Abstract
As emerging novel DNA-based methodologies are adopted, nucleic acid-based assays depend critically on the quality and quantity of extracted DNA. Formalin fixed, paraffin embedded (FFPE) tissue samples provide an invaluable resource for subsequent molecular studies of clinical phenotypes, but high quality DNA extraction from archival FFPE tissue specimen remains complex and time consuming. To address this challenge, we have developed a reliable rapid DNA extraction method for FFPE tissue specimens. It is based on deparaffinization at high temperature coupled with relieving crosslink in a pressure cooker. The DNA yield by this rapid method resulted in an average 1.8-fold increase in comparison with the commercial kit; O.D 260/280 ratios between 1.87 and 1.95. The DNA obtained by the rapid method was suitable for methylation analyses in colon cancer patients. These data suggest that this new DNA extraction method coupled with MSP can be used for epigenetic studies with the advantages of rapidity and high quality, and may contribute to the development of biomarkers in clinical studies.
Keywords: DNA extraction, DNA methylation, Methylation Sepcific PCR (MSP), Formalin-fixed paraffin-embedded tissues, Intraepithelial neoplasia
Introduction
Nucleic acid-based molecular assays have great utility in molecular medicine, particularly in the context of biomarker discovery and validation. Current nucleic acid-based assay approaches have been applied mainly to fresh and frozen biological samples due to superior DNA and RNA quality. For clinical application, nucleic acid assays need to utilize archival formalin fixed, paraffin embedded (FFPE) tissue as most hospitals and clinics lack the infrastructure to store and archive frozen tissue for nucleic acid extraction [1]. In the case of rare diseases, FFPE tissue is the invaluable nucleic acid resource for genetic analysis. However, the recovery of high quality DNA and RNA from FFPE tissue specimens remains challenging. It is well described that formalin fixation leads to protein-protein and protein-DNA cross-linkage, and formaldehyde within the tissue gradually changes to formic acid, inducing chain breaks [1; 2; 3; 4]. Despite these problems, it has been shown that DNA extracted from FFPE tissue is applicable for polymerase chain reaction (PCR) amplification using relatively short amplicons[5; 6]. Subsequently, studies on DNA extraction from FFPE tissue reported varying degrees of improvements such as increased amplicon length or increased effective amplifiable copy number [3; 7; 8]. However the current DNA extraction methods do not meet the practicability of a routine processing of the most common archival materials in the clinical laboratory.
Colorectal cancer (CRC) has been characterized as the consequence of an accumulation of genetic and epigenetic alterations. Investigations within the last few years indicate that this disease provides an excellent model for understanding the role of epigenetic changes in human colon cancer [9]. Most CRCs have epigenetic abnormalities, such as DNA methylation and core histone modifications. As a result, inactivation of many tumor suppressor genes such as, p16, Mlh1, etc. can occur. In the human genome, approximately half of the genes have CpG-rich promoter regions, also called CpG islands. Methylation of CpG islands in the gene promoter regions may be a epi-genetic process to inhibit transcription, but can also be associated with aberrant silencing and inactivation of tumor-suppressor genes [10]. Aberrant DNA methylation patterns are established early during cancer development, and remain relatively fixed, despite cell division [11]. Therefore, DNA methylation has been suggested as a potential early biomarker for clinical application in various cancers [11]. There is a wide variety of methods for analysis of methylated DNA that have been critically reviewed [12; 13]. Of these analytical methods, methylation specific PCR (MSP) is a common technique that many investigators employed [14]. The technique relies on sodium bisulfite treatment of DNA, which converts unmethylated cytosine to uracil while leaving methylated cytosine unaffected. The modified sequences are then amplified with specific primers, and the amplified products are identified using gel electrophoresis. More recently, a number of new hypermethylated genes have been identified in colon cancer tumors using a genome wide approach by chip technique [15]. Although there is a great deal of progress in assays for methylation of DNA, the recovery of DNA from FFPE tissue remains challenging. The classic DNA extraction method that was based on long-term enzymatic reaction combined with phenol-chloroform extraction is incompatible with the development of simplified extraction.
