Abstract
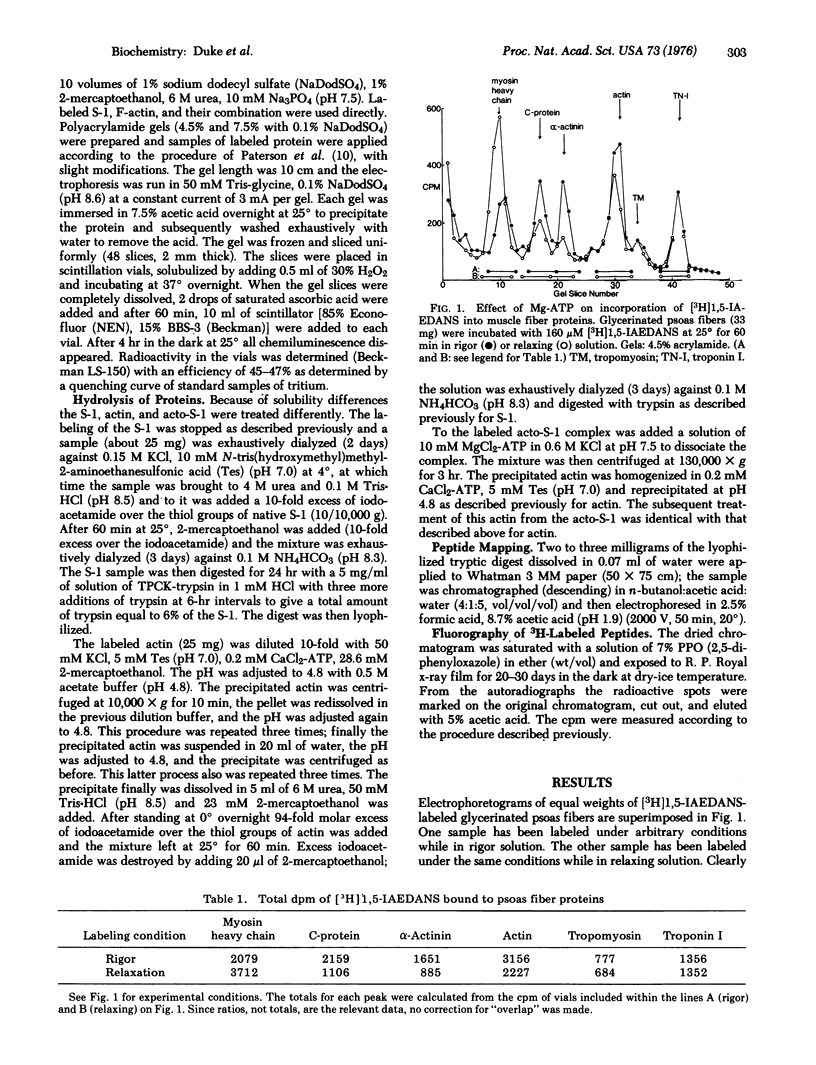
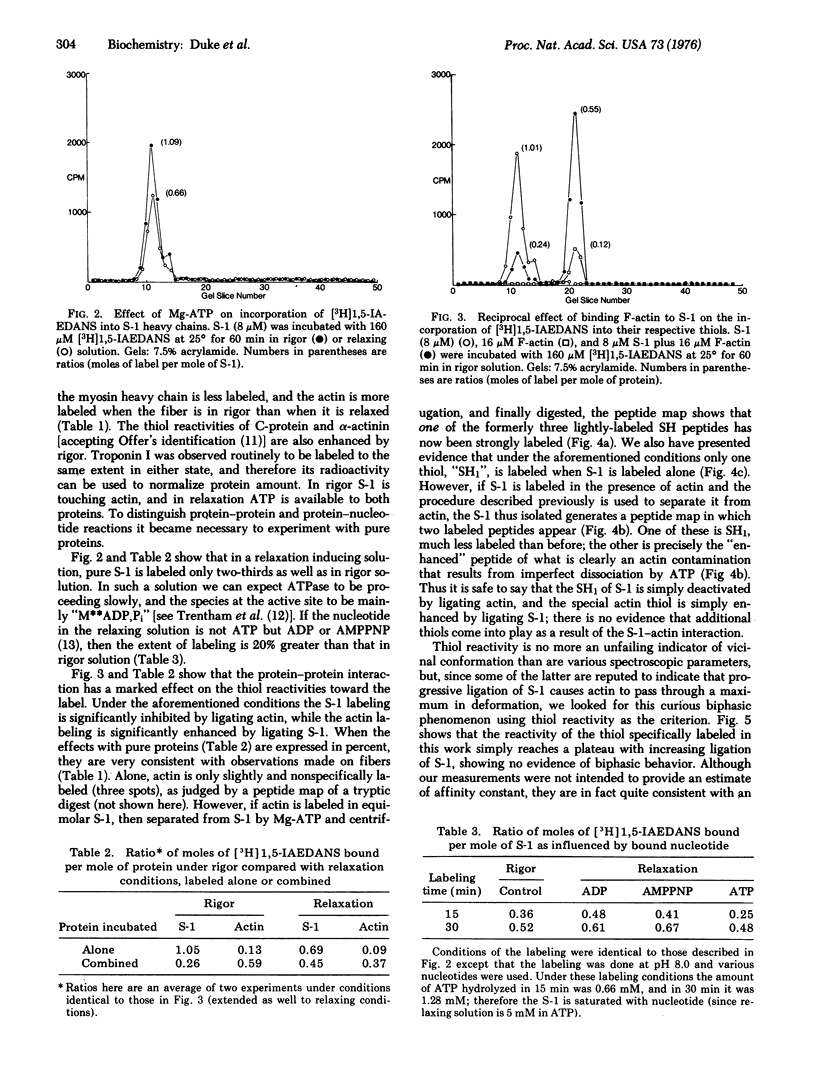
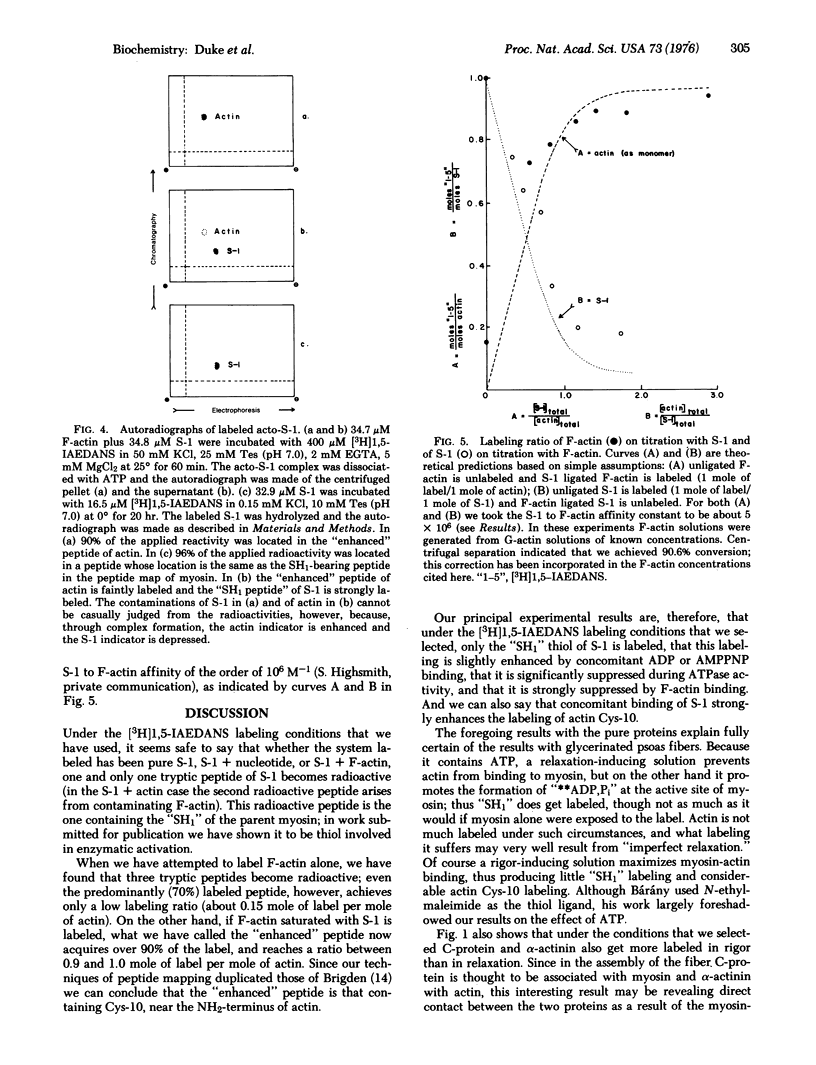
We report measurements of the reactivity (degree of labeling, as mole of ligand per mole of protein, at constant exposure time) of the reactive thiol, "SH1", of a subfragment of myosin (S-1), and of Cys-10 of F-actin under various conditions, using N-iodo-[3H]acetyl-N-(1-sulfo-5-naphthyl)ethylenediamine, a fluorescent radioactive iodoacetamide analog. When either ADP or adenyloyl imidodiphosphate (simulating unhydrolyzed ATP) is bound to the enzymatic site of S-1, the reactivity of "SH1" is slightly enhanced, but when active ATPase is going on, reactivity is reduced by about a third, presumably due to the species, (S-1) ADP,Pi. The reactivity of Cys-10 alone is very low. When the complex, (S-1)-F-actin, is formed, the reactivity of SH1 is strongly decreased, and the reactivity of Cys-10 is strongly increased. The foregoing results explain our further observation (on glycerol-treated rabbit psoas fibers) that when fibers labeled in relaxation solution are compared with fibers labeled in rigor solution, myosin is more reactive and actin is less reactive, in the former case; alpha-actinin and C-protein are also less reactive in the former case.