Abstract
A study has been conducted with the aim to provide researchers with general information on anti diabetic extracts based on relevant research articles collected from 34 reliable medical journals. The study showed that Asian and African continents have 56% and 17% share of the worldwide distribution of therapeutic herbal plants, respectively. In Asia, India and China are the leading countries in herbal plants research, and there has been an increase in medicinal research on plants extract for diabetes treatment since 1995 in these regions. The information collected shows that plant leaves are about 20% more favorable for storing active ingredients, as compared to other parts of herbal plants. A brief review on the extraction techniques for the mentioned parts is also included. Furthermore, the acting mechanisms for the anti diabetic activity were described, and the related active ingredients were identified. The findings reveal that most of the anti diabetic research is focused on the alteration of glucose metabolism to prevent diabetes.
Keywords: Active ingredient, Anti diabetic treatment, extraction technique, herbal plants extract
INTRODUCTION
Research on diabetes treatment is gaining ground as the world population with diabetes is rising each year, and is expected to hit 439 million adults by 2030.[1] The awareness on the issue has led to a vast discovery of new medications as well as natural products extracted from herbal plants. Many active ingredients extracted from herbal plants possess therapeutic values, i.e. hypoglycemic activity, antioxidant action, etc and they are yet to be discovered. In view of that, a study has been performed on anti diabetic plant extracts and the focuses are on: the global distribution of the plants; the parts in which therapeutic elements are located; and, also the acting mechanism for diabetic treatment. This study not only provides researchers with the information pertaining to anti diabetic plants, but also to evaluate their related research activities. The papers reviewed in this article are selected from the medicinal journals as tabulated in Table 1 due to their popularity and reliable reputation in medicinal research on herbal plant extract. The research papers were extracted from the selected journals under key words of “Plant extract for diabetes treatment”.
Table 1.
The selected 34 medicinal journals for the study

The information extracted from the 34 journals listed was compiled and arranged into respective sections as guidance for any interested parties.
GLOBAL DISTRIBUTION OF ANTI DIABETIC PLANTS
Anti diabetic plants were widely distributed in six continental regions, and some specific regions around the world such as in Caribbean, Mediterranean and Middle East. This section lists the areas of distribution of the anti diabetic plants with the intention to identify and deduce their locations. The worldwide distribution of anti diabetic plants is depicted in Figure 1. This figure shows that Asia (56%) and Africa (17%) dominated the global distribution of the anti diabetic plants. This is not surprising as the two continents are located in the tropic and sub-tropic regions, and have large coverage of tropical rain forests. Moreover, these regions have their long established traditional medicine systems. As the activity of herbal plant researches in certain regions is proportional to the plant distribution, American continent has a 10% research performed on the medicinal plants. European countries led by Germany are closely behind with 6%. Besides, some strategic regions such as Caribbean, Mediterranean and Middle East, have individually engaged to around 2 to 4% research on herbal plants, and Australian continent has contributed 1 % to the anti diabetic herbal plants research.
Figure 1.
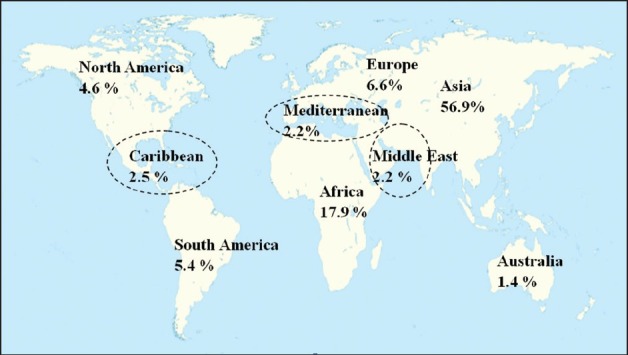
Distribution of plants for diabetes treatment as seen in the study
Based on the records for 20 years of research on plants possessing anti diabetic properties [Figure 2], Asia and Africa demonstrate an increase in the trends and research activities since 1995, and this rise is further expected to continue. Comparing between these two continents, the increment of the research on herbal plants in Asia is about 40% higher than that in the African continent. On the other hand, North America, South America and Europe show stable increase in the research activity towards the year 2010, whereas Australia remains unchanged in its research activity on anti diabetic plants for the last 20 years. From this figure, it is clear that Asia and Africa which are rich in plants effective for diabetes treatment, have carried out maximum research in the past few years.
Figure 2.
