Abstract
A method for blood collection from the jugular vein of mice without anesthesia was compared with a tail-incision technique. Jugular vein blood collection allowed withdrawal of almost 15% of the circulating blood volume at a time in less than 1 min. Hemolysis, hematocrit, and plasma thrombin–antithrombin complexes (a marker of blood coagulation) were higher in samples collected from the tail vein than the jugular vein. Mice produced similar plasma corticosterone levels after serial blood collection by either method. Tail incision led to a slight but significant increase in C-reactive protein levels. Using the jugular venipuncture technique, we then performed a pharmacokinetic study and an oral glucose tolerance test. Plasma concentrations of levofloxacin, an antimicrobial agent, were dose-dependently elevated after oral administration, and linear increases in Cmax and AUC were observed. We also confirmed that overall glucose excursion is significantly decreased in mice treated with exendin 4, a glucagon-like peptide 1 agonist. These results indicate that the jugular venipuncture is a useful technique from the point of view of no requirement for anesthetics, serial blood collection at short intervals, large volume of blood collection, quality of sample and animal welfare. This technique is of particular interest for studies that examine time-dependent changes in blood variables.
Abbreviation: CRP, C-reactive protein
Blood collection is one of the most common procedures performed on laboratory animals, and obtaining more than one sample per subject is often necessary. Serial blood collection from mice can be challenging because of animal size. Various methods for serial sampling from mice have been reported, including blood collection from the retroorbital sinus,6,7,18,23 the lateral tail vein,4,15 saphenous vein,1,9 sublingual vein,8,32 and the submandibular vein.6,7 However, these methods each have their own drawbacks, including associated tissue damage, a need for anesthesia, time-intensive, contamination with other tissue fluids, and limited blood volume. For example, retroorbital sinus bleeding requires anesthesia27 and carries the risk of secondary ocular injury.3,30 Saphenous vein puncture can be time-consuming and may not be feasible at short intervals of blood collection.10,12 Compared with saphenous venipuncture, submandibular bleeding required less time and allowed collection of a larger volume of blood, but was more stressful than saphenous venipuncture and greater tissue damage.12
Previous reports have described sampling from the jugular vein of mice.11,13 Although these reports described how to collect blood sample, it is unknown whether jugular venipuncture meets the following inclusion criteria: serial blood collection, large sample volume, high quality of sample, and animal welfare. The purpose of the current study was to compare jugular venipuncture with a method of tail incision, which has been used routinely at our institute. We evaluated the effect of the method of blood collection on various parameters, including hemoglobin, hematocrit, and levels of C-reactive protein (CRP), corticosterone, and thrombin–antithrombin complexes (a marker of blood coagulation. We also evaluated whether jugular vein blood collection is suitable for studies that require serial samples, such as pharmacokinetic studies and oral glucose tolerance testing.
Materials and Methods
Animals.
Male ICR and C57BL/6N mice (age, 5 wk) were obtained from Charles River Laboratories Japan (Yokohama, Japan). The mice were housed in a regulated environment (23 ± 2 °C, 55% ± 15% relative humidity) under a 12:12-h light:dark cycle (lights on, 0800 to 2000) and received food (F2; Funabashi Farm, Chiba, Japan) and tap water ad libitum. Blood collection was carried out on 6-wk-old mice. All experimental procedures were performed in accordance with the guidelines of the Daiichi–Sankyo IACUC.
Blood collection from the jugular vein and sample handling.