In the present study, we extracted DNA from 18 human colon FFPE tissues that contained low grade and high grade intraepithelial neoplasia as precursor lesions to invasive colon carcinoma. Using our DNA extraction method, we have tested MSP by two genes ( sFRP1 and TFPI2), which have been demonstrated to have a CpG island in promoter region that is highly methylated in colon cancer patients [16; 17; 18]. Also, we have confirmed these genes methylation status by bisulfite sequencing of the respective CpG loci. We show here the advantages and utility of our method and its impact on the quantity and quality of the DNA recovered.
Materials and Methods
FFPE samples
In order to establish a reliable DNA extraction, anonymized tissues were obtained from the Tissue Array Research Program, Laboratory of Pathology, NCI, NIH. The tissue blocks were approved for use in research by the Office of Human Subjects Protection of the NIH.
For the methylation study, 18 cases of colon adenoma were obtained from the University Hospital, RWTH Aachen, Germany. The tissues were used as anonymized samples with approval of the ethical board of the University Hospital, RWTH Aachen, Germany. As adenomas are recognized precursor lesions of colon carcinoma, our approach was to investigate the impact and accumulation of hypermethylated tumor suppressor genes. By clinical and pathological means, the group of adenomas was subdivided by morphologic and cytologic factors into two groups: low-grade and high-grade intraepithelial neoplasia. The selected cases did not show invasive growth, but histomorphological changes towards invasive carcinoma. In both groups, FFPE samples of 9 different patients were chosen. The 9 samples with low-grade intraepithelial neoplasia (LGIN) consisted of 3 female and 6 male patients with a mean age of 69 years. The 9 samples with high-grade intraepithelial neoplasia (HGIN) consisted of 5 female and 4 male patients with a mean age of 69 years. Removed tissue (generally by colonoscopy) was fixed in 10% buffered formalin, and subsequently paraffin embedded.
DNA extraction from FFPE tissue
For each paraffin block, two 10-μm-thick sections or a 1 mm tissue cores, obtained with a TMA core punch (Beecher Instruments Inc., Silver spring MD) were trimmed of excess wax and transferred to a 1.5 ml microcentrifuge tube. Deparaffinization was performed with Auto-Dewaxer (Open Biosystems, Gaithersburg, MD). The workflow of the presented study is described in Figure 1. The specimens were incubated in 1.2 mL of reagent for 15 minutes at 95°C with mild shaking in Thermomixer (Eppendorf, Hamburg, Germany), followed by centrifugation at room temperature for 2 minutes at 13,000 rpm. Each specimen was deparaffinized three times by the above mentioned procedures and then briefly washed with Auto-Alcohol (Open Biosystems). The deparaffinized specimens were air-dried, and then homogenized using a Disposable Pellet Mixers and Cordless Motor in 300 μ1 of lysis buffer (10 mM Tris.Cl, pH 8.0; 100 mM EDTA , pH 8.0; 0.5% SDS ), followed by incubation for 20 min at 124°C within a pressure cooker (Dako, Carpinteria, CA). After incubation and cooling steps , 15 μ1 proteinase-K (Qiagen, Valencia, CA, 1 mg/ml) and 2.5 μ1 RNase-A (Qiagen, 800 μg/ml) were added to the tissue lysate, followed by incubation for 2 hours at 60°C with shaking on the thermomixer (Eppendorf) at 500 rpm. Proteinase K was inactivated by heating at 95°C for 5 min. Addition of ice-cold 200 μ1 potassium acetate (5M) was followed by incubation on ice for 10 minutes and centrifugation at 4°C for 30 minutes at 13,000 rpm. The supernatant was transferred to a new microtube, and one volume ice-cold isopropanol was added, mixed by vortex, followed by incubation on ice for 15 minutes. The precipitated DNA was centrifuged at 13,000 rpm at 4°C. The supernatant was discarded, and the DNA precipitate washed once with 70% ethanol. The pelleted DNA was dissolved in 20 μ1 of DNase free water or TE buffer after complete drying in a fume hood. As a control, we also performed standard phenol-chloroform extraction method [19].
FIGURE 1.
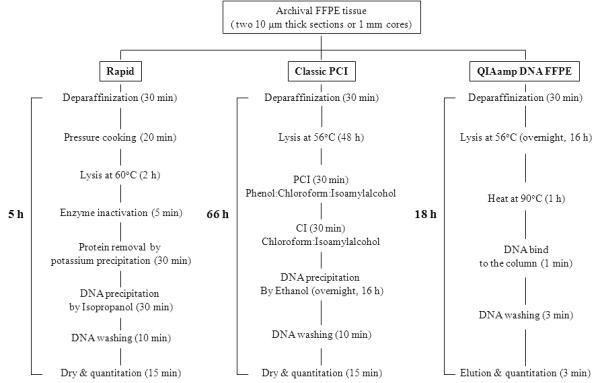
Schematic diagram of DNA extraction procedures from FFPE tissue section or core.