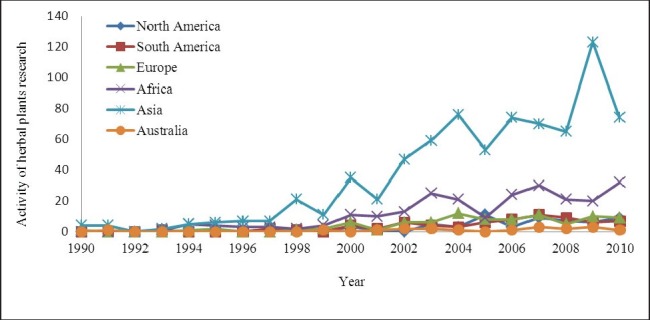
Activity of plant-based research for anti diabetic treatment in the past 20 years as seen in the study
The distribution in Figure 2 indicates that Asia has the most herbal plants which corresponds to the large numbers of researches conducted in the region. The detail distribution of the anti- diabetic herbal plants in this continent is charted in Figure 3. South Asia has a large distribution of 36% followed by East Asia and South East Asia which dominate 27% and 17% respectively. The leading countries for the herbal plant research here are India and China. Many of the research hypotheses have been based on traditional medicine system such as Chinese Herbology and Ayurveda’. These two traditional medicine systems are the foundation for the herbal plant medicinal research in their respective regions. Northern Asia has the least distribution of the herbal plants due to its temperate zone and Siberian influence whilst, South Asia and East Asia are the potential regions of herbal plants, and so is their intermediate, South East Asia.
Figure 3.
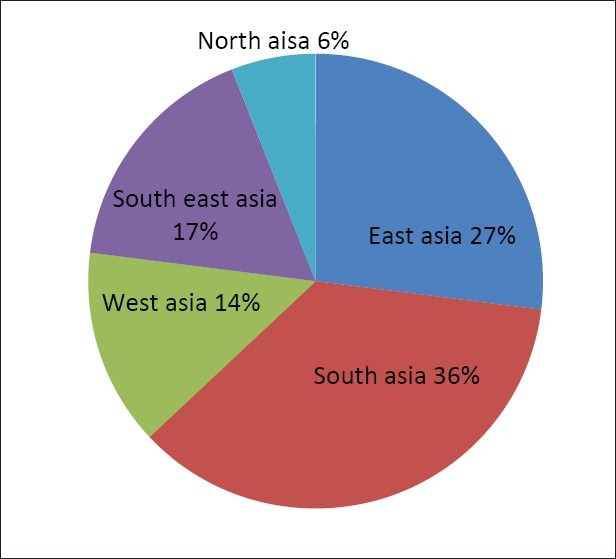
The distribution of anti diabetic plants in Asia as seen in the study
DISTRIBUTION OF ACTIVE ANTI DIABETIC COMPOUNDS IN PLANTS AND THEIR EXTRACTION TECHNIQUES
The parts of plants that possess active compounds for diabetic treatment and various types of extraction techniques applied are presented in this section. In some cases in which the active ingredients are scattered all over the plants, the entire plants were prepared and extracted for the desired ingredient. More than 80 plants with printed records from the 34 related journals reviewed in this manuscript involved the extraction of the whole plant for desired compounds in the last one decade. Generally, leaves are the favorable storage site for desired compounds and more than 35% of the plants extractions for diabetic treatment can be obtained from these parts as illustrated in Figure 4. Besides, fruits contain substantial amount of active ingredients, and thus, in many occasions they are consumed as juice via oral administration to obtain the desired compounds. Other parts of plants that can be extracted for therapeutic compounds are root, aerial parts, flowers, seeds, stem barks, etc.
Figure 4.