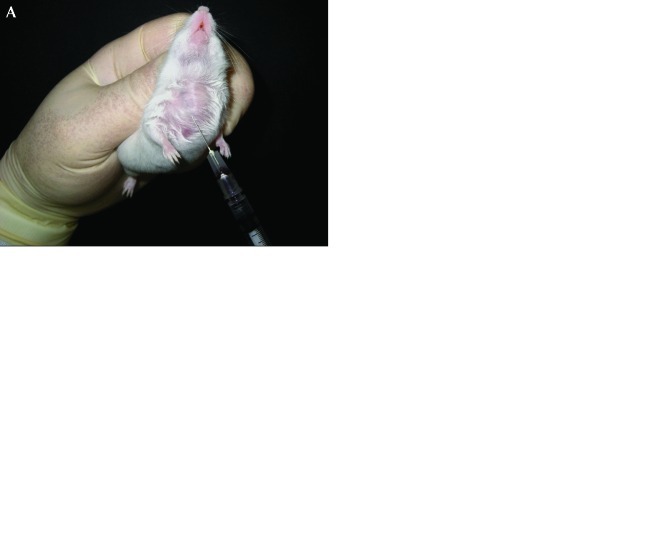
Jugular vein blood collection was performed by a modification of the method in previous reports.11,13 Operators required 0.5 to 1 d of training to master this bleeding technique. In the present study, blood collection was performed by 2 technicians. The mouse was held by grasping the loose skin of the back firmly with fingers, and its head was elevated without spreading the submaxillary gland. Petroleum jelly was applied to fur that had been wetted with alcohol, revealing the jugular vein as a blue, pulsating area (Figure 1 A). When pigmented mice were used, the fur around the thoracic region was removed by shaving (Figure 1 B). By using a 1-mL syringe, a 27-gauge needle was inserted into the jugular vein through the pectoral muscle below the sternoclavicular junction (Figure 1 A and B). Blood was withdrawn slowly to avoid the collapse of these vessels, and approximately 30 to 50 μL blood was collected at each time point. Immediately after blood collection, gentle pressure was applied to the site to stop the bleeding. The mean time from the beginning of animal handling to the end of blood collection was within 1 min (not including time for hair shaving), and approximately 15% of the total blood volume, the maximum allowed,3 was collected from each mouse. All procedures in both albino and pigmented mice were performed without skin section and anesthesia. After using a plastic tubing cutter (Bel-Art Products, Pequannock, NJ) to cut the syringe, the collected samples were transferred into heparin-coated capillary tubes unless otherwise noted (Figure 1 C and D) and centrifuged at 10,000 × g for 10 min (model 3220, Kubota, Tokyo, Japan). Plasma samples were stored at −80 °C until analyzed.
Figure 1.
Blood collection from the jugular veins of ICR albino and C57BL pigmented mice. (A) By applying petroleum jelly to fur wetted with alcohol, the jugular vein was visible as a blue, pulsating area. By using a syringe, a 27-gauge needle was inserted into the jugular vein through the pectoral muscle. (B) Hair around the thoracic region was removed by shaving. Blood sampling was performed by using a syringe with a 27-gauge needle. (C) Cutting the syringe with a plastic tubing cutter. (D) The collected blood sample was transferred into a capillary tube and then centrifuged.
Blood collection from the lateral tail vein.
Mice were placed in a conventional holder (ICN2, ICM, Tsukuba, Japan). Blood samples were obtained by obliquely incising the lateral tail vein by using a razor blade, and 30 to 50 μL of blood was collected into a heparinized capillary tube. After collection, bleeding was stopped by gentle compression on the wound for a few seconds. The length of time required for collecting 30 to 50 μL of blood was less than 1 min. This method was applicable to both albino and pigmented mice. For repeated sampling, the surface of the original wound was disrupted.
Assay of various parameters.
Heparinized plasma samples were used to determine hemoglobin, CRP, and corticosterone levels. Thrombin–antithrombin complexes were assayed by using plasma samples treated with 3.2% (w/v) sodium citrate. Hemoglobin concentration was determined by a colorimetric method based on a cyanohemoglobin method (Biochain, Hayward, CA). ELISA assays for CRP (Life Diagnostics West Chester, PA), corticosterone (Yanaihara, Shizuoka, Japan), and thrombin–antithrombin complexes (Siemens, Tokyo, Japan) were used to quantify concentrations in a microplate reader (Spectra Max 250, Molecular Devices, Sunnyvale, CA).
