Abstract
Context:
Posterior inferior cerebellar artery (PICA) aneurysms are associated with multiple anatomical variations of the parent vessel. Complexities in their surgical clipping relate to narrow corridors limited by brain-stem, petrous-occipital bones, and multiple neurovascular structures occupying the cerebellomedullary and cerebellopontine cisterns.
Aims:
The present study focuses on surgical considerations during clipping of saccular PICA aneurysms.
Setting and Design:
Tertiary care, retrospective study.
Materials and Methods:
In 20 patients with PICA aneurysms, CT angiogram/digital substraction angiogram was used to correlate the site and anatomical variations of aneurysms located on different segments of PICA with the approach selected, the difficulties encountered and the final outcome.
Statistical Analysis:
Comparison of means and percentages.
Results:
Aneurysms were located on PICA at: vertebral artery/basilar artery (VA/BA)-PICA (n=5); anterior medullary (n=4); lateral medullary (n=3); tonsillomedullary (n=4); and, telovelotonsillar (n=4) segments. The Hunt and Hess grade distribution was I in 15; II in 2; and, III in 3 patients (mean ictus-surgery interval: 23.5 days; range: 3-150 days). Eight patients had hydrocephalus. Anatomical variations included giant, thrombosed aneurysms; 2 PICA aneurysms proximal to an arteriovenous malformation; bilobed or multiple aneurysms; low PICA situated at the foramen magnum with a hypoplastic VA; and fenestrated PICA. The approaches included a retromastoid suboccipital craniectomy (n=9); midline suboccipital craniectomy (n=6); and far-lateral approach (n=5). At a follow-up (range 6 months-2.5 years), 13 patients had no deficits (modified Rankin score (mRS) 0); 2 were symptomatic with no significant disability (mRS1); 1 had mild disability (mRS2); 1 had moderately severe disability (mRS4); and 3 died (mRS6). Three mortalities were caused by vasospasm (2) and, rupture of unclipped second VA-BA junctional aneurysm (1).
Conclusions:
PICA aneurysms may present with only IVth ventricular blood without subarachnoid hemorrhage. PICA may have multiple anomalies and its aneurysms may be missed on CT angiograms. Surgical approach is influenced by VA-BA tortuosity and variations in anatomy, location of the VA-BA junction and the PICA aneurysm relative to the brain-stem, and the pattern of collateral supply. The special category of VA-PICA junctional aneurysms and its management; and, the multiple anatomical variations of PICA aneurysms, merit special surgical considerations and have been highlighted in this study.
Keywords: Anatomical variations, aneurysm, basilar artery, far lateral approach, posterior inferior cerebellar artery, radiology, subarachnoid hemorrhage, suboccipital craniectomy, surgery
Introduction
Vertebral artery- posterior inferior cerebellar artery (VA-PICA) aneurysms constitute 0.5-3% of all intracranial aneurysms. [1–3] Surgery for these aneurysms is extremely challenging. PICA has the most complex and variable course among all arteries of the posterior circulation. The complexities involved in the surgical approach to the PICA aneurysms relate to the narrow corridor limited by the brain-stem, petrous-occipital bones, and multiple neurovascular structures occupying the cerebellomedullary and cerebellopontine cisterns.[4] Moreover, an anomalous PICA is reported in 4-16% patients.[2,5]
PICA originates from the intracranial portion of VA in 80-95% patients, approximately 8.6 mm above the foramen magnum and 1cm proximal to the vertebrobasilar junction.[2,5] PICA aneurysms may take origin from one of its six segments and two loops (based on its relationship to the medulla oblongata and the cerebellum including: a) the BA-VA-PICA junction; b) the anterior medullary segment, from VA-PICA origin to the inferior olivary prominence; c) the lateral medullary segment, extending until the origin of IX-X-XIth cranial nerves; d) the tonsillomedullary segment, until the caudal portion of tonsils (including the caudal loop); e) the telovelotonsillar segment, from the midportion of its ascent along the medial surface of tonsil to the cortical cerebellar surface (including the cranial loop); and, f) the cortical segment, extending until the cerebellar vermis and hemisphere.[2,5–8]