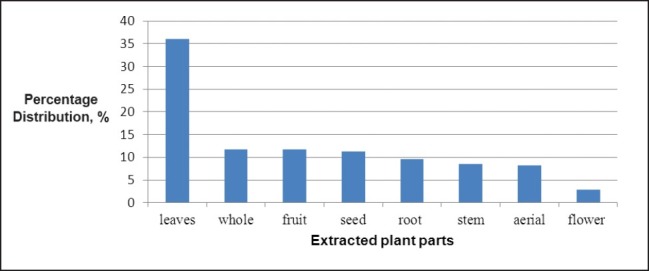
The percentage distribution of anti diabetic ingredients in plant parts as seen in the study
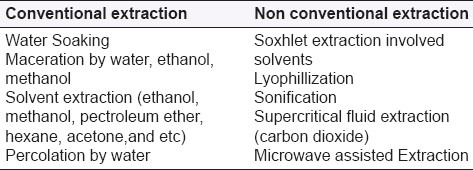
Most of the extractions used in plants extract research are associated with the conventional techniques. The techniques employed in the extraction are tabulated in Table 2. In conventional extraction, the release of the desired compounds traditionally required soaking and maceration in mild solvents. In traditional Chinese medicine practices, decoction in water is broadly employed and is an effective method to be considered in cases where the presence of a chemical solvent is undesired.[2–6] In addition to the soaking/maceration technique, percolations using methanol and ethanol on the stem were also applied.[7–12] Other solvents such as acetone, petroleum ether and hexane have also been used as solvents in the conventional extraction.[13–15] Moreover, extraction by liquid nitrogen was also witnessed in some research works.[16] The soxhlet technique for extracting anti-diabetic ingredient was not popular until 2005, after which the extraction technique was incorporated with ethanol and light petroleum.[17,18] Other than solvent extraction, techniques such as lyophilization[19,20] and sonification[21,22] have also been employed. Furthermore, supercritical fluid extraction and microwave assisted techniques have also been used in recent years. For instances, supercritical fluid extraction on lotus gem were carried out by Taiwanese research teams to investigate the antioxidant activity of the extract,[23] and microwave-assisted extraction was employed to investigate the bioactivity of tea flower polysaccharides.[24] These two advanced, non conventional techniques offer attractive advantages of short extraction time and solvent free active compounds, respectively. The extraction techniques normally used in plant research are lacking in the involvement of the engineering aspect as well, and hence the processes are not optimized. As a result, the therapeutic efficacy of the plants under investigation might be affected.
Table 2.
Various techniques employed in plant extraction as seen in the study
THE PLANT EXTRACTS FOR DIABETES TREATMENT AND RELATED ACTING MECHANISM
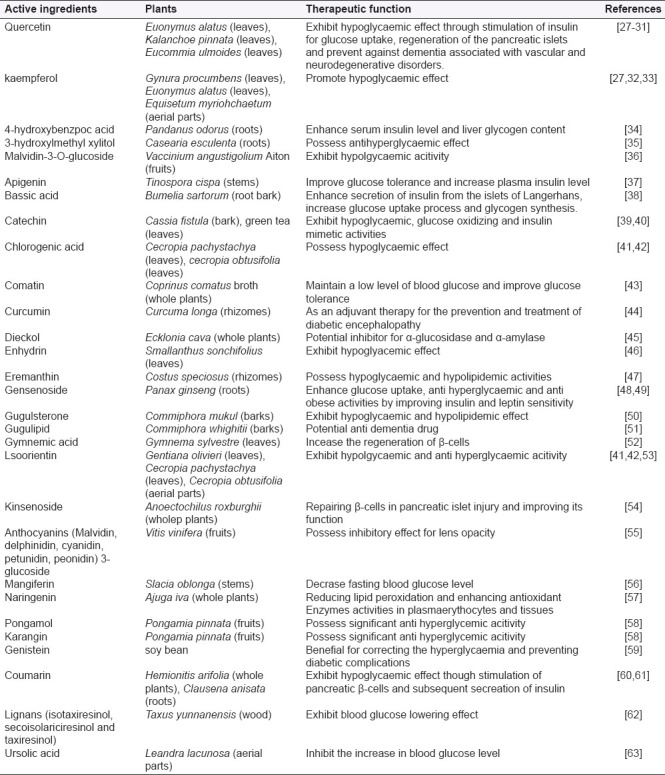
Most of the medicinal researches have aimed to evaluate the therapeutic value of plants and to identify the related active ingredients extracted. The active ingredients from flavonoids (e.g. quercetin and kaempferol), alkaloids such as dieckol, etc which are potential for diabetes treatment have been discovered and their therapeutic functions have been described in Table 3. Besides that, some polysaccharides[25,26] from plants are also beneficial to humans in fighting diabetes.
Table 3.
Potential anti diabetic active compounds extracted from plants in the study
In this study, the anti diabetic acting mechanisms of the active ingredients are categorized into 6 groups for the ease of compilation as shown in Figure 5. The acting mechanisms are namely: alteration of glucose metabolism; hypolipidemic effect; pancreatic effect; antioxidative effect; diabetes complication treatment; and, insulin-like effect. Figure 5 gives an overall picture on the trend of plant research for anti diabetic treatment based on the information gathered for the past 20 years. The figure indicates the percentage distribution of anti diabetic acting mechanisms possessed by herbal plant extracts in descending order.
