Abstract
Trichosanthes, a genus of family Cucurbitaceae, is an annual or perennial herb distributed in tropical Asia and Australia. Pointed gourd (Trichosanthes dioica Roxb.) is known by a common name of parwal and is cultivated mainly as a vegetable. Juice of leaves of T. dioica is used as tonic, febrifuge, in edema, alopecia, and in subacute cases of enlargement of liver. In Charaka Samhita, leaves and fruits find mention for treating alcoholism and jaundice. A lot of pharmacological work has been scientifically carried out on various parts of T. dioica, but some other traditionally important therapeutical uses are also remaining to proof till now scientifically. According to Ayurveda, leaves of the plant are used as antipyretic, diuretic, cardiotonic, laxative, antiulcer, etc. The various chemical constituents present in T. dioica are vitamin A, vitamin C, tannins, saponins, alkaloids, mixture of noval peptides, proteins tetra and pentacyclic triterpenes, etc.
Keywords: Cucurbitacin, diabetes, hepatoprotective, Trichosanthes dioica
INRODUCTION
The plants in Cucurbitaceae family is composed of about 110 genera and 640 species. The most important genera are Cucurbita, Cucumis, Ecballium, Citrullus, Luffa, Bryonia, Momordica, Trichosanthes, etc (more than 30 species).[1]
Trichosanthes, a genus of family Cucurbitaceae, is an annual or perennial herb distributed in tropical Asia, Polynesia, and Australia. Over 20 species are recorded in India of which two, namely T. anguina and T. dioica, are cultivated as vegetable. Other important species found throughout the world are T. palmata, T. cordata, T. nervifolia, T. cucumerina, T. wallichiana, T. cuspida, T. incisa, T. laciniosa, T. kirilowii, etc.[2]
BOTANICAL CLASSIFICATION
Ethnopharmacological uses
Pointed gourd (T. dioica) is known by the name of parwal, palwal, parmal, patol, and potala in different parts of India and Bangladesh and is one of the important vegetables of this region.[3] The fruits and leaves are the edible parts of the plant which are cooked in various ways either alone or in combination with other vegetables or meats.[4]
Juice of leaves of T. dioica is used as tonic, febrifuge, and in subacute cases of enlargement of liver and spleen;[5] in Charaka Samhita, leaves and fruits are used for treating alcoholism and jaundice. Leaves are used in edema and alopecia.[6] It is also used as antipyretic, diuretic, cardiotonic, and laxative. [Table 1].
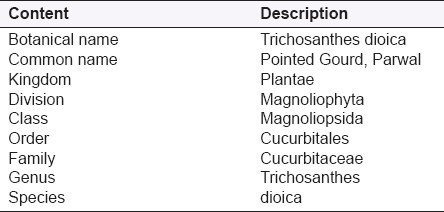
Table 1.
Botanical classification of Trichosanthes dioica
Distribution
Trichosanthes, a genus of family Cucurbitaceae, is an annual or perennial herb distributed in tropical Asia and Australia. T. dioica is cultivated throughout the plain of Northern India, extending to Assam and East Bengal.[3]
Cultivation
The pointed gourd is usually propagated through vine cuttings and root suckers. Seeds are not used in planting because of poor germination and inability to determine the sex of plants before flowering. As a result, crop established from seed may contain 50% nonfruiting male plants. Both prerooted and fresh vine cuttings are used for propagation. Vine cuttings are made in the fall of previous year and rooted during winter. Fresh vines used for field planting should have 8–10 nodes per cutting. The distance between plants is kept between 1.5–2.0 m × 1.5–2.0 m. A female:male ratio of 9:1 is optimum for ensuring maximum fruit set.[7]
Morphology
The plant is a perennial, dioecious, and grows as a vine. Vines are pencil thick in size with dark green cordate, ovate, oblong, not lobed, rigid, leaves. Roots are tuberous with long tap root system. Flowers are tubular white with 16–19 days initiation to anthesis time for pistillate flowers and 10–14 days for staminate flowers. Stigma remains viable for approximately 14 hours and 40–70% of flowers set fruit. Based on shape, size, and striation, fruits can be grouped into four categories:[7]
Long, dark green with white stripes, 10–13 cm long
Thick, dark green with very pale green stripes, 10–16 cm long
Roundish, dark green with white stripe, 5–8 cm long
Tapering, green and striped, 5–8 cm long
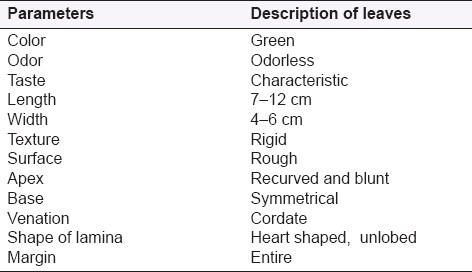
Some macroscopical characterstics of leaves of T. dioica are described in Table 2.