Pharmacokinetic study.
Levofloxacin, an antimicrobial agent (Daiichi–Sankyo, Tokyo, Japan), was administered orally at 3, 10, or 30 mg/kg to ICR mice. Blood was withdrawn at 15, 30, 60, 120, and 240 min after administration. All samples were measured by liquid chromatography with tandem mass spectrometry. Briefly, levofloxacin was extracted with acetonitrile and applied to a chromatograph (series 1100, Agilent Technologies, Santa Clara, CA) and tandem mass spectrometer (API 4000, Applied Biosystems, Foster City, CA). Chromatographic separation was performed on a Shim-pack XR-ODS column (inner diameter, 2 mm; length, 30 mm; Shimadzu, Kyoto, Japan). The analysis was performed in the multiple reaction monitoring mode with monitoring of precursor–product ion pairs of m/z 363→319 for levofloxacin and m/z 283→265 for nuflumic acid (Sigma-Aldrich, St Louis, MO) used as an internal standard.
Oral glucose tolerance testing.
Exendin 4 (5 μg/kg), a glucagon-like peptide-1 agonist (AnaSpec, San Jose, CA), was administered intraperitoneally 30 min before glucose administration. Oral glucose tolerance testing was performed by administering glucose at 2 g/kg to C57BL/6N mice, and collecting blood at 0, 15, 30, 60, and 120 min after glucose loading. The blood glucose levels were determined by using a Glucose CII-Test (Wako Pure Chemical Industries, Osaka, Japan), which measures the oxidation of β-D-glucose transformed by the action of mutarotase.
Statistical analysis.
All data are expressed as mean ± 1 SD. Statistical analyses were performed by using EXSAS version 7.5.2.2 (Arm, Osaka, Japan) which is based on SAS release 9.1.3 (SAS Institute Japan, Tokyo, Japan). Hematocrit, hemoglobin, and thrombin–antithrombin complexes were analyzed by unpaired Student t tests. Comparisons of changes in CRP, corticosterone, and glucose levels were analyzed by repeated-measures ANOVA, and time-dependent changes in CRP were compared by one-way ANOVA followed by the Dunnett test. The relationship between dose of levofloxacin and pharmacokinetic parameters was studied by linear regression analysis. Differences yielding a P value less than 0.05 were considered statistically significant.
Results
Comparison of jugular venipuncture and tail incision for collection of single blood samples.
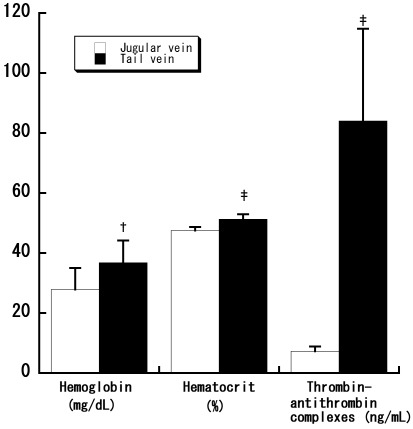
To characterize samples, we first measured plasma hemoglobin levels to assess collection-associated hemolysis of erythrocytes. Samples from the tail vein had significantly (P < 0.01) higher plasma hemoglobin levels than did those from the jugular vein (Figure 2), indicating increased hemolysis of erythrocytes by tail incision. In addition, hematocrit values were significantly (P < 0.001) higher in samples collected from the tail vein than the jugular vein (Figure 2). Furthermore, levels of plasma thrombin–antithrombin complexes were approximately 11-fold higher (P < 0.001; Figure 2) in samples from the tail vein compared with the jugular vein. These results suggest that jugular vein puncture leads to less coagulation than does incision of the tail.
Figure 2.