Figure 5.
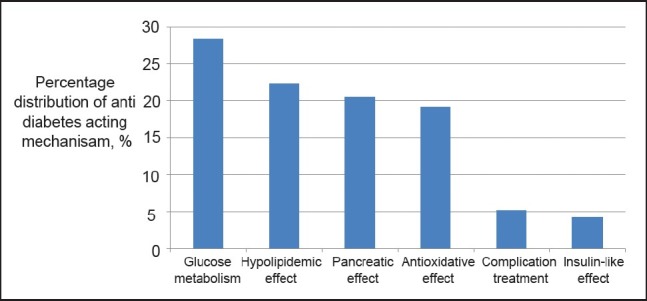
The percentage distribution of various anti diabetic acting mechanisms as seen in the study
Figure 5 show that 28% of the anti diabetic researches emphasized on the alteration of glucose metabolism. By altering the glucose metabolism, it helps to regulate the blood sugar levels to normal and thus prevent diabetes, whereas other anti diabetic mechanisms assist in treating diabetes and its complications. Several active ingredients belonging to this category are: bassic acid, an active compound isolated from Bumelia sartorum; and, natural flavonoids such as quercetin and kaempferol as they can promote hypoglycemia through increase glucose uptake and glycogen synthesis.[27,33,38] Recently, researchers found that dieckol, a compound isolated from Ecklonia cava, is a potential inhibitor for α -glucosidase and α-amylase. It exhibits hypoglycaemia by reducing the impact of carbohydrates on blood sugar and is claimed to be comparable to Acarbose, a medical anti diabetic drug.[45] In addition, the effect of enhancing glucose tolerance and homeostasis have been reported in the root extracts of Berberis aristata and in comatin, an active ingredient extracted from Coprinus comatus broth. Both extracts can prevent diabetes by reducing the severity of insulin resistance.[43,64]
The second, third and forth categories of the acting mechanisms respectively focus on hypolipidemic effect, pancreatic effect and antioxidative effect. The interactions among them are important focal points for anti diabetic research; for instance, a common secondary cause of hyperlipidemia is associated with diabetes. On one hand, some herbal plants extract such as guggulsterone isolated from Commiphora mukul and isoorientin obtained from Gentiana olivieri possess hypoglycemic and hypolipidemic properties which are suitable for obese diabetes patients;[50,53] on the other, herbal plants extract with pancreatic effect helps to enhance insulin secretion through insulin sensitizing mechanism. Furthermore, it has been reported that mangiferin and procyanidins, the natural compounds found in several plants like apple, grape and mango, exhibit hypoglycaemic activity through enhancing insulin signaling pathway.[65,66] As for antioxidative effect, plant extracts such as green tea, Ajuga iva aqueous extract and Chlorogenic acid from Cecropia pachystachya are capable of reducing oxidative stress and protecting against tissue damage in diabetes.[41,57,67] Another acting mechanism based on antioxidants such as kinsenoside, gymnemic acid and quercetin are helpful in preventing and treating Type I diabetes as they can regenerate the beta cells in islets of Langerhans in pancreas.[29,52,54,68]
The last two acting mechanisms associate with diabetic complication treatment and insulin-like active ingredients administration. There are a lot of diabetic complications; however, only a few are involved in research attributing to their severity. For example, Pterocarpus marsupium extract is more effective compared to Ocimum sanctum extract as it exhibits better anti cataract effect for diabetic complication treatment.[69] Moreover, some extracts, i.e. garlic and ginger extracts, can be used to prevent and attenuate the development of nephropathy.[70] In addition, extracts isolated from Syzigium plants, i.e. S. cumini and S. aromaticum, are potential active ingredients for insulin substitutes.[71,72]
CONCLUSIONS
The identified sources of plants with therapeutic value indicate that Asia has dominated more than 50% of the distribution followed by African continents which are estimated to be at 17%. Asian giants, e.g. India and China lead the research on anti diabetic plants, and correspondingly shown an increase in the related anti diabetic research trend. From the information gathered on various parts of plant, leaves are the most favorable storage sites for active ingredients. The extraction methods commonly employed in anti diabetic plant extraction are conventional methods involving solvents. However, the engineered extraction techniques such as supercritical extraction and microwave assisted extraction are gaining more attention due to the high efficiency of these techniques, and also bacuse they produce a better yield of the active ingredients. This review article also implies that the alteration of glucose metabolism by herbal plants is crucial as far as preventing diabetes is concerned.