Abstract
Background:
Female androgenetic alopecia (FAGA) is a frequent cause of hair loss in women. Standard diagnostic methods are clinical inspection, pull test, and trichogram. It has been suggested that scalp dermoscopy (trichoscopy) revealing diversity of hair shaft diameter >20% is diagnostic of FAGA.
Objective:
To evaluate the value of trichoscopy as compared to the trichogram for the diagnosis of FAGA.
Patients and Methods:
Retrospective case study of 162 women with the complaint of hair loss who underwent trichoscopic examination and trichograms.
Results:
Of all women diagnosed FAGA (55%), 62% were diagnosed by trichogram, 72% by trichoscopy with a cut-off point of 20%, and 100% irrespective of the degree of diversity of hair shaft diameter.
Conclusions:
Trichoscopy is a valuable and superior method to the trichogram for diagnosis of FAGA, especially in early cases, with the highest yield irrespective of the suggested cut-off of 20% diversity of hair shaft.
Keywords: Anisotrichosis, female pattern hair loss, trichogram, trichoscopy
INTRODUCTION
Androgenetic alopecia is a common cause of hair loss. It is a heritable, androgen-dependent condition that occurs in a defined pattern. It is assumed that the genetically predisposed hair follicles are the target for androgen-stimulated hair follicle miniaturization, leading to gradual replacement of large, pigmented terminal haairs by barely visible and depigmented vellus hairs in affected areas.[1] The result is a progressive decline in visible scalp hair density. While male androgenetic alopecia is characterized by its typical bitemporal recession of hair and balding vertex, female androgenetic alopecia (FAGA) is set apart by its more diffuse thinning of the crown area with an intact frontal hairline, usually beginning in women in their late 20s and affecting over 30% of women aged 70 years or more.[2]
Standard methods used to diagnose hair disorders are clinical inspection, pattern of hair loss, pull test, trichogram, biopsy, chronology of preceding events, and screening blood tests.[3] They vary in sensitivity, reproducibility, and invasiveness.
More recent studies have accumulated evidence that the use of dermoscopy of the hair and scalp (trichoscopy) in the clinical evaluation of hair disorders improves diagnostic capability beyond simple clinical inspection.[4–7] This method allows viewing of the hair and scalp at high magnifications. Since its introduction by A. Tosti for this purpose,[8] trichoscopy has gained popularity among dermatologists and a scientific basis in the evaluation of hair loss, owing to the advantages of being a quick and non-invasive, semi-quantitative method.
Trichoscopic features of FAGA are diversity of hair shaft diameter,[9] peripilar signs,[10] and empty follicles[11,12] [Figure 1], whereby it has been suggested that diversity of hair shaft diameter >20% is diagnostic of FAGA.[8,13] In analogy to anisocytosis in hematology, the term anisotrichosis has recently been proposed to describe diversity of hair shaft diameter.[14]
Figure 1.

Significant (>20%) diversity of hair shaft diameter (anisotrichosis). Note also peripilar signs (perifollicular halos) and empty follicles
It is the objective of this study to evaluate the value of trichoscopy as compared to the trichogram for the diagnosis of hair loss in women.
PATIENTS AND METHODS
Patients and diagnosis
Between June 2008 and October 2009, women who presented in the Hair Consultation of one of the authors (RMT) at the Department of Dermatology, University Hospital of Zurich, for assessment of hair loss underwent clinical examination, trichograms, and trichoscopy. As a standard, FAGA was diagnosed either clinically (Ludwig patterns I through III with or without pathologic trichogram) or on the basis of a pathologic trichogram with a frontal telogen rate >15% and a normal occipital telogen rate (<15%), diffuse telogen effluvium (TE) was diagnosed when the occipital telogen rate was above 15%. In the case of clinical diagnosis of FAGA occiptal telogen rate >15%, a diagnosis of FAGA in combination with TE was made.
Trichograms
For the trichogram, all patients had at least 50 hairs plucked from sampling sites on the frontal and occipital scalp, using a tightly closing epilation forceps. Uniformly, patients were instructed to leave the hair unwashed and untreated with any cosmetics for five days prior to the plucking of the hair for the trichogram. Immediately, after epilation the hair roots were embedded in Histokitt™ mounting medium which had previously been spread onto a glass slide for light microscopy. The hair roots were arranged neatly in a juxtaposition and covered with a coverslip. Quantification of the different hair roots on the basis of their morphological characteristics relating to the hair cycle was performed by the same investigator.
Trichoscopy
For trichoscopy, a simple dermatoscope (Heine Delta 20® or DermoGenius®) was used, with alcohol as the interface solution (Kodan® spray, Schulke and Mayr, Vienna, Austria), to assess the degree of anisotrichosis.
Evaluation of data
Clinical examination, trichogram, and trichoscopy data were collected from the patient records and reviewed.
RESULTS
A total of 162 women aged between 13 and 83 years (mean: 44.0 years) were examined [Figure 2]: Distribution of case counts by age).
Figure 2.
