Rev Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, lam K, Oltz E, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A, et al. RAG2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Nunes MC, Rashbaum WK, Schwartz TH, Goodman RA, McKhann G, Roy NS, Goldman SA. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nature Medicine. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- Yandava BD, Billinghurst LL, Snyder EY. “Global” cell replacement is feasible via neural stem cell transplantation: evidence from the dysmyelinated shiverer mouse brain. Proc Natl Acad Sci U S A. 1999;96:7029–7034. doi: 10.1073/pnas.96.12.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ogawa Y, Hedstrom K, Rasband M. βIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J Cell Biol. 2007;176:509–519. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.