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Das AK, Bag S, Sahu R, Dua TK, Sinha MK, Gangpodahyay M, et al. Protective effect of Corchorus olitorius leaves on sodium arsenite-induced toxicity in experimental rats. Food Chem Toxicol. 2010;48:326–35. doi: 10.1016/j.fct.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Harnafi H, Aziz M, Amrani S. Sweet basil (Ocimum basilicum L.) improves lipid metabolism in hypercholesterolemic rats. E Spen Eur E J Clin Nutr Metab. 2009;4:181–6. [Google Scholar]
- 4.Khan SA, Priyamvada S, Farooq N, Khan S, Khan MW, Yusufi AN. Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacol Res. 2009;59:254–62. doi: 10.1016/j.phrs.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Meddah B, Ducroc R, Faouzi ME, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121:419–24. doi: 10.1016/j.jep.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Sefi M, Fetoul H, Makni M, Zeghal N. Mitigating effects of antioxidant properties of Artemisia campestris leaf extract on hyperlipidemia, advanced glycation end products and oxidative stress in alloxan-induced diabetic rats. Food Chem Toxicol. 2010;48:1986–93. doi: 10.1016/j.fct.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Cunha AM, Menon S, Couto AG, Burger C, Biavatti MW. Hypoglycemic activity of dried extracts of Bauhinia forficata Link. Phytomedicine. 2010;17:37–41. doi: 10.1016/j.phymed.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Ghaisas M, Navghare V, Takawale A, Zope V, Tanwar M, Deshpande A. Effect of Tectona grandis Linn.on dexamethasone-induced insulin resistance in mice. J Ethnopharmacol. 2009;122:304–7. doi: 10.1016/j.jep.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Jung SH, Seol HJ, Son KH, Lee JR. Insulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza Bunge. Phytomedicine. 2009;16:327–35. doi: 10.1016/j.phymed.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Oh KS, Ryu SY, Lee S, Seo HW, Oh BK, Kim YS, et al. Melanin-concentrating hormone-1 receptor antagonism and anti-obesity effects of ethanolic extract from Morus alba leaves in diet-induced obese mice. J Ethnopharmacol. 2009;122:216–20. doi: 10.1016/j.jep.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Pandikumar P, Babu NP, Ignacimuthu S. Hypoglycemic and antihyperglycemic effect of Begonia malabarica Lam.in normal and streptozotocin induced diabetic rats. J Ethnopharmacol. 2009;124:111–5. doi: 10.1016/j.jep.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Paula FS, Kabeya LM, Kanashiro A, Figueriedo AS, Azzolini AE, Uyemura SA, et al. Modulation of human neutrophil oxidative metabolism and degranulation by extract of Tamarindus indica L. fruit pulp. Food Chem Toxicol. 2009;47:163–70. doi: 10.1016/j.fct.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Badole SL, Bodhankar SL. Investigation of antihyperglycaemic activity of aqueous and petroleum ether extract of stem bark of Pongamia pinnata on serum glucose level in diabetic mice. J Ethnopharmacol. 2009;123:115–20. doi: 10.1016/j.jep.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Bamuamba K, Gammon DW, Meyers P, Franca MG, Scott G. Anti-mycobacterial activity of five plant species used as traditional medicines in the Western Cape Province (South Africa) J Ethnopharmacol. 2008;117:385–90. doi: 10.1016/j.jep.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Ntandou GF, Banzouzi JT, Mbatchi B, Elion-Itou RD, Etou-Ossibi AW, Ramos S, et al. Analgesic and anti-inflammatory effects of Cassia siamea Lam.stem bark extracts. J Ethnopharmacol. 2010;127:108–11. doi: 10.1016/j.jep.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Sree BK, Rajendrakumar CS, Reddy AR. Aldose reductase in rice (Oryza sativa L.): Stree response and developmental specificity. Plant Sci. 2000;160:149–57. doi: 10.1016/s0168-9452(00)00376-9. [DOI] [PubMed] [Google Scholar]
- 17.Kurian GA, Paddikkala J. Oral delivery of insulin with Desmodium gangeticum root aqueous extract protects rat hearts against ischemia reperfusion injury in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2010;3:94–100. [Google Scholar]