In addition, the QIAamp DNA FFPE tissue kit (Qiagen) was used as a reference method. The extraction was performed according to the manufacturer’s instruction. Prior to the final elution step, 55 μ1 of elution buffer was applied to the column and incubated at RT for 5 minutes followed by centrifugation.
Determination of the quantity and the quality of DNA
In order to assess the quality and quantity of extracted DNA, we measured DNA concentration and purity (O.D, A260/A280 and A260/A230) by the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE).
Since the quality of DNA extracted from archival FFPE tissues varies widely, we further tested DNA quality using BioScoreTM Screening and amplification kit (Enzo Life Sciences, Farmingdale, NY). The kit was used to identify DNA extracted from FFPE tissues as being suitable for array analysis. The experimental procedure was performed according to the manufacturer’s instruction. Finally, the DNA quality was evaluated by amplified DNA amounts.
Methylation-specific PCR
Bisulfite modification of genomic DNA was carried out using the EZ DNA methylation kit (Zymo Research, Orange, CA). Methylation analysis of sFRP1 and TFPI2 gene promoters was performed using MSP primer pairs located close to the putative transcriptional start site in the 5 CpG island with 2 μl of bisulfite-treated DNA as template and JumpStart Red Taq DNA Polymerase (Sigma-Aldrich, St. Louis, MO) for PCR amplification. Primer sequences information for sFRP1 and TFPI2 genes have been described previously [16; 17; 18]. PCR conditions were as follows: 95°C for 5 min, 35 cycles of 95°C for 30 sec, 60°C for30 sec, and 72°C for 30 sec; and final extension of 5 min at 72°C.
Bisulfite sequencing
For bisulfite sequence analysis of the sFRP1 and TFPI2 promoter, primer sequences for bisulfite sequence analysis have been described previously [16; 18] and their location in the sFRP1 and TFPI2 promoter is from upstream region (from to −188 to +241: from −250 to +50, respectively) relative to transcriptional start site. PCR products were electrophoresed on 2% agarose gels and bands were excised using the QIAquick Gel Extraction kit following the manufacturer’s instructions (Qiagen). Purified DNA was cloned into the TOPO-TA II plasmid following the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and resulting colonies were screened for the presence of inserts by EcoRI restriction digestion. Plasmids with appropriate DNA inserts were sequenced at the JHU Sequencing Core Facility (http://biolchem.bs.jhmi.edu).
Statistical analyses
Statistical differences were calculated using independent-samples T test. P ≤0.05 was regarded as statistically significant. All statistical analyses were performed using the SPSS for Window (16.0) package (SPSS, Chicago, IL).
Results
Comparisons of different DNA extraction methods
We previously showed that the recovery of nucleic acid is closely linked to the deparaffinization process [20]. Based on our experience, we applied 3 cycles of deparaffinization at 95°C using the aqueous dewaxer. After deparaffinization, we treated the deparaffinized tissue with high temperature (124°C) and moderate pressure (15-22 psi) for facilitation of tissue lysis. As shown in Figure 2A, a strong deparaffinization in combination with high temperature and moderate pressure revealed DNA yield 181% (174% in tissue core; 5.17 ± 0.107 μg, 188% in tissue section, 4.21 ± 0.234 μg) in comparison with the QIAamp DNA FFPE tissue kit (tissue core; 2.96 ± 0.193 μg, tissue section; 2.24 ± 0.096 μg). The DNA extraction yield of tissue core was significantly higher than tissue section (p<0.05) when tissue volume was taken into account.
FIGURE 2.

Comparison of DNA extraction yield and quality between a new rapid method and the QIAamp DNA FFPE tissue kit. We performed DNA extraction from archival human liver cores or sections. Representative data were presented as a bar graph (A) and a gel image (B). The bar graph showed the average DNA yield and displayed as average ± SD (**, p<0.001; *, p<0.05). The amplicon were separated on a 1.2% agarose gel. 1, a rapid method; 2, the Qiagen kit; M1, 1 kb DNA ladder (Invitogen); M2, Ready-load 100 bp DNA ladder (Invitrogen).