Comparison of jugular venipuncture and tail incision for single blood collection. Blood collection from C57BL/6N mouse was performed at the timing of once per animals. Plasma samples were compared for hemolysis, hematocrit, and levels of thrombin–antithrombin complexes. Data represent the mean ± 1 SD (n = 10 in each group). Significant (†, P < 0.01; ‡, P < 0.001 [Student t test]) differences from value for jugular venipuncture are indicated.
Comparison of jugular venipuncture and tail incision for collection of serial blood samples.
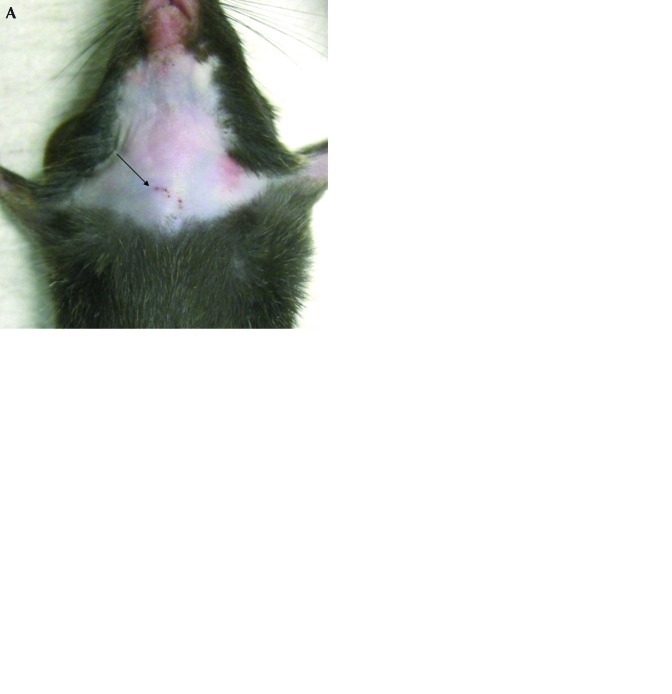
To examine the influence of sequential blood collection on animal stress and tissue damage, blood was obtained from either the jugular or lateral tail vein of mice once daily for 5 d. During the study period, a single technician collected blood samples at the same time each day. Serial blood collection was performed successfully by using the same jugular vein throughout the study, without having to access contralateral vessel. Mice that underwent jugular venipuncture had only slight hemorrhage and minimal tissue damage at the injection site (Figure 3 A and B). For tail incision, because serial blood collection was performed by disrupting the original wound, all mice had small incision wounds (data not shown). We evaluated the effect of serial blood collection on CRP, an acute-phase protein that is increased in plasma after acute tissue damage or inflammation.14,23,25 CRP levels in samples from the jugular vein were consistent throughout serial blood collection (Figure 3 C). However, blood samples from the tail vein showed slight but significant time-dependent increases in CRP (P < 0.05 for day 2 to 4; P < 0.001 for day 5; Figure 3 C). Tail incision led to CRP levels that were significantly (P < 0.001) higher than the mean for the jugular sampling method (Figure 3 C).
Figure 3.
Comparison of 2 sampling techniques for serial blood collection. Blood samples were collected daily for 5 d from C57BL/6N mice. (A and B) Tissue damage after serial sampling from the jugular vein. Arrows show injection sites. Plasma levels of (C) CRP and (D) corticosterone were compared between 2 techniques. Data represent the mean ± 1 SD (n = 10 in each group); significant differences from day 1 values (*, P < 0.05; ‡, P < 0.001 [one-way ANOVA followed by Dunnett test] and between jugular venipuncture and tail incision (+, P < 0.001[repeated-measures ANOVA]) are indicated.
Because handling-associated stress might differ between 2 sampling techniques, we measured plasma corticosterone as a key indicator of stress. Plasma corticosterone levels did not differ across the 5 d of blood collection for either blood collection technique (Figure 3 D). Furthermore, there was no statistical difference in corticosterone levels between the 2 sampling methods, suggesting similar stress responses in the 2 groups of mice (Figure 3 D).