We focus on the surgical management of saccular VA-PICA aneurysms with emphasis on the modifications in the approach based upon the location of aneurysms and variation in PICA, and also study the perioperative dilemmas encountered.
Materials and Methods
Patient spectrum
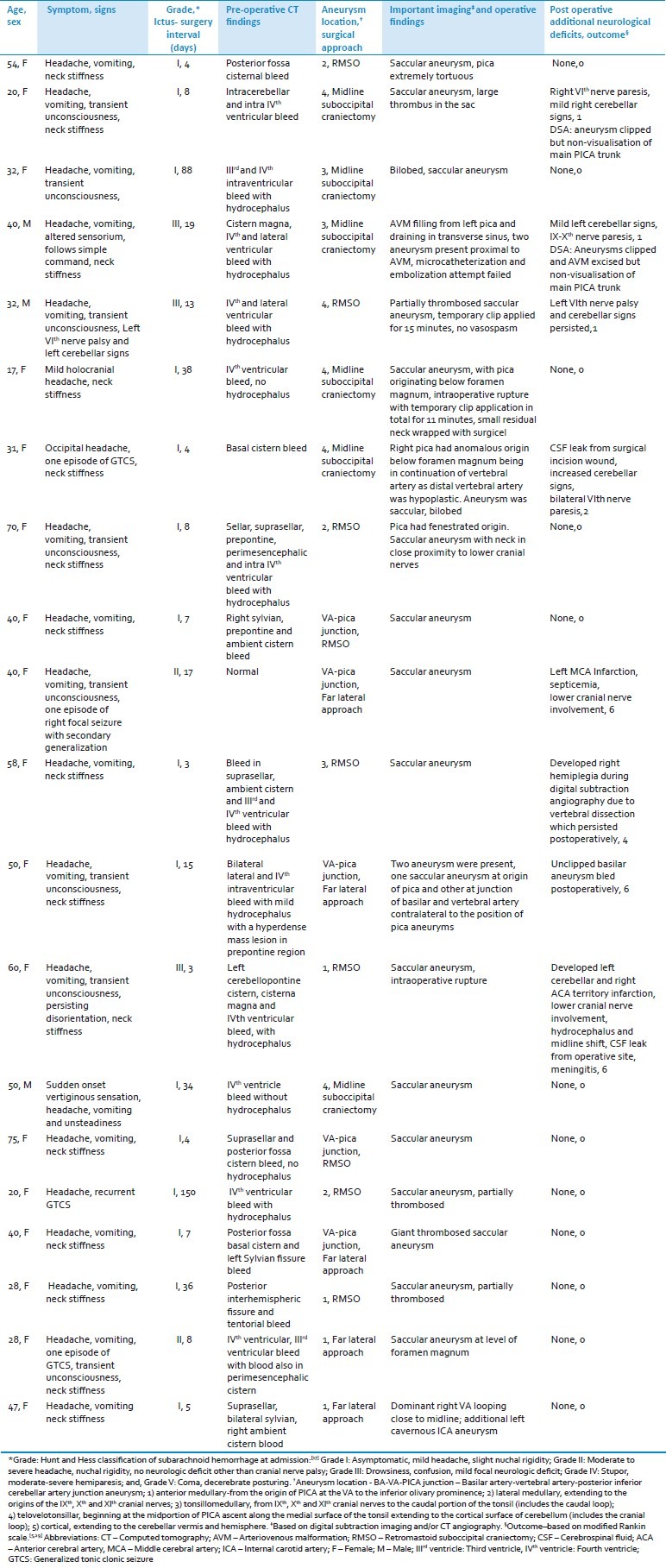
Twenty consecutive patients (mean age: 41.6 years, male: female ratio 3:17) admitted to Department of Neurosurgery and harboring ruptured PICA aneurysms underwent surgical management over an eight-year period. Their mean ictus-surgery interval was 23.5 days (range 3-150 days). Sudden onset severe holocranial headache was the presenting feature in 19 patients; 8 experienced transient loss of consciousness and 4 had associated seizures [generalized tonic clonic (n=2), focal with secondary generalization (n=1) and, tonic seizure without clonic movements (n=1)]. Neck stiffness was present in 15 patients. Their Hunt and Hess score (HandH) grade[9] at admission was I in 15; II in 2; and III in 3 patients[Table 1].
Table 1.
Spectrum of patients with PICA aneurysms
Radiographic features
All patients underwent a CT scan at the time of ictus [Table 2]. The site of hemorrhage was localized to posterior fossa cisterns in 5 (25%) patients; supra- and infra-tentorial cisterns in 6 (30%) patients; and in the cerebellar parenchyma, and in the interhemispheric/tentorial areas in one patient each, respectively. Third ventricular blood was seen in 2 (10%) patients; intra-IVth ventricular blood in 12 (60%) patients; and lateral ventricular blood in 3 (15%) patients. One patient (5%) had a normal CT scan. Among the 12 patients with intra-IVth ventricular hemorrhage, 6 each had purely ventricular or ventricular associated with cisternal hemorrhage, respectively. When IVth ventricular hemorrhage was not associated with cisternal hemorrhage, PICA aneurysms were usually located peripherally on the vessel (on lateral medullary (n=1), tonsillomedullary (n=1) and telovelotonsillar (n=4) segments, respectively) with blood entering the IVth ventricle either through the foramen of Luschka or Magendie. The PICA aneurysm was diagnosed on the basis of digital subtraction angiogram (DSA) in 16 patients (two of these also underwent an additional computed tomographic angiogram (CTA)); and only on CTA/MRA images in 4 patients. The location of PICA aneurysms was as follows: VA-BA-PICA junction (n=5); anterior medullary segment (n=4); lateral medullary segment (n=3); tonsillomedullary segment (n=4); and telovelotonsillar segment (n=4). There was no aneurysm located on the cortical segment of PICA. Notable findings on angiograms were large variations in the course of VA-PICA and the existence of numerous vascular anomalies [Table 3]. These included fenestrated or tortuous PICA; brain-stem compression by a giant PICA aneurysm [Figures 1 and 2]; two PICA aneurysms on a common trunk proximal to an arteriovenous malformation (AVM); bilobed or multiple aneurysms; low PICA situated at or below foramen magnum with or without a hypoplastic VA [Figures 3–5]; and partially or completely thrombosed aneurysms. In two patients, the preoperative CTA was unable to pick up the PICA aneurysm that was subsequently detected on DSA.
Table 2.
The radiological features seen on plain computed tomography at admission
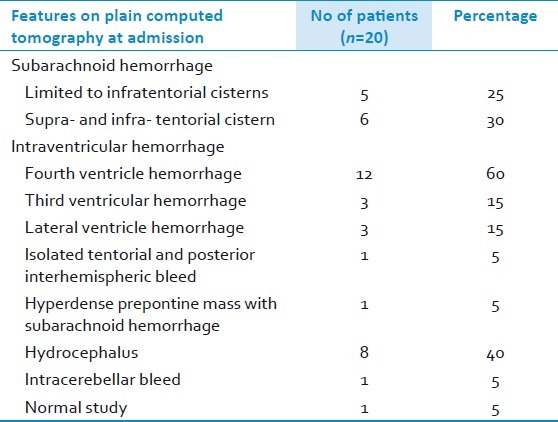
Table 3.
Radiological variations in PICA aneurysm and related anatomy
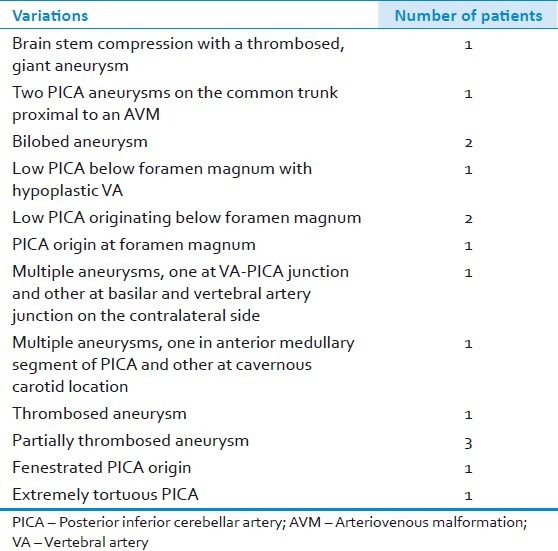
Figure 1.