Next, we analyzed the DNA extracted by 1.2% agarose gel electrophoresis in order to evaluate the integrity of the DNA obtained. The electrophoretic pattern of DNA extracted by the rapid method and the commercial kit revealed the broad range of DNA size (Figure 2B). The major band of DNA extracted by the rapid method was approximately 500 bp (ranging between 200 bp and 2,000 bp), suggesting that low molecular weight DNA was predominant, whereas the DNA obtained by the commercial kit was of larger size than DNA extracted by this method (Figure 2B).
Clinicopathological features of human colon FFPE tissue specimens
Clinicopathologic characteristics of cases are summarized in Table 1. The ages of the patients ranged from 38 to 89 years (mean, 68.9 ± 12 years). Eight cases were women and ten were men. Three cases presented other malignancies in the past, two patients with breast carcinoma and one with prostate carcinoma. The histopathological changes in LGIN cases demonstrated with mostly tubular adenoma with low nuclear variance and low hyperchromasia. The nuclei within the surface epithelium were all located on the basal part. Within the epithelium, lots of goblet cells were present (Figure 3A, 3B). In HGIN, the lesions presented as mostly tubulo-villous adenomas with high variance in nuclear size. The nuclei were hyperchromatic and located from the basal area up to the top of the epithelial cell. Some intraepithelial mitotic figures could be found, as well as near complete loss of goblet cells (Figure 3C, 3D).
Table 1.
Clinicopathologic characteristics of samples.
| No. | Diagnosis* | Age | Sex |
|---|---|---|---|
| 1 | HGIN | 74 | M |
| 2 | HGIN | 68 | F |
| 3 | HGIN | 55 | F |
| 4 | HGIN | 65 | M |
| 5 | HGIN | 82 | F |
| 6 | HGIN | 65 | M |
| 7 | HGIN | 72 | M |
| 8 | HGIN | 78 | M |
| 9 | HGIN | 62 | M |
| 10 | LGIN | 89 | F |
| 11 | LGIN | 86 | F |
| 12 | LGIN | 70 | F |
| 13 | LGIN | 38 | M |
| 14 | LGIN | 66 | F |
| 15 | LGIN | 65 | M |
| 16 | LGIN | 67 | M |
| 17 | LGIN | 82 | M |
| 18 | LGIN | 62 | F |
HGIN: high-grade intraepithelial neoplasia; LGIN: low-grade intraepithelial neoplasia
FIGURE 3.

The representative hematoxylin 6 eosin photomicrographs of two types of intraepithelial neoplasia, as precursor lesions to invasive colon carcinoma: low grade (A and B, LGIN) with a basal lining of almost normal nuclei and very few mitotic figures, high grade (C and D, HGIN) with atypical nuclei coming up to the cell apex with many mitotic figures (circle) (all H&E stains, in A and C: 40x, in B and D: 400x magnification).
DNA quantity and quality assessments by NanoDrop and the amplification kit
In order to determine the efficiency DNA recovery by the rapid method, we compared the quantity of DNA recovered from 18 archival colon FFPE specimens by rapid and the classic phenol-chloroform method. There was no difference between the two different methods with respect to quantity (Figure 4A). The DNA yield by the rapid method significantly correlated with that of the classic method (R2=0.946) (Figure 4B).
FIGURE 4.

Comparison of DNA extraction yield between a rapid extraction method and a long extraction method. We adopted a classic phenol-chloroform extraction method combined with proteinase-K digestion overnight as a reference DNA extraction method. The DNA extraction yield was expressed the mean of three replicated samples (A). Black bar and clear bar represent the result of rapid (■) and classic methods (□), representatively. The DNA yield by rapid and classic methods correlated strongly (B, R2 = 0.946).
The quality of the isolated DNA was assessed in terms of its contamination with proteins (A260/A280), and chaotropic salt and organic solvent value (A260/A230) using the spectrophotometer. The optimal A260/A280 ratio value (between 1.8 and 2.0) of DNA obtained by rapid method and classic method were presented 100% (ranging between 1.87 and 1.95) and 88.9% (ranging between 1.90 and 2.02), respectively. The A260/A230 value was in the optimal range (between 2.0 and 2.2) for 80.6% of the samples (83.3% by rapid method and 77.8% by classic method) (Figure 5).