Table 2.
Macroscopical characters of leaves of T. dioica
MICROSCOPY
Qualitative microscopy T.S. of leaf
T.S. of leaf [Figure 1] through the midrib shows the following characterstics:[8,9]
Figure 1.
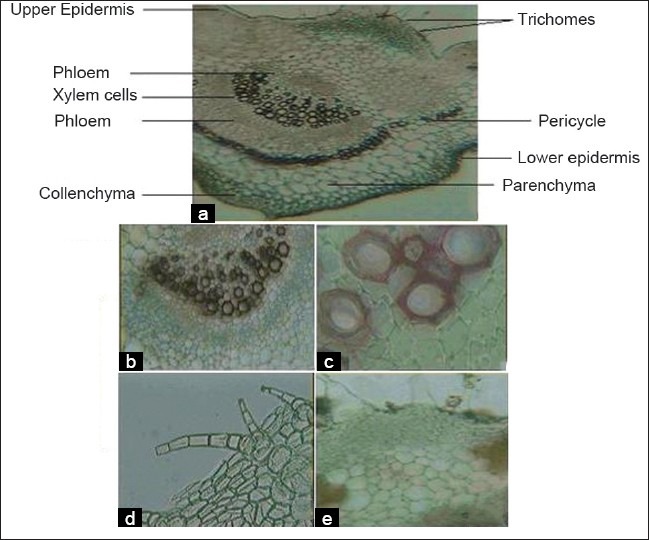
(a–e) Photographs of transverse sections of Trichosanthes dioica leaf, (a) Tranverse section showing Bicollateral vascular bundle (×4) of T. dioica leaves (b) Lignified Xylem and Phloem cells (×10),(c) Lignified xylem cells (×40), (d) Multicellular covering trichomes (×10), (e) Glandular trichomes (×10)
Presence of multicellular covering and glandular trichomes
Presence of upper and lower epidermis: Polygonal epidermal cells with cuticle
Bicollateral vascular bundle (xylem cells covered with phloem cells by both side)
Presence of pericycle at outside of phloem
Presence of parenchyma and collenchyma cells
Powder microscopy of leaves of T. dioica
On staining with different stains and after glycerin mounting, following powder characters were observed [Figures 2 and 3]:
Figure 2.
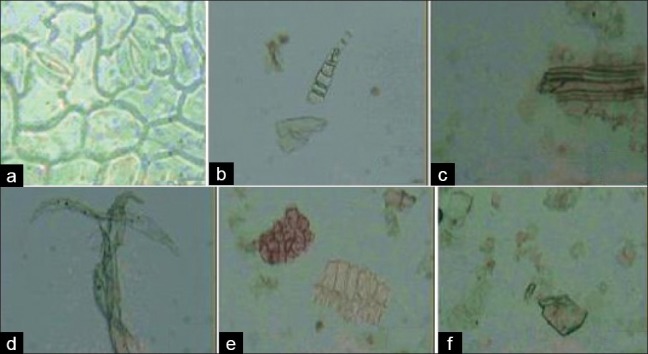
(a–f) Photographs of powder microscopic characterstics of T. dioica leaves, (a) Anomocytic stomata (×40), (b) Multicelluar trichome (×10), (c) Lignified spiral xylem vessels (×10), (b) Phloem fibires (×10), (e) Palisade cells (×10), (f) Prismatic calcium oxalate crystals (×10)
Figure 3.
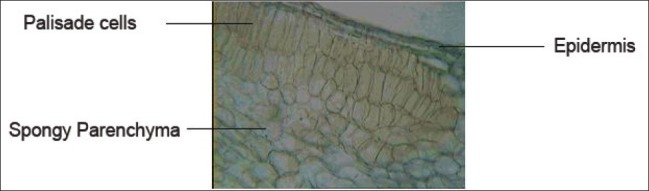
Mesophyll showing palisade and spongy parenchyma cells of T. dioica leaves (×10)
Anomocytic stomata
Muticellular trichomes
Lignified spiral xylem vessels
Phloem fibers with pits
Palisade cells
Prismatic shape calcium oxalate crystal
Mesophyll with 2–3 layered palisade cells and spongy parenchyma cell
Quantitative microscopy
With the help of camera lucida, leaf constants such as stomatal number, stomatal index, vein islets number, and palisade ratio were found. Stomata are present at both surfaces of the leaf [Figure 4].
