Abstract
Research investigators often choose to euthanize mice by cervical dislocation (CD) when other methods would interfere with the aims of a research project. Others choose CD to assure death in mice treated with injected or inhaled euthanasia agents. CD was first approved for mouse euthanasia in 1972 by the AVMA Panel on Euthanasia, although scientific assessment of its humaneness has been sparse. Here we compared 4 methods of spinal dislocation–3 targeting the cervical area (CD) and one the thoracic region–in regard to time to respiratory arrest in anesthetized mice. Of the 81 mice that underwent CD by 1 of the 3 methods tested, 17 (21%) continued to breathe, and euthanasia was scored as unsuccessful. Postmortem radiography revealed cervical spinal lesions in 5 of the 17 cases of unsuccessful CD euthanasia. In addition, 63 of the 64 successfully euthanized mice had radiographically visible lesions in the high cervical or atlantooccipital region. In addition, 50 of 64 (78%) mice euthanized successfully had radiographically visible thoracic or lumbar lesions or both. Intentionally creating a midthoracic dislocation in anesthetized mice failed to induce respiratory arrest and death in any of the 18 mice subjected to that procedure. We conclude that CD of mice holds the potential for unsuccessful euthanasia, that anesthesia could be valuable for CD skills training and assessment, and that postmortem radiography has minimal promise in quality-control assessments.
Abbreviation: ACD, anterograde cervical dislocation; HCD, hemostat-assisted cervical dislocation; MCD, manual cervical dislocation
In 1972, the AVMA updated its recommendations on animal euthanasia. Although striving for a science-based report in which “only those methods for which reliable information could be obtained were included,”9 the Panel nonetheless approved, without citation of supporting data, “disarticulation of the skull and cervical vertebrae” as a method of mouse euthanasia.19 Since then, with no studies of CD in mice to cite, the AVMA documents have linked cervical dislocation (CD) of mice to decapitation of rats, by extrapolating from the more plentiful literature for this technique and this species.2,5,18 CD's change in status from ‘approved’ to ‘conditionally approved’ in the AVMA guidelines rested almost entirely on data from and discussions of rat decapitation.2,5,9,11,15,18,20
The AVMA changed its recommendations on physical methods of rodent euthanasia (including decapitation and CD) from approved to conditionally approved after studies that showed that EEG waves persisted in the brains of rats for as long as 29.6 s after decapitation.15,18 Debate continues regarding the significance of these brain waves and of the visual evoked potentials (VEP) that can occur in decapitated and cervically dislocated mice.4,6,7 Although the extrapolation of data from rats to mice and from decapitation to CD may be informative, mice are not rats, and CD is not decapitation. Clear data on the CD of mice are essential.
In one report, 20% of mice that underwent euthanasia by CD had thoracic rather than cervical fractures, as assessed by radiography; data on the number of mice examined (possibly only 5 in total) or the time from dislocation to apparent death were not included.14 Another group of authors saw no cervical radiographic lesions in 9.6% of mice cervically dislocated, and 25% of the study population had thoracic as well as cervical lesions. In addition, the authors found no evidence that radiographic findings correlated with apparent quality of euthanasia or persistence of brain activity.7
The radiographic findings from the studies just cited7,14 suggest that CD could carry a high failure rate. If so, radiography could be a tool for quality-assurance and skills assessment. Failure could arise from poor skill or from the possibility that the mouse spine, like the proverbial chain, will break at its weakest point(s). Alternatively, we wondered whether pulling with sufficiently forceful tension anywhere along the length of the spinal cord would euthanize mice irrespective of where bony fractures or luxations occurred.
Here we sought to assess how quickly CD leads to respiratory arrest in mice and whether different techniques might differ in accomplishing that goal. We chose respiratory arrest as an easily measured surrogate marker of brain death. Although we recognize that high spinal injury alone might arrest respiration and leave brain function largely intact, this outcome seems unlikely with the sort of trauma the traction of cervical dislocation would place on spinal tissue.23 We sought to assess whether anatomic lesions seen on postmortem examination or radiography might correlate with successful CD and whether either evaluation might serve as a training and assessment tool for personnel.
Materials and Methods
Animals.
All experimental procedures were approved by the University of California San Francisco Institutional Animal Care and Use Committee. We obtained 99 adult mice of mixed strain and sex from several laboratory groups at our university. Several mouse genotypes were included, most of which were on C57Bl/6, Balb/c, or FVB background.