FIGURE 5.
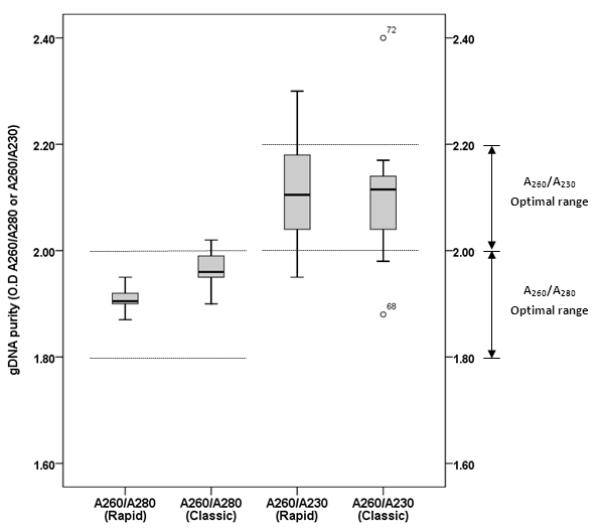
Analysis of the quality of the DNA extracted from archival human colon FFPE tissues by the Nanodrop spectrophotometer. The DNA quality was accessed by distribution of the ratio values (A260/A280 and A260/A230).
In addition to the spectrophotomic examine, we tested DNA obtained by the rapid method for array analysis suitability using the BioScoreTM Screening and amplification kit. Using a 100 ng DNA template prepared by the rapid method, we were able to amplify approximately 3.5 μg DNA of sufficient quality for nucleic acid array analysis. DNA obtained by the classic method and the QIAamp DNA FFPE tissue kit yielded approximately 3.3 μg and 3.7 μg DNA, respectively. These data suggest that DNA prepared by the rapid method has similar quality and quantity as compared to the classic DNA extraction method, with the advantage of high throughput fashion.
Application of MSP and bisulfite sequencing
sFRP1 and TFPI2 were selected for MSP analysis using 2 different genomic DNA extraction methods from FFPE samples. These genes have been reported to have high frequencies of methylation in colorectal cancer patients and are appropriate molecular biomarkers for methylation studies in CRCs [16; 17]. We then tested methylation of sFRP1 and TFPI2 genes in FFPE CRC tissues using rapid DNA extraction (Figure 6). They showed methylation in the majority of samples tested, which suggested that the rapid DNA extraction method could produce the same quality of data for methylation analysis as compared with previous studies [16; 18].
FIGURE 6.

MSP analysis of sFRP1 and TFPI2 genes in colon samples using the rapid and classic DNA extraction. Methylation pattern of (A) sFRP1 and (B) TFPI2 gene promoter region in 18 FFPE colon samples using rapid DNA extraction method. Colon FFPE tumor samples labeled 1 to 18 were extracted by a rapid method (R) and a classic phenol-chloroform method (C). For DNA samples extracted by classic method, we presented two representative samples (C10 and C11). Also, HCT 116 (colon cancer cell line) and DKO (DNMT1 and DNMT3b double knock out in HCT 116 cell) were extracted by classic phenol-chloroform method. PCR products recognizing unmethylated (U) and methylated (M) CpG sites were analyzed on 2% agarose gels visualized with GelStar nucleic acid gel stain (Cambrex Bio Science). IVD, in vitro methylated control; H2O, water control containing no DNA.
To confirm the methylation results, we took two (R1, C10) samples from the rapid and the classic DNA extraction and verified sFRP1 and TFPI2 methylation pattern by bisulfite sequencing (Figure 7). We confirmed that the two different DNA extraction methods did not show any difference in the 2 genes that we tested.
FIGURE 7.