Pharmacokinetic study and oral glucose tolerance test.
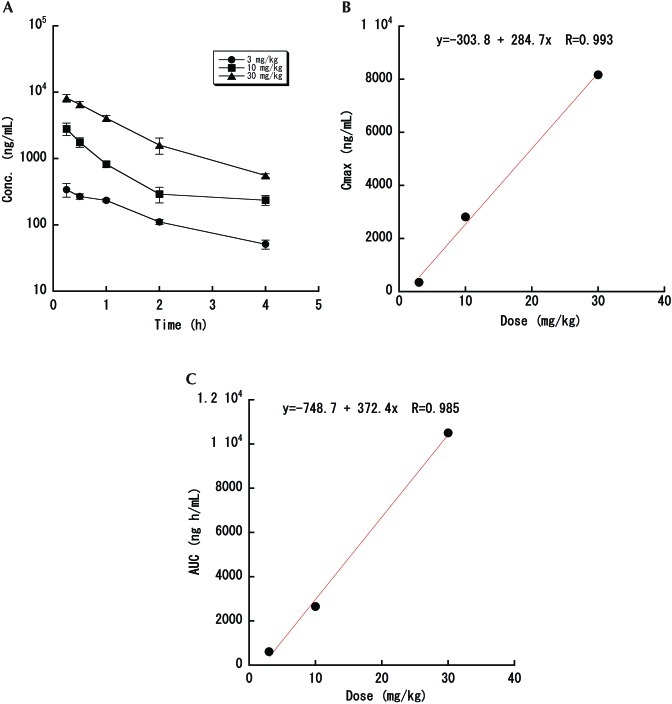
To further assess whether the jugular venipuncture technique is suitable for studies that require serial blood collection, we carried out a pharmacokinetic study and oral glucose tolerance testing. After oral administration of levofloxacin to mice, the plasma concentration of the drug increased dose-dependently (3 to 30 mg/kg) and then gradually decreased thereafter (Figure 4 A). Pharmacokinetic studies revealed that dose proportionality in Cmax (first sampling point) and AUC demonstrated high correlation coefficients of 0.993 and 0.985, respectively (Figure 4 B and C). In addition, blood glucose levels increased markedly in vehicle-treated mice after oral glucose challenge (Figure 5). Mice treated with exendin 4, a glucagon-like peptide-1 agonist, had significantly (P < 0.001) lower blood glucose levels than did mice that received vehicle alone.
Figure 4.
Pharmacokinetic study of levofloxacin. Levofloxacin was administered orally at 3, 10, or 30 mg/kg to ICR mice. Blood was drawn at 15, 30, 60, 120, and 240 min after administration. (A) Plasma concentration curve. (B) Cmax. (C) AUC. Data represent the mean ± 1 SD (n = 3 in each group).
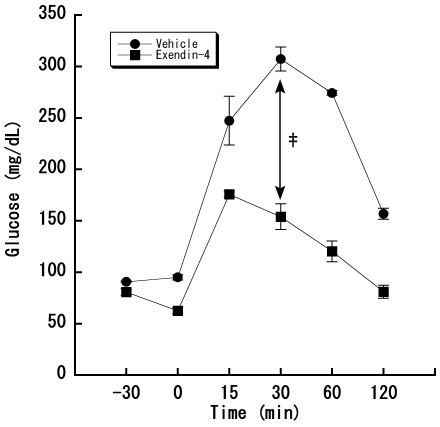
Figure 5.
Effect of exendin 4 on plasma glucose levels in mice. Exendin 4 (5 μg/kg) was administered intraperitoneally 30 min before glucose administration; oral glucose tolerance testing was performed by administering glucose (2 g/kg) to C57BL/6N mice and collecting blood at 0, 15, 30, 60, and 120 min after glucose loading. Data represent the mean ± 1 SD (n = 4 in each group). A significant (‡, P < 0.001 [repeated-measures ANOVA]) differences between vehicle-treated and exendin-4–treated groups is indicated.