All mice were in apparent good health, had not been used in experimentation, and were scheduled for euthanasia because their genotypes were inappropriate for other research projects.
Mice were housed at an AAALAC-accredited facility in isosexual groups of 2 to 5 until their scheduled use in this project. They were housed in a vivarium in which Helicobacter spp., Pasteurella pneumotropica, and murine norovirus are known to be enzootic. Common murine pathogens that are excluded, as determined by a soiled-bedding sentinel program, are mouse hepatitis virus, murine parvoviruses, Mycoplasma pulmonis, Sendai virus, pneumonia virus of mice, pinworms, and ectoparasites.
We randomly assigned each mouse to 1 of 4 conditions—manual cervical dislocation (MCD; n = 22), hemostat-assisted cervical dislocation (HCD; n = 37), anterograde cervical dislocation (ACD; n = 37), and thoracic dislocation (n = 18)—by drawing colored paperclips from a cup. An additional 6 mice were obtained for carbon dioxide (CO2) euthanasia without dislocation.
Euthanasia.
Two operators performed all euthanasia, flipping a coin to assign an operator to a mouse. Time to respiratory arrest was scored by both operators.
Mice were anesthetized in a chamber containing 2.5% isoflurane in oxygen, weighed while anesthetized, and then transferred to a face mask carrying the same concentration of isoflurane. Mice were placed in sternal recumbency, and a surgical plane of anesthesia was assured by firm bilateral foot pinch. When each mouse was fully anesthetized, the operator instructed the other scorer to turn away, performed 1of the 4 dislocation procedures, started the stopwatch, and alerted the scorer to turn back and assess respiration. Mice were kept with their nose in the anesthetic face-mask while their respiration was being scored and were maintained in the mask for at least 1 min past the last observed respiration.
HCD was performed by placing a large hemostat behind the base of the anesthetized mouse's skull and pulling back sharply on the tail. MCD was similar to HCD, except that thumb and forefingers were placed at the base of the skull instead of the hemostat. In ACD, the operator stabilized the anesthetized mouse’ tail with one hand and then pushed down and forward with some twist on the high cervical region, to manually pop the skull from the cervical vertebrae without breaking the skin. Thoracic dislocation was performed by placing the thumb and forefinger at the caudal end of the rib cage to hold the thorax in place and pulling sharply on the tail.
Time to respiratory arrest was measured in seconds. When mice never breathed after dislocation (time to respiratory arrest, 0 s), euthanasia was scored as successful. Any euthanasia attempt after which the mouse breathed (time to respiratory arrest, greater than 0 s) was scored as unsuccessful. Any mouse still breathing at 180 s either received intraperitoneal pentobarbital (1 to 2 mg; 39-mg/mL dilution [in sterile saline] of Euthasol, Virbac Animal Health, Fort Worth, TX) or was placed, anesthetized, in a prefilled CO2 chamber.
Six mice euthanized by CO2 inhalation were mixed in with those that underwent dislocation, and all 105 animals were examined postmortem by palpation, gross dissection to assess sites of subcutaneous and perispinal hemorrhage, and radiography.
Postmortem and radiographic examinations.
An experienced veterinarian, blinded to operator and euthanasia method, examined each mouse within 2 h of euthanasia. First, the spine was palpated to assess whether palpable fractures of the high or low cervical region, thoracolumbar junction, or other areas could be detected. Next, a dorsal midline skin incision was made with a scalpel to allow examination of the subcutaneous tissue above the spine and skull for hemorrhage.
In pilot work, plain-film radiography, with slight distraction of the spine, and CT without distraction both proved inconclusive and difficult to interpret. All carcasses were frozen and later thawed for digital radiography. Carcasses were clamped at nose and tail and gently stretched and digitally radiographed at 34 to 39 kV (Faxitron, Lincolnshire, IL). A veterinarian blinded to experimental conditions and findings scored the radiographs. Lesions were scored as ‘high cervical,’ which included atlantooccipital lesions, or ‘distal’ (midthoracic to midsacral). No caudal cervical or high thoracic lesions were seen.
Statistical analysis.