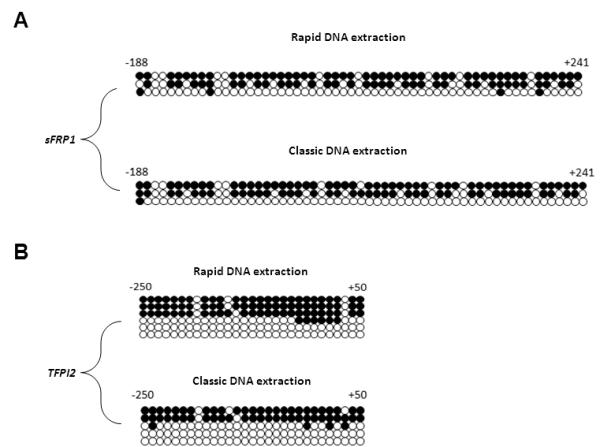
Bisulfite sequencing analysis of sFRP1 and TFPI2 genes in colon samples using rapid and classic DNA extraction methods. Methylation pattern of (A) sFRP1 (from −188 to +241 relative to transcriptional start site) and (B) TFPI2 (from −250 to +50 relative to transcriptional start site) gene promoter region in R1 and C10 FFPE CRC samples using the rapid and classic DNA extraction methods, respectively. Open circle (○) indicates unmethylated CpG residues and filled circle (●) indicates methylated CpG residues.
Discussion
Genomic DNA isolated from archived formalin fixed, paraffin embedded (FFPE) tissue potentially has important applications including new diagnostic assays, as well as retrospective genetic epidemiologic studies. FFPE tissues represent the most common tissue resource used for routine diagnosis as well as the largest source of archival biological material. Therefore it is very important to have a reliable DNA extraction method which yields DNA of high molecular weight and low level of fragmentation, as well as with high quality. The most commonly used procedure to isolate genomic DNA from archival FFPE specimens is based on deparaffinization in xylene, protein digestion, and followed by phenol-chloroform extraction; [3] however, the method is laborious and requires the use of organic solvents, in particular phenol which are carcinogens and require appropriate working environments as well as disposal protocols. Many studies show that fragmented DNA extracted from archival FFPE tissue, only allows PCR analysis of short amplicons, rarely exceeding 300 bp [4; 21; 22]. However, it is difficult to obtain consistent results because DNA extracted from the fixed tissue is not well preserved or is degraded. In addition, the extent of damage in DNA may depend on the type of fixative used and on the duration of fixation [1; 13; 23]. Many new methods have been developed and evaluated to address this challenge, with varying degrees of success. Subsequently, several commercial FFPE kits have introduced and applied a few studies which are tested on archival pathological tissue specimen [24]. However, using commercial FFPE kit is not practical and satisfies the goal of scientist in the advance of molecular techniques.
Previous some studies suggested that minimizing DNA manipulations may improve PCR efficiency [22; 25; 26]. In this context, we have established a rapid and reliable DNA extraction method for archival FFPE tissue specimens in this study. This approach is based on hot non-xylene-based deparaffinization reagents and subsequently exposed to, by means of a pressure cooker, to dewaxed tissue, followed by salting out procedure of deproteinization. In the previous study, we have shown that a pressure-cooking based method has speed up the protein extraction process [27]. The entire procedure takes less than five hours. Our approach results in DNA yield 1.7 and 1.9 fold greater than the QIAamp DNA FFPE tissue kit for tissue core and tissue section, respectively. As shown in Figure 2A, the DNA yield using tissue core is significantly improved (approximately 122% to 132%) in comparison with the traditional recovery from a tissue slide. This finding suggests that the tissue core method may be practical for large scale applications with the advantage of efficiency and tissue conservation. The DNA extraction from tumor cells or normal cells contained FFPE tissue is usually employed scalpel microdissection of tissue or laser capture microdissection for very small specimens. We have applied this extraction method to microdissected specimens and obtained comparable results (data not shown).
Our data demonstrate that DNA prepared by the rapid method has same quality and quantity as that of classic phenol-chloroform extraction method. As shown in Figure 4, there is strong correlation (R2=0.946) between rapid and classic methods with respect to DNA yield. In addition, both methods resulted in high quality DNA (Figure 5). Although the fragment length of DNA is shorter by this rapid method (Figure 2B), the quality is sufficient for downstream molecular analysis. The shorter DNA fragment length may be a consequence of the high energy approach to relieve crosslinks. It is sufficient for next-generation sequencing which provides insight to genome-wide genetic marker and genotyping with high throughput platform [28]. The evaluation of the DNA isolated in subsequent applications such as methylation-specific PCR and bisulfite sequencing reactions confirmed the potential of the protocol presented.