- 18.Lakshmi BV, Sudhakar M. Attenuation of acute and chronic restraint stress-induced perturbations in experimental animals by Zingiber officinale Roscoe. Food Chem Toxicol. 2010;48:530–5. doi: 10.1016/j.fct.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Chen HL, Wang CH, Chang CT, Wang TC. Effects of Taiwanese Yam (Dioscorea japonica Thunb var.pseudojaponica Yamamoto) on upper gut function and lipid metabolism in Balb/c mice. Nutrition. 2003;19:646–51. doi: 10.1016/s0899-9007(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 20.Grover JK, Vats V, Rathi SS. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2000;73:461–70. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 21.Chukwujekwu JC, Lategan CA, Smith PJ, Heerden FR, Staden VJ. Antiplasmodial and cytotoxic activity of isolated sesquiterpene lactones from the acetone leaf extract of Vernonia colorata. S Afr J Bot. 2009;75:176–9. [Google Scholar]
- 22.Yang B, Zhao M, Jiang Y. Anti-glycated activity of polysaccharides of longan (Dimocarpus longan Lour.) fruit pericarp treated by ultrasonic wave. Food Chem. 2009;114:629–33. [Google Scholar]
- 23.Li J, Zhang M, Zheng T. The in vitro antioxidant activity of lotus germ oil from supercritical fluid carbon dioxide extraction. Food Chem. 2009;115:939–44. [Google Scholar]
- 24.Wei X, Chen M, Xiao J, Yu L, Zhang H, Wang Y. Composition and bioacitivity of tea flower polysaccharides obtained by different methods. Carbohydr Polymers. 2010;79:418–422. [Google Scholar]
- 25.Yang N, Zhao M, Zhu B, Yang B, Chen C, Cui C, et al. Anti-diabetic effects of polysaccharides from Opuntia monacantha cladode in normal and streptozotocin-induced diabetic rats. Innov Food Sci Emerg Technol. 2008;9:570–4. [Google Scholar]
- 26.Mao X, Yu F, Wang N, Wu Y, Zou F, Wu K, et al. Hypoglycemic effect of polysaccharides enriched extract of Astragalus membranaceus in diet induced insulin resistant C57BL/6J mice and its potential mechanism. Phytomedicine. 2009;16:416–25. doi: 10.1016/j.phymed.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Fang XK, Gao J, Zhu DN. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82:615–22. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Tota S, Awasthi H, Kamat PK, Nath C, Hanif K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav Brain Res. 2010;209:73–9. doi: 10.1016/j.bbr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C. 2003;135:357–64. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim HY, Moon BH, Lee HJ, Choi DH. Flavonol glycosides from the leaves of Eucommina ulmoides O.with glucation inhibitory activity. J Ethnopharmacol. 2004;93:227–30. doi: 10.1016/j.jep.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 31.Cruz EA, Da-Silva SA, Muzitano MF, Silva PM, Costa SS, Rossi-Bergmann B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int Immunopharmacol. 2008;8:1616–21. doi: 10.1016/j.intimp.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Rosidah, Yam MF, Sadikun A, Ahmad M, Akowuah GA, Asmawi MZ. Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol. 2009;123:244–9. doi: 10.1016/j.jep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Cetto AA, Wiedenfeld H, Revilla MC. Hypoglycaemic effect of Equisetum myriochaetum aerial parts on streptozotocin diabetic rats. J Ethnopharmacol. 2000;72:129–33. doi: 10.1016/s0378-8741(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 34.Peungvicha P, Temsiririrkkul R, Prasain JK, Tezuba Y, Kadota S, Thirawarapan SS, et al. 4-hydroxybenzoic acid: A hypoglycemic constituent of aquous extract of Pandanus adorus root. J Ethnopharmacol. 1998;62:79–84. doi: 10.1016/s0378-8741(98)00061-0. [DOI] [PubMed] [Google Scholar]
- 35.Chandramohan G, Ignacimuthu S, Pugalendi KV. A novel compound from Casearia esculenta (Roxb.) root and its effect on carbohydrate metabolism in streptozotocin-diabetic rats. Eur J Pharmacol. 2008;590:437–43. doi: 10.1016/j.ejphar.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 36.Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, et al. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16:406–15. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noor H, Ashcroft SJ. Antidiabetic effects of Tinospora crispa in rats. J Ethnopharmacol. 1989;27:149–61. doi: 10.1016/0378-8741(89)90087-1. [DOI] [PubMed] [Google Scholar]