A statistician who was blinded to procedures and findings analyzed the data. Logistic regression was used to assess the relationship between the procedure and outcome (unsuccessful compared with successful euthanasia). Multivariable models were used to test variables selected a priori (sex, weight, and operator) as potential cofounders.
Results
Euthanasia success: overall and by individual method.
Table 1 displays characteristics of the mice by procedure. In the absence of any prior literature on the success of CD methods and any information regarding the influence of mouse neck size or strength on CD outcome, we determined whether these parameters were equally distributed across euthanasia methods and between the 2 operators. Among all procedure types and among those targeting the cervical region, there were no statistical differences between groups in the percentage of male mice or percentage of mice euthanized by operator. Because the groups did not have equal variances, a Kruskal–Wallis test was used to test differences in weight, and the results suggested a significant difference of group by weight across all groups (χ2 = 12.5, df = 3, P < 0.01) and across the 3 cervical-targeting methods (χ2 = 9.6, df = 2, P < 0.01). Post hoc comparisons were conducted by using Mann–Whitney tests and Bonferroni adjusted α levels of 0.0083 per test (0.05 ÷ 6, for the 6 comparisons) for all groups and 0.01667 per test (0.05 ÷ 3, for the 3 comparisons) for the cervical-targeting method groups. Among all groups, results revealed that weight was significantly greater for the MCD group than the ACD group (z = 2.9, P = 0.004) and for the ACD group compared with the thoracic dislocation group (z = 3.0, P = 0.003). In addition, among the cervical-targeting methods, weight was significantly (z = 2.6, P = 0.0086) greater for the HCD group than the ACD group.
Table 1.
Characteristics of the study population
| Cervical dislocation |
||||||
| Thoracic dislocation | Manual | Anterograde | Hemostat-assisted | Total | Total All | |
| No. of mice | 18 | 22 | 22 | 37 | 81 | 99 |
| % (no.) of male mice | 5.6 (1) | 27.3 (6) | 31.8 (7) | 21.6 (8) | 25.9 (21) | 22.2 (22) |
| % (no.) assigned to operator 1 | 44.4 (8) | 45.5 (10) | 59.1 (13) | 40.4 (21) | 54.3 (44) | 52.5 (52) |
| Weight (g; mean ± 1 SD)a | 26.6 ± 7.6 | 22.9 ± 4.2 | 20.6 ± 7.8 | 25.6 ± 10.2 | 23.5 ± 8.5 | 24.1 ± 8.4 |
Weight differed (P < 0.05, with Bonferroni correction) between groups: manual CD > ACD, thoracic dislocation > ACD; among the CD groups only: manual CD, hemostat-assisted CD > anterograde CD.
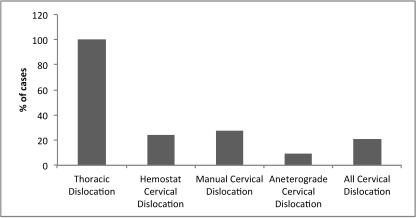
Figure 1 shows the proportion of euthanasias that were unsuccessful by method, including the proportion of unsuccessful procedures among all CD methods combined. None of the 18 euthanasias in the thoracic dislocation group were successful. Of these 18, 16 mice (88.9%) reached the 180-s cut-off; the remaining 2 (11.1%) died at 118 and 132 s after the procedure. Due to the failure of the thoracic dislocation procedure to yield any successful outcomes, the remaining analyses include only the CD procedures.
Figure 1.
Cases of unsuccessful euthanasia (%) by procedure. Group size: thoracic dislocation, n = 18; hemostat-assisted cervical dislocation (CD), n = 37; manual CD, n = 22; anterograde CD, n = 22; and all CD, n = 81.
Euthanasia success: cervical-region targeting methods.
Among the 81 euthanasias targeting the cervical region, 17 (21.0%) were unsuccessful. Eleven of these 17 mice (65%) survived beyond the 180-s cut-off, and the remaining 6 (35%) stopped breathing between 27 and 150 s.
Because HCD was the largest group, it was used as the referent in the model that regressed unsuccessful outcome on the 3 procedures.21 In an unadjusted logistic regression model with HCD as the referent procedure, neither MCD nor ACD significantly altered the odds of an outcome failure (odds ratio for MCD compared with HCD = 1.2, 95% confidence interval = 0.4 to 3.9; odds ratio for P compared with Y = 0.3, 95% confidence interval = 0.1 to 1.6).