Discussion
Repeated blood collection from individual animals would reduce animal use and decrease interindividual variability and variation. However, repeated sample collection is technically difficult in small animals such as mice due to their relatively small blood volume and the lack of easily accessible vessels. A common technique for blood sampling in mice involves collection from the lateral tail vein by transection or puncture.1,4,28 Although tail incision has been used routinely at our institute, this technique tends to yield small volumes of blood and is time-consuming. To provide an alternative method for serial blood collection in mice, we compared the jugular venipuncture with tail incision.
Both technicians were able to collect single blood samples with the same degree of technical skill for all samples. The mean time required for blood collection from the jugular vein was less than 1 min, and this sampling method enabled one-time collection of 15% of the circulating blood volume, the maximal amount allowed.3 Therefore, jugular venipuncture facilitates potential serial blood collection at short intervals and of large volumes. The time required to collect a small volume of blood (less than 50 μL) by tail incision was similar to that for the jugular vein puncture. However, the tail incision technique was time-consuming when more than 100 μL blood was collected.
The quality of blood samples influences the results of analyses of biochemical parameters. For example, increased hemolysis affects the values of some biochemical parameters, including AST and ALT.19 Blood samples obtained by tail incision exhibited greater hemolysis of erythrocytes than did jugular vein collection, although the differences in hemolysis were macroscopically unremarkable. Furthermore, hematocrit values and levels of plasma thrombin–antithrombin complexes were higher in samples collected from the lateral tail vein than the jugular vein, indicating that jugular vein bleeding causes less coagulation than does incision of the tail. Therefore, our results suggest that blood samples from the jugular vein are higher-quality than those from tail incision.
In regard to the health and welfare of the animals are taken into account, it is important to minimize the tissue damage incurred through repeated blood collection. The mice that underwent jugular vein puncture tolerated serial blood collection well and had only small hematomas and slight tissue damage at the injection site. For tail vein incision, although an incision wound was observed in all animals, the wound did not appear to produce tissue damage that affected animal wellbeing. Therefore, serial blood collection by both methods was well-tolerated. CRP is an acute-phase protein that is elevated in blood during inflammation-associated tissue injury.14,22,25 For example, CRP increases approximately 20-fold in mouse serum due to exposure to endotoxin.20 However, CRP increases only slightly during the acute-phase response.21,26 Samples from the jugular vein had no changes on CRP across the 5 d of blood collection, whereas blood collection by tail incision resulted in slight but significant increases in CRP concentration. Therefore, our data indicate that jugular venipuncture may induce less inflammatory tissue injury than does tail incision.
The stress induced by blood collection may be more conspicuous when the experimental design requires serial sample collection or when scientific concerns preclude the use of anesthetic agents. In addition, mice subjected to physical restraint may have different stress responses between 2 restraint procedures. We therefore measured plasma corticosterone as a key indicator of stress. Because basal corticosterone levels follow circadian rhythms,31 a single technician collected blood samples at the same time each day. Despite different restraint procedures, corticosterone levels were similar between the mice that underwent jugular venipuncture and those subjected to tail incision, indicating that handling-associated stress was comparable between the 2 collection methods. These similar stress responses may be, in part, due to the fact that the time required for blood collection by either method is similar, as has been suggested previously.17 Furthermore, corticosterone levels do not peak when blood is obtained in less than 2 to 3 min and therefore may not reflect full activation of the hypothalamic–pituitary axis.39 Given that the mean time of our blood sampling was within 1 min, the stress to the animals may be within acceptable levels, although the actual peak corticosterone level for each sampling method remains to be determined.