Figure 4.
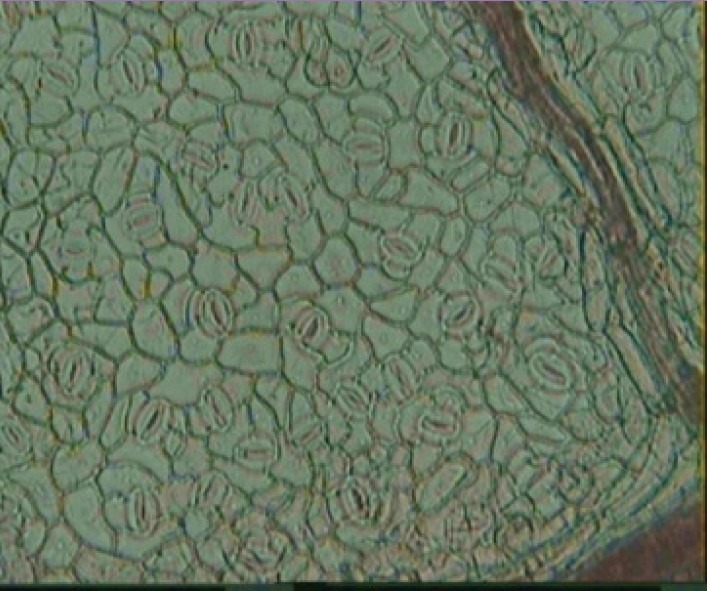
Stomata present in surface of T. dioica leaves (×10)
Lower surface of leaf was found to have more stomatal number than the upper surface. Palisade cells were found in the upper surface of the leaf.[10]
Stomatal number: 261–343 per mm2 (upper surface) and 400–441 per mm2 (lower surface)
Stomatal index: 19 (upper surface) and 30 (lower surface)
Vein islets number: 60–65 per mm2
Palisade ratio: 5–7
PHYSICOCHEMICAL EVALUATION[11]
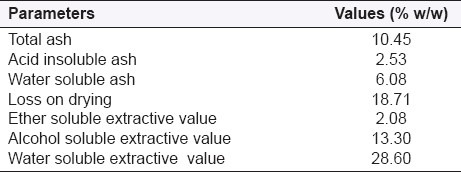
Various physicochemical parameters were analyzed by observations of three samples for each parameter and an average value of each parameter was determined as given in Table 3.
Table 3.
Physicochemical parameters of T. dioica leaves
CHEMICAL CONSTITUENTS
Early chemical study revealed that addition to a number of tetra and pentacyclic triterpenes, the toxic bitter principles cucurbitacins, a group of often highly oxygenated tetracyclic compounds with a unique carbon skeleton, and almost always a carbonyl group in ring C, could be consider as a taxonomic character in Cucurbitaceae. Structures of cucurbitacin B, which is found in T. dioica, are shown in Figure 5.
Figure 5.
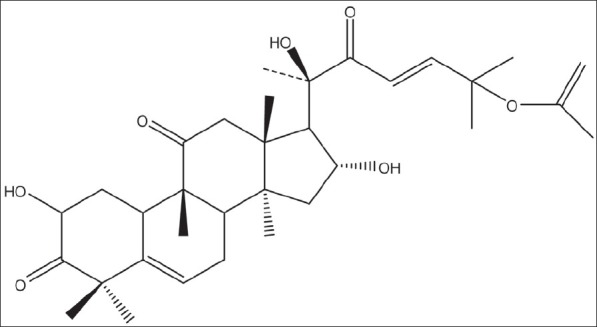
Cucurbitacin-B (molecular formula- C32H46O8)
Pointed gourd is rich in vitamins and contains 9.0 mg Mg, 2.6 mg Na, 83.0 mg K, 1.1 mg Cu, and 17.0 mg S per 100 g edible part.[12] The seeds of T. dioica contain a large amount of peptides. The seed peptides have the unique property of being resistant to the action of silver nitrate, a sensitive reagent commonly used to stain proteins.[13] The various chemical constituents present in T. dioica are vitamin A, vitamin C, tannins, and saponin.[14] Phytochemical evaluations of aqueous and ethanolic extracts have shown the presence of saponins and tannins.[15] The seed extract of T. dioica contains 7-oxidihydrokarounidol-3-benzoate as the most predominant component in the highly polar fraction of the nonsaponifiable lipid.[16]
Two main phytosterols present in T. dioica are, namely, 24α-ethylcholest-7-enol and 24β-ethylcholest-7-enol.[17] Seeds of T.dioica also contain lectin, a carbohydrate (specifically galactose) binding protein which is homologous to type-II ribosome inhibitory proteins (type-II RIP). Sultan and Kenoth (2004) have done purification, physicochemical characterization, saccharide specificity, and chemical modification of a Gal/GalNAc-specific lectin from the seeds of T. dioica.[18]
Kabir has evaluated that the seeds of T. dioica contain a large amount of peptides.[13] The seed peptides have the unique property of being resistant to the action of silver nitrate, a sensitive reagent commonly used to stain proteins. Sharma et al. have determined that the total phenolic content of T. dioica leaves was about two times more than that obtained from the fruits and seeds of M. olifera and E. officinalis, respectively.[19]
Preliminary phytochemical screening
The main phytochemical groups that were present were alkaloids, glycosides, flavonoids, carbohydrates, fixed oils, steroids, tannins, and phenols.[20] There was used four solvents viz. petroleum ether, chloroform, ethanol, and water on the basis of increasing polarity for successive extraction of dried leaves of T. dioica. The % yield of each extract and results of chemical screening are summarized in the Tables 4 and 5.