The likelihood ratio test was used to determine whether sex, weight, and operator confounded the main effect and therefore were required to be included in an adjusted model.21 This process yielded a model that predicted unsuccessful euthanasia based on procedure and operator (χ2 = 0.23, df = 3; P > 0.05) . As in the unadjusted model, the final adjusted model that controlled for operator indicated that the relative odds of method failure were not significantly different in either MCD or ACD compared with HCD (odds ratio for MCD compared with HCD = 1.5, 95% confidence interval = 0.4 to 5.4; odds ratio for ACD compared with HCD = 0.3, 95% confidence interval = 0.1 to 1.5). In addition, in the final model, procedures done by one operator were more likely to result in unsuccessful euthanasia than those performed by the other (odds ration = 6.2; 95% confidence interval = 1.6 to 24.8).
Postmortem evaluations.
Although some mice had easily palpable and visibly complete dislocations of the skull from the cervical spine, fractures or dislocations could not be confirmed by gross exam in most mice. Complete dissection or collection of tissue for histopathology would have precluded postmortem radiography and was not performed.
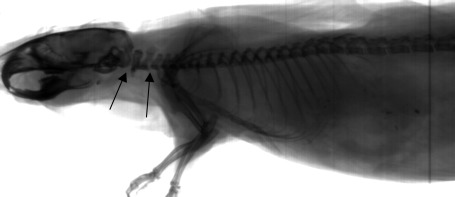
Radiographic lesions were scored as present or absent at the various anatomic locations, but as with gross examination, assessment was often difficult. In the thoracic dislocation group, all 18 euthanasia attempts were unsuccessful; all 18 mice had radiographic lesions in the thoracic, lumbar, or sacral (or multiple) regions; and none had evident cervical lesions. The 6 mice euthanized by CO2 had no radiographic lesions (Figure 2), thereby alleviating any concern that postmortem processing led to artifacts.
Figure 2.
Radiographs of a mouse that underwent euthanasia by CO2 asphyxiation but not cervical dislocation. Arrows show gaps at the atlantooccipital joint and between the C2 and C3 vertebral bodies, which that are most evident when the carcass is distracted for radiography.
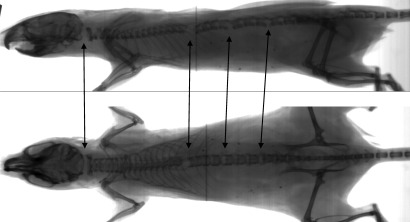
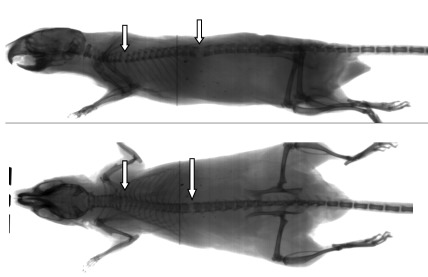
All 20 successful euthanasias in the ACD group had evident cervical fractures; 14 mice showed cervical fracture exclusively, whereas the remaining 6 also had distal lesions. ACD was the only method in which some of the successfully euthanized mice showed no thoracic, lumbar, or sacral fractures. Among the 59 HCD and MCD mice, 45 (76%) underwent successful euthanasia, all of which had lesions in the thoracic, lumbar, or sacral region (or combinations thereof), and 44 had one or more cervical lesions as well (Figure 3). A single animal in the MCD group had thoracic and lumbar lesions but no evident cervical lesion (Figure 4).
Figure 3.
Radiographs of a case of successful HCD euthanasia, with lesions in the cervical, thoracic, and lumbar regions (arrows).
Figure 4.
Radiographs of the one case of successful euthanasia in the MCD group that had no clear radiographic cervical lesion. Arrows show thoracic and lumbar lesions.
Although any breath after dislocation counted as unsuccessful euthanasia, 8 animals (8%) breathed for 27 to 143 s before reaching respiratory arrest, comprising 2 TD (11%) and 6 CD mice (7.4% overall, comprising 1 ACD [4.5% of this subpopulation], 4 HCD [10.8%] and 1 MCD [4.5%]). Radiography revealed cervical as well as more distal lesions in 5 of the 6 CD mice. The sixth animal (in the HCD group) reached respiratory arrest at 156 s; the rater did not see radiographic cervical lesions in this mouse.