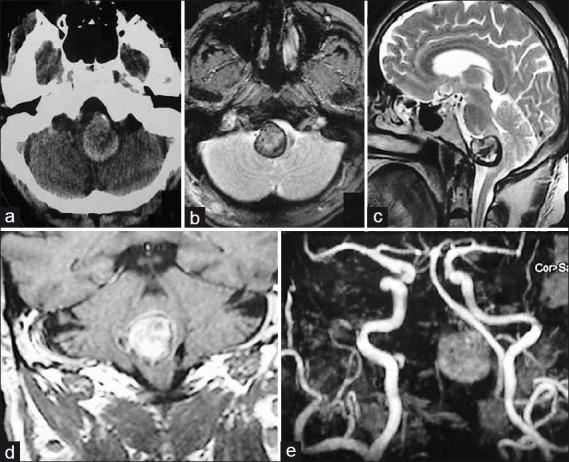
Patient 1– (a) Axial CT scan shows a giant, thrombosed PICA aneurysm causing brain-stem compression; (b) T2-axial and, (c) Sagittal MRI showing aneurysm surrounded by hemosiderin ring and having a laminated core; (d) Contrast coronal MR showing intimate relationship of aneurysm to brainstem; (e) MR angiogram showing the aneurysm lateral to midline
Figure 2.
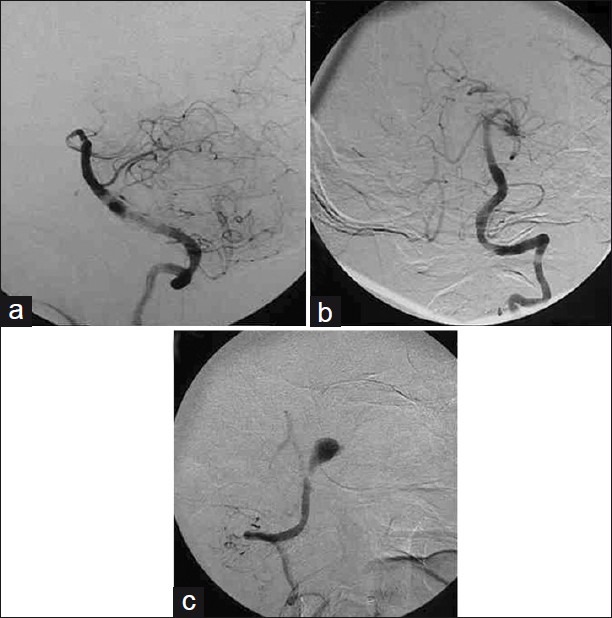
Patient 1 – Right vertebral angiogram (a) lateral; and, (b) anteroposterior view does not show any aneurysmal filling; (c) Left vertebral angiogram anteroposterior view shows partial filling of the BA-VA-PICA junction aneurysm
Figure 3.
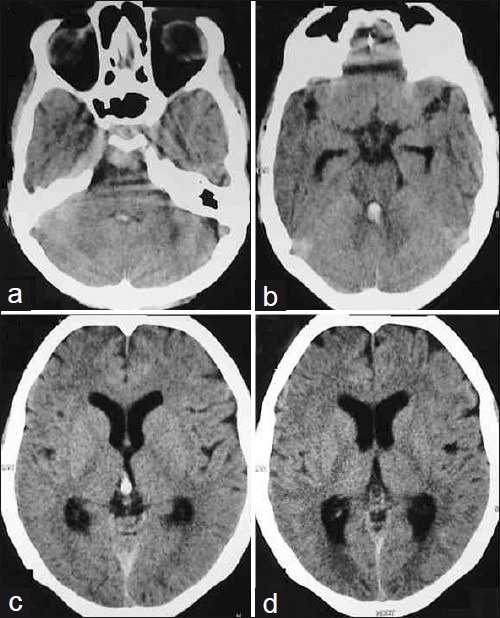
Patient 2 – (a-d) Plain axial CT scans showing SAH in the prepontine cistern with IVth and IIIrd ventricular blood
Figure 5.
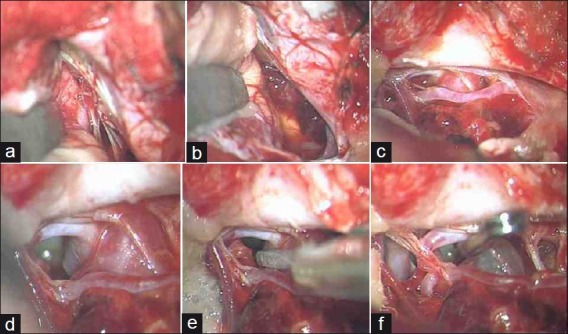
Patient 2 – Operative images showing (a) Retracted cerebellum exposing lower cranial nerves; (b) Clot in cerebellomedullary cistern; (c and d) PICA aneurysm from anteromedullary segment with draped lower cranial nerves; (e and f) Neck clipped sparing the origin of PICA
Figure 4.