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridgen J. The reactivity and function of thiol groups in trout actin. Biochem J. 1972 Jan;126(1):21–25. doi: 10.1042/bj1260021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Bárány K., Gaetjens E. Change in the reactivity of the head part of myosin during contraction of frog muscle. J Biol Chem. 1971 May 25;246(10):3241–3249. [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk T. W., Jr, Ue K. The measurement of actin concentration in solution: a comparison of methods. Anal Biochem. 1974 Nov;62(1):66–74. doi: 10.1016/0003-2697(74)90367-4. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Fasold H. Characterization of sulfhydryl groups of actin. Biochemistry. 1969 Jul;8(7):2933–2939. doi: 10.1021/bi00835a036. [DOI] [PubMed] [Google Scholar]
- Nihei T., Mendelson R. A., Botts J. Use of fluorescence polarization to observe changes in attitude of S-1 moieties in muscle fibers. Biophys J. 1974 Mar;14(3):236–242. doi: 10.1016/S0006-3495(74)85911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offer G., Moos C., Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and characterization. J Mol Biol. 1973 Mar 15;74(4):653–676. doi: 10.1016/0022-2836(73)90055-7. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin structure as revealed by simultaneous electrophoresis of heavy and light subunits. Biochemistry. 1970 Oct 13;9(21):4094–4105. doi: 10.1021/bi00823a010. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Tonomura Y., Appel P., Morales M. On the molecular weight of myosin. II. Biochemistry. 1966 Feb;5(2):515–521. doi: 10.1021/bi00866a017. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Bardsley R. G., Eccleston J. F., Weeds A. G. Elementary processes of the magnesium ion-dependent adenosine triphosphatase activity of heavy meromyosin. A transient kinetic approach to the study of kinases and adenosine triphosphatases and a colorimetric inorganic phosphate assay in situ. Biochem J. 1972 Feb;126(3):635–644. doi: 10.1042/bj1260635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. ON THE STRUCTURAL ASSEMBLY OF THE POLYPEPTIDE CHAINS OF HEAVY MEROMYOSIN. J Biol Chem. 1965 Jun;240:2428–2436. [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]