Table 4.
Percentage Yield on successive extraction in soxhlet apparatus

Table 5.
Phytochemical screening of various extracts of T. dioica leaves

CHROMATOGRAPHIC PROFILE
Thin layer chromatography
On performing thin layer chromatography (TLC) with appropriate mobile phases, the various extracts of leaves of T. dioica Roxb. showed the presence of three constituents in petroleum ether extract, six constituents in chloroform, and four constituents in ethanolic extracts.[21,22] The Rf values and observation of spots of these constituents are summarized in Table 6 and Figure 6.
Table 6.
TLC profile of various extracts for presence of maximum no. of constituents

Figure 6.
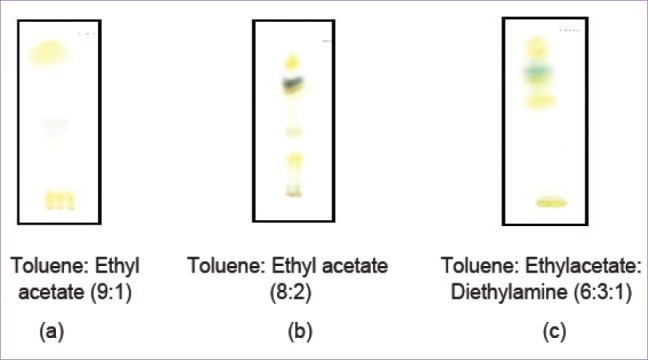
TLC for (a) petroleum ether extract; (b) chloroform extract; (c) ethanolic extract
Gas chromatography
In this order, first of all preparative chromatography of alcoholic (concentrated) extract was done by using general solvent system of alkaloid, toluene:ethylacetate:diethylamine (7:2:1). Then solvent was allowed to run up to appropriate height, the spots were marked with needle, and silica powder of these spots was carefully scraped and collected separately and dissolved in methanol. The gas chromatogram of the above prepared sample reveals four peaks having retention time 3.79, 14.59, 35.35, and 36.57; the peak corresponding to sample is the one having retention time 35.35 and 36.57 mm. Other peaks are representing some impurity/ contamination of the column. On further analyzing the compound emerging for gas chromatography (GC) column in mass spectrometer, the two probable compound identified were:[23]
Ferrocene, 1,1”,3,3’-tetrakis (1,1-dimethylethyl) with molecular formula C26H42Fe and molecular weight of 410
1,1’:2’,1”-Terphenyl, 3’,4’ - dimethyl - 5’,6’ - diphenyl with molecular formula C32H26 and molecular weight of 410
Spectral analysis (IR)
Looking to the spectral study of the newly synthesized (dihydropyridines) molecules, the carbonyl (> C=O) of the amidic functionality stretching was observed at 1655–1670 cm-1 and another ketonic group (ester or acetyl) was observed approximately at 1670–1690 cm-1 due to the conjugation with the DHP skeleton. The amide (>C–N stretching) is observed at 1300–400 cm-1. The stretching of secondary amine (>NH) appears approximately around 3100–3400 cm-1 which indicates the presence of –NH– in the compound. Here, in almost all the compounds, two –NH sis vibration are observed for since there are two secondary amine groups. One of them shows absorption at lower frequencies due to the carbonyl group attached to it. The stretching C–N appears at 1200–1400 cm-1 which further adds up the evidence of the presence of secondary amine group. T. dioica peak obtained from IR spectrum indicate the methyl overtone, amide, aromatic functional groups, etc.