Discussion
In the current study, we sought to assess whether CD of mice might carry a failure rate that translated to an animal welfare concern. We used time to respiratory arrest as our surrogate marker for the AVMA criterion of “time required to induce loss of consciousness.”1 We recognized that clean severance of the high cervical spinal column above C2 might induce respiratory arrest without unconsciousness, but the gross trauma of the various CD methods seemed unlikely to result in this outcome.
We set time to respiratory arrest equal to 0 s as our cutoff for defining successful euthanasia. We believe that any euthanasia method after which mice might continue breathing (regardless for how long) calls for caution. If performed on unanesthetized mice, incomplete euthanasia leaves a potentially conscious mouse experiencing a severe traumatic injury until an operator can repeat the procedure or switch to another euthanasia method. Complete midthoracic CNS severance may leave the caudal body analgesic, but injured tissue surrounding and cranial to the lesion could still be painful.17,25 Animals that have sufficient motor innervation to continue respiration likely would have sufficient afferent innervation for pain to occur.
In our pilot work, some mice continued breathing for as long as 15 min before we euthanized them with sodium pentobarbital; these mice likely could have continued breathing even longer. Had they been removed from anesthesia and regained consciousness, with multiple spinal injuries, their suffering could have been severe. Even as an adjunct to inhalant or injectable euthanasia agents, were a mouse to recover (possibly inside a carcass freezer) from a poorly performed dislocation technique, the potential for pain or distress would be compounded. It is essential that any method of CD meet the requirement that essentially 100% of animals permanently cease breathing immediately.
Our 21% unsuccessful percent for CD methods, based on time to respiratory arrest, was similar to the unsuccessful percent in reports of mice that underwent CD but lacked radiographically evident cervical spinal lesions.7,14 The authors of those previous studies7,14 do not detail their radiographic methods. Because no events of unsuccessful euthanasia were reported,7,14 the authors may have misread mice that had cervical and thoracic lesions but insufficient distraction during radiography as having thoracic lesions only.
In light of previous findings,7,14 we postulated that sufficiently forceful tension applied anywhere along the spinal cord could induce unconsciousness and respiratory arrest, even when the skeletal trauma was caudal to the recommended high-cervical region. Our 100% unsuccessful rate for the thoracic dislocation group does not support this hypothesis. We used thoracic dislocation in anesthetized mice to model the incorrect technique that a poorly trained operator might perform, and we have verified that correct technique is necessary for successful euthanasia of mice.
AVMA guidelines have treated CD generally, without specific description of the procedure, reflecting a dearth of comparative assessments of different CD techniques. Various publications describe assorted techniques for performing cervical dislocation.8,13,14,16,22 Others, including the AVMA Panel reports, do not distinguish among various CD techniques.3,10,12,24 In addition to the 3 methods we chose, authors have specified that MCD should be performed by pulling up at a 30° angle, in the hope that the odontoid process of the C2 vertebra will damage the spinal cord and thereby increase the efficacy of the technique.7 Fracture of this small bony process in a mouse is unlikely to be visible radiographically. Furthermore, some operators describe pulling until the skull is felt to be completely unattached to the spine, and this is the method we teach at our institution. This practice is reasonable when CD is used after other euthanasia techniques (for example, anesthetic overdose) but this longer, slower procedure may cause additional pain in a not-yet-unconscious animal.
Postmortem radiography could play a role in assessing the success of euthanasia after the event, but this technique is labor-intensive and must be interpreted cautiously. We were concerned that, without sufficient distraction, intact ligaments could hold the skull close enough to the spine to mask successful luxation in some cases. We found the postmortem radiographs easiest to interpret when tension was applied to stretch the carcass, but doing so then created a suspicious gap between the C2 and C3 vertebral bodies that required careful assessment. Negative CO2-only controls (Figure 2) were helpful in distinguishing lesions from normal. When we set time to respiratory arrest as our ‘gold standard,’ cervical radiography carried a positive predictive value of only 71%. Therefore, when radiography is used as a quality-control or skills assessment tool, some instances of unsuccessful euthanasia will be missed.
