Circulating tumor cells (CTCs) can be collected from peripheral blood and may provide pharmacodynamic information to aid therapeutic decision making.1 Furthermore, CTCs as a “virtual and real-time biopsy” have clear potential to facilitate exploration of the same prognostic or predictive biomarkers as those found in the primary tumor.2,3
The multitarget antifolate agent pemetrexed (PMX) currently administered to patients with nonsquamous non-small cell lung cancer (NSCLC). PMX primarily targets thymidylate synthase (TS), and TS is currently being investigated as a predictive biomarker for PMX-based chemotherapy.4,5
In November 2010, a 50-year-old woman was diagnosed with metastatic adenocarcinoma of the right lower lobe (cT4 cN3 cM1b, epidermal growth receptor factor wild type). First-line treatment with cisplatin and PMX was scheduled for December 3, 2010. Before administration, we collected 20 ml of peripheral venous blood to isolate CTCs. Mononuclear cells including CTCs were enriched using modified buoyant density gradient centrifugation method in Percoll PLUS solution (GE Healthcare GmbH, Munich, Germany). Hematopoietic cells were negatively selected from the sample using immunomagnetic beads with anti-CD15 and anti-CD456 monoclonal antibodies. Epithelial tumor cells were enriched using magnetic beads (Dynabead Epithelial Enrich, Invitrogen GmbH, Darmstadt, Germany) coated with the monoclonal antibody BerEP41 against the human epithelial antigen EpCAM.6 The resulting CTC-enriched cell population was dissolved in ThinPrep CytoLyt solution and was centrifuged at 150g for 10 minutes. The sediment was dissociated in fixating solution consisting of 50% ethanol and 50% phosphate-buffered saline-buffered formalin. Cells were fixed for a minimum of 20 minutes before being mounted on a ThinPrep slide.
TS protein expression was evaluated using the BenchMark XT autostainer (Ventana Medical Systems, Inc., Tucson, AZ). After pretreatment, CTC containing slides and 4 μm sections from the formalin-fixed paraffin-embedded primary tumor were incubated for 1 hour at 25°C with a mouse monoclonal anti-TS antibody (clone 4H4B1, Invitrogen, Carlsbad, CA) and counterstained with hematoxylin for 4 minutes. Negative control was obtained by using a nonimmunized ready-to-use mouse antibody as the primary antibody. Tissue section of colorectal carcinoma served as positive control.
Microscopic examination revealed several CTCs with varying TS expression ranging from weak to strong (Figures 1A–C). For estimation of the staining intensities, we compared the stained CTCs with TS-stained primary tumors (Fig. 2). In addition, a strongly TS-positive CTC adjacent immune magnetic beads was detected, whose shape is similar to a dividing tumor cell in the late prophase stage of mitosis (Figure 1A). This dividing CTC presents chromosome maturation with condensed chromosomes, which has rarely been observed in CTCs. To the best of our knowledge, this is the first report of circulating NSCLC cells in the peripheral blood, most of which stained strongly for TS.
FIGURE 1.
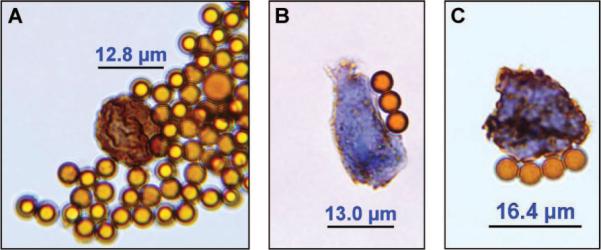
Circulating tumor cells (CTCs) isolated from the peripheral blood of a patient suffering from an adenocarcinoma of the lung stained with an antibody against thymidylate synthase (TS). A, Strongly TS expressing CTC which resembles a proliferating CTC in prophase of mitosis and displays chromosome maturation with condensed chromosomes. B, Weakly TS expressing CTC. C, Strongly TS expressing CTC. Magnification 1:1000 (using an oil immersion objective). The shown immunomagnetic beads have a diameter of 4.5 μm.
FIGURE 2.
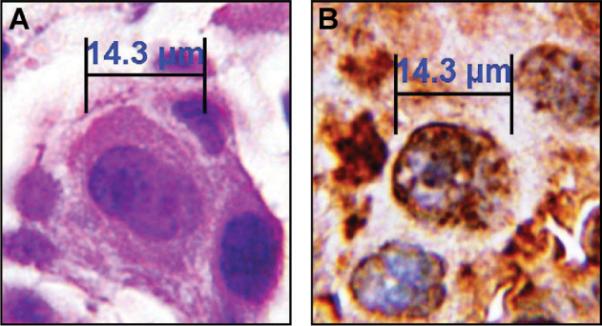
Adenocarcinoma cells of the lung from the same patient stained with hematoxylin/eosin (HE) or an antibody against thymidylate synthase (TS) on a 4-μm section of the formalin-fixed paraffin-embedded primary tumor. A, HE staining. B, TS staining. Magnification 1:1000 (using an oil immersion objective).
As high TS expression is associated with inferior clinical outcome to PMX-based therapy, we followed the clinical course of this patient. Until February, she received four cycles of combined chemotherapy with cisplatin and PMX resulting in a partial response per RECIST. Disease progression was diagnosed in multiple sites on May 9, 2011, which was 157 days after initiation of treatment and 70 days after the end of first-line treatment. The progression-free survival is compatible with the median progression-free survival reported in a large phase III trial of cisplatin and PMX.7 Despite receiving four additional lines of treatment (erlotinib, docetaxel, gemcitabine/vinorelbine, and mitomycin), the patient never achieved a second objective treatment response and showed disease progression notably under each line within 6 weeks. She was still alive on August 8, 2011.
In conclusion, our report established that immunocytochemical detection of biomarkers in CTCs from the peripheral blood of NSCLC patients is feasible, and CTCs acting as a surrogate for tumor biopsies hold promise for “real-time” monitoring of personalized systemic treatments for lung cancer.
ACKNOWLEDGMENTS
Supported by an IASLC Fellowship Award (to D.C.C). The authors acknowledge J. Eckelberger for critically reading the manuscript.
Disclosure: D. Christoph received travel support from Lilly Germany. T. Gauler received consulting fee or honorarium and travel support from Lilly Germany.
REFERENCES
- 1.Hou J-M, Krebs M, Ward T, et al. Circulating tumour cells, enumeration and beyond. Cancers. 2010;2:1236–1250. doi: 10.3390/cancers2021236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pestrin M, Bessi S, Galardi F, et al. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat. 2009;118:523–530. doi: 10.1007/s10549-009-0461-7. [DOI] [PubMed] [Google Scholar]
- 3.Marrinucci D, Bethel K, Luttgen M, et al. Circulating tumor cells from well-differentiated lung adenocarcinoma retain cytomorphologic features of primary tumor type. Arch Pathol Lab Med. 2009;133:1468–1471. doi: 10.1043/1543-2165-133.9.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CY, Chang YL, Shih JY, et al. Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer. 2011;74:132–138. doi: 10.1016/j.lungcan.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Sun J-M, Han J, Ahn JS. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. J Thorac Oncol. 2011;6:1392–1399. doi: 10.1097/JTO.0b013e3182208ea8. [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Yao F, Lou Y, et al. Detecting carcinoma cells in peripheral blood of patients with hepatocellular carcinoma by immunomagnetic beads and rt-PCR. J Clin Gastroenterol. 2011;41:783–788. doi: 10.1097/01.mcg.0000247996.19710.f2. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]