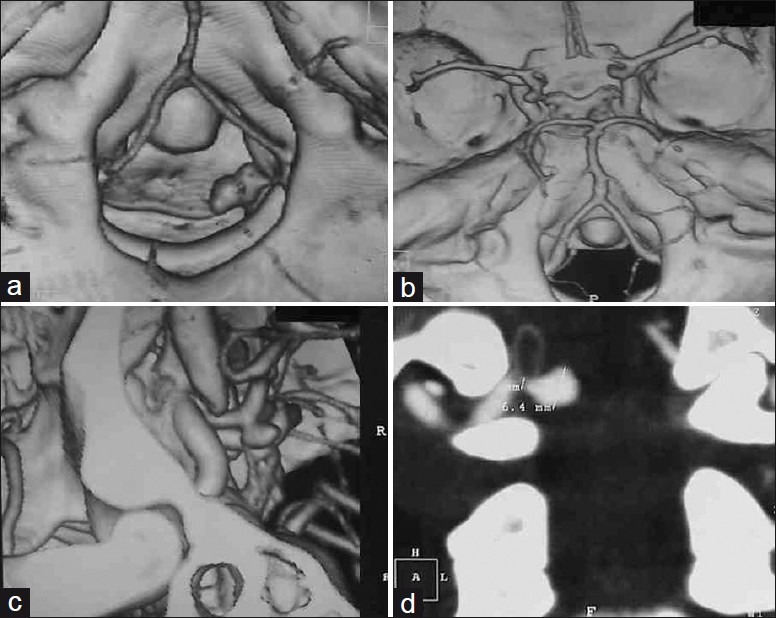
Patient 2 – (a and b) Axial; (c) Oblique; and, (d) Coronal CT angiogram showing anterior medullary PICA aneurysm at the level of foramen magnum. The aneurysm is situated laterally in cerebellomedullary cistern. PICA is arising dorsolateral to aneurysm
Surgical treatment
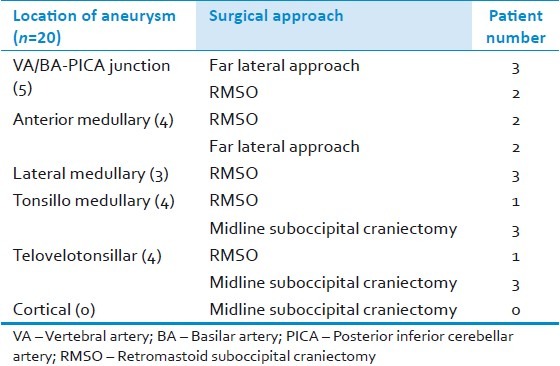
In patients who were admitted within 2 weeks of ictus, oral nimodipine and steroids (intravenous dexamethsone 4 mg 6 hourly) were instituted. All patients also received phenytoin (100 mg 6 hourly). Following angiographic confirmation of the aneurysm, surgery was performed usually as the first case the next day morning. The surgical approaches included a retromastoid suboccipital craniectomy (n=9; usually for aneurysms located lateral to brain stem); midline suboccipital craniectomy (n=6; for aneuryms of tonsillomedullary and telovelotonsilar segments); and, far-lateral approach (n=5; for aneurysms located close to midline anterior to the brain stem, on the anterior medullary segment [Figures 6 and 7] and BA-VA-PICA junctional aneurysms [Figures 8 and 9, Table 4]. The tortuosity of VA-BA complex also influenced the decision to use either a retromastoid suboccipital craniectomy or a far lateral approach while addressing PICA aneurysms of BA-VA-PICA junction (n=5) and anterior medullary segment (n=4). In these anteriorly situated aneurysms (that is, BA-VA-PICA as well as anterior medullary), a relatively straight segment of BA-VA in 5 patients favored utilization of the far lateral approach to access the aneurysm located close to the midline anterior to the brain stem; while the looping of the vessels toward the cerebellomedullary cistern made the aneurysm easily accessible using the conventional retromastoid approach in 4 other patients. Temporary clipping was utilized in two patients (patient 5 and 6). None of these patients had any additional neurological deficits in the postoperative period. Following surgery, nimodipine and hypertensive, hypervolemic, hemodilutional (triple H) therapy were continued for two weeks in 4 patients who were operated within a week of their ictus, had a transient neurological deterioration in the form of mild alteration in the level of consciousness immediately following surgery and whose preoperative angiogram had shown vasospasm. Prior to institution of the triple H therapy, they underwent a CT scan/MRI to rule out an infarct, hematoma, increased postoperative edema or hydrocephalus.
Figure 6.
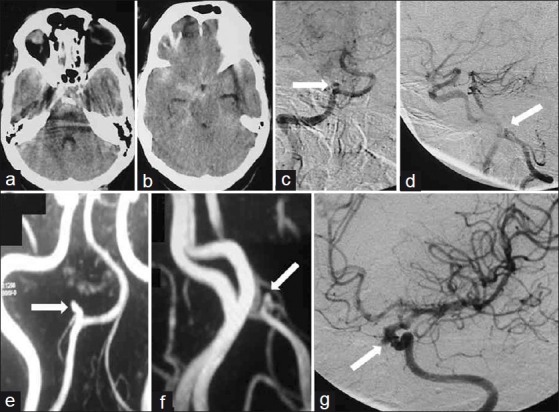
Patient 3 – (a and b) Axial CT scans showing SAH within suprasellar, sylvian, prepontine and ambient cisterns; (c) Anteroposterior; and (d) Lateral left vertebral angiogram showing PICA aneurysm near anatomical midline; (e and f) CTA shows dominant left VA harbouring aneurysm; (g) Oblique right carotid angiogram showing an additional cavernous ICA aneurysm
Figure 7.
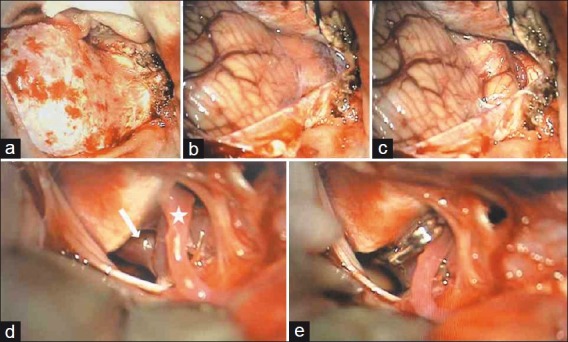
Patient 3 – Far lateral approach; (a) Dural exposure; (b) Exposed cerebellum and cistern magna; (c) Opening cistern magna reveals PICA below tonsil; (d) Cerebellar retraction exposes anterior medullary segment aneurysm anterior to lower cranial nerves and brain-stem with fundus attached to jugular tubercle; (e) Aneurysm clipped sparing PICA origin
Figure 8.
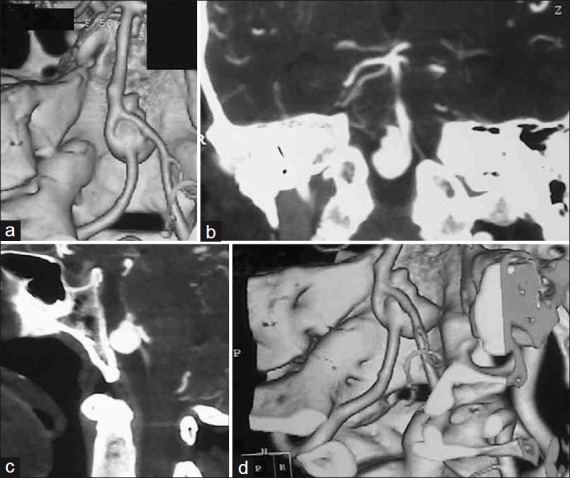
Patient 4 – (a) Three-dimensional reconstructed axial; (b) coronal; (c) sagittal; and (d) three dimensional reconstructed oblique CTA showing BA-VA-PICA aneurysm in a high, anterior location. Left VA-BA junction is incorporated in aneurysm wall. Right VA is draped over aneurysmal fundus
Figure 9.