DNA methylation analyses in patient tumor samples are routinely applied to small specimens, and high quality is essential. Bisulfite conversion for methylation analysis mostly depends on quality of genomic DNA. Here, our data suggested that Rapid method with its higher yield and equal quality of DNA will enable methylation analysis and of small lesions than currently feasible. Further study should be extended to test simplified extraction approach for genome-wide methylation analysis.
In conclusion, FFPE tissues processed with the protocol herein described yield high-quantity and high-quality genomic DNA. Using this rapid DNA extraction method provides greater yield than commercially available kits, while at the same time provide equal quality of DNA to traditional phenol-chloroform based extractions. Our results suggest that this new DNA extraction method is simple, and may be able to provide a high yield of DNA fragments that can be applied to methylation-specific PCR and bisulfite sequencing reactions. The method is more effective and sensitive than ordinary organic chemical reagent-based extraction and commercial spin-column based-extraction kit of formalin-fixed tissue samples. As a fundamental molecular tool, our methodology can be used to study epigenetic alterations such as generalized hypomethylation, gene-specific methylation changes in CpG islands of gene promoter regions, and histone modifications.
Acknowledgments
We thank Kris Ylaya for technical assistance.
Abbreviations used
- CRC
colorectal cancer
- FFPE
formalin-fixed and paraffin-embedded
- HGIN
high-grade intraepithelial neoplasia
- LGIN
low-grade intraepithelial neoplasia
- MSP
methylation specific PCR
- PCR
polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, Goralski TJ, Langmore JP, Raja RG, Williams PM, Palma JF, Warrington JA. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2008;132:1929–35. doi: 10.5858/132.12.1929. [DOI] [PubMed] [Google Scholar]
- [2].Feldman MY. Reactions of nucleic acids and nucleoproteins with formaldehyde. Prog Nucleic Acid Res Mol Biol. 1973;13:1–49. doi: 10.1016/s0079-6603(08)60099-9. [DOI] [PubMed] [Google Scholar]
- [3].Gilbert MT, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewell LD, Van Marck E, Worobey M. The isolation of nucleic acids from fixed, paraffin-embedded tissues-which methods are useful when? PLoS One. 2007;2:e537. doi: 10.1371/journal.pone.0000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pavelic J, Gall-Troselj K, Bosnar MH, Kardum MM, Pavelic K. PCR amplification of DNA from archival specimens. A methodological approach. Neoplasma. 1996;43:75–81. [PubMed] [Google Scholar]
- [5].Largey JS, Meltzer SJ, Yin J, Norris K, Sauk JJ, Archibald DW. Loss of heterozygosity of p53 in oral cancers demonstrated by the polymerase chain reaction. Cancer. 1993;71:1933–7. doi: 10.1002/1097-0142(19930315)71:6<1933::aid-cncr2820710602>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [6].Lewis F, Maughan NJ, Smith V, Hillan K, Quirke P. Unlocking the archive--gene expression in paraffin-embedded tissue. J Pathol. 2001;195:66–71. doi: 10.1002/1096-9896(200109)195:1<66::AID-PATH921>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [7].Poljak M, Seme K, Gale N. Rapid extraction of DNA from archival clinical specimens: our experiences. Pflugers Arch. 2000;439:R42–4. doi: 10.1007/s004240000085. [DOI] [PubMed] [Google Scholar]
- [8].Wang LT, Smith A, Iacopetta B, Wood DJ, Papadimitriou JM, Zheng MH. Nested PCR-SSCP assay for the detection of p53 mutations in paraffin wax embedded bone tumours: improvement of sensitivity and fidelity. Clin Mol Pathol. 1996;49:M176–8. doi: 10.1136/mp.49.3.m176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–7. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- [10].Laird PW. The power and the promise of DNA methylation markers. Nat Rev Cancer. 2003;3:253–66. doi: 10.1038/nrc1045. [DOI] [PubMed] [Google Scholar]