CLINICAL INVESTIGATIONS
Crude drug T. dioica is known to have antiulcerous effect in polyherbal preparation. Two formulations have been clinically investigated as given below:[24]
Rai and Tripathi (1968) have showed that Patoladi kasaya, a polyherbal formulation, consisted of 11 herbs viz., Patola, Haritaki, Bibhitaka, Amalaki, Kutaki, Cirayata, Amrta, Pittapapada, Sunthi, and Bhrngaraja exhibited complete improvement in 50% cases and partial improvement in 40% cases with peptic ulcer (10 patients case study).
Tripathi and Pathak (1975) have worked another Patoladi kasaya which consisted of only four herbs namely Sunthi, Patola, Amrta, and Kutaki in the 33 case study of duodenal ulcer. It kept the patients symptoms/complication free when given in dose of 40 ml/day in two divided doses. It normalized both hyper and hypoacidity of these patient.
Aryavansha et al. have studied the efficacy of single herb patola in 20 patients with duodenal ulcer. Effectivity of patola in duodenal ulcer was found to have 45% excellent response out of 20 cases.
PHARMACOLOGICAL PROPERTIES
Antidiabetic activity
Rai et al. have showed the glycemic attributes of an aqueous extract of T. dioica leaves in normal as well as various diabetic models. The variable doses of 250, 500, and 750 mg kg- 1 body weight of the extract were administered orally to normal and streptozotocin (STZ) induced sub- and mild-diabetic rats in order to define its glycemic potential. This evidence clearly indicates that the aqueous extract of T. dioica leaves has good hypoglycemic potential along with a high antidiabetic profile.[25]
Rai et al. have showed that in rats with STZ-induced severe diabetes mellitus, aqueous extract of T. dioica fruits dose of 1000 mg/kg body weight daily once for 28 days reduced the levels of fasting blood glucose, postprandial glucose, asparate amino transferase, alanine amino transferase, alkaline phosphatase, creatinine, urine sugar, and urine protein, whereas total protein and body weight were increased. No toxic effect was observed during LD50. This study suggests that further detailed toxicity studies and mechanism of action of T. dioica would be useful for undertaking human trials.[26]
Chandrasekhar et al. have reported that pointed gourd possesses the medicinal property of lowering blood sugar level in rats.[27]
Hepatoprotective activity
Ghaisas et al. have showed hepatoprotective activity of aqueous and ethanolic extracts of T. dioica (whole plant) in ferrous sulfate-induced liver injury. Ethanolic and aqueous extracts of T. dioica at different doses (100, 200, and 400 mg/kg) and silymarin (100 mg/kg) were administered orally for 10 days. The groups treated with 400 mg/kg aqueous and ethanolic extracts showed significant reduction in aspratate aminotransferase (AST), Alanine aminotransferase (ALT), and alkaline phosphotase (ALP) levels. The pretreatment with T. dioica extracts showed profound histopathological protection to liver cells as evident from histopathological studies. Hence, it can be concluded that T. dioica Roxb. has significant hepatoprotective activity.[15]
Cholesterol-lowering activity
Sharmila et al. have observed cholesterol-lowering activity of the aqueous fruit extract of T. dioica Roxb. in normal and STZ diabetic rats.[28]
Sharma and Pant have showed influence of alcoholic extract of whole fruit of T. dioica on blood sugar, serum lipids, lipoproteins, and fecal sterols in normal albino rabbits. Effects of oral administration of 2 ml per day of suspension (in water) of alcoholic extract of whole fruit of T. dioica (2%) with basal diet for 4 weeks have been studied in the normal albino rabbits. It was observed that this extract lowered the blood sugar, total cholesterol, low density lipoprotein cholesterol, and triglyceride levels and increased high density lipoprotein cholesterol, phospholipid, and fecal sterol levels.[29]
Antiinflammatory activity
Fulzul et al. have found antiinflammatory activity of polyherbal formulation “Jatyadi Ghrita,” the ingredients of Jatyadi Ghrita are Jasmine officinale, Azadirachta indica, Berberis aristata, Curcuma longa, Picrorrhiza kurroa, Rubia cordifolia, T. dioica, Aristolochia indica, Hemidesmus indicus, Randio spinosa, Glycyrrhiza glabra, and Cow's ghee.[30]
In skin disorder
Bhujbal has showed that polyherbal formulation including T. dioica is useful in skin disorder. Fifty cases of various skin diseases were treated with decoction of a mixture of Trichosanthes and other herbal crude drugs in a dose of 20–40 ml empty stomach with hot water and honey for 4–6 weeks. The drug was found to be useful in the entire patient and no side effect was observed.[31]
Antifungal activity
Hariti and Rathee have stated that the fixed oil of seeds of Trichosanthes species including T. dioica has antifungal property.[32]
Antibacterial activity
Hariti and Rathee have showed antibacterial activity of the unsaponifiable fraction of the fixed oil of T. dioica seeds against Bacilus anthracis and Xanthomonas malracearum.[33]
Rai et al. have reported the in vitro assessment of antimicrobial activity of different concentrations of extract of different parts of T. dioica. Five clinical isolates of different bacterial strains were used and the disc diffusion method was opted. The results reveal that leaves, fruits, and seeds, all three parts of T. dioica plant, can be used as antibacterial agents. Though the leaves extract was active against all five strains and the highest inhibition was observed against Mycobacterium smegmatis. Thus the leaves extract could be used for tuberculosis treatment.[34] Antimicrobial activity of different parts of T. dioica against certain pathogens is given in Table 7.