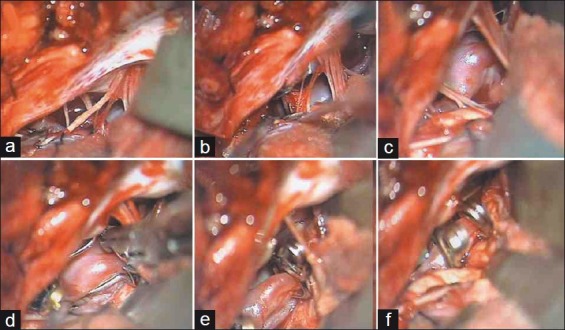
Patient 4 – Far lateral approach; (a) Cerebellar retraction exposes lower cranial nerves; (b) Aneurysm is anterior to lower cranial nerves; (c) Left VA trunk is incorporated in aneurysm neck; (d-f) Angulated fenestrated clip clips aneurysm and encloses proximal PICA within fenestration
Table 4.
Location of aneurysm on the PICA and the surgical approach adopted
Results
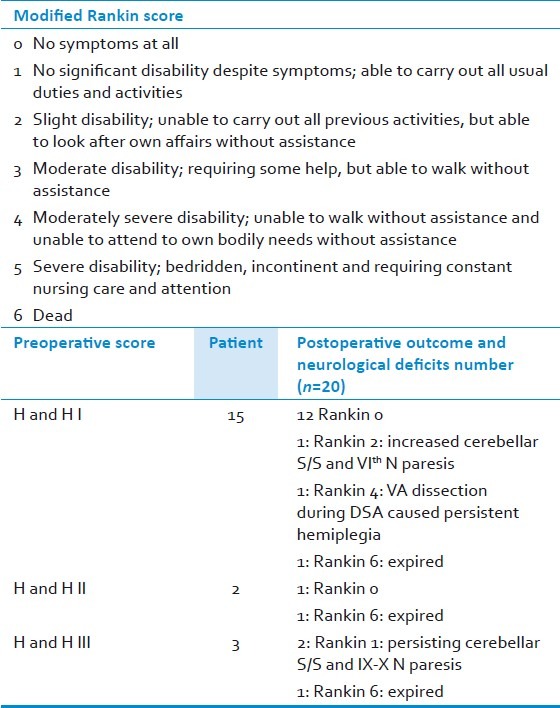
The patient outcome was assessed at discharge and at follow-up (range: 6 months-2.5 years) utilizing modified Rankin′s score (mRS) [Table 5].[10,11] Of the 15 patients in HandH grade I, 12 remained in mRS 0 (no symptoms); and one was in mRS 2 (mild disability, due to increased cerebellar signs in the form of appendicular ataxia and VIth nerve paresis). One patient in this group developed vertebral artery dissection with hemiplegia during the DSA. He had hemiparesis grade III at follow-up (mRS 4; moderately severe disability). Of the two patients in HandH grade II, one remained asymptomatic at follow-up (mRS 0). Among the three patients with HandH grade III, two improved to mRS 1 (no significant disability despite mild symptoms of cerebellar ataxia and occasional difficulty in swallowing at follow-up). Three patients (one each with preoperative modified HandH grade I, II, and III, respectively) expired in the perioperative period (mRS 6). A patient in HandH grade I had two aneurysms, a saccular aneurysm at the PICA origin and another at the BA-VA junction contralateral to the side of the PICA aneurysm. Following the uneventful clipping of the ruptured PICA aneurysm, this patient died suddenly in the intensive care unit probably because of rupture of the unclipped BA-VA aneurysm. The second patient with a VA-PICA junctional aneurysm and in preoperative HandH grade II developed patchy infarction in the MCA territory and increased lower cranial nerve paresis. He succumbed to aspiration pneumonitis and septicemia. Finally, a patient in HandH grade III had a VA-PICA junctional aneurysm and developed vasospasm leading to left cerebellar and right ACA territory infarction. He also developed cerebrospinal fluid leak through the operative wound and succumbed to meningitis. The postoperative DSA revealed non-visualization of the distal trunk of PICA in two patients, but fortunately without significant morbidity. In the first patient, a partially thrombosed aneurysm of the telovelotonsillar segment was successfully clipped and its luminal thrombus excised. In the second patient, an AVM was filling from the left PICA and draining into the transverse sinus. He also had two aneurysms present proximal to the AVM in the tonsillomedullary segment. Both the aneurysms were clipped and the AVM excised in the same setting.
Table 5.