Whereas all unsuccessful euthanasias had thoracic or lumbar lesions (or both), so did 50 of the 64 successful euthanasias. We conclude that the presence of thoracic or lumbar lesions is not diagnostic of poor technique. In addition, none of the 11 CD animals that went to the 180-s endpoint had observable cervical lesions. In contrast, 63 of 64 successful CD cases did so, and the mouse that lacked an observable cervical lesion (Figure 3) might reflect misdiagnosis of a subtle lesion or serve as evidence that sufficiently severe distal spinal cord trauma can cause respiratory arrest; indeed, 2 mice in the thoracic dislocation group did ultimately achieve respiratory arrest before another technique could be applied. In addition, 6 CD mice underwent unsuccessful euthanasia but died before the 180-s endpoint, even though radiography revealed cervical lesions in 5 of these mice. Radiographic misdiagnosis is possible, as is the possibility that intermediate injuries, although radiographically evident, can ultimately be fatal but are too prolonged to qualify the outcome as a ‘good death.’
We recognize some limitations to our study. We did not perform all described or possible variations of CD in mice. We made no attempt to use brain waves or other measurements to analyze intact brain function after dislocation. We did not remove breathing animals from the anesthesia mask to assess potential for return to consciousness. The presence of a time to respiratory arrest that exceeds 0 s suggests at least minimal intact brain function, a state that seems inconsistent with a ‘good death;’ we present no data on the quality of death when time to respiratory arrest is 0 s.
The operator effect that we noted requires further exploration. With only 2 operators, the effect could not be ascribed to differences in operator age, training, strength, or any other factor. Quite possibly, other operators would differ in their success rates. Follow-up study to more fully examine operator effect needs to account for various operators’ knowledge that they were being scored and the effect this knowledge might have on their performance. That the more successful operator still was unsuccessful in 8.1% of cases raises concern.
In addition, radiographic diagnosis could be subject to an interpreter effect. Without a ‘gold standard’ for correct radiographic diagnosis (for example, absence of a visible lesion in a successful euthanasia could mean either that spinal fracture or luxation at that site is not necessary for quick death, or that the radiographer missed the lesion), we could not explore the possibility of an interpreter effect. Pilot work with plain-film radiographs revealed modest disagreement in diagnosis among several veterinarians who read the radiographs.
Finally, all mice were dislocated under isoflurane anesthesia for humane reasons, because we knew we would be keeping unsuccessful cases alive and breathing for several minutes rather than repeating the procedure immediately. Because the anesthetic abolished muscle tone, this method may model postmortem CD better than it models CD as the sole method of euthanasia. Because it is a respiratory depressant, isoflurane may have hastened respiratory arrest in some intermediate cases. As a neuroprotective agent, isoflurane may have delayed death. Either way, the use of isoflurane prevented us from assessing potential consciousness in the still-breathing unsuccessful cases, because we never weaned them off of the anesthetic to make that assessment.
We conclude that 3 methods of CD can result in unsuccessful euthanasia in which mice continue breathing after the attempted dislocation. We cannot state whether consciousness or pain perception accompany this outcome. Practice on anesthetized mice could be a useful training method, with time to respiratory arrest set as the measure of trainee success. Radiography is a labor-intensive skills-assessment tool that appears to be no more informative than observation of the practice on anesthetized mice. If additional studies replicate our findings, recommended practices will need to change, whether to disallow CD of unanesthetized mice, identify a CD method with a higher success rate, or emphasize that mice must be watched closely after CD, and if still breathing (which can be difficult to ascertain given the motor discharge that often follows CD), immediately treated through the same or an alternate method of euthanasia.
Acknowledgments
We thank Patricia Ramsey, Michael Sheets, Stephanie Taylor, Rabbe Lindstrom, and Jeannie Bailey for their technical assistance. With appreciation to the mice who were euthanized in this project.