Table 7.
Extent of antimicrobial activity of Trichosanthes dioica against certain pathogens

Antioxidant activity
Shivhare et al. evaluated the antioxidant activity of fruits of T. dioica (cucurbitaceae) and compared with ascorbic acid (standard). Materials and Methods: Antioxidant activity of aqueous extract of T. dioica fruits was studied for its free-radical scavenging property in different in vitro methods as 1,1 diphenyl-2-picryl hydrazyl, nitric oxide, reducing power assay and hydrogen peroxide radical method. The findings could justify the inclusion of this plant in the management of antioxidant activity.[35]
The antioxidative potential of T. dioica in ferrous sulfate (FeSO4), hydrogen peroxide (H2O2), and carbon tetrachloride (CCl4) induced lipid peroxidation has been significantly proven.[36]
Wound healing activity
Shivhare et al. reported the wound healing potential of methanolic (MeOH) extract of T. dioica fruits.[37]
Shivhare et al. have used methanolic extract of the plant T. dioica for assessment of healing potential in the form of simple ointment using full thickness burn wound model in rats. The effect produced by the extract ointment showed significant healing when compared with the control and standard groups.[38]
Other significant studies on trichosanthes
Fluorescence quenching and time-resolved fluorescence studies have been carried out on the T. dioica seed lectin. The emission λmax of native T. dioica seed lectin, seen at 328 nm, shifts to 343 nm upon denaturation with 6 M guanidinium chloride. Quenching titrations were performed with neutral (acrylamide and succinimide) and ionic (I- and Cs+) quenchers in order to probe the exposure and accessibility of tryptophan residues of the protein. Maximum quenching was observed with acrylamide, followed by succinimide, iodide, and Cs+.[39]
Galactose-specific lectins have been purified from seeds and roots of Trichosanthes. The roots also contain an abortifacient protein, trichosanthin, which is a ribosome-inactivating protein (RIP), a similar RIP, trichokirin, was also found in the seeds of Trichosanthes.[40]
From the fruits of Trichosanthes 14 cucurbitane glycosides (khekadaengosides A–J, M–N, cucurbitacin J 2-O-bglucopyranoside and cucurbitacin K 2-O-b-glucopyranoside), a hexanorcucurbitane glucoside (khekadaengoside K) and octanorcucurbitane (khekadaengoside L) were isolated along with two known cucurbitane glucosides (cucurbitacin 2-O-b-glucopyranoside and 25-O-acetyl-cucurbitacin 2-O-b-glucopyranoside). Structural elucidations were based on chemical and spectroscopic analyses.[41]
CONCLUSION
Trichosanthes is an easily available common plant, the fruit of which is integral part of an average Indian diet, being consumed as a vegetable. The plant belongs to family Cucurbitaceae, which has given us many important medicinal plants such as Momordica charantia, Citrullus colocynthis, etc., from which important pharmacological activities and markers such as charantin and cucurbitacin have been reported and isolated; hence, it would not be wrong to state that still a lot has to be worked upon this important plant. Apart from old traditional texts, such as Charak Samhita mentioned the protective role of Trichosanthes on important body organs such as liver, spleen, heart, etc., many of which are now scientifically proven. Clinical investigation on peptic ulcer with polyherbal formulation, where Trichosanthes was an integral part, has shown promising results. The authors perceive that Trichosanthes may play a significant role in developing formulations for geriatric care as it is having almost all the properties of pharmaceutical care designed for the elderly, i.e., antioxidant property, antidiabetic property, cholesterol lowering, hepatoprotective, etc. In developing countries like ours one must fully explore this important medicinal plant which might provide us some important “leads/hits” in near future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Khare CP. Encyclopedia of Indian medicinal plants. Berlin, Heidelberg; New York: Springer-Verlag; 2004. p. 458. [Google Scholar]