Surgical outcome at discharge and follow up range of 6 months-2.5 years
Discussion
Pathogenesis
The size of saccular aneurysms of PICA that produced SAH ranged from 4mm to greater than 2.5cm. Some of them represented extremes of sizes of aneurysms that are usually not prone to bleeding at other locations. The complex and tortuous course of PICA with multiple acute angulations promoted increased luminal shear stress. This resulted in the development of aneurysms even where there were no visible bifurcation sites or branching vessels. Thus, these saccular aneurysms were on thinner and weaker vessel walls (commonly associated with fusiform and dissecting aneurysms) irrespective of whether the aneurysms were extremely small or giant.[5,12,13] They could, however, be adequately clipped (usually with parent vessel preservation) due to their saccular shape and a well defined neck. One of our patients had the unique presentation of two aneurysms on the parent vessel proximal to an arteriovenous malformation (AVM). This association has rarely been reported.[5,14–17] The risk of hemorrhage under these circumstances is high both from the AVM and the associated aneurysm.[5,14,15,17] Thus, they required simultaneous addressal during surgery in our patient with the aneurysms being treated before proceeding to the more distal AVM.[16,17]
Radiological features
Seventeen patients (85%) presented with intraventricular hemorrhage (IVH) that was associated nearly 50% of the time with cisternal blood. In the reported literature, the incidence of IVH due to PICA aneurysm rupture has ranged from 83-100%.[18–20] The high incidence of hydrocephalus in our patients (8 patients, 40%) was in all probability, consequent to the frequent presence of intra-ventricular bleed. The cisternal hemorrhage, when present, was not entirely confined to the posterior fossa, but often extended to the supratentorial cisterns. It resulted in severe cerebral ischemia due to anterior circulation vasospasm in two patients. These cases exemplify the fact that a ruptured PICA aneurysm cannot be discounted while encountering a patient with an extensive supratentorial subarachnoid hemorrhage (SAH).[19]
A unique subset of patients were those who had only IVth ventricular hemorrhage without any cisternal, cortical, or sulcal blood. This manifestation was due to seepage of blood through the foramen of Luschka or Magendie following aneurysmal rupture. These patients nearly always had distally located PICA aneurysms. One must be aware of this frequent association between a primary IVth ventricular hemorrhage and a distal PICA aneurysm in order not to miss these aneurysms that may rebleed later on if left uninvestigated and untreated.[5,19,21–23] In two of our patients, the CTAs missed a PICA aneurysm that was later detected on a DSA. Aneurysms of the posterior fossa may be missed on a CTA due to the beam hardening effect in the region.[24] An additional DSA is, therefore, mandatory before a PICA aneurysm may be unequivocally ruled out after a normal CTA. This is especially true when a high index of suspicion regarding the presence of a PICA aneurysm exists based upon the clinicoradiological manifestations. Numerous variations of PICA and its associated aneurysms were also evident in this series. Evaluation of the VA and the origin of PICA on both sides is useful in picking up additional aneurysms or anomalies in the vasculature on the side contralateral to the side of evident bleeding.[19,21,25] This is especially important when perimedullary cisternal hemorrhage, IVH, hydrocephalus or unilateral or bilateral VIth nerve paresis[21] are encountered in the clinical setting of SAH. These aneurysms may occasionally assume giant proportions mimicking a tumor or a large cavernous angioma. They may also thrombose spontaneously causing significant brain stem compression.[12,13,26,27] This was seen in one of our patients. The laminated appearance of the thrombus in various stages (the target sign) within the lumen of the lesion pointed towards the existence of an aneurysm rather than a tumor. An MRA and DSA further ruled out partial filling of this giant aneurysm. Removal of the clot, clipping of its neck and excision of its fundus relieved the brain stem distortion.
Surgical nuances
While planning the surgical trajectory, the location of the aneurysm based upon the segmental anatomy of PICA; its relationship to the anatomical midline (10 mm or less being considered significant), brainstem and cerebellum; and, the variability in the vessel′s origin should be carefully assessed.[23,28,29] The brain-stem and cranial nerves may be compromising the surgical view to these aneurysms. Moreover, one or more parent vessels at the VA-BA junctional region may be ending into or taking origin from the neck of this aneurysm. Application of clip may cause narrowing or twisting of these vessels leading to luminal compromise.
In VA-BA-PICA junctional and anterior medullary aneurysms situated close to midline anterior to the brain-stem, the far lateral or extreme lateral transcondylar approach may be utilized.[12,30–32] Removal of the posterior condyle,[32] C1 lateral mass,[12] and/or the jugular tubercle [33] help in widening the surgical corridor.[31] In those patients in whom far lateral approach was used, control of the proximal ipsilateral VA was obtained in its extradural portion in the suboccipital triangle after its emergence from C1 foramen transversarium. The VA has a fairly constant and well-localized position while traversing over the C1 posterior arch prior to its entry into the dura.[12,30] A tortuous VA with a high shoulder may be exposed from the contralateral “aneurysmal dome” side or by using the translabyrinthine approaches.[21,34]
PICA usually arises dorsal or lateral to the aneurysm; occasionally, however, it may be posterior or medial to its neck. The applied clip blades are usually pointing forwards, distal to VA-PICA junction parallel to the long axis of VA. Fenestrated clips may protect lower cranial nerves or the origin of PICA. Most of these anterior aneurysms are located ventral to lower cranial nerves.[21] While traversing the plane of the these nerves, an occasional patient may develop dysphagia, rhinolalia, hoarseness, nasal regurgitation or aspiration pneumonia related to their functional impairment even when the arachnoidal plane has been meticulously preserved.[1,35,36] The incidence of lower cranial nerve paresis following clipping of proximal PICA aneurysms has been reported to be varying between 10-45%;[1,35–37] in our series, it was seen in 3 (15%) patients. The related morbidity was fortunately self-limiting and usually improved in approximately three to six months. However, occasionally, lower cranial nerve paresis may lead to disastrous consequences particularly in moribund patients with poor preoperative HandH scores. The definite risk factors for the development of nosocomial pneumonia (that carries a mortality rate of 20-50%)[38] are low nutritional status, lower Glasgow Scale Score, vertebrobasilar stroke, and the need for mechanical ventilation.[35]
We concede that an endovascular intervention may have provided similar good results. These patients, when given an option between surgical clipping and endovascular approach chose the former due to the lesser expense of the surgical procedure. A complication rate of as high as 13% has been reported with embolization of PICA aneurysms.[39] As is well illustrated in this study, PICA often has extremely variable and tortuous course with multiple anomalies and variability in position of the aneurysm relative to the brain-stem.[28] Superselective catheterization of the PICA is often not feasible or may be associated with complications during the endovascular procedure as exemplified by our patient in whom vertebrobasilar dissection occurred during catheterization. Identification of the neck and selective catheterization may be difficult or impossible especially in peripheral aneurysms.[8] When aneurysms are small, the risk of abrupt “jumping” of the catheter or guide wire into the sac increases the chances of its rupture. Moreover, if the microcatheter tip does not conform favorably to the anatomy, the system may be very unstable and further coils may migrate or displace the catheter into the parent vessel.[40] It is apparent from this study that the mortality was related more to vasospasm as a result of subarachnoid hemorrhage rather than due to technical aspects of surgery. Thus, surgeons have a responsibility to be well-versed with the surgical approaches and nuances for clipping these aneurysms in case the endovascular treatment fails or is not possible; or, if salvage surgery is required for SAH occurring during the interim period between the endovascular coiling and the development of luminal thrombosis within the aneurysm.
Limitations
The medical personnel assessing outcome were not blinded to the initial clinicoradiological diagnosis and were participating in the care of the patients. The subjects included patients with saccular aneurysms amenable to surgical clipping; fusiform[5] or dissecting PICA aneurysms[41] do not form a part of the study. Trapping of PICA with occipital artery-PICA anastomosis and excision of aneurysm and end-to-end anastomosis[7,27,42–45] were not performed in any of the patients including the two in whom surgical clipping of the PICA aneurysm was associated with non-visualization of the parent blood vessel on postoperative DSA.