We then used jugular venipuncture of mice to obtain the samples needed for a pharmacokinetic study and oral glucose tolerance testing. Plasma concentrations of levofloxacin showed dose proportionality in Cmax and AUC, at similar mean levels of but smaller standard deviations to those previously obtained from single samples from anesthetized mice (data not shown). Exendin 4 enhances β-cell function, resulting in increased secretion of insulin and subsequently decreased glucose levels.2,16 We confirmed that the overall glucose excursion during oral glucose tolerance testing after jugular venipuncture is significantly reduced in mice treated with exendin 4, which is similar to previous reports demonstrating the effect of exendin 4 on oral glucose tolerance testing performed by bleeding the tail vein of mice.5,24 These results indicate that jugular venipuncture is potentially useful for examining time-dependent changes in blood variables, such as pharmacokinetic studies and glucose tolerance testing.
In conclusion, we found that the blood collection from the jugular vein of mice is a useful method that met the following inclusion criteria: no requirement for anesthetics, accommodation of serial blood collection, acquisition of large-volume samples of high quality, and the preservation of the health and wellbeing of animals. In light of our current observations, we consider that jugular venipuncture will contribute to the refinement of experimental technique.
Acknowledgments
We thank Ayumi Matsuura for technical support and Drs Fujio Sekiguchi and Fumi Ito for helpful discussion and advice.
References
- 1.Abatan OI, Welch KB, Nemzek JA. 2008. Evaluation of saphenous venipuncture and modified tail-clip blood collection in mice. J Am Assoc Lab Anim Sci 47:8–15 [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz A, Anderson GH. 2002. Exendin 4, a GLP1 receptor agonist, modulates the effect of macronutrients on food intake by rats. J Nutr 132:990–995 [DOI] [PubMed] [Google Scholar]
- 3.Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. 2001. A Good Practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23 [DOI] [PubMed] [Google Scholar]
- 4.Durschlag M, Wurbel H, Stauffacher M, Von Holst V. 1996. Repeated blood collection in the laboratory mouse by tail incision–modification of an old technique. Physiol Behav 60:1565–1568 [DOI] [PubMed] [Google Scholar]
- 5.Fan R, Kang Z, He L, Chan J, Xu G. 2011. Exendin 4 improves blood glucose control in both young and aging normal nondiabetic mice: possible contribution of β-cell-independent effects. PLoS ONE 6:e20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez I, Pena A, Del Teso N, Perez V, Rodriguez-Cuesta J. 2010. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci 49:202–206 [PMC free article] [PubMed] [Google Scholar]
- 7.Golde WT, Gollobin P, Rodriguez LL. 2005. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 34:39–43 [DOI] [PubMed] [Google Scholar]
- 8.Heimann M, Kasermann HP, Pfister R, Roth DR, Burki K. 2009. Blood collection from the sublingual vein in mice and hamsters: a suitable alternative to retrobulbar technique that provides large volumes and minimizes tissue damage. Lab Anim 43:255–260 [DOI] [PubMed] [Google Scholar]
- 9.Hem A, Smith AJ, Solberg P. 1998. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret, and mink. Lab Anim 32:364–368 [DOI] [PubMed] [Google Scholar]
- 10.Heo S. 2009. Facial bleeding: techniques and effects. J Am Assoc Lab Anim Sci 48:575 [Google Scholar]
- 11.Hoff J. 2000. Methods of blood collection in the mouse. Lab Anim 29:47–53 [Google Scholar]
- 12.Mazlan NH, Arndt SS, Van't Klooster J, Van Lith HA, Kirchhoff S, Hoekman MFM, Avsaroglu H, Ohl F. 2011. Comparison of 2 blood sampling methods in mice in order to increase animal welfare and the reliability of experimental results. Exp Anim 60:226 [Google Scholar]