- 38.Naik SR, Filho JM, Dhuley JN. Probable mechanism of hypoglycemic activity of bassic acid, a natural product isolated from Bumelia sartorum. J Ethnopharmacol. 1991;33:37–44. doi: 10.1016/0378-8741(91)90158-a. [DOI] [PubMed] [Google Scholar]
- 39.Daisy P, Balasubramanian K, Rajalakshmi M, Eliza J, Selvaraj J. Insulin mimetic impact of Catechin isolated from Cassia fistula on the glucose oxidation and molecular mechanisms of glucose uptake on Streptozotocin-induced diabetic Wistar rats. Phytomedicine. 2010;17:28–36. doi: 10.1016/j.phymed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Kamiyama O, Sanae F, Ikeda K, Higashi Y, Minami Y, Asano N, et al. In vitro inhibition of a-glucosidases and glycogen phosphorylase by catechin gallates in green tea. Food Chem. 2010;122:1061–6. [Google Scholar]
- 41.Aragao DM, Guariza L, Costa JC, Garcia RM, Scio E. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. J Ethnopharmacol. 2010;128:629–33. doi: 10.1016/j.jep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Toledo VM, Tellez MG, Sortibran AN, Cetto AA, Arnaiz RR. Genotoxicity testing of Cecropia obtusifolia extracts in two in vivo assays: The wing somatic mutation and recombination test of Drosophila and the human cytokinesis-block micronucleus test. J Ethnopharmacol. 2008;116:58–63. doi: 10.1016/j.jep.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 43.Ding Z, Lu Y, Lu Z, Lv F, Wang Y, Zhang K. Hypoglycaemic effect of comatin, an antidiabetic substance separated from Coprinus comatus broth, on alloxan-induced-diabetic rats. Food Chem. 2010;121:39–43. [Google Scholar]
- 44.Kuhad A, Chopra K. Curcumin attenuates diabetic encephalopathy in rats: Behavioral and biochemical evidences. Eur J Pharmacol. 2007;576:34–42. doi: 10.1016/j.ejphar.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Park MH, Heo SJ, Ko SC, Han JS, Jeon YJ. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem Toxicol. 2010;48:2633–7. doi: 10.1016/j.fct.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 46.Genta SB, Cabrera WM, Mercado MI, Grau A, Catalan CA, Sanchez SS. Hypoglycemic activity of leaf organic extracts from Smallanthus sonchifolius: Constituents of the most active fractions. Chem Biol Interact. 2010;185:143–52. doi: 10.1016/j.cbi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Eliza J, Daisy P, Ignacimuthu S, Duraipandiyan V. Antidiabetic and antilipidemic effect of eremanthin from Costus speciosus (Koen.)Sm., in STZ-induced diabetic rats. Chem Biol Interact. 2009;182:67–72. doi: 10.1016/j.cbi.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Lee MS, Hwang JT, Kim SH, Yoon S, Kim MS, Yang HJ, et al. Ginsenoside Rc, an active component of Panax ginseng, stimulates glucose uptake in C2C12 myotubes through an AMPK-dependent mechanism. J Ethnopharmacol. 2010;127:771–6. doi: 10.1016/j.jep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Yang C, Wang J, Zhao Y, Shen L, Jiang X, Xie Z, et al. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J Ethnopharmacol. 2010;2:231–6. doi: 10.1016/j.jep.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 50.Sharma B, Salunke R, Srivastava S, Majumder C, Roy P. Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem Toxicol. 2009;47:2631–9. doi: 10.1016/j.fct.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Saxena G, Singh SP, Pal R, Singh S, Pratap R, Nath C. Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav. 2007;86:797–805. doi: 10.1016/j.pbb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed AB, Rao AS, Rao MV. In vitro callus and in vivo leaf extract of Gymnema sylvestre stimulate β-cells regeneration and anti-diabetic activity in Wistar rats. Phytomedicine. 2010;17:1033–9. doi: 10.1016/j.phymed.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Sezik E, Aslan M, Yesilada E, Ito S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay- directed fractionation techniques. Life Sci. 2005;76:1223–38. doi: 10.1016/j.lfs.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Cai J, Ruan H, Pi H, Wu J. Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J Ethnopharmacol. 2007;114:141–5. doi: 10.1016/j.jep.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 55.Morimitsu Y, Kubota K, Tashiro T, Hashizume E, Kamiya T, Osawa T. Inhibitory effect of anthocyanins and colored rice on diabetic cataract formation in the rat lenses. Int Congr Ser. 2002;1245:503–8. [Google Scholar]