References
- 1.American Veterinary Medical Association. [Internet] 2007. AVMA guidelines on euthanasia, 2007 update. [Cited 15 August 2011]. Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf
- 2.Andrews EJ, Bennett BT, Clark JD, Houpt KA, Pascoe PJ, Robinson GW, Boyce JR. 1993. 1993 report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc 202:229–249 [PubMed] [Google Scholar]
- 3.Artwohl J, Brown P, Corning B, Stein S. 2006. Report of the ACLAM Task Force on Rodent Euthanasia. J Am Assoc Lab Anim Sci 45:98–105 [PubMed] [Google Scholar]
- 4.Bates G. 2010. Humane issues surrounding decapitation reconsidered. J Am Vet Med Assoc 237:1024–1026 [DOI] [PubMed] [Google Scholar]
- 5.Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, Bennett BT, Pascoe P, Shull E, Cork LC, Francis-Floyd R, Amass KD, Johnson R, Schmidt RH, Underwood W, Thornton GW, Kohn B. 2001. 2000 Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc 218:669–696 [DOI] [PubMed] [Google Scholar]
- 6.Carbone L. 2004. Death by decapitation: a case study, p 186–205. : What animals want: expertise and advocacy in laboratory animal welfare policy. New York (NY): Oxford University Press [Google Scholar]
- 7.Cartner SC, Barlow SC, Ness TJ. 2007. Loss of cortical function in mice after decapitation, cervical dislocation, potassium chloride injection, and CO2 inhalation. Comp Med 57:570–573 [PubMed] [Google Scholar]
- 8.Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C. 1997. Recommendations for euthanasia of experimental animals: part 2. Lab Anim 31:1–32 [DOI] [PubMed] [Google Scholar]
- 9.Derr RF. 1991. Pain perception in decapitated rat brain. Life Sci 49:1399–1402 [DOI] [PubMed] [Google Scholar]
- 10.Gografe SI, Fridland G. 2006. Laboratory mouse handbook. Memphis (TN): American Association for Laboratory Animal Science [Google Scholar]
- 11.Holson RR. 1992. Euthanasia by decapitation: evidence that this technique produces prompt, painless unconsciousness in laboratory rodents. Neurotoxicol Teratol 14:253–257 [DOI] [PubMed] [Google Scholar]
- 12.Howard HL, McLaughlin-Taylor E, Hill RL. 1990. The effect of mouse euthanasia technique on subsequent lymphocyte-proliferation and cell-mediated lympholysis assays. Lab Anim Sci 40:510–514 [PubMed] [Google Scholar]
- 13.Iwarsson K, Rehbinder C. 1993. A study of different euthanasia techniques in guinea pigs, rats, and mice. Animal response and postmortem findings. Scand J Lab Anim Sci 20:191–205 [Google Scholar]
- 14.Keller GL. 1982. Physical euthanasia methods. Lab Anim (NY) 11:20–26 [Google Scholar]
- 15.Mikeska JA, Klemm WR. 1975. EEG evaluation of humaneness of asphyxia and decapitation euthanasia of the laboratory rat. Lab Anim Sci 25:175–179 [PubMed] [Google Scholar]
- 16.Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2002. Manipulating the mouse embryo: a laboratory manual, 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press [Google Scholar]
- 17.Nakae A, Nakai K, Yano K, Hosokawa K, Shibata M, Mashimo T. 2011. The animal model of spinal cord injury as an experimental pain model. J Biomed Biotechnol 2011:939023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AW, Houpt KH, Kitchell RL, Kohn DF, McDonald LE, Passaglia M, Thurmon JC, Ames ER. 1986. 1986 Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc 188:252–268 [PubMed] [Google Scholar]
- 19.Smith CR, Booth NH, Fox MW, Jortner BS, Lumb WV, Moreland AF, Wass WM. 1972. Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc 160:761–772 [PubMed] [Google Scholar]
- 20.Vanderwolf CH, Buzsaki G, Cain DP, Cooley RK, Robertson B. 1988. Neocortical and hippocampal electrical activity following decapitation in the rat. Brain Res 451:340–344 [DOI] [PubMed] [Google Scholar]
- 21.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. 2005. Regression methods in biostatistics: linear. logistic, survival, and repeated-measures models (statistics for biology and health). New York (NY): Springer [Google Scholar]
- 22.Waynforth HB, Flecknell PA. 1992. Experimental and surgical technique in the rat. San Diego (CA): Academic Press [Google Scholar]
- 23.Winslow C, Rozovsky J. 2003. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 82:803–814 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Hasegawa H, Ikeda K, Ichiyama A. 1988. Cervical dislocation of mice induces rapid accumulation of platelet serotonin in the lung. Agents Actions 25:48–56 [DOI] [PubMed] [Google Scholar]
- 25.Yezierski RP. 1996. Pain following spinal cord injury: the clinical problem and experimental studies. Pain 68:185–194 [DOI] [PubMed] [Google Scholar]