- 2.A Dictionary of Indian medicinal plant's raw material and industrial products. New Delhi: CSIR; 1998. The Wealth of India; pp. 289–90. [Google Scholar]
- 3.Kirtikar KR, Basu BD. Indian medicinal plant. 2nd ed. Dehradun: Oriental enterprises; 2001. pp. 1543–4. [Google Scholar]
- 4.Singh BP, Wayne F. Whitehead Pointed gourd: Potential for temperate climates. J Janick. 1999;118:27–35. [Google Scholar]
- 5.Nadkarni AK. Indian Materia Medica. 3rd ed. Mumbai: Popular prakashan Pvt. Ltd; 1982. pp. 1236–7. [Google Scholar]
- 6.Khare CP. Encyclopedia of Indian medicinal plants. Berlin, Heidelberg; New York: Springer-Verlag; 2004. pp. 457–8. [Google Scholar]
- 7.Maurya S. Standardization of male plant population in pointed gourd. Ann Agr Sci. 1985;30:1405–11. [Google Scholar]
- 8.Evans WC. Trease and Evans Pharmacognosy. Edinburgh: W. B. Saunders; 2002. p. 150. [Google Scholar]
- 9.Khandelwal KR. Practical Pharmacognosy: Techniques and Experiments. Pune: Nirali Prakashan; 2005. pp. 149–53. [Google Scholar]
- 10.Kokate CK. Practical Pharmacognosy. New Delhi: Vallabh Prakashan; 1994. pp. 107–11. [Google Scholar]
- 11.WHO Guidelines. Quality Control Methods for Medicinal Plant Materials. [Last accessed on 2011 July 21]. Available from: http://www.google.com/who/pharm/92.559.rev.1.pdf .
- 12.Singh K. Pointed gourd (Trichosanthes dioica Roxb.) Indian Hort. 1989;33:35–8. [Google Scholar]
- 13.Kabir S. The novel peptide composition of the seeds of Trichosanthes dioica Roxb. Cytobios. 2000;103:121–31. [PubMed] [Google Scholar]
- 14.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal plants. 1st ed. New Delhi: CSIR; 2002. pp. 340–1. [Google Scholar]
- 15.Ghaisas MM, Tanwar MB, Ninave PB, Navghare VV, Deshpande T. Hepatoprotective activity of aqueous and ethanolic extract of T.dioica in ferrous sulphate induced liver injury. Pharmacologyonline. 2008;3:127–35. [Google Scholar]
- 16.Toshihiro A, Yumico K, Yoshimasa K, Kunio K, Thakur S, Tamura T. 7-oxodihydrokarounidiol-3-benzoate and other triterpenes from the seeds of Cucurbitaceae. Phytochemistry. 1997;46:1261–6. [Google Scholar]
- 17.Kongton S. Chemical study of bioactive constituents from Trichosanthes cucumerina root and fruit juice. Hong Kong: Thesis, Mahidol University; 2003. [Google Scholar]
- 18.Ali N, Mohammed S, Kenoth R, Swamy MJ. Purification, physicochemical characterization, saccharide specificity, and chemical modification of a Gal/GalNAc specific lectin from the seeds of Trichosanthes dioica. Arch Biochem Biophysics. 2004;432:212–21. doi: 10.1016/j.abb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Sharma RK, Chatterji S, Rai DK, Mehta S, Rai PK, Singh RK, et al. Antioxidant activities and phenolic contents of the aqueous extracts of some Indian medicinal plants. J Med Plants Res. 2009;3:944–8. [Google Scholar]
- 20.Mukherjee PK. Quality Control of Herbal Drugs. New Delhi: Business Horizons; 2002. pp. 164–71. [Google Scholar]
- 21.Wagner H, Bladt S. Plant drug analysis- A Thin Layer Chromatography Atlas. New Delhi: Thomson Press (India) Ltd; 2004. pp. 275–8. [Google Scholar]
- 22.Harborne JB. Phytochemical Methods: A Guide to modern techniques of plant analysis. Berlin, Heidelberg: Springer Science Business Media; 2005. pp. 40–278. [Google Scholar]