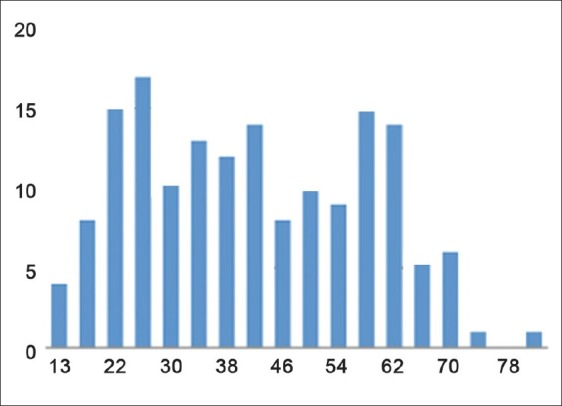
Distribution of case counts by age
The number of women with a diagnosis of FAGA was 89/162 (55%), TE 35/162 (22%), and a combination of FAGA and TE 36/162 (22%). In 2 (1%) women, there were no pathologic findings.
Depending on the diagnosis made on the basis of clinical inspection and the trichogramm, a distinction of five groups was made [Table 1]: Early FAGA, advanced FAGA, early and advanced FAGA in combination with TE, and TE. In each group, the percentage pathologic trichograms and trichoscopic findings were analyzed. With respect to the trichoscopic findings, three limits of anisotrichosis were defined: no anisotrichosis [Figure 3], any degree of anisotrichosis, and anisotrichosis >20% [Figure 1].
Table 1.
Pathologic trichogram and trichoscopic findings in relation to diagnosis
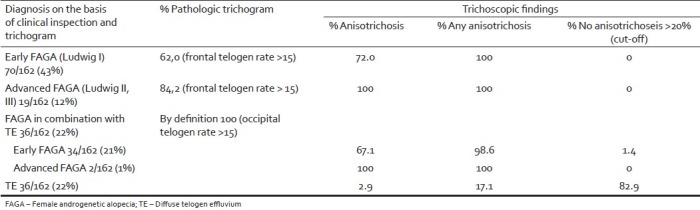
Figure 3.
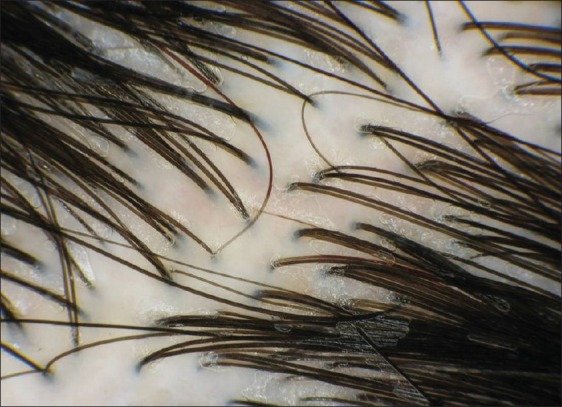
No diversity of hair shaft diameter
In early female pattern hair loss, the trichogram was pathologic in 62%, while trichoscopy revealed anisotrichosis >20% in 72%.
In advanced FPHL, the trichogram was pathologic in 84.2%, while trichoscopy revealed anisotrichosis >20% in 100%.
In TE, by definition, occipital telogen rates are >15%.
In early FPHL in combination with TE, trichoscopy revealed anisotrichosis >20% in 67.1%, and in advanced FPHL in combination with TE in 100%.
Irrespective of the cut-off of >20%, anisotrichosis was found in 100% of early and advanced FPHL and advanced FPHL in combination with TE, in 98.6% of early FPHL in combination with TE.
In TE, trichoscopy revealed anisotrichosis >20% in 2.9%, any degree of anisotrichosis in 17.1%, and no anisotrichosis in 82.9%.
DISCUSSION
FAGA is the result of a progressive shortening of the anagen phase of the hair growth cycle and miniaturization of the hair bulb, resulting in a telogen effluvium and terminal-to-vellus hair transformation in the affected scalp area.
Among the methods for diagnosis of FAGA, the trichogram reflects low-grade telogen effluvium of the affected frontal and centroparietal scalp, while the trichoscopic finding of anisotrichosis relates to the presence of hairs with different caliber as a consequence of progressive hair follicle miniaturization.
Originally it is has been suggested that diversity of hair shaft diameter >20% is diagnostic of FAGA.[8,13] More recently, more sophisticated diagnostic criteria of FAGA have been proposed based on trichoscopic imaging. Major criteria were: (1) ratio of more than four empty follicles in four images (at 70-fold magnification) in the frontal area, (2) lower average thickness in the frontal area compared to the occiput and (3) more than 10% of thin hairs (<0.03 mm in diameter) in the frontal area. Minor criteria were: (1) increased frontal to occipital ratio of single-hair pilosebaceous units, (2) vellus hairs, and (3) peripilar signs. Fulfillment of two major criteria or of one major and two minor criteria allow diagnosis FAGA with 98% specificity.[15]
It was the objective of this study to evaluate the value of trichoscopy in comparison to the trichogram in the diagnosis of FAGA.