- 13.Kassel R, Levitan S. 1953. A jugular technique for the repeated bleeding of small animals. Science 118:563–564 [DOI] [PubMed] [Google Scholar]
- 14.Kushner I. 1982. The phenomenon of the acute phase response. Ann N Y Acad Sci 389:39–48 [DOI] [PubMed] [Google Scholar]
- 15.Lewis VJ, Thacker WL, Mitchell SH, Baer GM. 1976. A new technic for obtaining blood from mice. Lab Anim Sci 26:211–213 [PubMed] [Google Scholar]
- 16.Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. 2003. Glucagon-like peptide 1 receptor signaling modulates β cell apoptosis. J Biol Chem 278:471–478 [DOI] [PubMed] [Google Scholar]
- 17.Liu JY, Diaz TG, Vadgama JV, Henry JP. 1996. Tail sectioning: a rapid and simple method for repeated blood sampling of the rat for corticosterone determination. Lab Anim Sci 46:243–245 [PubMed] [Google Scholar]
- 18.Mahl A, Heining P, Ulrich P, Jakubowski J, Bobadilla M, Zeller W, Bergmann R, Singer T, Meister L. 2000. Comparison of clinical pathology parameters with 2 different blood sampling techniques in rats: retrobulbar plexus versus sublingual vein. Lab Anim 34:351–361 [DOI] [PubMed] [Google Scholar]
- 19.Mazzaccara C, Labruna G, Cito G, Scarfo M, De Felice M, Pastore L, Sacchetti L. 2008. Age-related reference intervals of the main biochemical and hematological parameters in C57BL/6J, 129SV/EV, and C3H/HeJ mouse strains. PLoS ONE 3:e3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson LT, Higginbotham RD. 1965. Mouse C-reactive protein and endotoxin-induced resistance. J Bacteriol 90:1520–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepys MB, Baltz M, Gomer K, Davies AJ, Doenhoff M. 1979. Serum amyloid P component is an acute-phase reactant in the mouse. Nature 278:259–261 [DOI] [PubMed] [Google Scholar]
- 22.Pepys MB, Hirschfield GM. 2003. C-reactive protein: a critical update. J Clin Invest 111:1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riley V. 1960. Adaptation of orbital bleeding technic to rapid serial blood studies. Proc Soc Exp Biol Med 104:751–754 [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Sorenby A, Wernerson A, Efendic S, Kumagai-Braesch M, Tibell A.2006. Exedin 4 treatment improves metabolic control after rat islet transplantation to athymic mice with streptozotocin-induced diabetes. Diabetologia 49: 1247–1253. [DOI] [PubMed]
- 25.Schreiber G, Tsykin A, Aldred AR, Thomas T, Fung WP, Dickson PW, Cole T, Birch H, De Jong FA, Milland J. 1989. The acute-phase response in the rodent. Ann N Y Acad Sci 557:61–85 [DOI] [PubMed] [Google Scholar]
- 26.Siboo R, Kulisek E. 1978. A fluorescent immunoassay for the quantification of C-reactive protein. J Immunol Methods 23:59–67 [DOI] [PubMed] [Google Scholar]
- 27.Taylor R, Hayes KE, Toth LA. 2000. Evaluation of an anesthetic regimen for retroorbital blood collection from mice. Contemp Top Lab Anim Sci 39:14–17 [PubMed] [Google Scholar]
- 28.Tuli JS, Smith JA, Morton DB. 1995. Corticosterone, adrenal and spleen weight in mice after tail bleeding, and its effect on nearby animals. Lab Anim 29:90–95 [DOI] [PubMed] [Google Scholar]
- 29.Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. 2005. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–E828 [DOI] [PubMed] [Google Scholar]
- 30.Van Herck H, Baumans V, Van der Craats NR, Hesp AP, Meijer GW, Van Tintelen G, Walvoort HC, Beynen AC. 1992. Histological changes in the orbital region of rats after orbital puncture. Lab Anim 26:53–58 [DOI] [PubMed] [Google Scholar]
- 31.Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. 1998. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139:443–450 [DOI] [PubMed] [Google Scholar]
- 32.Zeller W, Weber H, Panoussis B, Burge T, Bergmann R. 1998. Refinement of blood sampling from the sublingual vein of rats. Lab Anim 32:369–376 [DOI] [PubMed] [Google Scholar]