- 23.Singh S, Machawal L, Chauhan MG. Pharmacognostic study of male leaves of Trichosanthes dioica Roxb. with special emphasis on microscopic technique. J Pharmacogn Phytother. 2010;2:71–5. doi: 10.4103/0975-7406.80780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh KP, Singh RH. Clinical investigation of importance of T. dioica. J Res Ayurveda Siddha. 1985;6:132–40. [Google Scholar]
- 25.Rai PK, Jaiswal D, Singh RK, Gupta RK, Watal G. Glycemic Properties of Trichosanthes dioica leaves. Pharmaceutical biology. 2008;46:894–9. [Google Scholar]
- 26.Rai DK, Rai PK, Jaiswal D, Sharma B, Watal G. Effect of water extract of Trichosanthes dioica fruits in streptozotocin induced diabetic rats. Indian J Clin Biochem. 2008;23:387–90. doi: 10.1007/s12291-008-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandrasekhar B, Mukherjee B, Mukherjee SK. Blood sugar lowering effect of Trichosanthes dioica Roxb. in experimental rat models. Int J Cru Drug Res. 1988;26:102–6. [Google Scholar]
- 28.Sharmila BG, Kumar G, Rajasekhara PM. Cholesterol-lowering activity of the aqueous fruit extract of Trichosanthes dioica in normal and streptozotocin diabetic rats. J Clin Dia Res. 2007;1:561–9. [Google Scholar]
- 29.Sharma G, Pant MC. Influence of alcoholic extract of whole fruit of T. dioica on blood sugar, serum lipids, lipoproteins and faecal sterols in normal albino rabbits. Indian J Clin Biochem. 1992;1:53–6. [Google Scholar]
- 30.Fulzule SV, Satturwar D, Joshi SB. Studies on anti-inflammatory activity of a polyherbal formulation- Jatydi Ghrita. Indian Drugs. 2001;39:42–4. [Google Scholar]
- 31.Bhujbal MM. Patoladi Quath in the management of skin disorder. Deerghayu. 1999;58:72–6. [Google Scholar]
- 32.Hariti M, Rathee PS. Antifungal activity of the unsaponifiable fraction of the fixed oil of Trichosanthes seeds. Asian J Chem. 1996;8:180–2. [Google Scholar]
- 33.Hariti M, Rathee PS. Antibacterial activity of the unsaponifiable fraction of the fixed oil of Trichosanthes seeds. Asian J Chem. 1995;7:909–11. [Google Scholar]
- 34.Rai PK, Mehta S, Gupta RK, Watal G. A Novel Antimicrobial Agents Trichosanthes dioica. Int J Pharma Bio Sci. 2010;1:1–9. [Google Scholar]
- 35.Shivhare Y, Singh P, Rajak H, Patil UK, Pawar RS. Antioxidant potential of Trichosanthes dioica Roxb. (fruits) Phcog J. 2010;2:107–11. doi: 10.1016/j.jep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Dixit Y, Kar A. Antioxidative activity of some vegetable peels determined in vitro by inducing liver lipid peroxidation. Food Res Int. 2009;42:1351–4. [Google Scholar]
- 37.Shivhare Y, Singour P, Patil UK, Pawar RS. Wound healing potential of methanolic extract of Trichosanthes dioica Roxb. (fruits) in rats. J Ethnopharmacol. 2010;127:614–9. doi: 10.1016/j.jep.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Shivhare Y, Singh P, Patil UK. Healing potential of Trichosanthes dioica Roxb. on burn wounds. Res J Pharmacol Pharmacodyn. 2010;2:168–71. [Google Scholar]
- 39.Ali N, Mohammed S, Swamy MJ. Fluorescence quenching and time-resolved fluorescence studies on Trichosanthes dioica seed lectin. J Photochem Photobiol B: Biol. 2005;80:93–100. doi: 10.1016/j.jphotobiol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Falasca AI, Abbondanza A, Barbieri L, Bolognesi A, Rossi CA, Stirpe F. Purification and partial characterization of a lectin from seeds of Trichosanthes kirilowii. Federation Eur Biochem Soc (FEB) 1989;246:159–62. doi: 10.1016/0014-5793(89)80274-1. [DOI] [PubMed] [Google Scholar]
- 41.Tripetch KB, Ryoji K, Yamasakia K. Cucurbitane, hexanorcucurbitane and octanorcucurbitane glycosides from fruits of Trichosanthes tricuspidata. Phytochemistry. 2002;59:215–28. doi: 10.1016/s0031-9422(01)00430-7. [DOI] [PubMed] [Google Scholar]