- [11].Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–54. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- [12].Clark SJ, Statham A, Stirzaker C, Molloy PL, Frommer M. DNA methylation: bisulphite modification and analysis. Nat Protoc. 2006;1:2353–64. doi: 10.1038/nprot.2006.324. [DOI] [PubMed] [Google Scholar]
- [13].Fraga MF, Esteller M. DNA methylation: a profile of methods and applications. Biotechniques. 2002;33:632, 634, 636–49. doi: 10.2144/02333rv01. [DOI] [PubMed] [Google Scholar]
- [14].Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schuebel KE, Chen W, Cope L, Glockner SC, Suzuki H, Yi JM, Chan TA, Van Neste L, Van Criekinge W, van den Bosch S, van Engeland M, Ting AH, Jair K, Yu W, Toyota M, Imai K, Ahuja N, Herman JG, Baylin SB. Comparing the DNA hypermethylome with gene mutations in human colorectal cancer. PLoS Genet. 2007;3:1709–23. doi: 10.1371/journal.pgen.0030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Glockner SC, Dhir M, Yi JM, McGarvey KE, Van Neste L, Louwagie J, Chan TA, Kleeberger W, de Bruine AP, Smits KM, Khalid-de Bakker CA, Jonkers DM, Stockbrugger RW, Meijer GA, Oort FA, Iacobuzio-Donahue C, Bierau K, Herman JG, Baylin SB, Van Engeland M, Schuebel KE, Ahuja N. Methylation of TFPI2 in stool DNA: a potential novel biomarker for the detection of colorectal cancer. Cancer Res. 2009;69:4691–9. doi: 10.1158/0008-5472.CAN-08-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Suzuki H, Gabrielson E, Chen W, Anbazhagan R, van Engeland M, Weijenberg MP, Herman JG, Baylin SB. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–9. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- [18].Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–22. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- [19].Greer CE, Wheeler CM, Manos MM. Sample preparation and PCR amplification from paraffin-embedded tissues. PCR Methods Appl. 1994;3:S113–22. doi: 10.1101/gr.3.6.s113. [DOI] [PubMed] [Google Scholar]
- [20].Chung JY, Braunschweig T, Hewitt SM. Optimization of recovery of RNA from formalin-fixed, paraffin-embedded tissue. Diagn Mol Pathol. 2006;15:229–36. doi: 10.1097/01.pdm.0000213468.91139.2d. [DOI] [PubMed] [Google Scholar]
- [21].Bonin S, Petrera F, Rosai J, Stanta G. DNA and RNA obtained from Bouin’s fixed tissues. J Clin Pathol. 2005;58:313–6. doi: 10.1136/jcp.2004.016477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shi SR, Datar R, Liu C, Wu L, Zhang Z, Cote RJ, Taylor CR. DNA extraction from archival formalin-fixed, paraffin-embedded tissues: heat-induced retrieval in alkaline solution. Histochem Cell Biol. 2004;122:211–8. doi: 10.1007/s00418-004-0693-x. [DOI] [PubMed] [Google Scholar]
- [23].Wu L, Patten N, Yamashiro CT, Chui B. Extraction and amplification of DNA from formalin-fixed, paraffin-embedded tissues. Appl Immunohistochem Mol Morphol. 2002;10:269–74. doi: 10.1097/00129039-200209000-00015. [DOI] [PubMed] [Google Scholar]
- [24].Okello JB, Zurek J, Devault AM, Kuch M, Okwi AL, Sewankambo NK, Bimenya GS, Poinar D, Poinar HN. Comparison of methods in the recovery of nucleic acids from archival formalin-fixed paraffin-embedded autopsy tissues. Anal Biochem. 2010;400:110–7. doi: 10.1016/j.ab.2010.01.014. [DOI] [PubMed] [Google Scholar]
- [25].Bielawski K, Zaczek A, Lisowska U, Dybikowska A, Kowalska A, Falkiewicz B. The suitability of DNA extracted from formalin-fixed, paraffin-embedded tissues for double differential polymerase chain reaction analysis. Int J Mol Med. 2001;8:573–8. doi: 10.3892/ijmm.8.5.573. [DOI] [PubMed] [Google Scholar]
- [26].Sato Y, Sugie R, Tsuchiya B, Kameya T, Natori M, Mukai K. Comparison of the DNA extraction methods for polymerase chain reaction amplification from formalin-fixed and paraffin-embedded tissues. Diagn Mol Pathol. 2001;10:265–71. doi: 10.1097/00019606-200112000-00009. [DOI] [PubMed] [Google Scholar]
- [27].Chung JY, Lee SJ, Kris Y, Braunschweig T, Traicoff JL, Hewitt SM. A well-based reverse-phase protein array applicable to extracts from formalin-fixed paraffin-embedded tissue. Proteomics Clin Appl. 2008;2:1539–47. doi: 10.1002/prca.200800005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]