Conclusion
This series presents the surgical experience in dealing with both proximal and distal, ruptured, saccular PICA aneurysms based on the anatomical segments of PICA. The patients often presented late with the majority being in a good preoperative HandH grade; this may be a major factor in the usually gratifying postoperative outcome seen. The presence of SAH in the supratentorial cisterns does not rule out the existence of a PICA aneurysm. Unilateral or bilateral VIth nerve palsy, intraventricular hemorrhage and hydrocephalus in the clinical setting of SAH mandates a four-vessel (rather than the conventional three-vessel) angiogram to assess the anatomy of VA-PICA complex on both sides especially since PICA had significant variations and is associated with numerous anomalies. VA-PICA junctional aneurysms require special surgical considerations based upon tortuosity of the main vessels and distance from the midline.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hudgins RJ, Day AL, Quisling RG, Rhoton AL, Jr, Sypert GW, Garcia-Bengochea F. Aneuryms of the posterior inferior cerebellar artery.A clinical and anatomical analysis. J Neurosurg. 1983;58:381–7. doi: 10.3171/jns.1983.58.3.0381. [DOI] [PubMed] [Google Scholar]
- 2.Lister JR, Rhoton AL, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170–99. [PubMed] [Google Scholar]
- 3.Weir B. Baltimore: Williams and Wilkins; 1987. Aneurysms affecting the nervous system; pp. 489–91. [Google Scholar]
- 4.Rodríguez-Hernández A, Lawton MT. Anatomical triangles defining surgical routes to posterior inferior cerebellar artery aneurysms. J Neurosurg. 2011;114:1088–94. doi: 10.3171/2010.8.JNS10759. [DOI] [PubMed] [Google Scholar]
- 5.Lewis SB, Chang DJ, Peace DA, Lafrentz PJ, Day AL. Distal posterior inferior cerebellar artery aneurysms: Clinical features and management. J Neurosurg. 2002;97:756–66. doi: 10.3171/jns.2002.97.4.0756. [DOI] [PubMed] [Google Scholar]
- 6.Drake CG. The treatment of aneurysms of the posterior circulation. Clin Neurosurg. 1979;26:96–144. doi: 10.1093/neurosurgery/26.cn_suppl_1.96. [DOI] [PubMed] [Google Scholar]
- 7.Krayenbuhl N, Guerrero C, Krisht AF. Technical strategies to approach aneurysms of the vertebral and posterior inferior cerebellar arteries. Neurosurg Focus. 2005:19–E4. doi: 10.3171/foc.2005.19.2.5. [DOI] [PubMed] [Google Scholar]
- 8.Margolis MT, Newton T. Borderlands of the normal and abnormal posterior inferior cerebellar artery. Acta Diagn (Stockh) 1972;13:163–76. doi: 10.1177/02841851720130p122. [DOI] [PubMed] [Google Scholar]
- 9.Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 10.Bonita R, Beaglehole R. Modification of Rankin Scale: Recovery of motor function after stroke. Stroke. 1988;19:1497–500. doi: 10.1161/01.str.19.12.1497. [DOI] [PubMed] [Google Scholar]
- 11.Rankin J. Cerebral vascular accidents in patients over the age of 60. Scott Med J. 1957;2:200–15. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 12.Heros RC. Lateral suboccipital approach for vertebral and vertebro-basilar artery aneurysms. J Neurosurg. 1986;64:559–62. doi: 10.3171/jns.1986.64.4.0559. [DOI] [PubMed] [Google Scholar]
- 13.Heros RC. Posterior inferior cerebellar artery. J Neurosurg. 2002;97:747–8. doi: 10.3171/jns.2002.97.4.0747. [DOI] [PubMed] [Google Scholar]
- 14.Azzam CJ. Growth of multiple peripheral high flow aneurysms of the posterior inferior cerebellar artery associated with a cerebellar arteriovenous malformation. Neurosurgery. 1987;21:934–9. doi: 10.1227/00006123-198712000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Higashi K, Hatano M, Yamashita T, Inoue S, Matsumura T. Coexistence of posterior inferior cerebellar artery aneurysm and arteriovenous malformation fed by the same artery. Surg Neurol. 1979;12:405–8. [PubMed] [Google Scholar]
- 16.Kaptain GJ, Lanzino G, Do HM, Kassell NF. Posterior inferior cerebellar artery aneurysms associated with posterior fossa arteriovenous malformation.Report of five cases and literature review. Surg Neurol. 1999;51:146–52. doi: 10.1016/s0090-3019(98)00037-8. [DOI] [PubMed] [Google Scholar]
- 17.Mintz A, Cosgrove GR. Multiple peripheral aneurysms of the posterior inferior cerebellar artery associated with a cerebellar arteriovenous malformation: Case report. Neurosurgery. 1990;26:533–7. doi: 10.1097/00006123-199003000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Andoh T, Shirakami S, Nakashima T, Nishimura Y, Sakai N, Yamada H, et al. Clinical analysis of a series of vertebral aneurysm cases. Neurosurgery. 1992;31:987–93. doi: 10.1227/00006123-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Kallmes DF, Lanzino G, Dix JE, Dion JE, Do H, Woodcock RJ, et al. Patterns of hemorrhage with ruptured posterior inferior cerebellar artery aneurysms.CT findings in 44 cases. AJR Am J Roentgenol. 1997;169:1169–71. doi: 10.2214/ajr.169.4.9308484. [DOI] [PubMed] [Google Scholar]
- 20.Sadato N, Numaguchi Y, Rigamonti D, Salcman M, Gellad FE, Kishikawa T. Bleeding patterns in ruptured posterior fossa aneurysms: a CT study. J Comput Assist Tomogr. 1991;15:612–7. doi: 10.1097/00004728-199107000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Salcman M, Rigamonti D, Numaguchi Y, Sadato N. Aneurysms of the posterior inferior cerebellar artery-vertebral artery complex.Variations on a theme. Neurosurgery. 1990;27:12–21. doi: 10.1097/00006123-199007000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tokimura H, Yamahata H, Kamezawa T, Tajitsu K, Nagayama T, Sugata S, et al. Clinical presentation and treatment of distal posterior inferior cerebellar artery aneurysms. Neurosurg Rev. 2011;34:57–67. doi: 10.1007/s10143-010-0296-z. [DOI] [PubMed] [Google Scholar]