- 56.Im R, Mano H, Matusuura T, Nakatani S, Shimizu J, Wada M. Mechanisms of blood glucose-lowering effect of aqueous extract from stems of Kothala himbutu (Salacia reticulata) in the mouse. J Ethnopharmacol. 2009;121:234–40. doi: 10.1016/j.jep.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 57.Senouci DT, Ghomari H, Krouf D, Bouderbala S, Prost J, Dubois MA, et al. Antioxidant effectof Ajuga iva aqueous extract in streptozotocin-induced diabetic rats. Phytomedicine. 2009;16:623–33. doi: 10.1016/j.phymed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Tamrakar AK, Yadav PP, Tiwari P, Maurya R, Srivastava AK. Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamia pinnata fruits. J Ethnopharmacol. 2008;118:435–9. doi: 10.1016/j.jep.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 59.Lee JS. Effects of soy protein and genistein on blood glucose, antioxidant enzyme activities, and lipid profile in streptozotocin-induced diabetic rats. Life Sci. 2006;79:1578–84. doi: 10.1016/j.lfs.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 60.Nair SA, Shylesh BS, Gopakumar B, Subramoniam A. Anti-diabetes and hypoglycaemic properties of Hemionitis arifolia (Burm.) Moore in rats. J Ethnopharmacol. 2006;106:192–7. doi: 10.1016/j.jep.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Ojewole JA. Hypoglycaemic effect of Clausena anisata (Willd) Hook methanolic root extract in rats. J Ethnopharmacol. 2002;81:231–7. doi: 10.1016/s0378-8741(02)00085-5. [DOI] [PubMed] [Google Scholar]
- 62.Banskota AH, Nguyen NT, Tezuka Y, Nobukawa T, Kadota S. Hypoglycemic effects of the wood of Taxus yunnanensis on streptozotocin-induced diabetic rats and its active components. Phytomedicine. 2006;13:109–14. doi: 10.1016/j.phymed.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 63.Cunha W, Arantes GM, Ferreira DS, Lucarini R, Silva ML, Furtado NA, et al. Hypoglicemic effect of Leandra lacunosa in normal and alloxan-induced diabetic rats. Fitoterapia. 2008;79:356–60. doi: 10.1016/j.fitote.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Singh J, Kakkar P. Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J Ethnopharmacol. 2009;123:22–6. doi: 10.1016/j.jep.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 65.Giron MD, Sevillano N, Salto R, Haidour A, Manzano M, Jumenez ML, et al. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: Role of mangiferin. Clin Nutr. 2009;28:565–74. doi: 10.1016/j.clnu.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 66.Montagut G, Onnockx S, Vaque M, Blade C, Blay M, Larrea J, et al. Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J Nutr Biochem. 2010;21:476–81. doi: 10.1016/j.jnutbio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 67.Juskiewicz J, Zdunczyk Z, Jurgonski A, Brzuzan L, Klos IG. Extract of green tea leaves partially attenuates streptozotocin-induced changes in antioxidant status and gastrointestinal functioning in rats. Nutr Res. 2008;28:343–9. doi: 10.1016/j.nutres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Baskaran K, Kizar Ahamath B, Shanmugasundaram Radha K, Shanmugasundaram ER. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol. 1990;30:295–305. doi: 10.1016/0378-8741(90)90108-6. [DOI] [PubMed] [Google Scholar]
- 69.Vats V, Yadav SP, Biswas NR, Grover JK. Anti-cataract activity of Pterocarpus marsupium bark and Trigonella foenum-graecum seeds extract in alloxan diabetic rats. J Ethnopharmacol. 2004;93:289–94. doi: 10.1016/j.jep.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 70.Al-Qattan K, Thomson M, Ali M. Garlic (Allium sativum) and ginger (Zingiber officinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. E Spen Eur E J Clin Nutr Metab. 2008;3:62–71. [Google Scholar]
- 71.Prasad RC, Herzog B, Boone B, Sims L, Law MW. An extract of Syzygium aromaticum represses genes encoding hepatic gluconeogenic enzymes. J Ethnopharmacology. 2005;96:295–301. doi: 10.1016/j.jep.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 72.Prince PS, Kamalakkannan N, Menon V. Antidiabetic and antihyperlipidaemic effect of alcoholic syzigium cumini seeds in alloxan induced diabetic albino rats. J Ethnopharmacol. 2004;91:209–13. doi: 10.1016/j.jep.2003.11.001. [DOI] [PubMed] [Google Scholar]