The study results confirm that trichoscopy represents a valuable tool for the diagnosis of FAGA. In addition, the data demonstrate superiority of trichoscopy as compared to the trichogram, especially when applied in early cases of FAGA and irrespective of the suggested cut-off of 20% anisotrichosis.
In early FAGA, the trichogram yielded a diagnostic accuracy of 62.0% and trichoscopy with a cut-off of >20%, anisotrichosis 72%. Albeit statistically non-significant (P=0.205), this tendency to diagnostic superiority of trichoscopy over the trichogram is confirmed when anisotrichosis is considered diagnostic irrespective of the cut-off of 20%. In this case, anisotrichosis was found in 100% (P>0.001).
The respective findings in early FAGA in combination with TE were practically identically with 67.1% and 98.6% anisotrichosis with a cut-off of >20% and irrespective of the cut-off, respectively. An advantage of trichoscopy in the case of a combination of FAGA and CT is that a diagnosis of FAGA can be made, while the trichogram shows elevated telogen rates due to TE.
With advanced FAGA, alone or in combination with TE, anisotrichosis was found to be 100% with a cut-off of 20% or not, while the trichogram was diagnostic in 84.2% (P<0.001).
Finally, anisotrichosis was found in TE (as diagnosed by the criteria above) in 2.9% with a cut-off > 20% anisotrichosis and 17.1% irrespective of the cut-off of 20%. This may either reflect undiagnosed cases of FAGA, or lesser specificity of trichoscopy for the diagnosis of FAGA irrespective of the cut-off of 20% anisotrichosis.
CONCLUSIONS
In conclusion, trichoscopy not only represents a valuable tool for the evaluation of hair loss in women, but seems to also be superior to the trichogram, particularly in early FAGA. Finally, we challenge the suggested cut-off of 20% anisotrichosis for the diagnosis of FAGA, since the presence of anisotrichosis irrespective of the cut-off of 20% shows a higher sensitivity (100%), though a somewhat lesser specificity cannot be excluded. It is conceivable that in these cases, specificity may be increased using the extended diagnostic criteria proposed by Rakowska et al.,[15]
Ultimately, early recognition and treatment of FAGA is mandatory, since treatment is more effective at arresting progression of hair loss than stimulating regrowth of hair.[16]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–7. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 2.Norwood O. Incidence of female androgenetic alopecia (female pattern alopecia) Dermatol Surg. 2001;27:53–4. [PubMed] [Google Scholar]
- 3.Trüeb RM. Systematic approach to hair loss in women. J Dtsch Dermatol Ges. 2010;8:284–97. doi: 10.1111/j.1610-0387.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 4.Ross E, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- 5.Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. doi: 10.1001/archderm.144.8.1007. [DOI] [PubMed] [Google Scholar]
- 6.Tosti A, Whiting D, Iorizzo M, Pazzaglia M, Misciali C, Vincenzi C, et al. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol. 2008;59:64–7. doi: 10.1016/j.jaad.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Toncić R, Lipozencić J, Pastar Z. Videodermoscopy in the evaluation of hair and scalp disorders. Acta Dermatovenerol Croat. 2007;15:116–8. [PubMed] [Google Scholar]
- 8.Tosti A, Torres F. Dermoscopy in the diagnosis of hair and scalp disorders. Actas Dermosifiliogr. 2009;100(Suppl 1):114–9. doi: 10.1016/s0001-7310(09)73176-x. [DOI] [PubMed] [Google Scholar]
- 9.de Lacharrière O, Deloche C, Misciali C, Piraccini B, Vincenzi C, Bastien P, et al. Hair diameter diversity: A clinical sign reflecting the follicle miniaturization. Arch Dermatol. 2001;137:641–6. [PubMed] [Google Scholar]
- 10.Deloche C, de Lacharrière O, Misciali C, Piraccini B, Vincenzi C, Bastien P, et al. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res. 2004;295:422–8. doi: 10.1007/s00403-003-0447-y. [DOI] [PubMed] [Google Scholar]
- 11.Guarrera M, Vecchio F, Rebora A. Do hair density and thickness correlate with the hamilton scale? Arch Dermatol. 2002;138:1099. doi: 10.1001/archderm.138.8.1099. author reply 1099. [DOI] [PubMed] [Google Scholar]
- 12.Guarrera M, Rebora A. Kenogen in female androgenetic alopecia. A longitudinal study. Dermatology. 2005;210:18–20. doi: 10.1159/000081477. [DOI] [PubMed] [Google Scholar]
- 13.Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in asian people. J Dermatol. 2009;36:82–5. doi: 10.1111/j.1346-8138.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 14.Sewell L, Elston D, Dorion R. “Anisotrichosis”: A novel term to describe pattern alopecia. J Am Acad Dermatol. 2007;56:856. doi: 10.1016/j.jaad.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Dermoscopy in female androgenic alopecia: Method standardization and diagnostic criteria. Int J Trichol. 2009;1:123–30. doi: 10.4103/0974-7753.58555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinh Q, Sinclair R. Female pattern hair loss: Current treatment concepts. Clin Interv Aging. 2007;2:189–99. [PMC free article] [PubMed] [Google Scholar]