- 23.Yeh HS, Tomsick TA, Tew JM., Jr Intraventricular hemorrhage due to aneurysm of the distal posterior inferior cerebellar artery.Report of three cases. J Neurosurg. 1991;62:772–5. doi: 10.3171/jns.1985.62.5.0772. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz RB, Tice HM, Hooten SM, Hsu L, Stieg PE. Evaluation of cerebral aneurysms with helical CT: correlation with conventional angiography and MR angiography. Radiology. 1994;192:717–22. doi: 10.1148/radiology.192.3.8058939. [DOI] [PubMed] [Google Scholar]
- 25.Rothman SL, Azar-Kia B, Kier EL, Schechter MM, Allen WE. The angiography of posterior inferior cerebellar artery aneurysms. Neuroradiology. 1973;6:1–7. doi: 10.1007/BF00338851. [DOI] [PubMed] [Google Scholar]
- 26.Dernbach PD, Sila CA, Little JR. Giant and multiple aneurysms of the distal posterior inferior cerebellar artery. Neurosurgery. 1988;22:309–12. doi: 10.1227/00006123-198802000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Madsen JR, Heros R. Giant peripheral aneurysms of the posterior inferior cerebellar artery treated with excision and end-to-end anastomosis. Surg Neurol. 1988;30:140–3. doi: 10.1016/0090-3019(88)90100-0. [DOI] [PubMed] [Google Scholar]
- 28.Cho YD, Han MH, Lee JY. Double origin of the posterior inferior cerebellar artery with juxta-proximal fenestration of caudal component. Surg Radiol Anat. 2011;33:271–3. doi: 10.1007/s00276-010-0747-9. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto J, Uemura S, Hayasaki A, Kimura H, Morioka M, Kuratsu J. Ruptured aneurysm at an anastomotic artery extending from the vertebral artery to the posterior inferior cerebellar artery: A case report. Acta Neurochir (Wien) 2011;153:931–5. doi: 10.1007/s00701-010-0854-8. [DOI] [PubMed] [Google Scholar]
- 30.Kawase T, Bertalanffy H, Otani M, Shiobara R, Toya S. Surgical approaches for vertebro-basilar trunk aneurysms located in the midline. Acta Neurochir (Wien) 1996;138:402–10. doi: 10.1007/BF01420302. [DOI] [PubMed] [Google Scholar]
- 31.Matsushima T, Matsukado K, Natori Y, Inamura T, Hitotsumatsu T, Fukui M. Surgery on a saccular vertebral-posterior inferior cerebellar artery aneurysm via the transcondylar fossa (supracondylar transjugular tubercle) approach or the transcondylar approach: Surgical results and indications for using two different lateral skull base approaches. J Neurosurg. 2001;95:268–74. doi: 10.3171/jns.2001.95.2.0268. [DOI] [PubMed] [Google Scholar]
- 32.Spetzler RF, Grahm TW. The far lateral approach to the inferior clivus and the upper cervical region: Technical note. Barrow Neurol Inst. 1997;6:35–8. [Google Scholar]
- 33.Bertalanffy H, Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991;29:815–21. doi: 10.1097/00006123-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Giannotta SL, Maceri DR. Retrolabyrinthine transigmoid approach to basilar trunk and vertebrobasilar artery junction aneurysms.Technical note. J Neurosurg. 1988;69:461–6. doi: 10.3171/jns.1988.69.3.0461. [DOI] [PubMed] [Google Scholar]
- 35.Al-Khayat H, Al-Khayat H, Beshay J, Manner D, White J. Vertebral artery posteroinferior cerebellar artery aneurysms: Clinical and lower cranial nerve outcomes in 52 patients. Neurosurgery. 2005;56:2–11. [PubMed] [Google Scholar]
- 36.Horowitz M, Kopitnik T, Landreneau F, Krummerman J, Batjer HH, Thomas G, et al. Posteroinferior cerebellar artery aneurysms: Surgical results for 38 patients. Neurosurgery. 1998;43:1026–32. doi: 10.1097/00006123-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Nourbakhsh A, Katira KM, Notarianni C, Vannemreddy P, Guthikonda B, Nanda A. Long-term follow-up of disability among patients with posterior inferior cerebellar artery aneurysm. J Clin Neurosci. 2010;17:980–3. doi: 10.1016/j.jocn.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 38.Fagon JY, Chastre J, Vuagnat A, Trouillet JL, Novara A, Gilbert C. Nosocomial pneumonia and mortality among patients in intensive care units. JAMA. 1996;275:866–9. [PubMed] [Google Scholar]
- 39.Mukonoweshuro W, Laitt RD, Hughes DG. Endovascular treatment of PICA aneurysms. Neuroradiology. 2003;45:188–92. doi: 10.1007/s00234-002-0913-9. [DOI] [PubMed] [Google Scholar]
- 40.Bradac GB, Bergui M. Endovascular treatment of the posterior inferior cerebellar artery aneurysms. Neuroradiology. 2004;46:1006–11. doi: 10.1007/s00234-004-1245-8. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki O, Ogawa H, Koike T, Koizumi T, Tanaka R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991;75:874–82. doi: 10.3171/jns.1991.75.6.0874. [DOI] [PubMed] [Google Scholar]
- 42.Dolenc V. End-to-end suture of the posterior inferior cerebellar artery after the excision of a large aneurysm: case report. Neurosurgery. 1982;11:690–3. doi: 10.1227/00006123-198211000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Korja M, Sen C, Langer D. Operative nuances of side-to-side in situ posterior inferior cerebellar artery-posterior inferior cerebellar artery bypass procedure. Neurosurgery. 2010;67(2 Suppl Operative):471–7. doi: 10.1227/NEU.0b013e3181f7420e. [DOI] [PubMed] [Google Scholar]
- 44.Yamaura A, Ise H, Makino H. Radiometric study on posterior inferior cerebellar aneurysms with special reference to accessibility by the lateral suboccipital approach. Neurol Med Chir (Tokyo) 1981;21:721–3. doi: 10.2176/nmc.21.721. [DOI] [PubMed] [Google Scholar]
- 45.Yamaura A. Diagnosis and treatment of vertebral aneurysms. J Neurosurg. 1988;69:345–9. doi: 10.3171/jns.1988.69.3.0345. [DOI] [PubMed] [Google Scholar]


